Abstract
Aims
The present study aims to describe real‐life outcomes in stable patients after‐myocardial infarction (MI) similar to those in the PEGASUS‐TIMI 54 trial (PEGASUS), which found long‐term benefits of ticagrelor in patients with a history of MI.
Methods
One‐year event‐free post‐MI patients were identified in the French claims database representative 1/97 sample (2005–2010) and followed for up to 3 years. A PEGASUS‐like (PL) population included patients with age ≥ 65 years, or age ≥ 50 and diabetes, renal dysfunction or prior MI, without stroke, end‐stage renal failure or oral anticoagulation. Outcomes were: a composite of all‐cause death or hospital admission for MI or stroke; individual events; major bleeding.
Results
There were 1585 post‐MI patients totalling 3926 person–years including 865 PL patients (2114 PY); 68% were male; mean age was 66 (standard deviation 15) in post‐MI, 74 (10) in PL. Outcomes per 100 person–years [95% confidence interval] were, respectively, in post‐MI and PL 6.3 [5.6–7.1] and 7.8 [6.7–8.9] for the composite outcome; 5.1 [4.4–5.8] and 6.5 [5.5–7.6] for death; 1.0 [0.7–1.3] and 1.0 [0.6–1.4] for MI; 0.6 [0.4–0.9] and 0.9 [0.5–1.2] for stroke; 1.3 [0.9–1.6] and 1.4 [0.9–1.9] for major bleeding. Event rates were stable over the 3 study years. Placebo patients in the PEGASUS‐TIMI54 Study were younger, more often male and had lower event rates, especially for all‐cause death and major bleeding.
Conclusions
Patients selected using the criteria described in PEGASUS were older with more comorbidities, resulting in higher all‐cause death and bleeding rates, but similar MI recurrence rates.
Keywords: coronary disease, epidemiology, haemorrhage, myocardial infarction, secondary prevention, stroke
What is Already Known about this Subject
The PEGASUS‐TIMI 54 trial studied the effects of long‐term treatment with ticagrelor in stable coronary patients, and found a benefit on cardiovascular events and mortality.
As with all clinical trials, the representativeness of patients included in the clinical trial is uncertain.
What this Study Adds
When the published PEGASUS patient selection criteria are applied to a representative sample of the French population, the patients who are selected are older and probably have more noncardiovascular diseases than the patients included in PEGASUS.
This results in higher all‐cause death and severe haemorrhage rates in the real‐life population compared to the clinical trial.
This might indeed affect the interpretation and applicability of PEGASUS in real‐life patients.
Introduction
Antiplatelet agents such as clopidogrel, ticagrelor or prasugrel have demonstrated benefits during the 1st year after myocardial infarction (MI). They are part of the standard post‐MI secondary prevention treatment 1, especially dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor antagonist. One‐year treatment provides a clear benefit, but the situation is still uncertain beyond 1 year. Some studies seem to indicate no added benefit beyond 1 year, others that there may be a benefit beyond 1 year DAPT or that stopping DAPT may be associated with increased risk 2, 3, 4, 5, 6, 7, 8. Most of these studies were of clopidogrel, a prodrug with many potential interactions that may reduce its benefits. Ticagrelor does not share these issues. For this reason, PEGASUS‐TIMI 54 (PEGASUS), a randomized placebo‐controlled clinical trial of two doses of ticagrelor vs. placebo in patients with at least 1‐year event‐free survival after MI, treated with aspirin, and with additional risk factors was set up. Its results showed a cardiovascular benefit of long‐term use of ticagrelor 9.
However, the real usefulness of a clinical trial depends upon its external validity, i.e., how the patients who are included in the trial relate to real‐life patients, and whether the real‐life baseline risks are similar to those in the control arm of the trial, so that the demonstrated benefit can be expected and modelled in real life. We therefore set up a cohort study in a representative sample of the French national claims database to assess the incidence of death, nonfatal MI, stroke and major bleeding in all patients surviving 1 year after an MI, and in those with similar inclusion criteria to PEGASUS.
Methods
Design (Figure 1)
Figure 1.
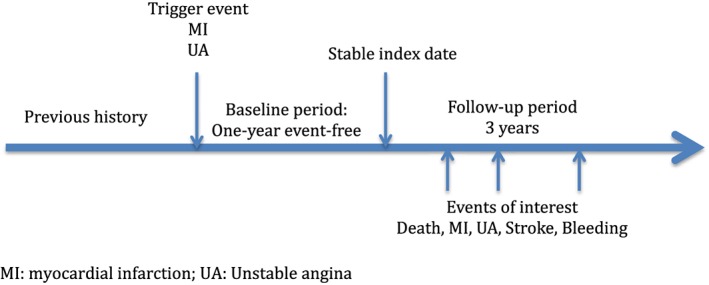
Study timelines
Cohort study of stable coronary patients with a 1‐year event‐free period after MI in a representative French health insurance and hospitalization claims database (Echantillon Généraliste de Bénéficiaires: EGB) with up to 3 years of further follow‐up until 31 December 2012, at a time when ticagrelor was not yet marketed in the country.
