Abstract
Aims
The aims of the study were to: 1) determine if a plasma oxypurinol concentration–response relationship or an allopurinol dose–response relationship best predicts the dose requirements of allopurinol in the treatment of gout; and 2) to construct a nomogram for calculating the optimum maintenance dose of allopurinol to achieve target serum urate (SU) concentrations.
Methods
A nonlinear regression analysis was used to examine the plasma oxypurinol concentration– and allopurinol dose–response relationships with serum urate. In 81 patients (205 samples), creatinine clearance (CLCR), concomitant diuretic use and SU concentrations before (UP) and during (UT) treatment were monitored across a range of allopurinol doses (D, 50–700 mg daily). Plasma concentrations of oxypurinol (C) were measured in 47 patients (98 samples). Models (n = 47 patients) and predictions from each relationship were compared using F‐tests, r 2 values and paired t‐tests. The best model was used to construct a nomogram.
Results
The final plasma oxypurinol concentration–response relationship (UT = UP – C*(UP – UR)/(ID50 + C), r 2 = 0.64) and allopurinol dose–response relationship (UT = UP – D*(UP – UR)/(ID50 + D), r 2 = 0.60) did not include CLCR or diuretic use as covariates. There was no difference (P = 0.87) between the predicted SU concentrations derived from the oxypurinol concentration– and allopurinol dose–response relationships. The nomogram constructed using the allopurinol dose–response relationship for all recruited patients (n = 81 patients) required pretreatment SU as the predictor of allopurinol maintenance dose.
Conclusions
Plasma oxypurinol concentrations, CLCR and diuretic status are not required to predict the maintenance dose of allopurinol. Using the nomogram, the maintenance dose of allopurinol estimated to reach target concentrations can be predicted from UP.
Keywords: allopurinol, creatinine clearance, diuretics, gout, oxypurinol, urate
What is Already Known about this Subject
Allopurinol is first‐line treatment for gout; its urate‐lowering effect is due to its active metabolite, oxypurinol.
Allopurinol should be initiated at a low dose, based on renal function, and then slowly titrated upwards until a target serum urate concentration is achieved.
The maintenance dose of allopurinol may be dependent on the pretreatment serum urate concentrations; however, there has yet to be any consideration of the influence of plasma oxypurinol concentrations.
What this Study Adds
The predicted serum urate concentrations derived from the plasma oxypurinol concentration– and allopurinol dose–response relationships were similar.
Overall, renal function and concomitant diuretic use did not influence the urate‐lowering effect of oxypurinol and allopurinol from baseline values.
A simple nomogram has been constructed that can be used to estimate the final maintenance dose of allopurinol needed to achieve a target serum urate concentration in an individual patient requiring only the patient's pretreatment serum urate concentration.
Tables of Links
| TARGETS |
|---|
| Enzymes 2 |
| Xanthine oxidoreductase |
| LIGANDS | |
|---|---|
| Allopurinol | Uric acid |
| Furosemide | Vitamin C |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http: //www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1, and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2.
Introduction
Allopurinol is the first‐line treatment for gout. It is metabolised by aldehyde oxidase to oxypurinol, which is an inhibitor of xanthine oxidoreductase (XOR), thereby decreasing the synthesis of uric acid (urate at physiological pH) 3. Gout is strongly associated with increasing serum urate (SU) concentrations above 0.42 mmol l–1 (~7 mg dl–1) 4, 5. The recommended SU target is <0.36 mmol l–1 (~6 mg dl–1; European League Against Rheumatism 6; American College of Rheumatology 7; 3e initiative 8) for recurrent gout attacks and SU < 0.30 mmol l–1 for chronic tophaceous gout 7, 8.
A low dosage (50–100 mg) of allopurinol is recommended on initiation of therapy and this starting dose should be based on renal function 7, 8. This guidance is based on a putative reduction in the risk of hypersensitivity to allopurinol when the starting dose is low and proportional to the creatinine clearance (CLCR) 9. However, guidance on how to predict the maintenance dose of allopurinol is less certain, particularly in patients with renal impairment. Following the observation of cutaneous adverse effects of allopurinol in patients, particularly in patients with renal impairment, Hande et al. 10 suggested a dosage regimen with a reduced dosage at lower creatinine clearances. However, this dosage regimen substantially reduces attainment of target SU concentrations and, more recently, it has been recommended that the daily dose of allopurinol should be titrated upwards until the chosen target SU concentration is achieved irrespective of renal function 11, 12.
We have previously published a dose–response model for allopurinol indicating that the maintenance dose required to achieve target SU concentrations is dependent on the pretreatment urate concentration (UP) 13. Our model revealed that there is a limit to the reduction of SU, indicating an apparent ‘resistance’ to allopurinol‐mediated inhibition of XOR (UR). Increasing the dose of allopurinol will not achieve concentrations of SU lower than this asymptotic level. This is perhaps due to the reduced binding of oxypurinol on glycosaminoglycan‐bound XOR 14.
Surprisingly, renal function (measured by CLCR) was not a significant covariate in our model. Also of note was the unexpected finding that diuretics do not significantly affect the dose–response relationship despite the known urate‐raising effects of diuretics 15, 16. These discrepancies may be due to the small number of patients with renal impairment and/or concomitant diuretics use in the cohort of patients used to construct the model. Furthermore, our former study did not include plasma oxypurinol concentrations that may improve the ability of the model to relate allopurinol maintenance dose to serum urate concentrations.
The aim of this study was to determine if a plasma oxypurinol concentration–response relationship or an allopurinol dose–response relationship better predicts the maintenance dose requirements of allopurinol in the treatment of gout. In order to undertake this comparison and extend our previous work, we have obtained data with a greater proportion of subjects with reduced renal function and concomitant diuretic use and included data on plasma concentrations of oxypurinol for a subset of these patients. An additional aim was to construct a nomogram to facilitate selection of an effective maintenance dose of allopurinol to achieve target SU for an individual patient.
Methods
Patients and study design
The data for the present study were sourced from four cohorts of patients with gout. All patients gave consent in accordance with the Declaration of Helsinki. All studies were approved by local ethics committees (St Vincent's Hospital Research Ethics Committee, New Zealand Upper South A Regional Ethics Committee, New Zealand Health and Disabilities Ethics Committee and the University of Otago Ethics Committee). The groups of patients were:
Forty‐six patients (111 blood samples) from an observational dose‐escalation study conducted in Sydney, Australia, in which patients had their dosage of allopurinol titrated after being on a fixed dose level for 14–31 days 13.
Eight patients (20 blood samples) from a randomised controlled trial conducted in Christchurch, New Zealand comparing the effects of vitamin C and allopurinol on serum urate concentrations 17.
