Abstract
Aims
Novel oral anticoagulants (NOACs) are alternatives to vitamin‐K antagonists (VKAs) for the prevention of thromboembolism. It is unclear how NOACs have been adopted in the UK since first introduced in 2008. The present study was conducted to describe the trends in the prescription of NOACs in the UK, including dabigatran, rivaroxaban and apixaban.
Methods
Using the UK's Clinical Practice Research Datalink, the rates of new use of NOACs and VKAs from 2009 to 2015 were calculated using Poisson regression. Patient characteristics associated with NOAC initiation were identified using multivariate logistic regression.
Results
The overall rate of oral anticoagulant initiation increased by 58% over the study period [rate ratio (RR) 1.58; 95% confidence interval (CI) 1.23, 2.03], even as the rate of new VKA use decreased by 31% (RR 0.69; 95% CI 0.52, 0.93). By contrast, the rate of initiation of NOAC increased, particularly from 2012 onwards, with a 17‐fold increase from 2012 to 2015 (RR 17.68; 95% CI 12.16, 25.71). In 2015, NOACs accounted for 56.5% of oral anticoagulant prescriptions, with rivaroxaban prescribed most frequently, followed by apixaban and then dabigatran. Compared to VKAs, new NOAC users were less likely to have congestive heart failure, coronary artery disease and peripheral vascular disease, and more likely to have a history of ischaemic stroke.
Conclusions
In the UK, the rate of initiation of NOACs has increased substantially since 2009, and these agents have now surpassed VKAs as the anticoagulant of choice. Moreover, the characteristics of patients initiated on NOACs have changed over time, and this should be accounted for in future studies comparing NOACs and VKAs.
Keywords: atrial fibrillation, oral anticoagulants, pharmacoepidemiology, prescription patterns, venous thromboembolism
What is Already Known about this Subject
Novel oral anticoagulants (NOACs) were first marketed in the UK in 2008, as effective and safe options for the prevention of thromboembolic events.
The present study was conducted in order to describe how NOACs have been adopted and prescribed in UK primary care since the time they were first introduced.
What this Study Adds
The number of patients receiving a first‐time oral anticoagulant prescription increased by 58% from 2009 to 2015.
New NOAC prescriptions have increased dramatically, and in 2015 accounted for 56% of first‐time oral anticoagulant prescriptions, with rivaroxaban prescribed most frequently.
New NOAC users present distinct characteristics which have changed over time.
Table of Links
This Table lists key ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1.
Introduction
For the past six decades, vitamin K antagonists (VKAs) have been the preventative treatment of choice for patients with atrial fibrillation (AF) and/or venous thromboembolism (VTE). Although clinically effective at reducing thromboembolic events 2, 3, VKAs have been associated with significant bleeding risks 4. The use of VKAs further requires close monitoring on account of their narrow therapeutic window and variable anticoagulant effects 5.
Novel oral anticoagulants (NOACs) are attractive alternatives for patients in whom traditional oral anticoagulant (OAC) therapy may be contraindicated or impractical. Clinical trials have reported NOACs to be non‐inferior, and in some cases superior to VKAs in reducing the risk of ischaemic stroke and VTE 6, 7, 8. In addition to having a potentially more favourable safety profile 9, 10, 11, NOACs have also been hailed as substantially more practical and easier to use 12. Accordingly, the first NOAC, dabigatran, was placed on the market throughout the European Union and in the UK in 2008, followed by rivaroxaban in the same year, and by apixaban in 2011.
The UK's National Health Services (NHS) has issued guidelines on the prescription of NOACs 13. Guidance documents on the use of these medications have also been published by the UK's National Institute for Health and Care Excellence (NICE), which recommends NOACs as possible alternatives to VKAs in specific subgroups of patients with AF or VTE 14, 15, 16. These include AF patients aged 75 years or older, and those with heart failure and a history of stroke or systemic embolism, among others. However, little is known about how these medications have been prescribed in everyday practice in the UK since their licensing and approval, and it remains unclear to what extent official recommendations and guidelines have been adopted by general practice (GP) clinicians.
The objective of the present study was to address these uncertainties, and to provide insight as to how the recent introduction of NOACs has affected the way that OACs are being received by primary care patients in the UK. To this end, the study examined the temporal trends in the rates of OAC initiation, and in the patient characteristics associated with a first prescription for NOACs as compared with VKAs.
