Abstract
Aims
Researchers in clinical and pharmacoepidemiology fields have adopted information technology (IT) and electronic data capture, but these remain underused despite the benefits. This review discusses electronic case report forms and electronic data capture, specifically within pharmacoepidemiology and clinical research.
Methods
The review used PubMed and the Institute of Electrical and Electronic Engineers library. Search terms used were agreed by the authors and documented. PubMed is medical and health based, whereas Institute of Electrical and Electronic Engineers is technology based. The review focuses on electronic case report forms and electronic data capture, but briefly considers other relevant topics; consent, ethics and security.
Results
There were 1126 papers found using the search terms. Manual filtering and reviewing of abstracts further condensed this number to 136 relevant manuscripts. The papers were further categorized: 17 contained study data; 40 observational data; 27 anecdotal data; 47 covering methodology or design of systems; one case study; one literature review; two feasibility studies; and one cost analysis.
Conclusion
Electronic case report forms, electronic data capture and IT in general are viewed with enthusiasm and are seen as a cost‐effective means of improving research efficiency, educating participants and improving trial recruitment, provided concerns about how data will be protected from misuse can be addressed. Clear operational guidelines and best practises are key for healthcare providers, and researchers adopting IT, and further work is needed on improving integration of new technologies with current systems. A robust method of evaluation for technical innovation is required.
Keywords: electronic case report form, electronic data capture
What is Already Known about this Subject
Information technology (IT) has tangible benefits to assisting high quality research.
Investment in IT is underfunded in healthcare and research.
What this Study Adds
A governance support framework is necessary to assist healthcare providers and researchers to maximize the benefits of IT.
Further work is required in improving interoperability between IT systems for research and pharmacoepidemiology.
An unambiguous legislative framework is needed to ensure high quality research can continue successfully whilst continuing to adhere to good clinical practice, data protection and ethics.
Generic and adaptable solutions are required to meet the software needs of researchers and healthcare providers.
Introduction
Information technology (IT) provides a fast and efficient way to collect scientific and clinical data and has become the most effective way to collaboratively share data. The benefits have underpinned the incremental introduction of electronic patient records in healthcare organizations which has been suggested as the principal reason for the increasing allocation of healthcare industry funding to IT; from 2% of total revenue, in the 1990s, to 5–7% in recent years 1. This in turn has contributed to investment in the use of IT and electronic case report forms (eCRFs) in clinical research. Whilst these systems are designed and used differently, they share a common goal of storing, and communicating in a safe and confidential way private clinical data in a structured format 2. Pharmacoepidemiology and clinical research have undoubtedly benefitted from IT; however, developments in these areas have continued to lag behind the healthcare sector, with investment limited due to various concerns. Reasons cited for not further using IT in research include: technical issues in setting up infrastructure, financing and maintaining the newest technology, and ethical fears 3. Additionally, different funding streams and personnel involved in development of electronic patient records used for healthcare purposes, and those used for data capture for research, make it difficult to integrate solutions that would satisfy both aims. The objectives of both types of system are often different, which can also lead to conflicts.
Different regulatory processes govern systems used in routine healthcare and research. However, clinical research relying on IT and electronic data capture (EDC) often depends on interfacing with healthcare IT systems, which generally comprise numerous dissimilar software systems and storage formats for storing patient data. Clinical research also often operates over large geographical areas, incorporating several different healthcare providers, further compounding challenges when interfacing with diverse local systems. Although there is a drive towards IT unification in the National Health Service primary care practises and hospital trusts in the UK are under no obligation to use collaborative IT systems or storage formats, nor are they required to make these data available for research purposes. While the need to exploit healthcare data for research to cost effectively drive healthcare improvements has never been greater, it is largely for these reasons that the task of collecting, storing and amalgamating health service data is likely to become increasingly difficult in the future.
Objective
The objective of this review is to assess the advantages and disadvantages of eCRF and EDC technologies in pharmacoepidemiology and clinical research, and to explore where further research should be best directed. For the purpose of this paper the term eCRF will refer to a system used to capture clinical data for research and EDC will refer to the generic process of data capture.
Methods
A literature review was conducted to identify articles pertaining to pharmacoepidemiology (drug epidemiology) and clinical research, and their use of eCRFs and EDC. Whilst the use of IT in routine healthcare is increasingly commonplace, the emphasis of this review was on the use of EDC and eCRFs in the conduct of clinical research. Common themes relating to these topics emerged covering a broad range of issues including technical and practical matters, consent, ethics, and security. PubMed and the Institute of Electrical and Electronic Engineers (IEEE) libraries were searched using to cast a wide net over the subject area; electronic case report form, eCRF, electronic data capture, and electronic data collection. Filters were applied to search terms to condense results to relevant articles (see Appendix). The search was conducted between 2014 and 2015 with a final analysis of the literature completed in August 2016. PubMed is a clinical library while IEEE is technology based.
All returned abstracts were read and articles deemed irrelevant to eCRFs and EDC, or articles that did not involve pharmacoepidemiology or clinical research, were excluded. Unlike clinical studies, IT has no universally accepted quality scoring system for academic papers. Therefore, it was decided that any published and peer reviewed article that was returned from the IEEE or PubMed search would be included. Exceptions to this were where there was an overt conflict of interest or the journal was not available in English. Figure 1 depicts a flow diagram of the review process. The authors endeavoured to adhere fully to the PRISMA checklist 4 in structuring this review; however, the nonstandard output of technical papers made this impractical. The included papers were sorted by relevance, and categorized according to whether they contained opinion or data. Papers reporting data included anecdotal data, observational data from selected data sources, observational data in population‐based studies, prospective observational data and experimental data such as clinical trials. Papers were analysed to identify reported positive and negative aspects of the IT tools being discussed.
Figure 1.
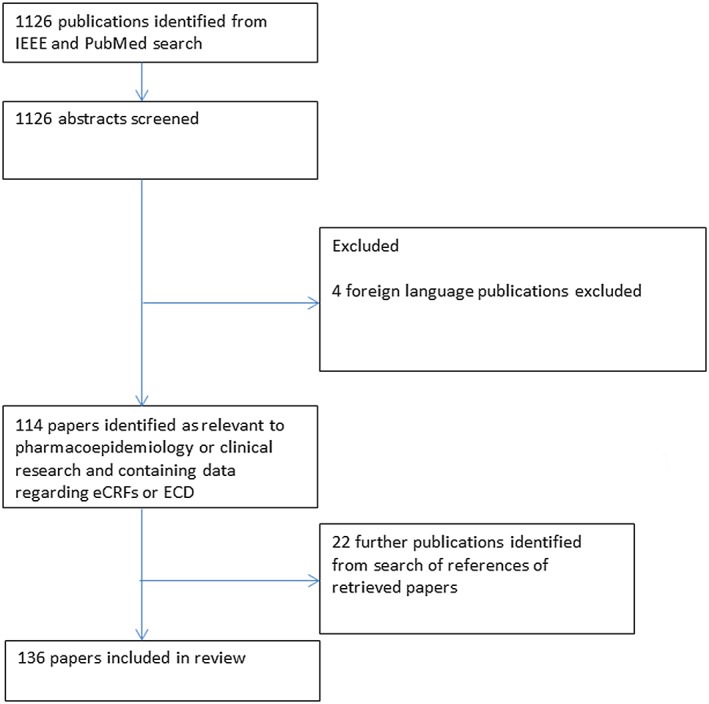
Flow diagram of review
Results
A total of 1126 papers were returned from all search topics. After review and consideration, 136 manuscripts were deemed relevant to the review. Each topic was further separated into manuscript types. There were 17 papers documenting a study or clinical trial that used EDC where the system was the primary focus of the manuscript; 40 papers discussed observational studies comparing or evaluating EDC; 27 papers contained anecdotal evidence or opinion regarding EDC; 47 papers detailed EDC models or designs. There was one literature review, one cost benefit analysis, two feasibility studies, and one case study comparing the use of EDC in five studies (Table 1). During this review, papers were further discarded that were found to be of poor overall quality or adding little to the topic. For a list of all included publications see Table 2.
Table 1.
Characteristics of journal papers
| Report characteristics | n | % |
|---|---|---|
| Report included one or more benefit/disadvantage of EDC | 136 | |
| Main objective(s) of report: | ||
| Studies using EDC | 17 | 12.5 |
| Observational studies evaluating EDC use | 40 | 29.4 |
| Opinion/discussion piece | 27 | 19.9 |
| Description of model EDC system | 47 | 34.6 |
| Feasibility studies | 2 | 1.5 |
| Literature review | 1 | 0.7 |
| Cost–benefit analysis | 1 | 0.7 |
| Case study | 1 | 0.7 |
Table 2.