Eight patients (29 blood samples) from an unpublished observational dose‐escalation study conducted in Christchurch, New Zealand where the dose of allopurinol was titrated until the target SU concentration of <0.36 mmol l–1 was achieved.
Nineteen patients (45 blood samples) from a study that examined the urate‐lowering response upon initiation of allopurinol therapy conducted in Christchurch, New Zealand 18, 19.
In total, data (pretreatment and during allopurinol treatment) were available for 81 patients (205 samples). Patients were on a stable dose of allopurinol for at least 1 month prior to blood collections. Blood was collected prior to each dose titration of allopurinol.
Analysis of plasma and serum
For the study conducted in Australia, SU concentrations were determined using standard methodologies on the Roche/Hitachi Modular P Analyser Platform, Roche Diagnostics, Australia (Douglass Hanly Moir Pathology Laboratories and SydPath, St Vincent's Hospital, Sydney). The SU concentrations from the New Zealand studies were measured using a modified Trinder method by Canterbury Health Laboratories, Christchurch, New Zealand 20.
Plasma oxypurinol concentrations for all studies were determined by a high‐performance liquid chromatography assay described by Stocker et al. 21 (Australian study) and Stamp et al. 22 (New Zealand studies). The intra‐ and interday variation for both assays were <10.1% and the lower limit of quantification was 2 mg l–1 (13.1 μmol l–1) 21 and 0.1 mg l–1 (0.7 μmol l–1) 22. Interlaboratory blinded analyses of six plasma oxypurinol samples in Australia and New Zealand were very similar, with differences ranging from 1.5 to 7.3%.
Mean plasma concentrations of oxypurinol over a dosage interval
To determine the mean steady‐state plasma oxypurinol concentrations in each individual patient at each dosage rate, the pharmacokinetic parameter estimates from a published population pharmacokinetic model were put into a Bayesian forecasting program, TCIWorks version 1.0 20, 23. Demographics (age, sex, height and weight), allopurinol dosage history and plasma oxypurinol concentrations (including the time of blood sampling) for each patient were entered into TCIWorks, from which an individualised oxypurinol clearance (CLOXY) was predicted by the program. Mean steady state oxypurinol concentrations (C) over a dosage interval were then estimated using Equation (1):
| (1) |
where CLOXY, is the individualised clearance of oxypurinol (l h–1); D, the dose of allopurinol (mg day–1); C, the mean steady‐state oxypurinol concentration (mg l–1). This technique allows the estimation of the mean plasma concentration of oxypurinol at any known time. As CLOXY was predicted through Bayesian forecasting methods, the time of collection does not have to be prespecified.
CLCR
CLCR was used as the estimate of the glomerular filtration rate for each patient, and was calculated using the Cockcroft and Gault formula 24 and using fat‐free mass as a body size descriptor 25.
Nonlinear modelling
Through nonlinear regression, we used our previously published, modified, maximum‐inhibition model 13 to examine the relationships between oxypurinol concentration and allopurinol dose with pretreatment SU concentrations (UP) and SU concentrations achieved during treatment with allopurinol, UT (Equations (2) and (3), respectively).
| (2) |
Equation (2) is the oxypurinol concentration–response relationship. C is the mean steady state plasma oxypurinol concentration in each patient (mg l–1); IC50, concentration of oxypurinol that has reduced the inhibitable urate concentration (UP – UR) by 50% (mg l–1); UR, resistant serum urate concentration (mmol l–1). UT and UP were clinical measures obtained from each patient. UR and IC50 are mean optimal parameters estimated for the whole group of patients.
| (3) |
Equation (3) is the allopurinol dose–response relationship. D is the daily dose of allopurinol (mg day−1) which inhibits the inhibitable urate by 50% (mg day−1). Other parameters are described above.
Effect of UR on the response to allopurinol:
UR is a term to describe an apparent SU concentration that exists during maximal inhibition of XOR with allopurinol. To test if the UR is a significant term for the concentration–response relationship and in the dose–response relationship, the fits to Equations (2) and (3) were compared with and without the UR term (i.e. UR = 0), respectively.
Effect of CLCR on the response to allopurinol:
To examine whether renal function influenced the plasma oxypurinol concentration– and allopurinol dose–response relationships, respectively, an additive CLCR covariate (k* CLCR) was tested, where k was an estimated linear correction variable (Equations (4) and (5)). Other parameters are described above.
| (4) |
| (5) |
In addition to incorporating CLCR as a covariate in the modelling procedure, UT values were compared in two groups of patients; those with CLCR < 60 ml min–1 and those with CLCR > 60 ml min–1.
Effect of concomitant diuretics on the response to allopurinol:
To examine whether concomitant diuretics (thiazide and furosemide) influenced the SU lowering effect of allopurinol, a fractional effect variable (+ d*DIU, where d is an estimated constant, DIU = 0 for patients not taking a diuretic and 1 for patients taking a diuretic) was added to the dose‐ and concentration–response equations (Equations (6) and (7)). From this analysis, the mean estimates and 95% CIs for UR and ID50 were examined for significant changes.
| (6) |
| (7) |
where d is a covariate constant for fractional effect of diuretic; DIU is a binary variable for concomitant diuretic use. Other parameters are described above. The optimal oxypurinol concentration– and allopurinol dose–response relationships were compared to determine any differences in their SU predictions.
Nomogram construction
The optimal oxypurinol concentration– and allopurinol dose–response relationships were compared to determine any differences in their SU predictions. From this comparison, the most suitable model was used to construct a nomogram. The dose of allopurinol predicted to reach target serum urate concentrations was calculated through a rearrangement of the concentration– or dose–response relationship.
Statistical analysis
The regression parameter estimates (ID50, UR, k, d) describing the relationships between UT, UP, C and D and the objective function value (also known as the residual sum of squares, RSS) were obtained using R version 3.2.3 26. The correlation coefficients (r) between the parameter estimates were also determined using R version 3.2.3. The r 2 (coefficient of determination) was calculated based on its definition: r 2 = 1 – (RSS/TSS), where RSS is the residual sum of squares and TSS is the total sum of squares. Statistical differences between regressions, baseline urate concentrations and predicted serum urate concentrations were determined by F‐tests using the objective function values, unpaired and paired t‐tests, respectively. Also, a nonparametric bootstrap analysis with 1000 replicates was conducted using R version 3.2.3 in order to determine the robustness of the model. All analyses were conducted on pooled data (i.e. from all doses in all patients).