Methods
Data source
The study was conducted using the UK Clinical Practice Research Datalink (CPRD). The data within the CPRD are documented by trained GPs, and include information related to patient demographics, medical diagnoses and procedures, referrals and drug prescriptions. As of 2013, with over 11 million registered patients from over 670 medical practices, the CPRD comprises approximately 7% of the total UK population, of which it is broadly considered to be representative with respect to age, sex and ethnicity 17. As one of the world's largest databases of electronic medical records, the CPRD has been used extensively for observational research, including pharmacoepidemiological studies of drug safety and utilization 18, 19. The completeness and quality of CPRD data have been validated previously 20, 21, 22.
Study population
A cohort was defined comprising CPRD patients aged 18 years or older and registered with a GP for at least 1 day between 1 January 2009 and 31 December 2015. The study period began in 2009 so as to analyse only complete years of prescription data since NOACs were introduced in the UK in March 2008. The cohort was limited to OAC‐naïve patients with no record of an OAC prescription in the 12 months prior to the start of follow‐up. Follow‐up began at the latest of the study start date (1 January 2009), the patient's 18th birthday, 1 year after the patient's registration date with the general practice or 1 year after the date that the practice started to contribute up‐to‐standard data to the CPRD. Follow‐up ended at the earliest of the study end date (31 December 2015), or the patient's death or transfer out of the practice.
Oral anticoagulants
All OACs available in the UK over the course of the study period were identified. VKAs included warfarin, phenindione and acenocoumarol, and NOACs included dabigatran, rivaroxaban and apixaban. The NOAC edoxaban was licensed throughout the European Union in June 2015. Considering the study timeframe, edoxaban was not analysed in the context of the present study, and first‐time edoxaban users were censored at the time of first prescription.
Study covariates
The following patient characteristics were identified at the time of first OAC prescription: age and sex; the comorbidities obesity, smoking, hyperlipidaemia, hypertension, diabetes, coronary artery disease (including myocardial infarction and ischaemic heart disease), congestive heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, cancer, liver disease and a history of bleeding and ischaemic stroke/transient ischaemic attack (TIA); concomitant use of antiplatelet agents, antihypertensive drugs, nonsteroidal anti‐inflammatory drugs (NSAIDs) and lipid‐lowering drugs; and number of physician visits as a measure of healthcare utilization. All patient characteristics were identified based on CPRD records from the 12 months prior to first OAC prescription.
In patients with AF, a CHADS2 score (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke/TIA) and a CHA2DS2‐VASc score (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke/TIA, vascular disease, age 65–74 years, sex) were calculated as measures of the risk of stroke 23, 24. Finally, a modified HAS‐BLED score [hypertension, abnormal renal and/or liver function, stroke/TIA, bleeding, labile international normalized ratio (INR), age > 65 years, antiplatelet/NSAID use or alcohol abuse] was estimated as a measure of the risk of major bleeding 25. Labile INR was omitted from the HAS‐BLED score in the present study, considering that new OAC users are unlikely to have an extensive history of INR results, and that INR monitoring is irrelevant in NOAC treatment.
Statistical analyses
Using a Poisson model, the rates of OAC initiation were calculated for VKAs and NOACs separately, and for each year of study as the number of new OAC users divided by the person‐time of follow‐up from all cohort members, up to their first OAC prescription. These rates were also estimated for each individual NOAC, and were further stratified by age, sex and OAC indication in secondary analyses. The OAC indication was identified as either AF or VTE using an algorithm developed after a blinded review of the records of a random sample of patients. Briefly, READ codes related to AF and VTE were identified in the 6 months and 1 month prior to OAC initiation, respectively. Rate ratios (RRs) were estimated to compare the annual rate of OAC initiation to 2009, as well as to the preceding year. Temporal changes in the distribution of new prescriptions between NOACs and VKAs were evaluated using a chi‐squared test for trend. Multivariate logistic regression models were fitted with the aforementioned covariates to identify predictors of NOAC initiation, and stratified by individual NOAC and calendar period (2009–2012, 2013–2014, and 2015). Predictors of NOAC initiation were also estimated separately for patients with AF and patients with VTE for 2015. CHADS2, CHA2DS2‐VASc and HAS‐BLED scores were excluded from these models, as each score component was included individually. Confidence intervals (CI) were calculated for all estimates using a 5% significance level. All statistical procedures were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA).
The study protocol (No. 16_167R) was approved by the independent scientific advisory committee of the CPRD, and the research ethics committee of the Jewish General Hospital (Montreal, Canada), and was made available to journal reviewers.
Results
After applying all selection criteria, 5 417 063 patients were included in the study cohort, contributing a total of 21 962 610 person‐years of follow‐up. Within this cohort, 89 626 patients were newly prescribed an OAC during the study period, among whom 18 (<0.1%) were further excluded for having received two first prescriptions on the same day. Of the remaining and final 89 608 new users, 74 767 (83.4%) were initiated on a VKA and 14 841 (16.6%) on a NOAC. AF and VTE were identified as the primary OAC indication in 53 843 (60.1%) and 27 155 (30.3%) new users, respectively. The indication remained unknown for 8610 (9.6%) patients.