Data source
The database used in this study was the EGB, a permanent 1/97 representative sample of Système National d'Informations Interrégimes de l'Assurance Maladie (SNIIRAM), the nationwide healthcare insurance database linked to the national hospital‐discharge summary database and the national death registry 10, 11, 12. SNIIRAM includes 66 million people, over 97% of the French population from birth (or immigration) to death (or emigration). Subjects remain followed even if they change health insurance, occupation, socioeconomic status or retire. EGB includes approximately 700 000 persons, and contains anonymized data on demographic characteristics (sex, year of birth, date of death), long‐term diseases (ALD) resulting in full insurance coverage; reimbursed outpatient healthcare expenditures (visits, medical procedures, laboratory tests, dispensed drugs, medical devices), as well as hospital procedures and discharge summaries (ICD‐10).
Populations
Source population
Adults with main hospital discharge diagnosis of MI (ICD‐10 code I21) between 01 January 2005 and 31 December 2010 (trigger event), with database follow‐up data until death or 31 December 2012.
Stable post‐MI study population
All patients in the source population who were alive and free of MI recurrence 1 year after trigger MI. Index date for inclusion in the study cohort was the first anniversary of admission for initial MI (Figure 1).
PEGASUS‐TIMI 54‐like population
The PEGASUS‐TIMI 54‐like population (PL) population was made of all patients in the stable post‐MI study population aged ≥65 years, or ≥50 years with diabetes mellitus, previous history of MI before trigger event (ICD‐10 codes I21, I22) or renal disease, but without history of stroke or dialysis, and without use of oral anticoagulants within 30 days of the index date.
Diabetes mellitus was identified by ALD registration for diabetes, hospital discharge diagnosis of diabetes, or use of any oral antidiabetic agent or insulin before index date 13.
Previous history of MI was identified from ALD registration for MI before trigger event, or hospital discharge diagnosis mentioning acute or chronic MI before trigger event.
Renal disease was identified by ALD registration for chronic renal failure, or hospital discharge summary mentioning chronic renal failure as main or secondary diagnosis, or dialysis (Figure 2).
Figure 2.
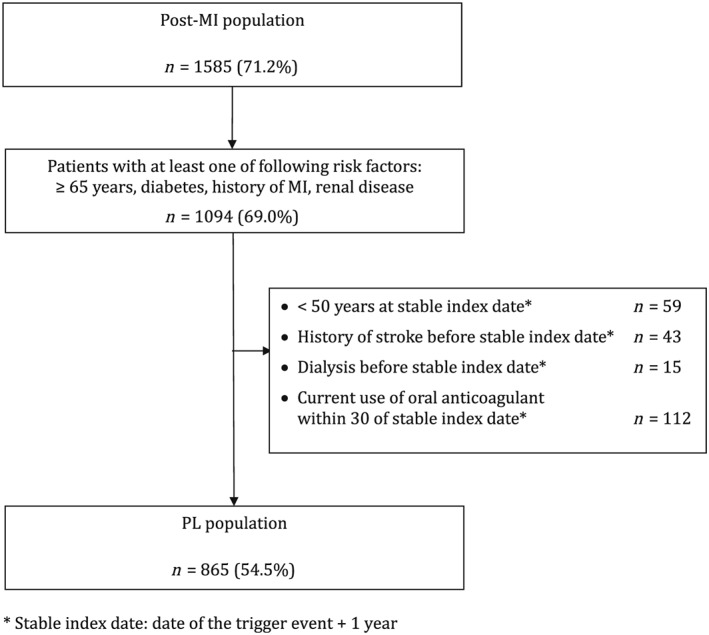
Patient flowchart
Outcomes
Primary outcome was a composite of:
All‐cause death (cause of death was not available in the database);
MI, defined as hospital admission with a primary diagnosis of MI (ICD‐10 code I21);
Ischemic or undefined stroke (ICD‐10 codes I63, I64).
Secondary outcomes were:
Each composite outcome event separately;
Acute coronary disease (ACS) defined as hospital admission with a primary diagnosis of MI (ICD‐10 code I21) or of unstable angina (ICD‐10 Code I20.0);
Major bleeding, defined as hospitalization with primary diagnosis of bleeding events including haemorrhagic stroke, using the relevant ICD‐10 codes 12.
Study periods (Figure 1)
The time periods considered were: the period before the trigger event (previous history); the 1‐year period between the trigger event and the index date (baseline period); the period following the index date (follow‐up period).
Drug exposures
Drug exposures were identified by the dispensing of the relevant drug by ATC code. Mean medication possession rate was measured as the number of defined daily doses dispensed compared to the total number of days in the period considered. In patients with events, the period for the computation of medication possession rate was the time from index date to occurrence of event of interest.
Statistical analysis
Risk factors that were considered in the analysis were age (in years), sex, year of trigger event, existence of diabetes (as defined above), previous history of coronary disease (before trigger event), stroke, atrial fibrillation, heart failure, renal disease, hypertension, hospitalization for bleeding, chronic obstructive pulmonary disease (COPD), cancer, peripheral arterial disease. Diagnoses were identified from registration for chronic diseases (ALD) or hospital admissions. Drugs for secondary prevention (antiplatelet agents, aspirin, β‐blockers, angiotensin converting enzyme inhibitors and angiotensin receptor blockers, and statins) were used as adjustment variables.
Cumulative incidences of clinical outcomes were estimated using Kaplan–Meier estimates and 95% confidence interval (CI) for each of the populations. Risk factors were analysed using multiple Cox proportional hazards models, fully adjusted on risk factors and exposure to drugs in the baseline and follow‐up periods.
The main analysis concerned the post‐MI population, using MI as outcome. Sensitivity analyses used MI or unstable angina as outcomes.
All analyses were performed using SAS version 9.4.