Results
Patient demographics
Baseline demographics for cohorts from Australia, New Zealand and combined are detailed in Table 1. The most common daily doses were 100, 200 and 300 mg allopurinol (Figure 1). Nine of these patients were maintained on one dose rate, and the remaining patients were studied at two (n = 30), three (n = 33), four (n = 6) and five (n = 3) different doses with blood samples taken at each dose rate. Oxypurinol concentrations were measured in 47 patients (98 blood samples). The mean (± standard deviation) plasma oxypurinol concentration was 10.8 (± 7.4) mg l–1 and plasma oxypurinol concentrations ranged from 2.8–37.8 mg l–1. Nineteen (23%) patients achieved a target urate concentration of ≤0.30 mmol l–1, 56 (69%) patients achieved serum urate concentrations ≤0.36 mmol l–1, and 68 patients (84%) achieved serum concentrations of urate ≤0.42 mmol l–1.
Table 1.
Demographics of Australian and New Zealand gout patients
| Australia (n = 46) | New Zealand (n = 35) | All patients (n = 81) | |
|---|---|---|---|
| Age, years (range) | 57.5 (17–80) | 60.6 (34–82) | 58.8 (17–82) |
| Sex (m: f) | 41: 5 | 27: 8 | 68: 13 |
| CL CR , ml min –1 , n (%) | |||
| >90 | 9 (20) | 2 (6) | 11 (14) |
| 60–89 | 21 (46) | 13 (37) | 34 (42) |
| 45–59 | 8 (17) | 6 (17) | 14 (17) |
| 30–44 | 4 (9) | 10 (29) | 14 (17) |
| 15–29 | 3 (7) | 4 (11) | 7 (9) |
| <15 | 1 (2) | 0 (0) | 1 (1) |
| Diuretics, n (%) | 10 (22) | 12 (34) | 22 (27) |
| Maximum allopurinol dose, mg day –1 , mean (standard deviation) | 310 (151) | 251 (134) | 285 (147) |
| Serum urate (SU) concentration mmol l –1 , mean (standard deviation) | 0.58 (0.13) | 0.58 (0.10) | 0.58 (0.12) |
Figure 1.
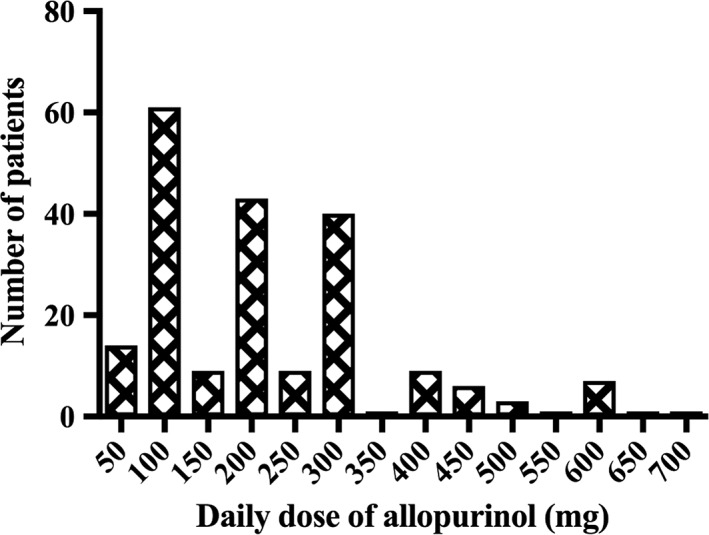
Distribution of allopurinol dosage in gout patients. A total of 205 dosage regimens were studied in 81 patients. The doses were increased in most patients
In patients taking diuretics, the mean UP was significantly higher than in patients not taking diuretics (Table 2). UP values were lower in patients with low renal function (< 60 ml min–1) than in patients with higher renal function (> 60 ml min–1). There was a clear correlation between Coxy and CLCR (Figure 2A) but a much weaker correlation between UP and CLCR (Figure 2B).
Table 2.
Effect of diuretics and creatinine clearance (CLCR) on baseline SU. Statistical significance determined by unpaired t‐test
| Diuretic or renal function | SU concentrations before dosage with allopurinol (U P ) Mean (95% CI, number of patients). |
|---|---|
| Diuretic dosage | |
| No | 0.54 (0.52–0.57, 59) |
| Yes | 0.67 (0.60–0.73, 22) |
| P < 0.0001 | |
| CL CR ≤ 60 ml min –1 | 0.61 (0.56–0.66, 37) |
| CL CR > 60 ml min –1 | 0.55 (0.52–0.57, 44) |
| P = 0.02 |
Figure 2.
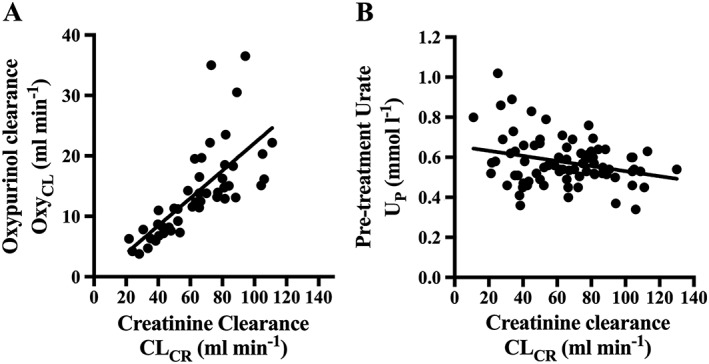
Correlations of creatinine clearance (CLCR) with A) the clearance of oxypurinol (CLOXY) and B) serum urate (SU) concentration. The black line indicates the linear regression line. A) r 2 = 0.51, P < 0.0001; B) r 2 = 0.078, P = 0.012
Oxypurinol concentration–response modelling
In the patients for whom plasma oxypurinol concentrations were measured (n = 47 patients, 98 data points), the plasma oxypurinol concentration–response relationships with SU were analysed.
Effect of UR :
The concentration–response models had a significantly improved fit when the (UR) concentration term was estimated by equation (2). This was in contrast to the fit without the term (i.e. fixing UR = 0; Table 3). For equation (2) (including UR, r 2 = 0.64), the mean IC50 was 11.3 mg and the UR was 0.17 mmol l–1. There was a good correlation between the observed and the predicted concentrations of urate during treatment with allopurinol for the concentration–response relationship (slope = 1.01, Figure 3A) Subsequent modelling of the plasma oxypurinol concentration–response relationship included the UR term.
Table 3.