The crude rate of OAC initiators increased by approximately 58% from 2009 to 2015 (RR 1.58; 95% CI 1.23, 2.03), as shown in Figure 1. During this time, there was a 31% decrease in the rate of new VKA use (RR 0.69; 95% CI 0.52, 0.93). By contrast, the rate of new NOAC use increased substantially over the study period (Table S1), and particularly from 2012 onwards, with a 17‐fold increase from 2012 to 2015 (RR 17.68; 95% CI 12.16, 25.71). Accordingly, NOACs accounted for 56.5% (95% CI 55.6, 57.3) of all OAC prescriptions in 2015 (P < 0.0001 for trend) (Figure S1). These NOAC prescriptions were primarily attributable to rivaroxaban (64.8%), followed by apixaban (29.3%) and dabigatran (5.9%). Whereas the rate of new dabigatran use was relatively low throughout the study period, the rates of rivaroxaban and apixaban initiation increased prominently, up to 200.1 (95% CI 181.8, 220.3) and 90.7 (95% CI 81.9, 100.4) new users per 100 000 persons per year in 2015, respectively (Figure 2).
Figure 1.
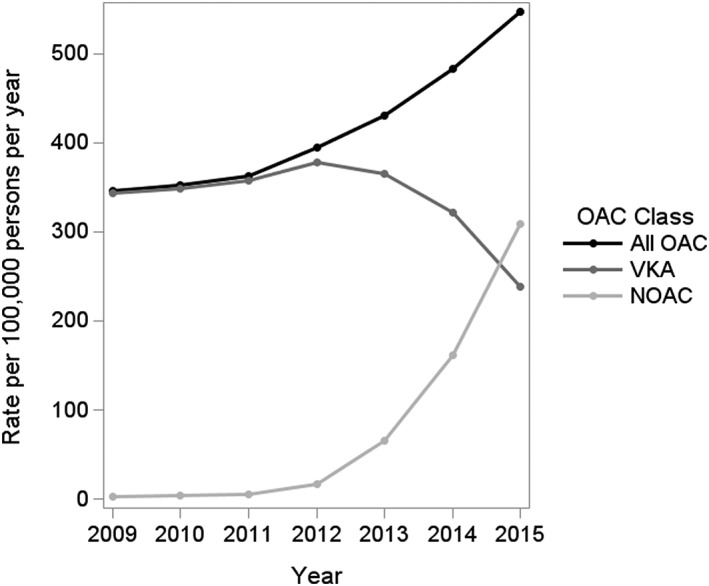
Rates of new use of oral anticoagulants (OAC) in the UK Clinical Practice Research Datalink, from 2009 to 2015. NOAC, novel oral anticoagulants; VKA, vitamin K antagonists
Figure 2.
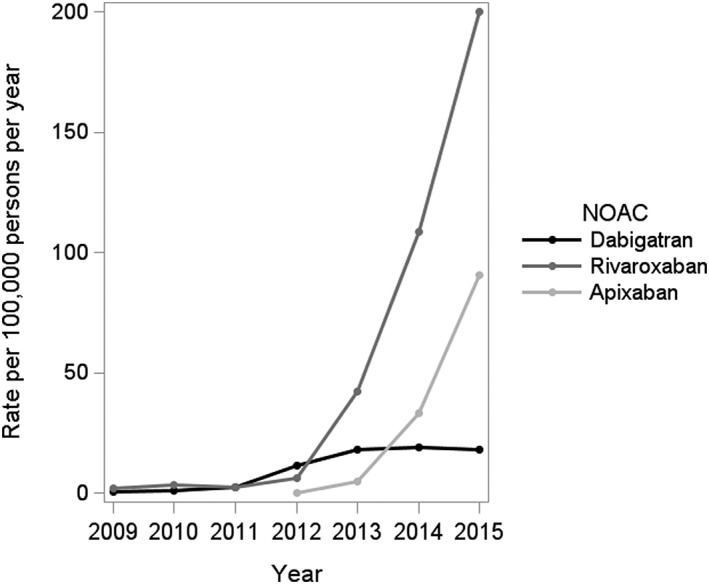
Rates of new use of individual novel oral anticoagulants (NOAC) in the UK Clinical Practice Research Datalink, from 2009 to 2015
For both VKAs and NOACs, the rates of initiation increased with age, and the most notable temporal changes occurred primarily among the elderly (aged 75 years and older) (Figure 3). Although the rate of new OAC use in patients with AF was considerably higher than for those with VTE, the temporal initiation patterns suggest an increasing rate of NOAC initiation over time for both indications (Figure 4). For VTE patients, this increase was primarily attributable to first‐time prescriptions of rivaroxaban (Figure S2). By contrast, there was an increased rate of initiation for all three NOACs in AF patients, which was more marked for both rivaroxaban and apixaban. There was no difference in the prescription trends between men and women, although men had slightly higher rates of OAC initiation overall (data not shown).