Publication review list
| Authors | Title | Journal | Year | Paper Type |
|---|---|---|---|---|
| Aiello EJ, Taplin S, Reid R, Hobbs M, Seger D, Kamel H, et al. | In a randomized controlled trial, patients preferred electronic data collection of breast cancer risk‐factor information in a mammography setting 58 | J Clin Epidemiol | 2006 | Observational |
| Alexander I. | The impact of future trends in electronic data collection on musculoskeletal research and evidence‐based orthopaedic care 71. | Arthroscopy | 2003 | Anecdotal |
| Ariza AJ, Binns HJ, Christoffel KK, Paediatric Practice Research Group. | Evaluating computer capabilities in a primary care practice‐based research network. | Ann Fam Med | 2004 | Observational |
| Ashar R, Lewis S, Blazes DL, Chretien JP. | Applying information and communications technologies to collect health data from remote settings: A systematic assessment of current technologies | J Biomed Inform | 2010 | Anecdotal |
| Ashley L, Jones H, Thomas J, Newsham A, Downing A, Morris E, et al. | Integrating patient reported outcomes with clinical cancer registry data: a feasibility study of the electronic Patient‐Reported Outcomes From Cancer Survivors (ePOCS) system. | J Med Internet Res | 2013 | Observational |
| Atreja A, Achkar JP, Jain AK, Harris CM, Lashner BA. | Using technology to promote gastrointestinal outcomes research: a case for electronic health records. | J Gastroenterol | 2008 | Anecdotal |
| Ayatollahi H, Mirani N, Haghani H. | Electronic health records: what are the most important barriers? 3 | Perspect Health Inf Manag | 2014 | Observational |
| Azad T, Kalani M, Wolf T, Kearney A, Lee Y, Flannery L, et al. | Building an electronic health record integrated quality of life outcomes registry for spine surgery. | J Neurosur Spine | 2016 | Observational |
| Bellamy N, Wilson C, Hendrikz J, Patel B, Dennison S. | Electronic data capture (EDC) using cellular technology: implications for clinical trials and practice, and preliminary experience with the m‐Womac® Index in hip and knee OA patients. | Inflammopharmacology | 2009 | Model |
| Bellary S, Krishnankutty B, Latha MS. | Basics of case report form designing in clinical research 9. | Perspect Clin Res | 2014 | Model |
| Bock M, Moore D, Hwang J, Shumay D, Lawson L, Hamolsky D, et al. | The impact of an electronic health questionnaire on symptom management and behaviour reporting for breast cancer survivors. | Breast Cancer Res Treat | 2012 | Observational |
| Borlawsky TB, Lele O, Jensen D, Hood NE, Wewers ME. | Enabling distributed electronic research data collection for a rural Appalachian tobacco cessation study. | J Am Med Inform Assoc | 2011 | Study |
| Brandt CA, Cohen DB, Shifman MA, Miller PL, Nadkarni PM, Frawley SJ. | Approaches and informatics tools to assist in the integration of similar clinical research questionnaires. | Methods Inf Med | 2014 | Model |
| Brewster W, Gibbs T, Lacroix K, Murray A, Tydeman M, Almenoff J. | Evolving paradigms in pharmacovigilance. | Curr Drug Saf | 2006 | Anecdotal |
| Bruland P, Forster C, Breil B, Ständer S, Dugas M, Fritz F. | Does single‐source create an added value? Evaluating the impact of introducing x4T into the clinical routine on workflow modifications, data quality and cost‐benefit. | Int J Med Inform | 2014 | Model |
| Burnstead B, Furlan G. | Unifying drug safety and clinical databases 72. | Curr Drug Saf | 2013 | Anecdotal |
| Bushnell DM, Martin ML, Parasuraman B. | Electronic versus paper questionnaires: a further comparison in persons with asthma 23. | J Asthma | 2003 | Observational |
| Bushnell DM, Reilly MC, Galani C, Martin ML, Ricci JF, Patrick DL, et al. | Validation of electronic data capture of the Irritable Bowel Syndrome – Quality of Life Measure, the Work Productivity and Activity Impairment Questionnaire for Irritable Bowel Syndrome and the EuroQol. | Value Health | 2003 | Observational |
| Carvalho JC, Bottenberg P, Declerck D, van Nieuwenhuysen JP, Vanobbergen J, Nyssen M. | Validity of an information and communication technology system for data capture in epidemiological studies 66. | Caries Res | 2011 | Observational |
| Cleland J, Caldow J, Ryan D. | A qualitative study of the attitudes of patients and staff to the use of mobile phone technology for recording and gathering asthma data. | J Telemed Telecare | 2007 | Study |
| Collins M, Ross E, Meropol NJ, Lazev AB. | Using metadata to generate web‐based Electronic Data Capture Forms. | AMIA Annu Symp Proc | 2006 | Model |
| Coons SJ, Gwaltney CJ, Hays RD, Lundy JJ, Sloan JA, Revicki DA, Lenderking WR, Cella D BEI ePRO TF. | Recommendations on evidence needed to support measurement equivalence between electronic and paper‐based patient‐reported outcome (PRO) measures: ISPOR ePRO Good Research Practises Task Force report. | Value Health | 2009 | Model |
| Courtney KL, Craven CK. | Factors to weigh when considering electronic data collection. | Can J Nurs Res | 2005 | Anecdotal |
| Crichton C, Davies J, Gibbons J, Harris S, Tsui A, Brenton J, et al. | Metadata‐driven software for clinical trials 10. | SEHC | 2009 | Model |
| Curcin V, Soljak M, Majeed A. | Managing and exploiting routinely collected NHS data for research 6. | Inform Prim Care | 2012 | Anecdotal |
| Curcin V, Woodcock T, Poots AJ, Majeed A, Bell D. | Model‐driven approach to data collection and reporting for quality improvement 17. | J Biomed Inform | 2014 | Model |
| Dale EL, Mueller MA, Wang L, Fogerty MD, Guy JS, Nthumba PM. | Epidemiology of operative burns at Kijabe Hospital from 2006 to 2010: pilot study of a web‐based tool for creation of the Kenya Burn Repository. | Burns | 2013 | Model |
| Dillon DG, Pirie F, Rice S, Pomilla C, Sandhu MS, Motala AA, et al. | Open‐source electronic data capture system offered increased accuracy and cost‐effectiveness compared with paper methods in Africa 52. | Clin Epidemiol | 2014 | Observational |
| Dugas M, Dugas‐Breit S, Getz K, Hearn J, Sullivan R, Stewart D, et al. | Integrated data management for clinical studies: automatic transformation of data models with semantic annotations for principal investigators, data managers and statisticians. | PLoS One | 2014 | Model |
| Dunsmuir DT, Payne BA, Cloete G, Petersen CL, Görges M, Lim J, et al. | Development of mHealth applications for pre‐eclampsia triage. | IEEE J Biomed Heal informatics | 2014 | Model |
| Dupont A, Wheeler J, Herndon JE, Coan A, Zafar SY, Hood L, et al. | Use of tablet personal computers for sensitive patient‐reported information 64. | J Support Oncol | 2009 | Observational |
| Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A, Hudak PL, et al. | The use of a tablet computer to complete the DASH questionnaire 24. | J Hand Surg Am | 2012 | Observational |
| Eisenstein EL, Collins R, Cracknell BS, Podesta O, Reid ED, Sandercock P, et al. | Sensible approaches for reducing clinical trial costs. | Clin Trials | 2008 | Study |
| El Emam K, Jonker E, Sampson M, Krleza‐Jerić K, Neisa A. | The use of electronic data capture tools in clinical trials: web‐survey of 259 Canadian trials. | J Med Internet Res | 2009 | Observational |
| El Fadly A, Lucas N, Rance B, Verplancke P, Lastic P‐Y, Daniel C. | The REUSE project: EHR as single datasource for biomedical research. | Stud Health Technol Inform | 2010 | Model |
| El Fadly A, Rance B, Lucas N, Mead C, Chatellier G, Lastic P‐Y, et al. | Integrating clinical research with the Healthcare Enterprise: from the RE‐USE project to the EHR4CR platform 57. | J Biomed Inform | 2011 | Model |
| Ene‐Iordache B, Carminati S, Antiga L, Rubis N, Ruggenenti P, Remuzzi G, et al. | Developing regulatory‐compliant electronic case report forms for clinical trials: experience with the demand trial 53. | J Am Med Inform Assoc | 2009 | Model |
| Farnell DJJ, Routledge J, Hannon R, Logue JP, Cowan RA, Wylie JP, et al. | Efficacy of data capture for patient‐reported toxicity following radiotherapy for prostate or cervical cancer. | Eur J Cancer | 2010 | Observational |
| Faulds MC, Bauchmuller K, Miller D, Rosser JH, Shuker K, Wrench I, et al. | The feasibility of using “bring your own device” (BYOD) technology for electronic data capture in multicentre medical audit and research. | Anaesthesia | 2016 | Feasibility Study |
| Fontaine P, Mendenhall TJ, Peterson K, Speedie SM. | The “Measuring Outcomes of Clinical Connectivity” (MOCC) trial: investigating data entry errors in the Electronic Primary Care Research Network (ePCRN). | J Am Board Fam Med | 2007 | Observational |