Best‐fit parameters for the oxypurinol concentration– and allopurinol dose–response relationships with serum urate in gout patients (47 patients, 98 plasma samples)
| ID 50 [Allopurinol mg dose‐resp, mg] or IC 50 [Oxypurinol mg l –1 conc–response] (95% CI) | U R , mmol l –1 (95% CI) | k or d a (95% CI) | r 2 , OFV | P‐value | |
|---|---|---|---|---|---|
| Concentration–response | |||||
| No U R (Eqn (2) , U R = 0) | 21.0 (19.2–23.0) | 0 | ‐ | 0.60, 0.29 | ‐ |
| With U R (Eqn (2) ) | 11.3 (8.0–16.2) | 0.17 (0.09–0.23) | ‐ | 0.64, 0.26 | 7.71 × 10–4 b |
| With U R , k*CL CR (Eqn (4) ) | 11.7 (8.1–17.1) | 0.18 (0.09–0.25) | –1.0 × 10–4 (–4.0 × 10–4 – 2.5 × 10–4) | 0.66, 0.25 | 0.51c |
| With U R , d*DIU ( Eqn (6) ) | 10.6 (7.4–15.3) | 0.18 (0.10–0.24) | 0.01 (–0.01–0.04) | 0.64, 0.25 | 0.26c |
| Dose–response | |||||
| No U R (Eqn (3) , U R = 0) | 417 (371–472) | 0 | ‐ | 0.36, 0.47 | ‐ |
| With U R (Eqn (3) ) | 117 (82–163) | 0.26 (0.22–0.30) | ‐ | 0.60, 0.29 | 7.27 × 10–12 d |
| With U R , k*CL CR (Eqn (5) ) | 112 (81–153) | 0.22 (0.17–0.27) | 4.5 × 10–4 (4.8 × 10–5 – 8.6 × 10–4) | 0.62, 0.28 | 0.03e |
| With U R , d * DIU ( Eqn (7) ) | 121 (83–176) | 0.26 (0.22–0.30) | ‐0.01 (–0.03–0.02) | 0.59, 0.29 | 0.67e |
CLCR, creatinine clearance; d, covariate constant for diuretic use; DIU, concomitant diuretic use; Eqn, equation; IC50, the concentration of oxypurinol that has reduced the inhibitable urate concentration (UP – UR) by 50%; ID50, the dose of allopurinol that has reduced the inhibitable urate concentration (UP – UR) by 50%; k, covariate constant for CLCR; OFV, objective function value; r, correlation coefficient; r 2, coefficient of determination; UP, pretreatment serum urate concentration; UR, ‘resistant’ serum urate concentration.
covariate constants depending on whether CLCR (k) or DIU (d) were entered as covariates.
P‐value compared to fit to Equation (2) where UR = 0.
P‐value compared to fit to Equation (2).
P‐value compared the fit to Equation (3) where UR = 0.
p‐value compared the fit to Equation (3).
Figure 3.
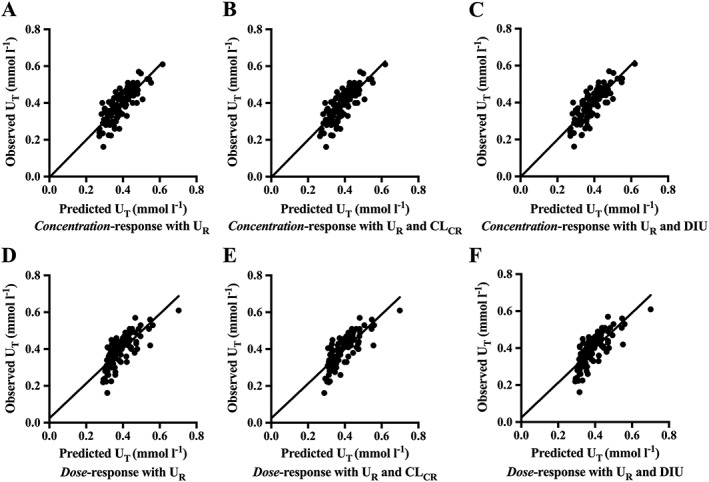
Comparisons of the observed vs. predicted serum urate concentrations during treatment (UT) of the oxypurinol concentration– and allopurinol dose–response relationships with serum urate in gout patients (47 patients, 98 data points). The black line indicates the regression line. The y‐intercepts for Figures 3A–F were not significantly different to zero. A) oxypurinol concentration–response model with UR, r 2 = 0.64, slope = 1.01 (95% CI 0.86–1.17); B) oxypurinol concentration–response model with UR and CLCR, r 2 = 0.65, slope = 1.02 (95% CI 0.86–1.17); C) oxypurinol concentration–response model with UR and DIU, r 2 = 0.65, slope = 1.01 (95% CI 0.86–1.17); D) allopurinol dose–response model with UR, r 2 = 0.60; slope = 0.94 (95% CI 0.79–1.10); E) allopurinol dose–response model with UR and CLCR, r 2 = 0.62, slope = 0.94 (95% CI 0.79–1.09); F) allopurinol dose–response model with UR and DIU, r 2 = 0.60; slope = 0.94 (95% CI 0.79–1.10). CLCR, creatinine clearance; DIU, binary concomitant diuretic use variable; r 2, coefficient of determination; UR, ‘resistant’ serum urate concentration
Effect of CLCR:
Incorporating CLCR (Equation (4)) did not improve the fit of the oxypurinol concentration–response model (Table 3). The estimated linear correction variable, k, was not significantly different from 0 (Table 3). Visually, the agreement between the observed and the predicted concentrations of urate during treatment with allopurinol was unchanged when CLCR was included as a covariate (Figure 3B vs. Figure 3A). The increase in the coefficient of determination was also very small (r 2 = 0.65 vs. 0.64). Furthermore, the fit to equation (3) in patients with CLCR > 60 ml min–1 vs. CLCR ≤ 60 ml min–1, and the IC50 and UR values in both groups were similar (Supplementary Table S1).
Effect of concomitant diuretic use:
The addition of concomitant diuretic use as a covariate in the concentration–response relationship (Equation (6)) did not improve the fit (Table 3). The estimated fractional effect constant (d) of taking a diuretic was 0.01 (95% CI: –0.01 to 0.04). The correlation between the observed and the predicted concentrations of urate during treatment with allopurinol was similar when concomitant diuretic use was and was not included as a covariate in the plasma concentration–response relationship (r 2 = 0.65, Figure 3C).
Therefore, the final oxypurinol concentration–response relationship included the UR term but did not include CLCR or concomitant diuretic use as covariates.
Allopurinol dose–response modelling
To allow for appropriate comparison of the predicted serum urate concentration obtained with the oxypurinol concentration–response relationship and the allopurinol dose–response relationship, the model building process was conducted using the same cohort of patients (n = 47 patients; i.e. the subset of patients with plasma oxypurinol concentrations measured).
Effect of UR:
The allopurinol dose–response relationship yielded a significantly improved fit to Equation (3) when UR was estimated as opposed to being fixed to 0, i.e. UR = 0 (Table 3). In this dose–response relationship, the mean ID50 was 117 mg day–1. The observed vs. predicted UT for equation (3) fitted well (r 2 = 0.60, slope = 0.94, Figure 3D).