Figure 3.
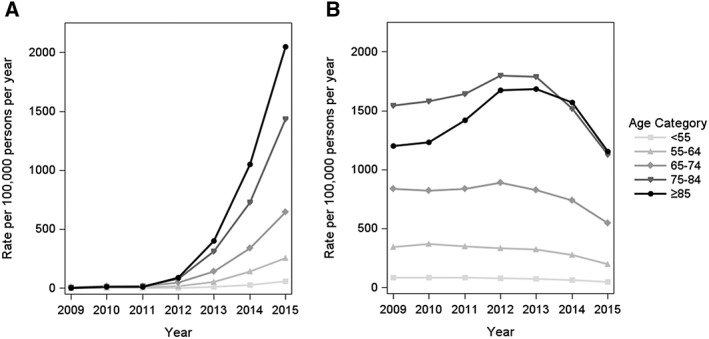
Age‐stratified rates of new use of novel oral anticoagulants (A) and vitamin K antagonists (B) in the UK Clinical Practice Research Datalink, from 2009 to 2015
Figure 4.
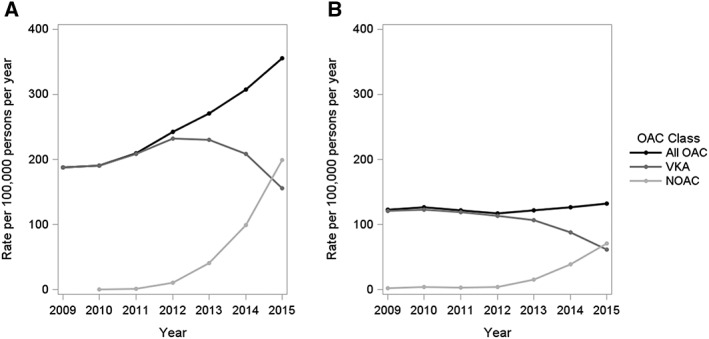
Rates of new use of oral anticoagulants (OAC) with an indication for atrial fibrillation (A) and venous thromboembolism (B) in the UK Clinical Practice Research Datalink, from 2009 to 2015. NOAC, novel oral anticoagulants; VKA, vitamin K antagonists
The baseline characteristics of first‐time NOAC users changed over the course of the study period (Table 1) and furthermore differed between individual NOACs (Table S2). Based on the logistic regression analyses, patients initiated on NOACs in 2015 were more likely to have a history of stroke/TIA, and less likely to have cardiovascular conditions such as peripheral vascular disease, congestive heart failure and coronary artery disease, compared with patients initiating VKAs (Table 2). Importantly, the baseline profile of new NOAC users changed substantially from the time that NOACs were first introduced. For instance, patients with chronic kidney disease or cancer were less likely to be prescribed NOACs over VKAs early after the former were introduced onto the market, whereas these characteristics were not associated with choice of OAC class in 2015. The baseline profile of new NOAC users also differed between AF and VTE patients (Table S3). Notably, among patients with AF, and compared with new users of VKAs, new users of NOACs were less likely to have congestive heart failure and coronary artery disease, and more likely to have had a previous stroke/TIA. These characteristics were not associated with NOAC initiation in new users with VTE.
Table 1.