| Fraccaro P, Dentone C, Fenoglio D, Giacomini M. | Multicentre clinical trials' data management: a hybrid solution to exploit the strengths of electronic data capture and electronic health records systems. | Informatics Heal Soc Care | 2013 | Model |
| Franklin JD, Guidry A, Brinkley JF. | A partnership approach for Electronic Data Capture in small‐scale clinical trials 18. | J Biomed Inform | 2011 | Anecdotal |
| Fritz F, Tilahun B, Dugas M. | Success criteria for electronic medical record implementations in low‐resource settings: a systematic review 68. | J Am Med Inform Assoc | 2015 | Review |
| Fu L, Ding S, Chen T. | Clinical Data Management System. | 2010 International Conference on Biomedical Engineering and Computer Science | 2010 | Model |
| Gallagher SA, Smith AB, Matthews JE, Potter CW, Woods ME, Raynor M, et al. | Roadmap for the development of the University of North Carolina at Chapel Hill Genitourinary Oncology Database – UNC GOLD. | Urol Oncol | 2014 | Anecdotal |
| Galliher JM, Stewart T V, Pathak PK, Werner JJ, Dickinson LM, Hickner JM. | Data collection outcomes comparing paper forms with PDA forms in an office‐based patient survey. | Ann Fam Med | 2008 | Observational |
| Gibbons C, Caudwell P, Finlayson G, King N, Blundell J. | Validation of a new hand‐held electronic data capture method for continuous monitoring of subjective appetite sensations. | Int J Behav Nutr Phys Act | 2011 | Study |
| Gioli‐Pereira L, Bernardez‐Pereira S, Goulart Marcondes‐Braga F, Rocha Spina JM, Muniz Miranda da Silva R, Evangelista Ferreira N, et al. | Genetic and Electronic medical records to predict outcomes in Heart Failure patients (GENIUS‐HF) – design and rationale. | BMC Cardiovasc Disord | 2014 | Study |
| Goodman K, Krueger J, Crowley J. | The automatic clinical trial: leveraging the electronic medical record in multisite cancer clinical trials 5. | Curr Oncol Rep | 2012 | Model |
| Gupta SK. | Paperless clinical trials: myth or reality? 2 | Indian J Pharmacol | 2015 | Anecdotal |
| Haak D, Samsel C, Gehlen J, Jonas S, Deserno TM. | Simplifying electronic data capture in clinical trials: workflow embedded image and biosignal file integration and analysis via web services 11. | J Digit Imaging | 2014 | Model |
| Haller G, Haller DM, Courvoisier DS, Lovis C. | Handheld vs. laptop computers for electronic data collection in clinical research: a crossover randomized trial 67. | J Am Med Inform Assoc | 2009 | Observational |
| Hammond WE, Bailey C, Boucher P, Spohr M, Whitaker P. | Connecting information to improve health. | Health Aff | 2010 | Anecdotal |
| HARDING JP, HAMM LR, EHSANULLAH RSB, HEATH AT, SORRELLS SC, HAW J, et al. | Use of a novel electronic data collection system in multicentre studies of irritable bowel syndrome. | Aliment Pharmacol Ther | 1997 | Study |
| Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. | Research electronic data capture (REDCap) – a metadata‐driven methodology and workflow process for providing translational research informatics support 44. | J Biomed Inform | 2009 | Model |
| Haskew J, Kenyi V, William J, Alum R, Puri A, Mostafa Y, et al. | Use of Mobile Information Technology during Planning, Implementation and Evaluation of a Polio Campaign in South Sudan. | PLoS One | 2015 | Observational |
| Hensel DJ, Fortenberry JD, Harezlak J, Craig D. | The feasibility of cell phone based electronic diaries for STI/HIV research 65. | BMC Med Res Methodol | 2012 | Study |
| Hetland ML. | DANBIO – powerful research database and electronic patient record 54. | Rheumatology (Oxford) | 2011 | Model |
| Holzner B, Giesinger JM, Pinggera J, Zugal S, Schöpf F, Oberguggenberger AS, et al. | The Computer‐based Health Evaluation Software (CHES): a software for electronic patient‐reported outcome monitoring 12. | BMC Med Inform Decis Mak | 2012 | Model |
| Huffstutter J, David Craig W, Schimizzi G, Harshbarger J, Lisse J, Kasle S, et al. | A multicentre, randomized, open study to evaluate the impact of an electronic data capture system on the care of patients with rheumatoid arthritis. | Curr Med Res Opin | 2007 | Study |
| Hye RJ, Inui TS, Anthony FF, Kiley M‐L, Chang RW, Rehring TF, et al. | A multiregional registry experience using an electronic medical record to optimize data capture for longitudinal outcomes in endovascular abdominal aortic aneurysm repair. | J Vasc Surg | 2015 | Study |
| Installé AJ, Van den Bosch T, De Moor B, Timmerman D. | Clinical data miner: an electronic case report form system with integrated data preprocessing and machine‐learning libraries supporting clinical diagnostic model research. | JMIR Med informatics | 2014 | Model |
| Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. | Electronic diaries for monitoring chronic pain: 1‐year validation study 69. | Pain | 2001 | Study |
| Jamison RN, Raymond SA, Slawsby EA, McHugo GJ, Baird JC. | Pain assessment in patients with low back pain: comparison of weekly recall and momentary electronic data 22. | J Pain | 2006 | Observational |
| Jansen ME, Kollbaum PS, McKay FD, Rickert ME. | Factors influencing the electronic capture of patient‐reported contact lens performance data. | Cont Lens Anterior Eye | 2013 | Observational |
| Jensen RE, Rothrock NE, DeWitt EM, Spiegel B, Tucker CA, Crane HM, et al. | The role of technical advances in the adoption and integration of patient‐reported outcomes in clinical care. | Med Care | 2015 | Case Study |
| Katzan I, Speck M, Dopler C, Urchek J, Bielawski K, Dunphy C, et al. | The Knowledge Program: an innovative, comprehensive electronic data capture system and warehouse. | AMIA Annu Symp Proc | 2011 | Model |
| Kessel KA, Bohn C, Engelmann U, Oetzel D, Bougatf N, Bendl R, et al. | Five‐year experience with setup and implementation of an integrated database system for clinical documentation and research. | Comput Methods Programs Biomed | 2014 | Model |
| Kho A, Zafar A, Tierney W. | Information technology in PBRNs: the Indiana University Medical Group Research Network (IUMG ResNet) experience. | J Am Board Fam Med | 2007 | Anecdotal |
| King C, Hall J, Banda M, Beard J, Bird J, Kazembe P, et al. | Electronic data capture in a rural African setting: evaluating experiences with different systems in Malawi. | Glob Health Action | 2014 | Model |
| King JD, Buolamwini J, Cromwell EA, Panfel A, Teferi T, Zerihun M, et al. | A novel electronic data collection system for large‐scale surveys of neglected tropical diseases. | PLoS One | 2013 | Observational |
| Kinnula S, Renko M, Tapiainen T, Pokka T, Uhari M. | Post‐discharge follow‐up of hospital‐associated infections in paediatric patients with conventional questionnaires and electronic surveillance 51. | J Hosp Infect | 2012 | Observational |
| Kohl CD, Garde S, Knaup P. | Facilitating secondary use of medical data by using openEHR archetypes. | Stud Health Technol Inform | 2010 | Model |
| Kuchinke W, Ohmann C, Yang Q, Salas N, Lauritsen J, Gueyffier F, et al. | Heterogeneity prevails: the state of clinical trial data management in Europe ‐ results of a survey of ECRIN centres. | Trials | 2010 | Observational |
| Kush R, Alschuler L, Ruggeri R, Cassells S, Gupta N, Bain L, et al. | Implementing Single Source: the STARBRITE proof‐of‐concept study. | J Am Med Inform Assoc | 2007 | Feasibility Study |
| Laird‐Maddox M, Mitchell SB, Hoffman M. | Integrating research data capture into the electronic health record workflow: real‐world experience to advance innovation 8. | Perspect Health Inf Manag | 2014 | Model |
| Le Jeannic A, Quelen C, Alberti C, Durand‐Zaleski I. | Comparison of two data collection processes in clinical studies: electronic and paper case report forms 45. | BMC Med Res Methodol | 2014 | Observational |
| Levin E, Levin A. | Evaluation of spoken dialogue technology for real‐time health data collection. | J Med Internet Res | 2006 | Model |
| Lin CH, Wu NY, Liou DM. | A multi‐technique approach to bridge electronic case report form design and data standard adoption. | J Biomed Inform | 2014 | Model |
| Long MD, Kappelman MD, Martin CF, Lewis JD, Mayer L, Kinneer PM, et al. | Development of an internet‐based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results 13. | Inflamm Bowel Dis | 2012 | Model |
| López‐Carrero C, Arriaza E, Bolaños E, Ciudad A, Municio M, Ramos J, et al. | Internet in clinical research based on a pilot experience 55. | Contemp Clin Trials | 2005 | Model |
| Lu M, Rupp LB, Moorman AC, Li J, Zhang T, Lamerato LE, et al. | Comparative effectiveness research of chronic hepatitis B and C cohort study (CHeCS): improving data collection and cohort identification. | Dig Dis Sci | 2014 | Observational |