Effect of CLCR:
Incorporating clearance (k*CLCR) into the relationship improved the fit of the allopurinol dose–response equation (Equation (5) vs. Equation (3), Table 3). Visually, the fit to Equations (5) and (3) were very similar (Figure D,E). The linearity of the observed vs. predicted UT slope remained unchanged and the r2 improved to 0.62 from 0.60 when CLCR was entered as a covariate (Figure 3E). The similarity of the fits to Equations (5) and (3) was examined quantitatively by comparing the predicted values of UT for the fits to these equations. In this case, the predicted mean difference (95% CI) in the UT values was 0.0015 mmol l–1 (–0.0009 to 0.003) and was not statistically significant (P = 0.23, paired t test).
There were no significant differences in the ID50 and UR when Equation (3) was tested in patients with CLCR ≤ 60 ml min–1 vs. CLCR > 60 ml min–1 (Supplementary Table S1).
Effect of concomitant diuretic use:
Concomitant diuretic use as a covariate did not significantly improve the dose–response relationship (Table 3). The fractional effect constant (95% CI) for diuretics was –0.01 (–0.03 to 0.02; Table 3). Visually, there was no difference in the regressions between the observed and predicted UT during treatment with allopurinol when concomitant diuretic use was and was not included as a covariate (r 2 = 0.60, Figure 3F vs. Figure 3D).
Therefore, the final allopurinol dose–response model including only UR was used in subsequent analyses.
Comparison of the oxypurinol concentration– and allopurinol dose–response relationships
The observed vs. the model‐predicted UT in the final concentration– and dose–response relationships (Equations (2) and (3)) were compared against each other (Figure 3A,D). The slopes for both the plasma oxypurinol concentration– and allopurinol dose–response relationships were not significantly different to 1, indicating near unity between the observed and predicted concentrations of SU. Furthermore, the y‐intercepts for all fits to the model equations were not significantly different to 0. The concentration–response equation predicted the UT values better than the dose–response (r 2 = 0.64 vs. 0.60). However, predicted UT value for each equation were not significantly different from one another (mean difference, 95% CI: 0.0005, –0.006 to 0.007, P = 0.87, paired t test). Hence, the dose–response model, as the simpler of the two models, was deemed more suitable to describe the urate‐lowering effect of allopurinol in clinical practice.
Dose–response model in all patients
As the allopurinol dose–response model was shown to be the most suitable in describing the urate‐lowering ability of allopurinol, the relationship was re‐examined, with the addition of data from patients without plasma oxypurinol concentrations measured. The total number of patients was n = 81 with 205 measurements of SU.
Effect of UR:
The UR component significantly improved the fit to the model (Equation (3)) compared to when UR was set to 0 (Table 4, P < 2.2x10–16). The mean ID50 was 150 mg and the mean value of UR was 0.25 mmol l–1. The correlation between ID50 and UR was high but acceptable with respect to collinearity between parameters (r = – 0.90, Supplementary Table S2). There was very close agreement between the UT concentrations predicted by Equation (4) and the UP serum concentrations of urate (slope = 0.99, Figure 4A). Similar results were found in Australian and New Zealand cohorts (Figure 4B,C).
Table 4.
Best fit parameters for the allopurinol dose–response relationship with all serum urate concentrations in gout patients (n = 81)
| Group | ID 50 , mg (95% CI) | U R , mmol l –1 (95% CI) | k or d a (95% CI) | r 2 , OFV | P‐value |
|---|---|---|---|---|---|
| No U R ( Eqn (3) , U R = 0) | 420 (389–455) | 0 | ‐ | 0.49, 0.98 | ‐ |
| With U R (Eqn (3) ) | 150 (119–188) | 0.25 (0.21–0.28) | ‐ | 0.64, 0.69 | < 2.2 × 10–16 b |
| With U R , k * CL CR (Eqn (5) ) | 146 (119–179) | 0.18 (0.14–0.23) | 6.1 × 10–4 (3.4 × 10–4–8.9 × 10–4) | 0.67, 0.63 | 1.45 × 10–5 c |
| With U R , d * DIU ( Eqn (7) ) | 178 (137–232) | 0.24 (0.20–0.27) | –0.02 (–0.04 to –0.01) | 0.65, 0.67 | 0.01c |
| With U R in CL CR < 60 ml (Eqn (3) ) d | 151 (109–210) | 0.21 (0.14–0.27) | ‐ | 0.68, 0.37 | ‐ |
| With U R in CL CR > 60 ml min –1 (Eqn (3) ) e | 257 (180–370) | 0.20 (0.12–0.25) | ‐ | 0.62, 0.27 | ‐ |
CLCR, creatinine clearance; d, covariate constant for diuretic use; DIU, binary concomitant diuretic use variable; Eqn, Equation; ID50, the dose of allopurinol that has reduced the inhibitable urate concentration (UP – UR) by 50%; k, covariate constant for CLCR; OFV, objective function value; r 2, coefficient of determination; UP, pretreatment serum urate concentration; UR, ‘resistant’ serum urate concentration.
Covariate constants depending on whether CLCR (k) or DIU (d) were entered as covariates.
P‐value compared the fit to when UR set to 0.
P‐value compared the fit to Equation (3).
n = 37 subjects;
n = 44 patients.
Figure 4.