Temporal changes in the baseline characteristics of patients newly prescribed novel oral anticoagulants in the UK Clinical Practice Research Datalink, from 2009 to 2015
| 2009–2012 (n = 974) | 2013–2014 (n = 6548) | 2015 (n = 7319) | |
|---|---|---|---|
| Age (years), mean (SD) | 69.8 (12.5) | 71.9 (14.1) | 72.1 (13.9) |
| <55 | 108 (11.1) | 743 (11.3) | 840 (11.5) |
| 55–64 | 175 (18.0) | 849 (13.0) | 933 (12.7) |
| 65–74 | 329 (33.8) | 1725 (26.3) | 1922 (26.3) |
| 75–84 | 258 (26.5) | 2080 (31.8) | 2322 (31.7) |
| ≥85 | 104 (10.7) | 1151 (17.6) | 1302 (17.8) |
| sex, male | 498 (51.1) | 3426 (52.3) | 3820 (52.2) |
| Physician visits, mean (SD) | 9.8 (8.8) | 10.7 (8.9) | 10.6 (9.2) |
| 0 | 67 (6.9) | 281 (4.3) | 301 (4.1) |
| 1–6 | 358 (36.8) | 2179 (33.3) | 2606 (35.6) |
| 7–12 | 281 (28.9) | 1999 (30.5) | 2118 (28.9) |
| 13–24 | 215 (22.1) | 1584 (24.2) | 1741 (23.8) |
| ≥ 25 | 53 (5.4) | 505 (7.7) | 553 (7.5) |
| Indication | |||
| Atrial fibrillation | 391 (40.1) | 4050 (61.9) | 4727 (64.6) |
| Venous thromboembolism | 421 (43.2) | 1578 (24.1) | 1668 (22.8) |
| Unknown | 162 (16.6) | 920 (14.1) | 924 (12.6) |
| Comorbidities and risk factors | |||
| Congestive heart failure | 35 (3.6) | 402 (6.1) | 465 (6.4) |
| Coronary artery disease | 87 (8.9) | 608 (9.3) | 741 (10.1) |
| Peripheral vascular disease | 6 (0.6) | 70 (1.1) | 85 (1.2) |
| Hypertension | 672 (69.0) | 5234 (79.9) | 5829 (79.6) |
| Ischaemic stroke/TIA | 92 (9.4) | 733 (11.2) | 700 (9.6) |
| Chronic kidney disease | 48 (4.9) | 363 (5.5) | 477 (6.5) |
| Diabetes | 139 (14.3) | 1111 (17.0) | 1283 (17.5) |
| Bleeding | 57 (5.9) | 353 (5.4) | 354 (4.8) |
| Hyperlipidaemia | 436 (44.8) | 3352 (51.2) | 3767 (51.5) |
| Cancer | 47 (4.8) | 378 (5.8) | 391 (5.3) |
| Chronic obstructive pulmonary disease | 49 (5.0) | 512 (7.8) | 609 (8.3) |
| Liver disease | 5 (0.5) | 11 (0.2) | 13 (0.2) |
| Obesity | |||
| Obese | 200 (20.5) | 1325 (20.2) | 1386 (18.9) |
| Not obese | 269 (27.6) | 2077 (31.7) | 2205 (30.1) |
| Unknown | 505 (51.8) | 3146 (48.0) | 3728 (50.9) |
| Smoking | |||
| Never smoker | 219 (22.5) | 1720 (26.3) | 1720 (23.5) |
| Former/current smoker | 372 (38.2) | 2499 (38.2) | 2654 (36.3) |
| Unknown | 383 (39.3) | 2329 (35.6) | 2945 (40.2) |
| Medications | |||
| Antihypertensive drugs | 671 (68.9) | 5207 (79.5) | 5805 (79.3) |
| Antiplatelet agents | 447 (45.9) | 3367 (51.4) | 3421 (46.7) |
| Lipid‐lowering drugs | 433 (44.5) | 3322 (50.7) | 3733 (51.0) |
| Non‐steroidal anti‐inflammatory drugs | 352 (36.1) | 1199 (18.3) | 1206 (16.5) |
| CHADS 2 a | |||
| 0 | 17 (4.3) | 135 (3.3) | 167 (3.5) |
| 1 | 120 (30.7) | 1202 (29.7) | 1452 (30.7) |
| ≥ 2 | 254 (65.0) | 2713 (67.0) | 3108 (65.7) |
| CHA 2 DS 2 ‐VASc a | |||
| 0 | 6 (1.5) | 47 (1.2) | 39 (0.8) |
| 1 | 40 (10.2) | 347 (8.6) | 429 (9.1) |
| ≥ 2 | 345 (88.2) | 3656 (90.3) | 4259 (90.1) |
| Modified HAS‐BLED | |||
| ≤ 2 | 592 (60.8) | 3727 (56.9) | 4349 (59.4) |
| > 2 | 382 (39.2) | 2821 (43.1) | 2970 (40.6) |
All values are expressed as n (%), unless otherwise specified. CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/TIA; CHA2DS2‐VASc, congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke/TIA, vascular disease, age 65–74 years, sex; modified HAS‐BLED, hypertension, abnormal renal and/or liver function, stroke/TIA, bleeding, age >65 years, antiplatelet/non‐steroidal anti‐inflammatory drug use or alcohol abuse. SD, standard deviation; TIA, transient ischaemic attack
CHADS2 and CHA2DS2‐VASc were calculated for patients with atrial fibrillation only
Table 2.