| Lu Z. | Technical challenges in designing post‐marketing eCRFs to address clinical safety and pharmacovigilance needs 14. | Contemp Clin Trials | 2010 | Anecdotal |
| Lu Z. | Electronic Data‐Capturing Technology for Clinical Trials. | IEEE Engineering in Medicine and Biology Magazine | 2010 | Anecdotal |
| Mahaffey KW, Wampole JL, Stebbins A, Berdan LG, McAfee D, Rorick TL, et al. | Strategic lessons from the clinical event classification process for the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX‐AMI) trial. | Contemp Clin Trials | 2011 | Model |
| Mall S, Akmatov MK, Schultze A, Ahrens W, Obi N, Pessler F, et al. | Web‐based questionnaires to capture acute infections in long‐term cohorts: findings of a feasibility study. | Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz | 2014 | Observational |
| Maokola W, Willey BA, Shirima K, Chemba M, Armstrong Schellenberg JRM, Mshinda H, et al. | Enhancing the routine health information system in rural southern Tanzania: successes, challenges and lessons learned. | Trop Med Int Heal | 2011 | Model |
| Marley JE. | Safety and efficacy of nifedipine 20 mg tablets in hypertension using electronic data collection in general practice. | J R Soc Med | 1989 | Study |
| Matza LS, Patrick DL, Riley AW, Alexander JJ, Rajmil L, Pleil AM, et al. | Paediatric patient‐reported outcome instruments for research to support medical product labelling: report of the ISPOR PRO good research practises for the assessment of children and adolescents task force. | Value Heal | 2013 | Anecdotal |
| Meyer J, Fredrich D, Piegsa J, Habes M, van den Berg N, Hoffmann W. | A mobile and asynchronous electronic data capture system for epidemiologic studies 15. | Comput Methods Programs Biomed | 2013 | Model |
| Middleton RJ, Gavin AT, Reid JS, O′Reilly D. | Accuracy of hospital discharge data for cancer registration and epidemiological research in Northern Ireland. | Cancer Causes Control | 2000 | Study |
| Mitchel JT, Kim YJ, Choi J, Park G, Cappi S, Horn D, et al. | Evaluation of data entry errors and data changes to an electronic data capture clinical trial database 46. | Drug Inf J | 2013 | Observational |
| Nahm ML, Pieper CF, Cunningham MM. | Quantifying data quality for clinical trials using electronic data capture 63. | PLoS One | 2008 | Anecdotal |
| Ndume V, Nkansah‐Gyekye Y, Lyatu I, Ko J. | A methodology for data collection and integration of e‐Health records: A case study of Ifakara Health Institute in Tanzania. | In: 2013 Pan African International Conference on Information Science, Computing and Telecommunications (PACT) | 2013 | Model |
| Nesbitt G, McKenna K, Mays V, Carpenter A, Miller K, Williams M, et al. | The Epilepsy Phenome/Genome Project (EPGP) informatics platform. | Int J Med Inform | 2013 | Model |
| Newman ED, Lerch V, Billet J, Berger A, Kirchner HL. | Improving the quality of care of patients with rheumatic disease using patient‐centric electronic redesign software. | Arthritis Care Res | 2015 | Observational |
| Nichols BN, Pohl KM. | Neuroinformatics Software Applications Supporting Electronic Data Capture, Management, and Sharing for the Neuroimaging Community. | Neuropsychol | 2015 | Anecdotal |
| Njuguna HN, Caselton DL, Arunga GO, Emukule GO, Kinyanjui DK, Kalani RM, et al. | A comparison of smartphones to paper‐based questionnaires for routine influenza sentinel surveillance, Kenya, 2011–2012 21. | BMC Med Inform Decis Mak | 2014 | Comparative study |
| Noble NE, Paul CL, Carey ML, Sanson‐Fisher RW, Blunden S V, Stewart JM, et al. | A cross‐sectional survey assessing the acceptability and feasibility of self‐report electronic data collection about health risks from patients attending an Aboriginal Community Controlled Health Service. | BMC Med Inform Decis Mak | 2014 | Observational |
| Nyholm D, Kowalski J, Aquilonius S‐M. | Wireless real‐time electronic data capture for self‐assessment of motor function and quality of life in Parkinson's disease. | Mov Disord | 2004 | Observational |
| Ohmann C, Kuchinke W. | Future developments of medical informatics from the viewpoint of networked clinical research. | Methods Inf Med | 2009 | Anecdotal |
| Olsen IC, Haavardsholm EA, Moholt E, Kvien TK, Lie E. | NOR‐DMARD data management: implementation of data capture from electronic health records. | Clin Exp Rheumatol | 2014 | Model |
| P. O′Halloran J, S. Kemp A, P. Salmon D, N. Tariot P, S. Schneider L. | Psychometric comparison of standard and computerized administration of the alzheimers disease assessment scale – cognitive subscale (ADASCog). | Curr Alzheimer Res | 2011 | Observational |
| Pace WD, Staton EW. | Electronic data collection options for practice‐based research networks. | Ann Fam Med | 2005 | Anecdotal |
| Palmblad M, Tiplady B. | Electronic diaries and questionnaires: designing user interfaces that are easy for all patients to use. | Qual Life Res | 2004 | Model |
| Pavlović I, Kern T, Miklavčič D. | Comparison of paper‐based and electronic data collection process in clinical trials: Costs simulation study. | Contemp Clin Trials | 2009 | Model |
| Pavlovic I, Lazarevic I. | Two models used as a basis for development of electronic data collection software to support clinical trials. | In: Twentieth IEEE International Symposium on Computer‐Based Medical Systems (CBMS'07) | 2007 | Model |
| Pavlovic I, Miklavcic D. | Web‐based electronic data collection system to support electrochemotherapy clinical trial 1. | IEEE Trans Inf Technol Biomed | 2007 | Model |
| Pawellek I, Richardsen T, Oberle D, Grote V, Koletzko B. | Use of electronic data capture in a clinical trial on infant feeding. | Eur J Clin Nutr | 2012 | Study |
| Proctor SJ, Wilkinson J. | A web‐based study concept designed to progress clinical research for “orphan” disease areas in haematological oncology in the elderly: the SHIELD programme. | Crit Rev Oncol Hematol | 2007 | Model |
| Pyke‐Grimm KA, Kelly KP, Stewart JL, Meza J. | Feasibility, acceptability, and usability of web‐based data collection in parents of children with cancer 59. | Oncol Nurs Forum | 2011 | Observational |
| Rhodes SD, DiClemente RJ, Cecil H, Hergenrather KC, Yee LJ. | Risk among men who have sex with men in the United States: a comparison of an Internet sample and a conventional outreach sample. | AIDS Educ Prev | 2002 | Study |
| Salaffi F, Gasparini S, Ciapetti A, Gutierrez M, Grassi W. | Usability of an innovative and interactive electronic system for collection of patient‐reported data in axial spondyloarthritis: comparison with the traditional paper‐administered format 62. | Rheumatology (Oxford) | 2013 | Observational |
| SanJoaquin MA, Allain TJ, Molyneux ME, Benjamin L, Everett DB, Gadabu O, et al. | Surveillance Programme of IN‐patients and Epidemiology (SPINE): implementation of an electronic data collection tool within a large hospital in Malawi. | PLoS Med | 2013 | Model |
| Sargious A, Lee SJ. | Remote collection of questionnaires 60. | Clin Exp Rheumatol | 2014 | Anecdotal |
| Schmier JK, Kane DW, Halpern MT. | Practical applications of usability theory to electronic data collection for clinical trials. | Contemp Clin Trials | 2005 | Anecdotal |
| Schreier G, Messmer J, Rauchegger G, Modre‐Osprian R, Ladenstein R. | A Web‐based platform for interdisciplinary biomedical research 42. | Front Biosci (Landmark Ed) | 2009 | Model |
| Schrimpf D, Haag M, Pilz LR. | Possible combinations of electronic data capture and randomization systems. Principles and the realization with RANDI2 and OpenClinica. | Methods Inf Med | 2014 | Anecdotal |
| Shah J, Rajgor D, Pradhan S, McCready M, Zaveri A, Pietrobon R. | Electronic data capture for registries and clinical trials in orthopaedic surgery: open source versus commercial systems. | Clin Orthop Relat Res | 2010 | Anecdotal |
| Taylor MJ, Stables R, Matata B, Lisboa PJG, Laws A, Almond P. | Website design: technical, social and medical issues for self‐reporting by elderly patients 16. | Health Informatics J | 2014 | Observational |
| Thriemer K, Ley B, Ame SM, Puri MK, Hashim R, Chang NY, et al. | Replacing paper data collection forms with electronic data entry in the field: findings from a study of community‐acquired bloodstream infections in Pemba, Zanzibar 47. | BMC Res Notes | 2012 | Observational |
| Thwin SS, Clough‐Gorr KM, McCarty MC, Lash TL, Alford SH, Buist DSM, et al. | Automated inter‐rater reliability assessment and electronic data collection in a multi‐center breast cancer study 50. | BMC Med Res Methodo | 2007 | Study |