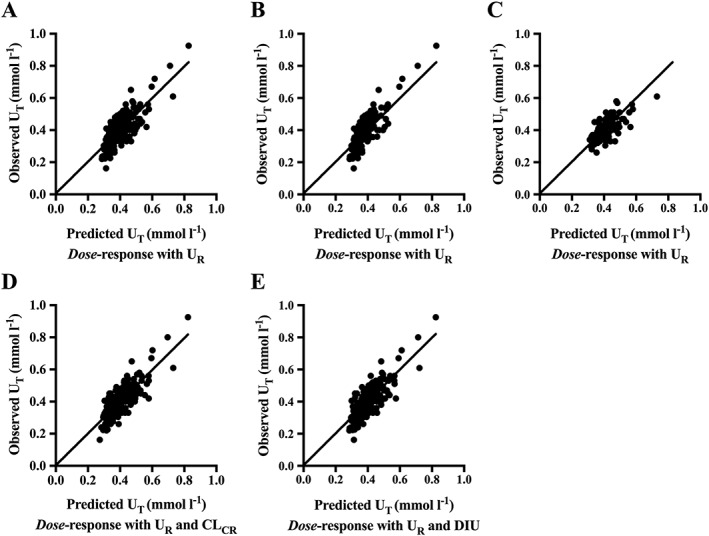
Comparisons of the observed vs. predicted outputs of the allopurinol dose–response relationship with serum urate in gout patients (81 patients, 205 data points). The black line indicates the regression line. The y‐intercepts for Figures 4A–C were not significantly different to zero. A) allopurinol dose–response model with UR , r 2 = 0.64, slope = 0.99 (95% CI 0.89–1.09); B) allopurinol dose–response model with UR in the gout patients from Australia, the regression line is the same as in Figure 4A,C) allopurinol dose–response model with UR in the gout patients from New Zealand, the regression line is the same as in Figure 4A,D) allopurinol dose–response model with UR and CLCR, r 2 = 0.67, slope = 0.99 (95% CI 0.89–1.09); E) allopurinol dose–response model with UR and DIU, r 2 = 0.65, slope = 0.99 (95% CI 0.89–1.09). CLCR, creatinine clearance; DIU, binary concomitant diuretic use variable; r 2, coefficient of determination; UR, ‘resistant’ serum urate concentration; UT, serum urate concentration achieved during treatment with allopurinol
Effect of CLCR:
Adding CLCR as a covariate to the allopurinol dose–response equation (Equation (5) vs. Equation (3)) improved the fit significantly (P = 1.45 × 10–5, Table 4). However, there was no significant difference between UT predicted from Equations (5) and (3) (mean difference, 95% CI: 0.002, –0.0004 to 0.0004, P = 0.11, Figure 4D vs. Figure 4A). There were also no significant differences in the ID50 and UR values estimated using the dose–response relationship (Equation (3)) in patients with CLCR ≤ 60 ml min–1 relative to those with CLCR > 60 ml min–1 (Table 4).
Effect of diuretics:
The addition of diuretic as a covariate alone (+d*DIU) in Equation (3) (i.e. Equation 7) improved the fit to the model (P = 0.01). As in previous estimates of the influence of covariates, the visual comparisons showed no contrasts (Figure 4E vs. Figure 4A). There were no significant differences between UT predicted from Equations (7) and (3) for all data (mean difference, 95% CI: 8.34 × 10–5, –0.001 to 0.001). When the paired t‐test was repeated in the subset of samples where diuretics were present, the mean difference (95% CI) was again very small [0.01 mmol l–1, (0.01– to 0.02)] but on this occasion significant (P < 2.2 × 10–16).
Allopurinol dose individualization
A bootstrap analysis conducted for the allopurinol dose–response model (Equation (3)) indicated a robust fit; no differences were observed between the mean and median U R or ID 50 values derived from the model and the bootstrap, respectively (Supplementary Table S3). Thus, the final estimates of UR (0.25 mmol l–1) and ID50 (150 mg) were carried forward for construction of the nomogram.
The dose of allopurinol predicted to reach target serum urate concentrations was calculated through a rearrangement of the dose–response relationship. The equation becomes:
| (8) |
in which the values UR and ID50 were calculated as the best fit mean parameters from the analysis of all dose–response data.
We constructed a nomogram to describe the relationship between UP and the daily dose of allopurinol to achieve a specific UT with allopurinol. In Equation (8), the UT was set to the target serum urate concentrations of 0.36 or 0.30 mmol l–1. The maintenance dose required to reach the target UT for a particular UP can be read off the y‐axis (Figure 5).
Figure 5.
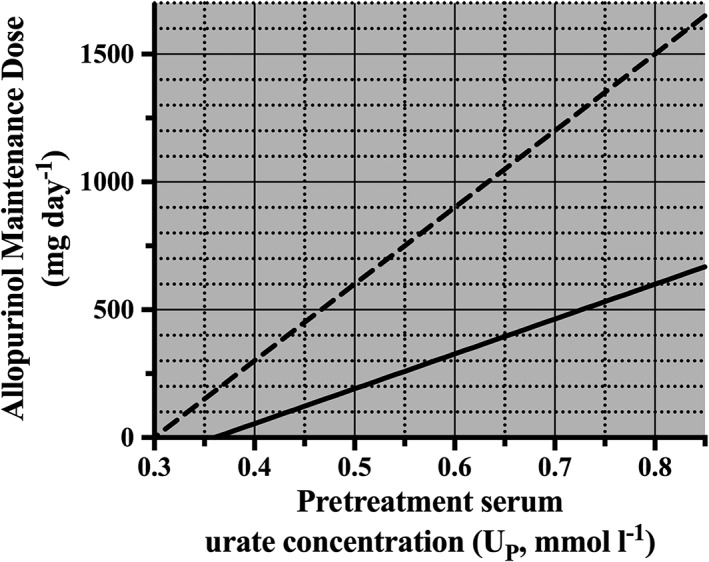
Nomogram describing the relationship between pretreatment serum urate concentration (UP) and the maintenance dose (D) required to achieve a target serum urate concentration (UT) of 0.36 mmol l–1 (solid line) and 0.30 mmol l–1 (dashed line), respectively
Discussion
This study has shown that our allopurinol dose–response relationship predicts serum urate concentrations just as well as an oxypurinol concentration–response relationship. This indicates that measuring plasma oxypurinol concentrations is not needed to determine the appropriate dose of allopurinol.
Additionally, this study has confirmed our previous findings, that the dose of allopurinol required to achieve a target SU is strongly dependent on the pretreatment concentration of SU. The nomogram developed can be used to estimate the appropriate maintenance dose of allopurinol required to reach the chosen therapeutic target SU concentration based on an individual's pretreatment SU concentrations.
By increasing the number of individuals with renal impairment (n = 37) and those taking diuretics (n = 21) in the present study that contrasts with our previous publication (n = 17 and 10, respectively) 13, we have increased confidence regarding the lack of influence of renal function and concomitant diuretics in the dose–response relationship of allopurinol.
Our results indicate that renal function and concomitant diuretic medication have no meaningful influence on the plasma oxypurinol concentration– and allopurinol dose–response relationships with SU concentrations. It should be noted that diuretics and decreasing CLCR both increase UP values. However, our analysis indicates that, once the UP is established, diuretic use and CLCR have little further influence on the response to allopurinol.
The kidney is responsible for the clearance of both oxypurinol and the majority of urate produced in the body. Hence, reduced CLCR is expected to impair the renal clearance of both oxypurinol and urate. Consequently, increased inhibition of urate production resulting from the increased plasma oxypurinol concentration largely negates the influence of concomitant decreased renal clearance of urate.