Odds ratios (95% confidence intervals) for the association between patient characteristics and the initiation of novel oral anticoagulants in the UK Clinical Practice Research Datalink, from 2009 to 2015
| 2009–2012 (n = 49 662) | 2013–2014 (n = 26 987) | 2015 (n = 12 959) | |
|---|---|---|---|
| Age (years) (vs. under 45 years) | |||
| 45–54 | 2.07 (1.38, 3.11) | 0.88 (0.75, 1.04) | 0.98 (0.80, 1.20) |
| 55–64 | 2.94 (2.02, 4.26) | 1.03 (0.89, 1.20) | 1.09 (0.90, 1.32) |
| 65–74 | 3.56 (2.48, 5.13) | 1.00 (0.87, 1.15) | 0.97 (0.81, 1.17) |
| 75–84 | 2.61 (1.79, 3.79) | 1.02 (0.89, 1.18) | 1.03 (0.86, 1.23) |
| ≥85 | 3.21 (2.14, 4.81) | 1.44 (1.24, 1.68) | 1.42 (1.17, 1.73) |
| Male (vs. female) | 0.88 (0.77, 1.01) | 0.98 (0.93, 1.04) | 0.95 (0.88, 1.02) |
| Physician visits | 1.01 (1.00, 1.01) | 1.00 (0.99, 1.00) | 1.00 (0.99, 1.00) |
| Comorbidities and risk factors | |||
| Congestive heart failure | 0.55 (0.39, 0.77) | 0.83 (0.74, 0.93) | 0.84 (0.73, 0.97) |
| Coronary artery disease | 0.90 (0.71, 1.14) | 0.75 (0.68, 0.83) | 0.80 (0.71, 0.91) |
| Peripheral vascular disease | 0.38 (0.17, 0.86) | 0.64 (0.49, 0.83) | 0.72 (0.53, 0.97) |
| Hypertension | 0.67 (0.57, 0.79) | 1.02 (0.94, 1.11) | 0.99 (0.90, 1.10) |
| Ischaemic stroke/TIA | 1.16 (0.93, 1.46) | 1.51 (1.37, 1.66) | 1.61 (1.40, 1.86) |
| Chronic kidney disease | 0.75 (0.56, 1.01) | 0.85 (0.75, 0.96) | 0.96 (0.83, 1.10) |
| Diabetes | 1.03 (0.84, 1.26) | 0.97 (0.89, 1.05) | 0.97 (0.88, 1.07) |
| Bleeding | 1.13 (0.86, 1.48) | 1.03 (0.91, 1.17) | 0.98 (0.83, 1.16) |
| Hyperlipidaemia | 0.98 (0.84, 1.15) | 1.04 (0.97, 1.11) | 1.12 (1.03, 1.22) |
| Cancer | 0.63 (0.46, 0.85) | 1.07 (0.95, 1.21) | 0.97 (0.83, 1.14) |
| Chronic obstructive pulmonary disease | 0.70 (0.52, 0.94) | 0.96 (0.86, 1.07) | 1.01 (0.89, 1.16) |
| Liver disease | 2.45 (0.99, 6.06) | 0.66 (0.35, 1.27) | 0.69 (0.33, 1.45) |
| Obesity | 1.00 (0.83, 1.21) | 0.99 (0.91, 1.07) | 0.91 (0.82, 1.01) |
| Smoking | 1.15 (0.97, 1.36) | 0.97 (0.90, 1.05) | 0.95 (0.87, 1.05) |
| Concomitant medication use a | |||
| Antiplatelets | 0.90 (0.77, 1.05) | 1.02 (0.95, 1.08) | 1.08 (0.99, 1.17) |
| Non‐steroidal anti‐inflammatory drugs | 2.11 (1.85, 2.42) | 1.12 (1.04, 1.21) | 1.11 (1.00, 1.22) |
TIA, transient ischaemic attack
Concomitant use of antihypertensive and lipid‐lowering drugs were included in all models under the hypertension and hyperlipidaemia covariates, respectively
Discussion
In the present large population‐based study, the rates of OAC initiation in the UK increased steadily from 2009 to 2015. NOACs were increasingly prescribed throughout the study period and accounted for over 50% of all new OAC prescriptions in 2015, while a substantial decrease in the rate of new VKA users was noted. Among NOACs, rivaroxaban was prescribed most frequently, followed by apixaban and dabigatran. Furthermore, the profile of patients who were prescribed NOACs changed significantly over time, as did the characteristics associated with initiating NOACs over VKAs.