Ethics and data protection
The EGB database contains only anonymous data, and its access is legally authorized without having to refer to the national data protection agency (CNIL). The study protocol was submitted to the appropriate INSERM and CNAMTS entities, as legally required. In addition, the study was declared to the European Network of Centres of Pharmacovigilance and Pharmacoepidemiology, ENCEPP (www.encepp.eu) at the European Medicines Agency, before the first data extraction (Study acronym HORUS, EUPAS 5816).
Independent data access and analysis
All data were extracted, managed, analysed and interpreted by the Bordeaux PharmacoEpi platform, INSERM CIC1401, University of Bordeaux.
Results
Study populations (Figure 2)
Post‐MI population
Among the 2226 adults with a hospital admission diagnosis of MI during 2005–2010 in EGB, 1585 patients (71% of the source population) had a 1‐year MI recurrence‐free period and were included in the stable post‐MI population, resulting in 3926 patient–years (PY) follow‐up.
PL subpopulation
Of the post‐MI patients above, 865 (55%) were included in the PL population, accruing 2114 PY during the follow‐up period.
Description of the populations: (Table 1)
Table 1.
Baseline characteristics in patients 1 year after trigger myocardial infarction (post‐MI), and in the PEGASUS‐like population (PL), compared to the placebo group in PEGASUS‐TIMI 54 9
| Post‐MI population n = 1585 | PL population n = 865 |
PEGASUS‐TIMI 54 placebo population 9
n = 7067 |
|
|---|---|---|---|
| Male: n (%) | 1085 (68.5) | 525 (60.7) | 5359 (75.7) |
| Mean age at index date (in years) (± SD) | 66.1 (14.5) | 73.8 (10.2) | 65.4 (8.3) |
| At least one of the following procedures, n (%) | 211 (13.3) | 116 (13.4) | / |
| Angiography, n (%) | 109 (6.9) | 63 (7.3) | |
| Percutaneous coronary intervention (PCI), n (%) | 104 (6.6) | 59 (6.8) | |
| Coronary artery bypass graft, n (%) | 31 (2.0) | 16 (1.8) | |
| Comorbidities before index date: n (%) | |||
| MI (prior to trigger event) | 243 (15.3) | 175 (20.2) | 1188 (16.8) |
| Hypertension | 833 (52.6) | 537 (62.1) | 5484 (77.6) |
| Diabetes | 365 (23.0) | 279 (32.3) | 2257 (31.9) |
| Heart failure | 358 (22.6) | 219 (25.3) | / |
| Atrial fibrillation | 203 (12.8) | 103 (11.9) | / |
| Cancer | 183 (11.5) | 119 (13.8) | / |
| Peripheral arterial disease (PAD) | 136 (8.6) | 74 (8.6) | 404 (5.7) |
| Chronic obstructive pulmonary disease (COPD) | 132 (8.3) | 89 (10.3) | current smoker |
| 1143 (16.2) | |||
| Hospitalized bleeding (any) | 123 (7.8) | 73 (8.4) | / |
| Drug exposure during the baseline period: | |||
| Coronary prevention treatment (ATC codes B01, C07, C09, C10), n (%) | 1556 (98.2) | 852 (98.5) | |
| Aspirin, n (%) | 1402 (88.5) | 772 (89.2) | 7057 (99.9%) |
| β‐blockers (ATC code C07), n (%) | 1364 (86.1) | 731 (84.5) | 5878 (83.2) |
| ACEI or ARBs (ATC code C09), n (%) | 1300 (82.0) | 719 (83.1) | 5697 (80.6) |
| Statins, n (%) | 1440 (90.9) | 781 (90.3) | 6583 (93.2) |
MI: myocardial infarction; PL population: PEGASUS‐TIMI 54‐like subpopulation; ACEI: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers
/: not applicable or not indicated in the PEGASUS publication. 9
At index date, 68% of patients in the stable post‐MI population were men, and the mean age was 66.1 years. Patients in the PL population were older on average (73.8 years), and 61% were men (Table 1). In the PEGASUS trial placebo arm, the mean age was 65 and 76% were male.
Nine percent of the patients had had myocardial revascularization (percutaneous coronary intervention, coronary artery bypass graft) during the baseline period between the trigger event and index date. This was similar in the MI and PL populations.
The most common comorbidities in the MI population were hypertension (53%), diabetes (23%), heart failure (23%), and a history of MI prior to trigger event (15%). Other risk factors such as atrial fibrillation, peripheral arterial disease or COPD were less common, around 13% or less.
Generally, the PL population had higher frequencies of risk factors, consistent with its selection process. In the PEGASUS study, the distribution of the reported risk factors was not very different, taking into account different diagnostic processes or coding systems.
Ninety‐eight percent of post‐MI patients received at least one medication recommended for secondary prevention during the baseline period. Individual drugs are shown in table 1. Post‐MI drug coverage at inclusion in PEGASUS was not different.
Over the follow‐up period, the exposure to secondary prevention drugs remained stable. 58% had clopidogrel with a mean possession ratio above 80%, mostly as dual antiplatelet treatment with aspirin, and 7% received anticoagulation with vitamin K antagonists.
Outcomes
MI and PL populations
The incidence rate of the composite criterion of all‐cause death, hospitalization for MI or stroke was 6.34 per 100 PY [95% CI (5.58–7.11)] for the entire post‐MI population, and 7.80 per 100 PY (6.66–8.95) in the PL population (Table 2). In PEGASUS the composite event rate (including cardiovascular rather than all‐cause death) was 3.01%.
Table 2.