As was the case with reduced renal function, we found that concomitant use of diuretics did not significantly alter the concentration–response to oxypurinol or dose–response relationship to allopurinol. Diuretics decrease not only the renal transport of urate (via inhibition of proximal tubule transporters 27, 28, 29, 30) but also the clearance of oxypurinol 19, 20, 31, 32. Similar to the effect of renal impairment on urate and oxypurinol clearance, the reductions in oxypurinol clearance results in higher plasma concentrations, which negates the decrease in urate clearance. Thus, the maintenance dose of allopurinol in gout patients with diuretics needs to be determined by their pretreatment serum urate concentration.
To our knowledge, our nomogram is the first for guiding maintenance allopurinol dosage. Our approach is to predict the maintenance dosage of allopurinol from a measurement of SU namely Up. By comparison, other dose‐guiding tools have been tables of doses of allopurinol based upon CLCR and the use of diuretics with no measurement of UP 10, 13, 19. We aim to conduct further work to compare the usefulness of the two approaches.
The nomogram indicates that the higher the UP, the higher the dose of allopurinol required to achieve a target urate. Informing the patient of the treatment goals (target urate concentration and predicted dose to achieve target) may improve adherence and, thus, improve treatment outcomes 33. Knowing the optimal maintenance dose beforehand may prompt the clinician to be proactive about dose escalation.
Lowering SU may have additional benefits in reducing the risks related to cardiovascular disease including reducing all‐cause mortality 34, 35. Consequently, adjustment of dosage of allopurinol to levels that should prevent gout may improve overall health particularly in patients with cardiovascular disease.
It is important to note that the additional NZ data increased our estimate of UR from 0.20 to 0.26 mmol l–1, due to the higher concentrations of SU observed during treatment with allopurinol, UT in the New Zealand cohort. The UR is a mean value and individuals may have lower or higher UR concentrations. The lower the individual UR, the lower the dose required to reach target SU. Conversely, the higher the UR, the larger the dose of allopurinol required to reach target SU as this concentration gets closer to the asymptotic UR level. In the extreme case where UR could exceed the target urate concentration, then allopurinol cannot be used, at least alone, to achieve target.
There are some limitations to the present study. Firstly, the analyses were on pooled data. An individual could be represented several times in the regression analyses due to having SU measurements at different dosage rates. A second limitation is that the nomogram may overestimate the predicted allopurinol maintenance dose required to reach a SU ≤ 0.30 mmol l–1 as the estimated mean UR (0.25 mmol l–1) is quite close to this target. As the proportion of patients reaching a SU < 0.30 mmol l–1 was relatively small (23%), a greater number of patients reaching this serum urate concentration target, indicative of intensive therapy, is required to enable a more accurate prediction of the required maintenance dose of allopurinol in these patients.
Variable adherence to allopurinol therapy is a third limitation of the study. Although variable adherence is a likely problem in gout patients taking allopurinol 36, oxypurinol was detected in all available plasma samples. However, adherence could not be determined in the 34 patients in whom plasma concentrations were not assayed but participation of all patients in clinical research studies is likely to enhance adherence.
Finally, the nomogram may be less accurate at allopurinol doses >700 mg day–1 as this exceeds the highest dose studied. While prescribers should increase the dose of allopurinol until target urate concentrations are achieved, if a predicted dose is >700 mg day–1, it may indicate that a combination of urate lowering therapies (e.g. combination with a uricosuric) could be considered to achieve target serum urate concentrations.
In summary, there were no significant differences between the allopurinol dose– and oxypurinol concentration–response relationship with serum urate concentration with respect to the accuracy of prediction of the allopurinol dose required to achieve target urate concentrations. Further, the dose–response relationship is more practical, as there is no need to measure plasma oxypurinol concentrations. The renal function and concomitant diuretics did not influence the maintenance dose required to achieve target urate concentrations. We have constructed a nomogram that can be used as a guide to predict a suitable maintenance dose of allopurinol to achieve target urate concentrations in patients with gout. However, a prospective study is required to validate the nomogram for routine clinical use in predicting the final maintenance dosage of allopurinol in patients with gout, especially in those with reduced renal function or taking diuretics.
Competing Interests
All authors have completed the Unified Competing Interest form at http: //www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
The authors thank Drs Mona Manghani and Darren Roberts and Ms Michelle Hennessey for their assistance in recruiting patients for the present study. The work was supported by an Arthritis Australia National Research Grant (Ray and Pam Robinson Award for Rheumatology, 2008); the National Health and Medical Research Council Program Grants 568 612 and 1 054 146, and the Lexy Davies Bequest.
Contributors
All authors contributed to the writing and editing of the manuscript. D.R.W.K., G.G.G., S.L.S., I.P., K.P., K.M.W. and R.O.D. designed the study conducted in Sydney, where R.O.D. was the principal investigator. D.F.B.W., M.L.B. and L.K.S. designed the studies conducted in New Zealand, where L.K.S. was the principal investigator. D.R.W.K., D.F.B.W., S.L.S., I.P., K.P., M.L.P. and L.K.S. collected the data. D.R.W.K. and G.G.G. conducted the data analysis.