Increasing rates of OAC prescription have been described in several previous reports from Europe and Canada, in line with our results 26, 27, 28. The observed increase in our study may be explained by the introduction and adoption of NOACs. Indeed, previous studies had repeatedly shown that VKAs were underutilized in AF, especially among vulnerable patients, such as those with a high risk of bleeding 29, 30. NOACs being potentially safer than VKAs, as shown in some clinical trials, these at‐risk AF patients would have been newly able to receive treatment when NOACs were introduced, and are likely to have contributed significantly to the increasing number of new OAC users. Accordingly, the rate of OAC initiation increased almost solely in AF patients, who also constituted the majority of the new users in the present study. This rate was also highest and most prominent in men and the elderly, which is further in keeping with the incidence of AF being higher in men and increasing with age 31. Therefore, the introduction of NOACs may have overcome some of the barriers to using OAC therapy in AF. Future studies should re‐evaluate the extent to which AF remains undertreated and explore any possible underlying reasons.
As expected, new prescriptions of NOACs increased over the study period. Interestingly, there was a delay in the adoption of NOACs, with new user rates remaining negligible until after 2012. This may be explained, in part, by the fact that the indications for NOACs were initially limited to the primary prevention of VTE in postoperative hip and knee patients. It was not until 2011 that the indications were officially expanded to include nonvalvular AF, and not until 2012 that recommendations from the UK's NICE were published in light of this amendment. This may be a reason for the prominent increase in NOAC prescriptions from 2012 onwards. Similar trends have been observed in Canada and France, where the proportion of OAC prescriptions attributable to NOACs also remained relatively low until NOACs were approved for stroke prevention in AF patients 26, 28. In the USA and Denmark, NOACs increased to account for approximately 50% of all new OAC prescriptions within 2 years following approval for AF 32, 33. Although comparable, this is slightly faster than the time taken for NOACs to surpass VKAs in our study. These differences in timing may be attributable to a number of factors that influence prescribing practices and that can vary substantially between countries, such as official prescription guidelines, medication costs and reimbursement rates, or even pharmaceutical marketing strategies 34.
Overall, the rate of dabigatran initiation was the lowest among the three NOACs. Previous research has suggested similar patterns in which, over time, dabigatran prescriptions plateau and are eventually overtaken by rivaroxaban 26, 28, 32, 35, or in some cases by both rivaroxaban and apixaban 36. In guidance documents issued by the UK's NHS, rivaroxaban and apixaban are cited as suitable for most patients with nonvalvular AF, whereas in some situations dabigatran is not preferred or even contraindicated 37, 38. Rivaroxaban is furthermore identified as the NOAC of choice for the treatment and prevention of VTE in several UK counties 39, 40. These recommendations offer possible explanations for the observed differences between the rates of initiation of individual NOACs, and, indeed, our results suggest that these guidelines have been well adopted by UK GPs. Dabigatran also differs from both rivaroxaban and apixaban in terms of its mechanism of action and other pharmacological characteristics. Notably, dabigatran has a longer half‐life and is also primarily cleared renally 41. A longer half‐life heightens the risk of overdose, which may be further exacerbated in those with any form of renal impairment, and dabigatran may therefore also be prescribed infrequently, for precautionary reasons. Conversely, a dramatic increase in new apixaban users was observed. Data on the temporal trends of apixaban initiation remain sparse, considering its more recent introduction as compared with dabigatran and rivaroxaban. Nevertheless, in Denmark, apixaban was found to be the most frequently prescribed among new users of NOACs in 2015 36. Future studies in the UK and in other countries will further inform the evolution of the initiation of individual NOACs over time.
Our results suggest that the patient profile associated with NOAC initiation has changed over time. NOAC may have been initially prescribed with greater caution owing to preliminary uncertainties with regard to their effectiveness and safety in primary care. Indeed, over time, patients initiated on NOACs and those initiated on VKAs were more similar in profile. Some patient characteristics were nonetheless significantly associated with a first‐time NOAC prescription. For instance, in partial keeping with NICE guidelines, NOACs were preferentially initiated in elderly patients from 2009 to 2012, and in those with a history of stroke/TIA in 2015. Interestingly, NICE also recommends NOACs in AF patients with congestive heart failure; however, these patients were less likely to initiate NOACs in our study. Older age has been both positively and negatively associated with first‐time NOAC use in previous studies in other countries, and conflicting conclusions have also been drawn with respect to the effect of patient sex, and history of bleeding and stroke/TIA 32, 33, 42, 43, 44. As already mentioned, the decision to initiate a patient on either NOACs or VKAs may be affected by how recently NOACs were marketed and introduced, and this time effect may also offer some explanation as to the differences in profile that can be observed across studies. The differences between first‐time users of NOACs and VKAs and the changes in these differences over time should be taken into consideration in any analyses comparing these distinct patients groups.