Outcomes and incidence rates per 100 person–years during a 3‐year follow‐up after 1‐year event‐free survival post‐MI in the post‐MI or PEGASUS‐like (PL) populations, and in the PEGASUS‐TIMI 54 trial placebo arm
| Post‐MI population n = 3926 PY | PL population n = 2114 PY | PEGASUS‐TIMI 54 placebo population per 100 PYa | |
|---|---|---|---|
| Death, hospitalization for MI or stroke, n [% (95% CI)] | 249 [6.34 (5.58–7.11)] | 165 [7.80 (6.66–8.95)] | 3.01b |
| Death (all‐cause), n [% (95% CI)] | 199 [5.07 (4.38–5.76)] | 138 [6.53 (5.47–7.58)] | 1.72 |
| Myocardial infarction, n [% (95% CI)] | 40 [1.02 (0.70–1.33)] | 21 [0.99 (0.57–1.42)] | 1.75 |
| Acute coronary syndrome (MI or UA), n [% (95% CI)] | 98 [2.50 (2.01–2.98)] | 54 [2.55 (1.88–3.23)] | / |
| Stroke, n [% (95% CI)] | 24 [0.61 (0.37–0.86)] | 18 [0.85 (0.46–1.24)] | 0.65 |
| Severe bleeding, n [% (95% CI)] | 49 [1.25 (0.90–1.60)] | 29 [1.37 (0.88–1.87)] | 0.35 |
Data in PEGASUS are given as percent over 3 years, and have been recomputed to per 100 patient–years (PY).
Cardiovascular death, MI or stroke.
MI: myocardial infarction; UA: unstable angina;
PL population: PEGASUS‐TIMI 54 equivalent subpopulation; / data not provided
Death was the most frequent individual event, respectively 5.07 and 6.53 per 100 PY for the MI and PL populations. All‐cause death in the placebo arm of the PEGASUS trial was 1.72%, i.e., four times lower.
Rates of MI were similar: 1.02 and 0.99 per 100 PY in the MI and PL populations, and 1.75 in the placebo arm of the PEGASUS trial. Adding unstable angina as an outcome, there were 2.50 and 2.55 per 100 PY in MI and PL respectively. This was not provided for PEGASUS.
Rates of stroke were 0.61 and 0.85 per 100 PY in MI and PL, compared to 0.65 in PEGASUS trial.
The incidence of hospitalization for bleeding was respectively 1.25 and 1.37 per 100 PY for the MI and PL populations, and 0.35 in the placebo arm of PEGASUS (Table 2). It was 0.8 per 100 PY in the active arm of PEGASUS 9.
Death rate was very constant over time, whereas rates of MI and ACS tended to flatten out after 18 months (Figure 3). Bleeding rates were also very stable over time (Figure 4).
Figure 3.
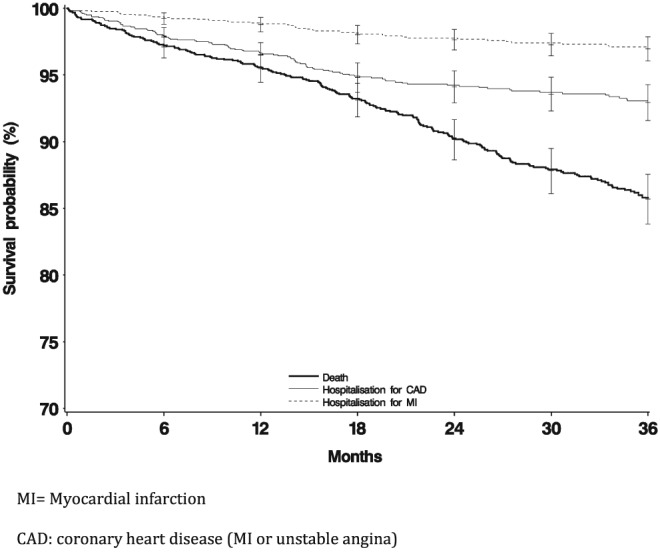
Event‐free survival in patients after myocardial infarction for death or coronary events
Figure 4.
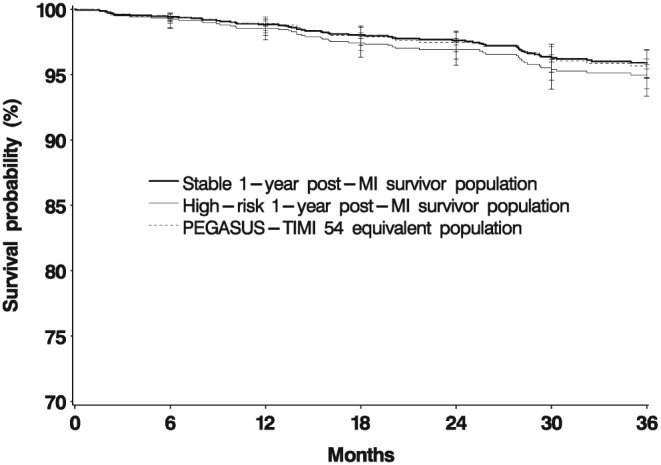
Event‐free survival in patients after myocardial infarction for major bleeding
Event predictors in the entire post‐MI population (Table 3)
Table 3.