Supporting information
Table S1 Comparison of the plasma oxypurinol concentration– and allopurinol dose–response relationships in gout patients with normal (n = 28 patients) and low renal function (n = 19 patients)
Table S2 Correlations between parameters in the plasma concentration– (n = 47), allopurinol dose‐ (n = 47) and full allopurinol dose–response relationships (n = 81)
Table S3 Final mean parameter estimates and median estimates from nonparametric bootstrap (n = 1000 replicates) analysis of the final allopurinol dose–response model (Equation (3))
Kannangara, D. R. W. , Graham, G. G. , Wright, D. F. B. , Stocker, S. L. , Portek, I. , Pile, K. D. , Barclay, M. L. , Williams, K. M. , Stamp, L. K. , and Day, R. O. (2017) Individualising the dose of allopurinol in patients with gout. Br J Clin Pharmacol, 83: 2015–2026. doi: 10.1111/bcp.13307.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Day RO, Graham GG, Hicks M, McLachlan AJ, Stocker SL, Williams KM. Clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol. Clin Pharmacokinet 2007; 46: 623–644. [DOI] [PubMed] [Google Scholar]
- 4. Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am J Med 1987; 82: 421–426. [DOI] [PubMed] [Google Scholar]
- 5. Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK. Serum uric acid and the risk of incident and recurrent gout: a systematic review. J Rheumatol 2017; https://doi.org/10.3899/jrheum.160452. [DOI] [PubMed] [Google Scholar]
- 6. Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda‐Sanabria J, et al. 2016 updated EULAR evidence‐based recommendations for the management of gout. Ann Rheum Dis 2017; 76: 29–42. [DOI] [PubMed] [Google Scholar]
- 7. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 2012; 64: 1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graf SW, Whittle SL, Wechalekar MD, Moi JH, Barrett C, Hill CL, et al. Australian and New Zealand recommendations for the diagnosis and management of gout: Integrating systematic literature review and expert opinion in the 3e initiative. Int J Rheum Dis 2015; 18: 341–351. [DOI] [PubMed] [Google Scholar]
- 9. Stamp LK, Taylor WJ, Jones PB, Dockerty JL, Drake J, Frampton C, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: a proposed safe starting dose of allopurinol. Arthritis Rheum 2012; 64: 2529–2536. [DOI] [PubMed] [Google Scholar]
- 10. Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med 1984; 76: 47–56. [DOI] [PubMed] [Google Scholar]
- 11. Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol 2006; 33: 1646–1650. [PubMed] [Google Scholar]
- 12. Stamp LK, O'Donnell JL, Zhang M, James J, Frampton C, Barclay ML, et al. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum 2011; 63: 412–421. [DOI] [PubMed] [Google Scholar]
- 13. Graham GG, Kannangara DR, Stocker SL, Portek I, Pile KD, Indraratna PL, et al. Understanding the dose–response relationship of allopurinol: predicting the optimal dosage. Br J Clin Pharmacol 2013; 76: 932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelley EE, Trostchansky A, Rubbo H, Freeman BA, Radi R, Tarpey MM. Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol. J Biol Chem 2004; 279: 37231–37234. [DOI] [PubMed] [Google Scholar]
- 15. Roberts CJ, Homeida M, Roberts F, Bogie W. Effects of piretanide, bumetanide and frusemide on electrolyte and urate excretion in normal subjects. Br J Clin Pharmacol 1978; 6: 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manuel MA, Steele TH. Changes in renal urate handling after prolonged thiazide treatment. Am J Med 1974; 57: 741–746. [DOI] [PubMed] [Google Scholar]
- 17. Stamp LK, O'Donnell JL, Frampton C, Drake JM, Zhang M, Chapman PT. Clinically insignificant effect of supplemental vitamin C on serum urate in patients with gout: A pilot randomized controlled trial. Arthritis Rheum 2013; 65: 1636–1642. [DOI] [PubMed] [Google Scholar]
- 18. Wright DF, Doogue MP, Barclay ML, Chapman PT, Cross NB, Irvine JH, et al. A population pharmacokinetic model to predict oxypurinol exposure in patients on haemodialysis. Eur J Clin Pharmacol 2016; 73: 71–78. [DOI] [PubMed] [Google Scholar]
- 19. Wright DF, Duffull SB, Merriman TR, Dalbeth N, Barclay ML, Stamp LK. Predicting allopurinol response in patients with gout. Br J Clin Pharmacol 2016; 81: 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wright DF, Stamp LK, Merriman TR, Barclay ML, Duffull SB, Holford NH. The population pharmacokinetics of allopurinol and oxypurinol in patients with gout. Eur J Clin Pharmacol 2013; 69: 1411–1421. [DOI] [PubMed] [Google Scholar]
- 21. Stocker SL, Williams KM, McLachlan AJ, Graham GG, Day RO. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in healthy subjects. Clin Pharmacokinet 2008; 47: 111–118. [DOI] [PubMed] [Google Scholar]
- 22. Stamp LK, Barclay ML, O'Donnell JL, Zhang M, Drake J, Frampton C, et al. Relationship between serum urate and plasma oxypurinol in the management of gout: Determination of minimum plasma oxypurinol concentration to achieve a target serum urate level. Clin Pharmacol Ther 2011; 90: 392–398. [DOI] [PubMed] [Google Scholar]
- 23. Kirkpatrick CM, Van Den Berg L, Duffull SB. TCIWORKS ‐ target concentration intervention software. The Australian Health & Medical Research Congress; Melbourne Convention Centre, Melbourne. Melbourne: NHMRC; 2006; 420.
- 24. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 25. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet 2005; 44: 1051–1065. [DOI] [PubMed] [Google Scholar]
- 26. R Core Team . R: a language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing, 2012. [Google Scholar]
- 27. Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol 2007; 18: 430–439. [DOI] [PubMed] [Google Scholar]
- 28. Jutabha P, Anzai N, Kitamura K, Taniguchi A, Kaneko S, Yan K, et al. Human sodium phosphate transporter 4 (hNPT4/SLC17A3) as a common renal secretory pathway for drugs and urate. J Biol Chem 2010; 285: 35123–35132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jutabha P, Anzai N, Wempe MF, Wakui S, Endou H, Sakurai H. Apical voltage‐driven urate efflux transporter NPT4 in renal proximal tubule. Nucleosides Nucleotides Nucleic Acids 2011; 30: 1302–1311. [DOI] [PubMed] [Google Scholar]
- 30. El‐Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol 2008; 155: 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stocker SL, McLachlan AJ, Savic RM, Kirkpatrick CM, Graham GG, Williams KM, et al. The pharmacokinetics of oxypurinol in people with gout. Br J Clin Pharmacol 2012; 74: 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stamp LK, Barclay ML, O'Donnell JL, Zhang M, Drake J, Frampton C, et al. Furosemide increases plasma oxypurinol without lowering serum urate‐‐a complex drug interaction: Implications for clinical practice. Rheumatology 2012; 51: 1670–1676. [DOI] [PubMed] [Google Scholar]
- 33. Rees F, Jenkins W, Doherty M. Patients with gout adhere to curative treatment if informed appropriately: proof‐of‐concept observational study. Ann Rheum Dis 2013; 72: 826–830. [DOI] [PubMed] [Google Scholar]
- 34. Robinson P, Taylor W, Merriman T. A systematic review of the prevalence of gout and hyperuricemia in Australia. Intern Med J 2012; 42: 997–1007. [DOI] [PubMed] [Google Scholar]
- 35. Kuo CF, See LC, Yu KH, Chou IJ, Chiou MJ, Luo SF. Significance of serum uric acid levels on the risk of all‐cause and cardiovascular mortality. Rheumatology (Oxford) 2013; 52: 127–134. [DOI] [PubMed] [Google Scholar]
- 36. Zandman‐Goddard G, Amital H, Shamrayevsky N, Raz R, Shalev V, Chodick G. Rates of adherence and persistence with allopurinol therapy among gout patients in Israel. Rheumatology (Oxford) 2013; 52: 1126–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Comparison of the plasma oxypurinol concentration– and allopurinol dose–response relationships in gout patients with normal (n = 28 patients) and low renal function (n = 19 patients)
Table S2 Correlations between parameters in the plasma concentration– (n = 47), allopurinol dose‐ (n = 47) and full allopurinol dose–response relationships (n = 81)
Table S3 Final mean parameter estimates and median estimates from nonparametric bootstrap (n = 1000 replicates) analysis of the final allopurinol dose–response model (Equation (3))