| Trachtenberg FL, Martin M, Green S, Oliveros O, Carson S, Gerstenberger E, et al. | Use of electronic data collection to assess pain in thalassaemia: a feasibility study. | Int J Palliat Nurs | 2012 | Observational |
| Vahabzadeh M, Mezghanni M, Gupman AE, Schmittner J, Preston KL. | An adaptable assessment generation system for clinical trials complementing human research information system 43. | 18th IEEE Symposium on Computer‐Based Medical Systems (CBMS'05) | 2005 | Model |
| Walther B, Hossin S, Townend J, Abernethy N, Parker D, Jeffries D. | Comparison of electronic data capture (EDC) with the standard data capture method for clinical trial data 48. | PLoS One | 2011 | Observational |
| Wang SJ, Middleton B, Prosser LA, Bardon CG, Spurr CD, Carchidi PJ, et al. | A cost–benefit analysis of electronic medical records in primary care 56. | Am J Med | 2003 | Cost benefit analysis? |
| Weiler K, Christ AM, Woodworth GG, Weiler RL, Weiler JM, Meltzer E, et al. | Quality of patient‐reported outcome data captured using paper and interactive voice response diaries in an allergic rhinitis study: is electronic data capture really better? | Ann Allergy, Asthma Immunol | 2004 | Observational |
| Welker JA, Cooper‐Dehoff R, Handberg E, Heissenberg C, Johnson K, Hyde AW, et al. | Implementation of electronic data capture systems: barriers and solutions. | Contemp Clin Trials | 2007 | Anecdotal |
| Whalen CJ, Donnell D, Tartakovsky M. | Supporting research sites in resource‐limited settings: challenges in implementing information technology infrastructure. | J Acquir Immune Defic Syndr | 2014 | Anecdotal |
| Wildeman MA, Zandbergen J, Vincent A, Herdini C, Middeldorp JM, Fles R, et al. | Can an online clinical data management service help in improving data collection and data quality in a developing country setting? 49 | Trials | 2011 | Observational |
| Wintner LM, Giesinger JM, Zabernigg A, Rumpold G, Sztankay M, Oberguggenberger AS, et al. | Evaluation of electronic patient‐reported outcome assessment with cancer patients in the hospital and at home. | BMC Med Inform Decis Mak | 2015 | Observational |
| Xu W, Guan Z, Sun J, Wang Z, Geng Y. | Development of an open metadata schema for prospective clinical research (openPCR) in China. | Methods Inf Med | 2014 | Model |
| Yamamoto K, Yamanaka K, Hatano E, Sumi E, Ishii T, Taura K, et al. | An eClinical trial system for cancer that integrates with clinical pathways and electronic medical records 7. | Clin Trials | 2012 | Model |
| Yuksel UC, Anabtawi AGM, Cam A, Poddar K, Agarwal S, Goel S, et al. | Predictive value of renal resistive index in percutaneous renal interventions for atherosclerotic renal artery stenosis. | J Invasive Cardiol | 2012 | Study |
| Zbrozek A, Hebert J, Gogates G, Thorell R, Dell C, Molsen E, et al. | Validation of electronic systems to collect patient‐reported outcome (PRO) data—recommendations for clinical trial teams: report of the ISPOR ePRO systems validation good research practises task force. | Value Health | 2013 | Anecdotal |
Research has been conducted into ways to maximize data accuracy and efficiency using IT. Trials have taken data from patient's electronic medical records (EMRs) and transferred these directly into eCRFs. The cost savings, quality improvements, and reduction of data entry errors, were significant 5, 6, 7. Whilst not all required data ARE available from the patient EMR, studies have found varying results with as much as 69% of data required being found and used to prepopulate trial eCRFs 8. Discussions around the design and theoretical modelling of EMRs, eCRFs and ECD were prevalent within the included papers 9, 10, 11, 12, 13, 14, 15, 16, 17. The electronic systems reported vary in quality, with some being used in mock environments and others being purely theoretical. Commercial software packages are available, but are generally not cost effective and in some circumstances it is unclear who owns the data entered into them 18, 19, 20. Observational studies have compared paper based systems against EDC or canvassed opinion on the use of EDC systems 21, 22, 23, 24. These papers were overwhelmingly in favour of EDC as long as security could be maintained.
Obtaining patient consent is an ethical necessity, and up until recently, has almost always required a physical signature. Varnhagen et al. 25 considered obtaining informed consent online and questioned whether it is ethical to obtain consent electronically. Recently, electronic consent has been accepted by the National Health Service as a viable alternative to a written signature 26. This review found one trial where consent had successfully been captured online 27. Collecting participant consent electronically is a novel, and largely unexplored, method that invites further innovation. There are ethical implications of conducting research entirely online. IT is advancing faster than ethical review panels can address and there is a need for greater ethical consideration of conducting research online and how we share data between IT systems and within organizations 28, 29, 30, 31, 32, 33, 34, 35. Government attempts to legislate – the Health Insurance and Portability Act 36 in the USA, and the Data Protection Act 37 in the UK, have had little impact on alleviating public scepticism 38. Patient privacy is critical and despite the well‐intentioned zeal for the mass adoption of IT within healthcare, serious security concerns remain 39, 40. However, patients are open to technology being used to store their medical data if trust and privacy concerns can be addressed 41.
Clinical research and pharmacoepidemiology often involve interdisciplinary research. This not only means that various researchers deal with different data sources and formats but also that they have different workflows and organizational structures. As a consequence, there are no off‐the‐shelf solutions to facilitate this. This often results in individual solutions being developed that, over time, evolve and are ultimately difficult to maintain 42. Unfortunately, it is often much easier to change time points, interventions, and assessment tools on paper than it is to suddenly change the programming of a computerized system. The reality demands future IT systems be flexible and adaptable with more automation 43.
Advantages and disadvantages
There are distinct advantages to EDC in research and pharmacoepidemiology. However, there are pragmatic concerns that need to be addressed. The role of clinical research and pharmacoepidemiology is to improve healthcare by generating and providing access to high quality data. Due to the limitations of paper based records this is not possible with the status quo 44. The objectives of ECD are to reduce medical errors, improve communication between healthcare providers, collect information for educational and research purposes and to gather complete and accurate data whilst avoiding duplication.
EDC's distinct advantage over paper‐based systems of research is that it is able to detect protocol violations and data outside the normal range at the time of entry and not days, weeks or months after. EDC systems have been shown to improve the quality of clinical trials, halt the development of ineffective or unsafe drugs earlier, reduce unnecessary work, reduce cost, and accelerate time to market of new drugs 45, 46, 47, 48, 49, 50, 51. There are also benefits in relation to data quality, performance, productivity and costs in clinical trial management 52, 53, 54, 55, 56. Observational data suggest that it is now considered a preferred method of data capture in clinical research 57, 58, 59. It is well accepted by users and has been shown to contribute to patient empowerment, allowing them to be more engaged in research and to take direct control of their own data 60, 61, 62. By contrast, paper‐based questionnaires can suffer from incomplete forms, questions being answered twice or skipped questions. Paper forms are considered time consuming, require dual checking, and data cleansing 63, whereas EDC can alert people to missing answers before any attempt to proceed, and can be easily incorporated into electronic health records. Remote data collection offers the additional advantage of convenience to patients, particularly those who are incapacitated or live far from the nearest clinic 47, and may provide a safer environment for questionnaires than paper‐based methods eliciting the answers to potentially sensitive questions 64, 65.