Cox proportional hazards for the cardiac outcomes in the MI population in a fully adjusted model, n = 1585
| Death | Myocardial infarction | Acute coronary syndrome | |
|---|---|---|---|
| Outcomes: | n = 199 | n = 40 | n = 98 |
| Risk factors | HR (95%CI) | HR (95%CI) | HR (95%CI) |
| Age at index date (per year) | 1.05 (1.03–1.06) | 1.02 (0.99–1.04) | 1.01 (0.99–1.03) |
| Female | 0.80 (0.58–1.11) | 0.83 (0.40–1.71) | 0.72 (0.44–1.16) |
| Year of trigger event | 1.04 (0.95–1.14) | 1.02 (0.84–1.24) | 1.07 (0.95–1.21) |
| Diabetes | 1.53 (1.13–2.08) | 1.69 (0.85–3.39) | 1.65 (1.05–2.59) |
| ACS prior to trigger event | 1.40 (1.00–1.97) | 1.12 (0.52–2.40) | 1.33 (0.84–2.12) |
| Stroke | 0.94 (0.51–1.72) | / | 0.53 (0.13–2.18) |
| Atrial fibrillation | 1.49 (1.06–2.10) | 1.65 (0.69–3.92) | 0.86 (0.45–1.64) |
| Heart failure | 1.56 (1.14–2.14) | 1.09 (0.50–2.33) | 1.33 (0.82–2.17) |
| Renal disease | 1.59 (1.05–2.39) | 1.09 (0.30–3.90) | 0.86 (0.36–2.09) |
| Hypertension | 1.09 (0.78–1.53) | 0.74 (0.37–1.50) | 0.87 (0.56–1.37) |
| Hospitalized bleeding | 1.02 (0.65–1.58) | 0.52 (0.12–2.30) | 0.75 (0.34–1.67) |
| COPD | 1.58 (1.08–2.31) | 1.93 (0.78–4.74) | 2.33 (1.34–4.07) |
| Cancer | 2.01 (1.43–2.83) | 0.14 (0.02–1.06) | 0.64 (0.32–1.31) |
| PAD | 1.75 (1.18–2.61) | 2.13 (0.89–5.11) | 1.25 (0.66–2.38) |
HR: hazard ratio; CI: confidence interval.
MI: myocardial infarction; ACS: MI or unstable angina; HR: hazards ratio; COPD: chronic obstructive pulmonary disease; PAD: peripheral arterial disease.
Exposure to medication cited in Table 1 is included in the adjustment model.
In bold: hazard ratios whose lower 95% confidence interval does not overlap 1.
Death
All‐cause death was associated with age at inclusion [hazard ratio (HR) per year: 1.05, 95%CI (1.03–1.06)], cancer [HR 2.01 (1.43–2.83)], peripheral arterial disease [1.75 (1.18–2.61)], renal disease [1.59 (1.05–2.39)], COPD [1.58 (1.08–2.31)], heart failure [1.56 (1.14–2.14], diabetes mellitus [1.53 (1.13–2.08)], atrial fibrillation [1.49 (1.06–2.10)], and coronary disease prior to trigger event [1.40 (1.00–1.97)].
Coronary events
Recurrence of MI during follow‐up was not significantly affected by the studied risk factors, probably because of the small number of events (40 only); ACS recurrence (98 events) was associated with COPD [2.33 (1.34–4.07)] and diabetes [1.65 (1.05–2.59)].
Stroke and bleeding were not common enough to allow for an analysis of risk factors.
Discussion
This study includes two main information items:
A comparison of patients and outcomes in the PEGASUS‐TIMI 54 placebo arm and the similarly selected PL population in the present study.
Outcomes in patients with MI, beyond 1 year of event‐free survival, and factors associated with these outcomes.
In patients with chronic coronary heart disease, defined as event‐free for 1 year after the trigger MI, over 98% of the patients had some type of secondary coronary prevention treatment during the baseline period and during follow‐up. This is typical of the post‐MI population in France 14, 15, 16, 17, 18, 19, 20. Death was the most common outcome, around 4–5 per 100 PY. There was one recurrence of MI per 100 PY, and about 2.5 recurrences of ACS. Stroke rate was around 0.5 per 100 PY, lower than in other countries 21, 22, 23. Bleeding resulting in hospital admission occurred at a frequency of 1.5 per 100 PY. The event rates were generally stable over time.
Risk of death during follow‐up was increased by previous coronary history and by a number of other disease states such as heart failure, atrial fibrillation, peripheral arterial disease, diabetes, COPD or cancer. Diabetes and COPD, the latter certainly a proxy for chronic smoking (present or past), were associated with increased recurrence of ACS 24, whereas coronary history previous to the index MI was not significantly associated beyond 1 year.
We did not assess the effect of secondary prevention treatments in this study because of the very small number of nonusers, which would require much larger populations and specific statistical methods for each treatment class. However, they were included in the adjustment variables for the effect of risk factors. We did not attempt to compare the possible effect of long‐term exposure to antiplatelet drugs to the results of PEGASUS, because ticagrelor, the drug tested in PEGASUS, was not marketed in France at the time in this indication.
PL patients represented about one half of the stable post‐MI population. They were 8 years older than the rest of the population with proportionally more associated chronic diseases, as a consequence of the selection criteria. Patients in the PL population had a higher incidence of the composite outcome, which was related mostly to death, and to a lesser extent to stroke, as expected from the age difference. There was no difference between the whole population and the PL subpopulation in the incidence of repeat MI, which was around 1%.
Compared to the placebo arm of the PEGASUS‐TIMI 54 trial 9, we found that similarly selected patients in a database representative of the French general population were older and had higher risks of all‐cause death or bleeding, a lower risk of myocardial infarction, and the same risk of stroke. This was also found when comparing PEGASUS to a population of patients selected from intensive care units 20, who were in turn younger with a more favourable prognosis than patients from the national population database 18.