Despite the advantages, EDC has not been universally accepted. Perceived disadvantages and concerns regarding EDC include: a lack of available technical support, a lack of investigator motivation, complexity of installation, maintenance of software, high initial investment cost, and complexity of use 66, 67. Reliable data handling methods, effective project management, and expert technical architecture and infrastructure are all key factors for successful implementation, and should not be underestimated 68. There are concerns over patient privacy and the need for computer literacy, which may affect generalisability of any research findings 60. Study retention is considered to be higher where there is direct patient interaction because of the explicit alignment of patient incentives; the patient learns about the study directly, understands what is required, self‐consents to participate, and then self‐reports study information 69. Jamison et al. 70 found better rates of compliance with electronic patient reported outcomes (PROs) than paper based PROs. Despite data suggesting benefits of EDC use, Alexander 71 reports that physicians lack motivation and will only use structured electronic records if the system reduces overhead while at the same time minimizing their work load. In the UK, it has been suggested that development of these technologies suffers from the lack of a clear national direction towards unifying clinical and medical data, with no common format for all data systems. Not only would EDC benefit clinical research, but pharmacovigilance and drug safety regulation could also be improved 72.
Discussion
IT and how it is used in pharmacoepidemiology and clinical research is a relatively new field with no substantial guidelines in place and few recommendations. There is a consensus that EDC has clear benefits for use in research but there are fears over security and data protection which must be addressed. IT offers an opportunity to improve pharmacoepidemiology and clinical research and to facilitate the continual improvement of healthcare. If the use of IT in research is to succeed fully, change is required: specifically, investment in infrastructure and the provision of support for integration of interoperable systems. Further efforts will be essential to alleviate healthcare providers and users legitimate concerns regarding IT. Policy makers will need to find ways to supply adequate financial resources to IT to counter a historical lack of investment within the public sector.
Healthcare providers and researchers require a governance‐led support network of technology experts to assist in integrating ever more complex systems and providing guidance on compliance and security. IT security is a challenging and fast moving field and requires careful consideration. There is a need for clearer and more consistent policies and more trained data managers, software architects and semantic web specialists working in medical research groups. These architectures will need ongoing support from a robust legal system protecting patient privacy.
Furthermore, the exchange of information between systems is essential. Data format differences need to be resolved, and a solution found for interoperability between healthcare systems. Motivating software vendors, healthcare providers and researchers to agree on a common path will be difficult but worthwhile endeavour. Future technical development needs to focus on creating adaptable and generic software that can be tailored to specific trial needs without major re‐development.
Limitations
This work has several limitations. Firstly, a publication bias is very likely as less successful IT projects are unlikely to be reported in published literature. This review only searched the IEEE library and the PubMed database and we did not include papers in non‐English languages. In addition, researchers from low‐income countries are known to have lower publication rates. The relative novelty of the field means that evaluation studies, in particular, are missing and rapid developments in the field may not yet have been published at the time of conducting the literature search. There are currently no widely accepted methods to evaluate technical publications in the same way as has been developed for reports of clinical trials, for example. Therefore, subjective interpretation had to be used to decide if a journal was of sufficient quality to be referenced. The authors took steps to avoid selection and objectivity bias by including all peer reviewed and published articles. The only exceptions authors made were where there was an overt conflict of interest, or the journal was not available in English. This review aimed to capture the full range of reported advantages and benefits of IT use. It did not measure the relative frequency or impact of individual factors of the utility of EDC and eCRFs. Despite the limitations detailed above, the authors believe this review to be an unbiased appraisal of publications on EDC and eCRFs in pharmacoepidemiology and clinical research.
Conclusion
It is apparent from the results of this literature review that the following areas would benefit from further development:
Clearer legislation and operational frameworks governing electronic health records.
Guidelines and best practises for researchers to follow in the use of IT and EDC.
Standard methods of reporting and evaluating technical innovation to facilitate comparison.
Regardless of the challenges, it is the imperative that healthcare organizations ensure that patients receive safe medications. Effective clinical research and pharmacoepidemiology are essential to this process.
Competing Interests
There are no competing interests to declare.
The authors acknowledge the support of the Medicines Monitoring Unit, University of Dundee.
Contributors
D.R. conceived the idea with input from T.M.. The initial draft of the manuscript was created by D.R. T.M., R.F., A.D., K.G., I.M. and A.R. analysed and reviewed the manuscript. All listed authors fulfil the requirements for authorship and agree to submission of the manuscript in its current form.
Table A1.
Electronic data capture – IEEE
| Search term used | Results | Search criteria and filters | Date |
|---|---|---|---|
| electronic case report form | 221 | NA | 27/07/2016 |
| (“Abstract”: electronic data capture) | 18 | NA | 27/07/2016 |
| ecrf | 14 | NA | 27/07/2016 |
| (“Abstract”: electronic data collection) | 15 | NA | 27/07/2016 |
Table A2.
Electronic data capture – PubMed
| Search term used | Results | Search criteria and filters | Date |
|---|---|---|---|
| (electronic data capture[Title/Abstract]) | 170 | Abstract available, Humans | 27/07/2016 |
| electronic case report form | 546 | Abstract available, Humans | 27/07/2016 |
| ecrf | 20 | Abstract available, Humans | 27/07/2016 |
| (electronic data collection[Title/Abstract]) | 122 | Abstract available, Humans | 27/07/2016 |
Rorie, D. A. , Flynn, R. W. V. , Grieve, K. , Doney, A. , Mackenzie, I. , MacDonald, T. M. , and Rogers, A. (2017) Electronic case report forms and electronic data capture within clinical trials and pharmacoepidemiology. Br J Clin Pharmacol, 83: 1880–1895. doi: 10.1111/bcp.13285.
References
- 1. Pavlovic I, Miklavcic D. Web‐based electronic data collection system to support electrochemotherapy clinical trial. IEEE Trans Inf Technol Biomed 2007; [cited 2014 Jun 23]11: 222–230. Available from: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=4118193 [DOI] [PubMed] [Google Scholar]
- 2. Gupta SK. Paperless clinical trials: myth or reality? Indian J Pharmacol. [cited 2016 Jul 27]; 47: 349–353. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26288464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ayatollahi H, Mirani N, Haghani H. Electronic health records: what are the most important barriers? Perspect Health Inf Manag 2014; [cited 2015 Nov 16]11: 1c Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4272437&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 4. PRISMA . [cited 2017 Jan 18]. Available from: http://prisma‐statement.org/PRISMAStatement/Checklist.aspx
- 5. Goodman K, Krueger J, Crowley J. The automatic clinical trial: leveraging the electronic medical record in multisite cancer clinical trials. Curr Oncol Rep 2012; [cited 2013 Sep 24]14: 502–508. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22907283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Curcin V, Soljak M, Majeed A. Managing and exploiting routinely collected NHS data for research. Inform Prim Care 2012; [cited 2015 Mar 30]20: 225–231. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23890333 [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto K, Yamanaka K, Hatano E, Sumi E, Ishii T, Taura K, et al. An eClinical trial system for cancer that integrates with clinical pathways and electronic medical records. Clin Trials 2012; [cited 2013 Sep 24]9: 408–417. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22605791 [DOI] [PubMed] [Google Scholar]
- 8. Laird‐Maddox M, Mitchell SB, Hoffman M. Integrating research data capture into the electronic health record workflow: real‐world experience to advance innovation. Perspect Health Inf Manag 2014; [cited 2015 Mar 24]11: 1e Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4272439&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 9. Bellary S, Krishnankutty B, Latha MS. Basics of case report form designing in clinical research. Perspect Clin Res 2014; [cited 2015 Mar 24]5: 159–166. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4170533/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crichton C, Davies J, Gibbons J, Harris S, Tsui A, Brenton J. Metadata‐driven software for clinical trials In: 2009 ICSE Workshop on Software Engineering in Health Care [Internet]. IEEE; 2009. [cited 2016 Apr 4]. p. 1–11. Available from: http://ieeexplore.ieee.org/document/5069600/ [Google Scholar]
- 11. Haak D, Samsel C, Gehlen J, Jonas S, Deserno TM. Simplifying electronic data capture in clinical trials: workflow embedded image and biosignal file integration and analysis via web services. J Digit Imaging 2014; [cited 2015 Nov 17]27: 571–580. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4171435&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holzner B, Giesinger JM, Pinggera J, Zugal S, Schöpf F, Oberguggenberger AS, et al. The computer‐based health evaluation software (CHES): a software for electronic patient‐reported outcome monitoring. BMC Med Inform Decis Mak 2012; [cited 2013 Sep 24]12: 126 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3529695&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Long MD, Kappelman MD, Martin CF, Lewis JD, Mayer L, Kinneer PM, et al. Development of an Internet‐based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflamm Bowel Dis 2012; [cited 2013 Aug 8]18: 2099–2106. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22287300 [DOI] [PubMed] [Google Scholar]
- 14. Lu Z. Technical challenges in designing post‐marketing eCRFs to address clinical safety and pharmacovigilance needs. Contemp Clin Trials 2010; [cited 2014 May 27]31: 108–118. Available from: http://www.sciencedirect.com/science/article/pii/S1551714409001803 [DOI] [PubMed] [Google Scholar]
- 15. Meyer J, Fredrich D, Piegsa J, Habes M, van den Berg N, Hoffmann W. A mobile and asynchronous electronic data capture system for epidemiologic studies. Comput Methods Programs Biomed Elsevier Ireland Ltd2013; [cited 2013 Sep 24]110: 369–379. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23195493 [DOI] [PubMed] [Google Scholar]
- 16. Taylor MJ, Stables R, Matata B, Lisboa PJG, Laws A, Almond P. Website design: technical, social and medical issues for self‐reporting by elderly patients. Health Informatics J 2014; [cited 2015 Mar 31]20: 136–150. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24047573 [DOI] [PubMed] [Google Scholar]
- 17. Curcin V, Woodcock T, Poots AJ, Majeed A, Bell D. Model‐driven approach to data collection and reporting for quality improvement. J Biomed Inform 2014; 52: 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franklin JD, Guidry A, Brinkley JF. A partnership approach for electronic data capture in small‐scale clinical trials. J Biomed Inform 2011; 44 (Suppl 1): S103–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leveille SG, Walker J, Ralston JD, Ross SE, Elmore JG, Delbanco T. Evaluating the impact of patients' online access to doctors' visit notes: designing and executing the OpenNotes project. BMC Med Inform Decis Mak BioMed Central Ltd2012; [cited 2013 Aug 14]12: 32 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3351950&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obeid JS, McGraw CA, Minor BL, Conde JG, Pawluk R, Lin M, et al. Procurement of shared data instruments for research electronic data capture (REDCap). J Biomed Inform 2013; [cited 2013 Sep 24]46: 259–265. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23149159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Njuguna HN, Caselton DL, Arunga GO, Emukule GO, Kinyanjui DK, Kalani RM, et al. A comparison of smartphones to paper‐based questionnaires for routine influenza sentinel surveillance, Kenya, 2011‐2012. BMC Med Inform Decis Mak BioMed Central2014; [cited 2016 Aug 9]14: 107. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25539745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jamison RN, Raymond SA, Slawsby EA, McHugo GJ, Baird JC. Pain assessment in patients with low back pain: comparison of weekly recall and momentary electronic data. J Pain 2006; 7: 192–199. [DOI] [PubMed] [Google Scholar]
- 23. Bushnell DM, Martin ML, Parasuraman B. Electronic versus paper questionnaires: a further comparison in persons with asthma. J Asthma 2003; [cited 2016 Aug 6]40: 751–762. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14626331 [DOI] [PubMed] [Google Scholar]
- 24. Dy CJ, Schmicker T, Tran Q, Chadwick B, Daluiski A, Hudak PL, et al. The use of a tablet computer to complete the DASH questionnaire. J Hand Surg Am Elsevier2012; [cited 2016 Aug 6]37: 2589–2594. Available from: http://linkinghub.elsevier.com/retrieve/pii/S036350231201372X [DOI] [PubMed] [Google Scholar]
- 25. Varnhagen CK, Gushta M, Daniels J, Peters TC, Parmar N, Law D, et al. How informed is online informed consent? Ethics Behav 2005; 15: 37–48. [DOI] [PubMed] [Google Scholar]
- 26. UK Approval Of Informed Electronic Consent A Pivotal Moment In Enhancing Trial Enrollment [Internet]. [cited 2015. Mar 6]. Available from: https://www.clinicalleader.com/doc/u-k-approval-of-informed-electronic-consent-a-pivotal-moment-in-enhancing-trial-enrollment-0001
- 27. Orri M, Lipset CH, Jacobs BP, Costello AJ, Cummings SR. Web‐based trial to evaluate the efficacy and safety of tolterodine ER 4mg in participants with overactive bladder: REMOTE trial. Contemp Clin Trials 2014; [cited 2015 Oct 20]38: 190–197. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24792229 [DOI] [PubMed] [Google Scholar]
- 28. David Y. Ethical issues in the clinical engineering profession. IEEE Eng Med Biol Mag 1988; 7: 83–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18244061 [DOI] [PubMed] [Google Scholar]
- 29. Saha PS, Saha S. Clinical trials of medical devices and implants: ethical concerns. IEEE Eng Med Biol Mag 1988; 7: 85–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18244062 [DOI] [PubMed] [Google Scholar]
- 30. Monzon JE, Monzon‐Wyngaard A. Professional ethics in biomedical engineering practice and research. Conf Proc IEEE Eng Med Biol Soc 2008; 2008: 2893–2896. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19163310 [DOI] [PubMed] [Google Scholar]
- 31. Anderson JG. The role of ethics in information technology decisions: a case‐based approach to biomedical informatics education. Int J Med Inform 2004; [cited 2013 Aug 14]73: 145–150. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15063373 [DOI] [PubMed] [Google Scholar]
- 32. Novossiolova T, Sture J. Towards the responsible conduct of scientific research: is ethics education enough? Med Confl Surviv 2012; 28: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hofmann BM. Why ethics should be part of health technology assessment. Int J Technol Assess Health Care 2008; [cited 2013 Aug 14]24: 423–429. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18828936 [DOI] [PubMed] [Google Scholar]
- 34. Munzel H. Towards an ethical Foundation of Green Software Engineering. In: 2015 IEEE 10th International Conference on Global Software Engineering Workshops [Internet]. IEEE; 2015. [cited 2015 Nov 16]. p. 23–6. Available from: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=7227529
- 35. Jumelle AKL, Ispas I, Thuernmler C, Mival OH, Kosta E, Casla P, et al Ethical assessment in e‐health. In: 2014 IEEE 16th International Conference on e‐Health Networking, Applications and Services (Healthcom) [Internet]. IEEE; 2014. [cited 2015 Nov 16]. p. 262–8. Available from: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=7001852
- 36. Health Information Privacy | HHS.gov [Internet]. [cited 2017 Jan 18]. Available from: https: //www.hhs.gov/hipaa/index.html
- 37. Data protection act 1998 [Internet]. Statute Law Database; [cited 2015 Nov 9]. Available from: http://www.legislation.gov.uk/ukpga/1998/29/contents
- 38. Piliouras T, Xin Tian, Desai D, Patel A, Shah D, Yang Su, et al Impacts of legislation on electronic health records systems and security implementation In: 2012 IEEE Long Island Systems, Applications and Technology Conference (LISAT) [Internet]. IEEE; 2012. [cited 2016 Apr 6]. p. 1–7. Available from: http://ieeexplore.ieee.org/document/6223106/ [Google Scholar]