Even though they were in principle selected on the same high‐risk criteria, compared to our PEGASUS‐like population, patients in the PEGASUS trial were younger (age 65 vs. 74 years). Many of the factors we found associated with the occurrence of death, such as atrial fibrillation, heart failure or cancer, were not documented in the PEGASUS trial study results 9. The age difference could explain a doubling of the risk of death. Differences in other risk factors would be needed to explain the 4‐fold difference in death rates. Cancer is not mentioned in the noninclusion criteria for PEGASUS, but there is no mention of cancer rates among patients included in the study 9, 25. Cancer was a major driver for death here. In contrast, the event rate for MI was higher in the PEGASUS trial than in our comparable population, but lower than our rate for ACS. This may be related to diagnostic criteria and differences in coding: many patients with ACS might be adjudicated as MI based on for instance highly sensitive troponin. The main risk factors we found for the recurrence of MI or ACS were age, diabetes and COPD. The rate of diabetes was about the same here as in PEGASUS. COPD was not described in the PEGASUS trial, but the authors did provide rates of current smokers, which were indeed higher than our rates of COPD. It is not certain how one might translate into the other, but it is certain that the main cause for chronic bronchitis and COPD is chronic heavy smoking. If indeed COPD is a proxy for chronic smoking and for the tissue damage related to chronic smoking, then perhaps the population in PEGASUS was more at risk on this point than our population. Stroke was not different in both populations, and hypertension, the main driver for stroke, had similar rates (although the diagnostic criteria might be different).
Finally, the safety event, hospitalized bleeding, was rarer in the PEGASUS placebo population, and even in the PEGASUS active treatment population, than in our general population. This might be related to a selection of lower bleeding risk patients in the trial, since these patients were to be potentially randomized to an active antiplatelet agent. By contrast, 60% of our patients had clopidogrel during the follow‐up so one might expect that contraindications to the use of powerful antiplatelet agents would also be respected. Patients in a clinical trial might also be more actively and carefully monitored during treatment than real‐life patients. Against this hypothesis, in another study we found no difference in bleeding rates in patients with vitamin K antagonists in real life and in clinical trials 12.
These suggest that the application of selection criteria from PEGASUS to the French general population selected a different patient population, resulting in more deaths and bleeding in our PL population than in the PEGASUS placebo arm. It is another example of the uncertain applicability of clinical trial results in the general population (poor external validity). Patients with a short life expectancy 25 or potentially life‐threatening diseases are commonly not included in clinical trials. Similar results were found when the PEGASUS selection criteria were applied to British population databases 26, or to French patients recruited in intensive care units 20.
These differences do not alter the positive results found in PEGASUS, of course, especially for the occurrence of MI, but prescribers may find higher rates of death or bleeding in real‐life than expected, related to a different patient population in the clinical trial and in real‐life. It still remains to be shown whether the benefits found in the clinical trial are confirmed in these real‐life populations 26.
Study strengths and limitations
The advantages or strengths of our study are those of population‐wide claims databases based on national public healthcare systems: the database population is representative of the whole country population. There is no patient selection bias. Data acquisition is continuous and prospective, independent of the study and study objectives. The use of a single health registration number ensures continuous recording of all data pertaining to individual patients, even in the case of a change in employment status or nature. Because of the French healthcare structure, most medication are reimbursed and present in the database, including for instance low‐dose aspirin for cardiovascular prevention, and all inhabitants in the country are covered in the same way, irrespective of age or economic conditions.
EGB is the 1/97 permanent representative sample of the full SNIIRAM, which included 98.8% of the French population in 2016. There is therefore no question concerning the exhaustiveness or the representativeness of the database 10, 11.
Both EGB and SNIIRAM have been regularly used in pharmacoepidemiological studies, including in the cardiovascular field 12, 14, 18, 27, 28, 29.
The limitations are also those of claims databases: the data are primarily used for healthcare reimbursement purposes, and do not contain direct clinical information on items such as weight, BMI, smoking status, the results of lab tests or the indications for drug prescriptions. However, there is information on a number of chronic diseases that warrant full insurance coverage. Social deprivation is identified.
Hospital discharge diagnoses include main, secondary and associated diagnoses. The latter will include information on patient severity modifiers, such as organ failure or chronic diseases. In addition, various combinations of hospital diagnoses, chronic diseases, marker drugs and laboratory tests can give precise diagnostic indications 12, 13, 27, 28, 30. The validity of ICD‐10 codes for myocardial infarction and ACS has been verified 31. Other disease diagnoses have been similarly validated and the algorithms for their identification have been published 12, 28, 32, 33.
Outcomes were not individually adjudicated, which could result in differential classification. This might be especially apparent for myocardial infarction, the rate of which in PEGASUS falls between our event rates for MI and ACS. There may be some classification of MI as ACS in the present study, especially since there have been changes over time of the diagnostic criteria for MI 34.
Some known risk factors such as smoking or BMI are not documented, but proxies may be present such as COPD for smoking, or type 2 diabetes for BMI. Both were associated with an increased risk of recurrence of acute coronary syndrome.