- 39. Wilkowska W, Ziefle M. Privacy and data security in E‐health: requirements from the user's perspective. Health Informatics J 2012; [cited 2013 Aug 14]18: 191–201. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23011814 [DOI] [PubMed] [Google Scholar]
- 40. Burns AJ, Johnson ME. Securing health information. IT Prof 2015; [cited 2015 Feb 6]17: 23–29. Available from: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=7030196 [Google Scholar]
- 41. Chhanabhai P, Holt A. Consumers are ready to accept the transition to online and electronic records if they can be assured of the security measures. MedGenMed : Medscape general medicine 2007; [cited 2013 Sep 11]9: 8 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1924980/ [PMC free article] [PubMed] [Google Scholar]
- 42. Schreier G, Messmer J, Rauchegger G, Modre‐Osprian R, Ladenstein R. A web‐based platform for interdisciplinary biomedical research. Front Biosci Landmark Ed2009; [cited 2015 Mar 30]14: 2738–2746. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19273232 [DOI] [PubMed] [Google Scholar]
- 43. Vahabzadeh M, Mezghanni M, Gupman AE, Schmittner J, Preston KL. An adaptable assessment generation system for clinical trials complementing human research information system. In: 18th IEEE Symposium on Computer‐Based Medical Systems (CBMS'05) [Internet]. IEEE; 2005. [cited 2014 Jun 24]. p. 191–6. Available from: http://ieeexplore.ieee.org/lpdocs/epic03/wrapper.htm?arnumber=1467689
- 44. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; [cited 2014 Nov 20]42: 377–381. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2700030&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Le Jeannic A, Quelen C, Alberti C, Durand‐Zaleski I. Comparison of two data collection processes in clinical studies: electronic and paper case report forms. BMC Med Res Methodol 2014; [cited 2015 Mar 31]14: 7 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3909932&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mitchel JT, Kim YJ, Choi J, Park G, Cappi S, Horn D, et al. Evaluation of data entry errors and data changes to an electronic data capture clinical trial database. Drug Inf J 2013; 45: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thriemer K, Ley B, Ame SM, Puri MK, Hashim R, Chang NY, et al. Replacing paper data collection forms with electronic data entry in the field: findings from a study of community‐acquired bloodstream infections in Pemba, Zanzibar. BMC Res Notes BioMed Central Ltd2012; [cited 2013 Sep 26]5: 113 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3392743&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walther B, Hossin S, Townend J, Abernethy N, Parker D, Jeffries D. Comparison of electronic data capture (EDC) with the standard data capture method for clinical trial data. PLoS One 2011; [cited 2013 Sep 21]6: e25348 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3179496&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wildeman MA, Zandbergen J, Vincent A, Herdini C, Middeldorp JM, Fles R, et al. Can an online clinical data management service help in improving data collection and data quality in a developing country setting? Trials 2011; [cited 2014 Jun 25]12: 190 Available from: http://www.trialsjournal.com/content/12/1/190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thwin SS, Clough‐Gorr KM, McCarty MC, Lash TL, Alford SH, Buist DSM, et al. Automated inter‐rater reliability assessment and electronic data collection in a multi‐center breast cancer study. BMC Med Res Methodol BioMed Central2007; [cited 2016 Aug 12]7: 23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17577410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kinnula S, Renko M, Tapiainen T, Pokka T, Uhari M. Post‐discharge follow‐up of hospital‐associated infections in paediatric patients with conventional questionnaires and electronic surveillance. J Hosp Infect 2012; 80: 13–16. [DOI] [PubMed] [Google Scholar]
- 52. Dillon DG, Pirie F, Rice S, Pomilla C, Sandhu MS, Motala AA, et al. Open‐source electronic data capture system offered increased accuracy and cost‐effectiveness compared with paper methods in Africa. J Clin Epidemiol 2014; [cited 2015 Mar 12]67: 1358–1363. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4271740&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ene‐Iordache B, Carminati S, Antiga L, Rubis N, Ruggenenti P, Remuzzi G, et al. Developing regulatory‐compliant electronic case report forms for clinical trials: experience with the demand trial. J Am Med Inform Assoc 2009; [cited 2013 Sep 26]16: 404–408. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2732224&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hetland ML. DANBIO – powerful research database and electronic patient record. Rheumatology (Oxford) 2011; [cited 2015 Mar 30]50: 69–77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21148154 [DOI] [PubMed] [Google Scholar]
- 55. López‐Carrero C, Arriaza E, Bolaños E, Ciudad A, Municio M, Ramos J, et al. Internet in clinical research based on a pilot experience. Contemp Clin Trials 2005; [cited 2015 Mar 25]26: 234–243. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15837443 [DOI] [PubMed] [Google Scholar]
- 56. Wang SJ, Middleton B, Prosser LA, Bardon CG, Spurr CD, Carchidi PJ, et al. A cost‐benefit analysis of electronic medical records in primary care. Am J Med Elsevier2003; [cited 2015 May 11]114: 397–403. Available from: http://www.amjmed.com/article/S0002934303000573/fulltext [DOI] [PubMed] [Google Scholar]
- 57. El Fadly A, Rance B, Lucas N, Mead C, Chatellier G, Lastic P‐Y, et al. Integrating clinical research with the healthcare Enterprise: from the RE‐USE project to the EHR4CR platform. J Biomed Inform Elsevier Inc2011; [cited 2013 Oct 1]44 (Suppl 1): S94–102. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21888989 [DOI] [PubMed] [Google Scholar]
- 58. Aiello EJ, Taplin S, Reid R, Hobbs M, Seger D, Kamel H, et al. In a randomized controlled trial, patients preferred electronic data collection of breast cancer risk‐factor information in a mammography setting. J Clin Epidemiol 2006; 59: 77–81. [DOI] [PubMed] [Google Scholar]
- 59. Pyke‐Grimm KA, Kelly KP, Stewart JL, Meza J. Feasibility, acceptability, and usability of web‐based data collection in parents of children with cancer. Oncol Nurs Forum Oncology Nursing Society2011; [cited 2016 Aug 11]38: 428–435. Available from: http://onf.ons.org/onf/38/4/feasibility‐acceptability‐and‐usability‐web‐based‐data‐collection‐parents‐children‐cancer [DOI] [PubMed] [Google Scholar]
- 60. Sargious A, Lee SJ. Remote collection of questionnaires. Clin Exp Rheumatol 2014; [cited 2015 Mar 18]32 (5 Suppl 85) S‐168‐72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25365110 [PubMed] [Google Scholar]
- 61. Mathieu E, Barratt A, Carter SM, Jamtvedt G. Internet trials: participant experiences and perspectives. BMC Med Res Methodol 2012; [cited 2013 Aug 14]12: 162 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3533967&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salaffi F, Gasparini S, Ciapetti A, Gutierrez M, Grassi W. Usability of an innovative and interactive electronic system for collection of patient‐reported data in axial spondyloarthritis: comparison with the traditional paper‐administered format. Rheumatology (Oxford) 2013; [cited 2013 Sep 24]276: 1–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23955646 [DOI] [PubMed] [Google Scholar]
- 63. Nahm ML, Pieper CF, Cunningham MM. Quantifying data quality for clinical trials using electronic data capture. PLoS One 2008; [cited 2013 Sep 24]3: e3049 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2516178&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dupont A, Wheeler J, Herndon JE, Coan A, Zafar SY, Hood L, et al. Use of tablet personal computers for sensitive patient‐reported information. J Support Oncol [cited 2016 Aug 12]7: 91–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19507456 [PubMed] [Google Scholar]
- 65. Hensel DJ, Fortenberry JD, Harezlak J, Craig D. The feasibility of cell phone based electronic diaries for STI/HIV research. BMC Med Res Methodol BioMed Central2012; [cited 2016 Aug 11]12: 75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22691189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carvalho JC, Bottenberg P, Declerck D, van Nieuwenhuysen JP, Vanobbergen J, Nyssen M. Validity of an information and communication technology system for data capture in epidemiological studies. Caries Res 2011; [cited 2015 Mar 30]45: 287–293. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21625125 [DOI] [PubMed] [Google Scholar]
- 67. Haller G, Haller DM, Courvoisier DS, Lovis C. Handheld vs. laptop computers for electronic data collection in clinical research: a crossover randomized trial. J Am Med Inform Assoc American Medical Informatics Association2009; [cited 2016 Aug 12]16: 651–659 http://www.ncbi.nlm.nih.gov/pubmed/19567799.Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fritz F, Tilahun B, Dugas M. Success criteria for electronic medical record implementations in low‐resource settings: a systematic review. J Am Med Inform Assoc 2015; [cited 2015 Sep 16]22: 479–488. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25769683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cascade E, Marr P, Winslow M, Burgess A, Nixon M. Conducting research on the internet: medical record data integration with patient‐reported outcomes. J Med Internet Res Journal of Medical Internet Research2012; [cited 2013 Aug 27]14: e137 Available from: http://www.jmir.org/2012/5/e137/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. Electronic diaries for monitoring chronic pain: 1‐year validation study. Pain 2001; [cited 2016 Aug 15]91: 277–285. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP: landingpage&an=00006396‐200104000‐00012 [DOI] [PubMed] [Google Scholar]
- 71. Alexander I. The impact of future trends in electronic data collection on musculoskeletal research and evidence‐based orthopaedic care. Arthroscopy 2003; [cited 2016 Aug 15]19: 1007–1011. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14608322 [DOI] [PubMed] [Google Scholar]
- 72. Burnstead B, Furlan G. Unifying drug safety and clinical databases. Curr Drug Saf 2013; 8: 56–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23656448 [DOI] [PubMed] [Google Scholar]