Conclusion
Patients selected using the criteria described in PEGASUS were older with more comorbidities, resulting in higher all‐cause death and bleeding rates, but similar MI recurrence rates. Further studies will assess the applicability of the PEGASUS results in real‐life, when the drug has been on the market long enough for such information to accrue in the full national database.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: F.T.D. is a salaried employee of Astra‐Zeneca. N.M. is a salaried employee of University of Bordeaux and Bordeaux University Hospitals. N.D. is a salaried employee of University of Paris V and of the Paris Public Hospitals (Assistance Publique—Hôpitaux de Paris). The other authors are salaried personnel of ADERA (Association pour le Développement de l'Enseignement et de la Recherche en Aquitaine) a nonprofit organization that accompanies the Universities in Aquitaine, France. None of the authors received personal compensation for the study or this paper.
N.M. provided methodological advice on post‐authorisation studies to Sanofi in the previous 3–5 years. N.D. provided advice to Sanofi in the previous 3–5 years. Other authors report no direct financial relationships with any organization that might have an interest in the submitted work in the previous 3 years. There are other studies on‐going on the subject of secondary prevention after myocardial infarction, including with, but not limited to, AstraZeneca. In addition, the Department of Pharmacology has worked over the years on many topics with most pharmaceutical companies, including companies manufacturing or selling some of the drugs described in this study. All studies are based on contractual agreements between the sponsor and University of Bordeaux, and are consistent with the ENCEPP code of conduct.
The study was performed in collaboration with and funded by AstraZeneca, to provide information on the potential generalizability of the PEGASUS‐TIMI 54 Study. However, AstraZeneca had no influence on the methods or analysis of the study, nor on its interpretation. Data were analysed by Bordeaux PharmacoEpi, and are available upon request according to the ENCEPP principles. N.M. and P.B. had full access to all the study data, and wielded decisional power over data acquisition, and analysis, the decision to publish and the content of this paper.
The authors wish to thank all the persons inside and outside our department who have provided advice and insights making this study better.
The authors also thank ADERA, a nonprofit organisation that provides administrative, legal and human resources support for our research team, and without which this study would not have been possible.
Contributors
N.M., P.B. and F.T.D. had the initial idea for the study. P.B., N.M., R.L. and C.D.P. designed the study; J.J. and R.L. acquired, managed and analysed the data. C.D.P., N.D., N.M. and P.B. contributed to guiding study analysis and interpreting the results, and to the different drafts of the paper. All authors read and approved the final version of the paper.
Blin, P. , Dureau‐Pournin, C. , Lassalle, R. , Jové, J. , Thomas‐Delecourt, F. , Droz‐Perroteau, C. , Danchin, N. , and Moore, N. (2017) Outcomes in patients after myocardial infarction similar to those of the PEGASUS‐TIMI 54 trial: A cohort study in the French national claims database. Br J Clin Pharmacol, 83: 2056–2065. doi: 10.1111/bcp.13291.
Study registration: EUPAS 5816, European Medicines Agency, www.encepp.eu
References
- 1. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130: e344–e426. [DOI] [PubMed] [Google Scholar]
- 2. Boggon R, van Staa TP, Timmis A, Hemingway H, Ray KK, Begg A, et al. Clopidogrel discontinuation after acute coronary syndromes: frequency, predictors and associations with death and myocardial infarction – a hospital registry‐primary care linked cohort (MINAP‐GPRD). Eur Heart J 2011; 32: 2376–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stephenson JJ, Chang CL, Devecchis Wygant G, Hauch O, Cziraky MJ. Incidence of death and recurring acute coronary syndrome after stopping clopidogrel therapy in a large commercially‐insured population in the US. Curr Med Res Opin 2011; 27: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 4. Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354: 1706–1717. [DOI] [PubMed] [Google Scholar]
- 5. Berger JS, Bhatt DL, Steg PG, Steinhubl SR, Montalescot G, Shao M, et al. Bleeding, mortality, and antiplatelet therapy: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J 2011; 162: 98–105 e1. [DOI] [PubMed] [Google Scholar]
- 6. Berger PB, Bhatt DL, Fuster V, Steg PG, Fox KA, Shao M, et al. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 2010; 121: 2575–2583. [DOI] [PubMed] [Google Scholar]
- 7. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med 2014; 371: 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49: 1982–1988. [DOI] [PubMed] [Google Scholar]
- 9. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 10. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique 2010; 58: 286–290. [DOI] [PubMed] [Google Scholar]
- 11. Moulis G, Lapeyre‐Mestre M, Palmaro A, Pugnet G, Montastruc JL, Sailler L. French health insurance databases: what interest for medical research? Rev Med Interne 2015; 36: 411–417. [DOI] [PubMed] [Google Scholar]
- 12. Blin P, Dureau‐Pournin C, Lassalle R, Abouelfath A, Droz‐Perroteau C, Moore N. A population database study of outcomes associated with vitamin K antagonists in atrial fibrillation before DOAC. Br J Clin Pharmacol 2016; 81: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blin P, Lassalle R, Dureau‐Pournin C, Ambrosino B, Bernard MA, Abouelfath A, et al. Insulin glargine and risk of cancer: a cohort study in the French National Healthcare Insurance Database. Diabetologia 2012; 55: 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bezin J, Pariente A, Lassalle R, Dureau‐Pournin C, Abouelfath A, Robinson P, et al. Use of the recommended drug combination for secondary prevention after a first occurrence of acute coronary syndrome in France. Eur J Clin Pharmacol 2014; 70: 429–436. [DOI] [PubMed] [Google Scholar]
- 15. Avendano M, Glymour MM, Banks J, Mackenbach JP. Health disadvantage in US adults aged 50 to 74 years: a comparison of the health of rich and poor Americans with that of Europeans. Am J Public Health 2009; 99: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tuppin P, Neumann A, Danchin N, de Peretti C, Weill A, Ricordeau P, et al. Evidence‐based pharmacotherapy after myocardial infarction in France: adherence‐associated factors and relationship with 30‐month mortality and rehospitalization. Arch Cardiovasc Dis 2010; 103: 363–375. [DOI] [PubMed] [Google Scholar]
- 17. Tuppin P, Neumann A, Danchin N, Weill A, Ricordeau P, de Peretti C, et al. Combined secondary prevention after hospitalization for myocardial infarction in France: analysis from a large administrative database. Arch Cardiovasc Dis 2009; 102: 279–292. [DOI] [PubMed] [Google Scholar]
- 18. Massoullie G, Wintzer‐Wehekind J, Chenaf C, Mulliez A, Pereira B, Authier N, et al. Prognosis and management of myocardial infarction: Comparisons between the French FAST‐MI 2010 registry and the French public health database. Arch Cardiovasc Dis 2016; 109: 303–310. [DOI] [PubMed] [Google Scholar]
- 19. Puymirat E, Riant E, Aissoui N, Soria A, Ducrocq G, Coste P, et al. Beta blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ 2016; 354: i4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puymirat E, Schiele F, Zeller M, Jacquemin L, Leclercq F, Marcaggi X, et al. Do randomized clinical trial selection criteria reflect levels of risk as observed in a general population of acute myocardial infarction survivors? The PEGASUS trial in the light of the FAST‐MI 2005 registry. Int J Cardiol 2016; 223: 604–610. [DOI] [PubMed] [Google Scholar]
- 21. Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, et al. Acute myocardial infarction: a comparison of short‐term survival in national outcome registries in Sweden and the UK. Lancet 2014; 383: 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rapsomaniki E, Thuresson M, Yang E, Blin P, Hunt P, Chung SC, et al. Using big data from health records from four countries to evaluate chronic disease outcomes: a study in 114 364 survivors of myocardial infarction. Eur Heart J—Quality of Care and Clinical Outcomes 2016; 2: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jernberg T, Hasvold P, Henriksson M, Hjelm H, Thuresson M, Janzon M. Cardiovascular risk in post‐myocardial infarction patients: nationwide real world data demonstrate the importance of a long‐term perspective. Eur Heart J 2015; 36: 1163–1170. [DOI] [PubMed] [Google Scholar]
- 24. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades‐Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 2015; 3: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonaca MP, Bhatt DL, Braunwald E, Cohen M, Steg PG, Storey RF, et al. Design and rationale for the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin‐Thrombolysis in Myocardial nfarction 54 (PEGASUS‐TIMI 54) trial. Am Heart J 2014; 167: 437–444 e5. [DOI] [PubMed] [Google Scholar]
- 26. Timmis A, Rapsomaniki E, Chung SC, Pujades‐Rodriguez M, Moayyeri A, Stogiannis D, et al. Prolonged dual antiplatelet therapy in stable coronary disease: comparative observational study of benefits and harms in unselected versus trial populations. BMJ 2016; 353: i3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neumann A, Maura G, Weill A, Ricordeau P, Alla F, Allemand H. Comparative effectiveness of rosuvastatin versus simvastatin in primary prevention among new users: a cohort study in the French national health insurance database. Pharmacoepidemiol Drug Saf 2014; 23: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tuppin P, Cuerq A, de Peretti C, Fagot‐Campagna A, Danchin N, Juilliere Y, et al. Two‐year outcome of patients after a first hospitalization for heart failure: a national observational study. Arch Cardiovasc Dis 2014; 107: 158–168. [DOI] [PubMed] [Google Scholar]
- 29. Maura G, Blotiere PO, Bouillon K, Billionnet C, Ricordeau P, Alla F, et al. Comparison of the short‐term risk of bleeding and arterial thromboembolic events in nonvalvular atrial fibrillation patients newly treated with dabigatran or rivaroxaban versus vitamin K antagonists: a French nationwide propensity‐matched cohort study. Circulation 2015; 132: 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bernard MA, Benichou J, Blin P, Weill A, Begaud B, Abouelfath A, et al. Use of health insurance claim patterns to identify patients using nonsteroidal anti‐inflammatory drugs for rheumatoid arthritis. Pharmacoepidemiol Drug Saf 2012; 21: 573–583. [DOI] [PubMed] [Google Scholar]
- 31. Bezin J, Girodet PO, Rambelomanana S, Touya M, Ferreira P, Gilleron V, et al. Choice of ICD‐10 codes for the identification of acute coronary syndrome in the French hospitalization database. Fundam Clin Pharmacol 2015; 29: 586–591. [DOI] [PubMed] [Google Scholar]
- 32. Weill A, Paita M, Tuppin P, Fagot JP, Neumann A, Simon D, et al. Benfluorex and valvular heart disease: a cohort study of a million people with diabetes mellitus. Pharmacoepidemiol Drug Saf 2010; 19: 1256–1262. [DOI] [PubMed] [Google Scholar]
- 33. Tuppin P, Cuerq A, de Peretti C, Fagot‐Campagna A, Danchin N, Juilliere Y, et al. First hospitalization for heart failure in France in 2009: patient characteristics and 30‐day follow‐up. Arch Cardiovasc Dis 2013; 106: 570–585. [DOI] [PubMed] [Google Scholar]
- 34. Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009; 361: 858–867. [DOI] [PubMed] [Google Scholar]