The present study was conducted using the CPRD, which provided a large and representative study population and thereby allowed for an accurate depiction of the use of OACs in the UK. Furthermore, the 7‐year study timeframe surpassed that of many previous studies, thus permitting a more thorough analysis of the longitudinal trends in OAC prescription, including more recent NOACs such as apixaban. A limitation of the study was that the CPRD contains only records of medications prescribed by primary care physicians. Nevertheless, GPs in the UK typically follow up on medications prescribed in secondary or tertiary care, and the trends described herein may still be considered accurate and informative with respect to the global patterns of OAC use. Additionally, in primary care databases such as the CPRD, diagnoses are not systematically recorded in tandem with issued prescriptions. It was therefore not possible to analyse all new OAC users when stratifying by indication. Finally, no differentiation was made between the different doses of OAC in the context of the present study. As it is often recommended that NOAC doses be adjusted under specific clinical conditions, further stratifying patients by prescribed dose could provide a more detailed depiction of their baseline profile.
In conclusion, the overall rate of OAC initiation increased in the UK from 2009 to 2015, primarily among AF patients, and with NOAC prescriptions now having surpassed those for VKA. The profile of patients initiating these medications has changed further over time. These trends are likely to reflect the interplay of several factors influencing prescribing practices, such as changes in the perceived utility and safety of NOACs, and/or official guidelines, among others. Further studies will explore the impact of these individual factors on OAC prescription trends, and will also establish the safety and effectiveness of NOACs in UK primary care. This will ultimately provide clinicians with more guidance in determining which NOAC is more suitable to prescribe to individual patients.
Competing Interests
There are no competing interests to declare.
This study was funded by an operating grant [ MOP‐341510 ] from the Canadian Institutes of Health Research (awarded to C.R.). S.L. is the recipient of a Frederick Banting and Charles Best Canada Graduate Scholarship from the Canadian Institutes of Health Research. C.R. is the recipient of a Chercheur–Boursier Award from the Fonds de la recherche en santé du Québec (FRSQ).
Contributors
S.L. contributed to the study design, analysed and interpreted the data, and wrote and revised the manuscript. S.D. contributed to the study design, analysed and interpreted the data, and reviewed the manuscript. L.H. reviewed the study design, interpreted the data, and reviewed the manuscript. C.R. conceived and designed the study, provided supervision and funding, analysed and interpreted the data, and revised the manuscript.
Supporting information
Figure S1 Distribution of first‐time oral anticoagulant users in the UK Clinical Practice Research Datalink, from 2009 to 2015
Figure S2 Rates of new users of novel oral anticoagulants with an indication for atrial fibrillation (left) and venous thromboembolism (right) in the UK Clinical Practice Research Datalink, from 2009 to 2015
Table S1 Temporal trends in the rate of new users of novel oral anticoagulants in the UK Clinical Practice Research Datalink, from 2009 to 2015
Table S2 Baseline characteristics of patients newly prescribed novel oral anticoagulants in the UK Clinical Practice Research Datalink in 2015
Table S3 Odds ratios (95% confidence intervals) for the association between patient characteristics and the initiation of novel oral anticoagulants in the UK Clinical Practice Research Datalink in 2015, stratified by oral anticoagulant indication
Loo, S. Y. , Dell'Aniello, S. , Huiart, L. , and Renoux, C. (2017) Trends in the prescription of novel oral anticoagulants in UK primary care. Br J Clin Pharmacol, 83: 2096–2106. doi: 10.1111/bcp.13299.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857–867. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Goldhaber SZ, Danielson E, Rosenberg Y, Eby CS, Deitcher SR, et al., PREVENT Investigators . Long‐term, low‐intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003; 348: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 4. Hylek EM, Evans‐Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007; 115: 2689–2696. [DOI] [PubMed] [Google Scholar]
- 5. Shameem R, Ansell J. Disadvantages of VKA and requirements for novel anticoagulants. Best Pract Res Clin Haematol 2013; 26: 103–114. [DOI] [PubMed] [Google Scholar]
- 6. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. & the RE‐LY Steering Committee investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 7. The EINSTEIN Investigators , Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363: 2499–2510. [DOI] [PubMed] [Google Scholar]
- 8. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369: 799–808. [DOI] [PubMed] [Google Scholar]
- 9. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al., the ROCKET AF Steering Committee . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 11. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361: 2342–2352. [DOI] [PubMed] [Google Scholar]
- 12. Eikelboom JW, Weitz JI. New anticoagulants. Circulation 2010; 121: 1523–1532. [DOI] [PubMed] [Google Scholar]
- 13. National Health Services: Lancashire Medicines Management Group (2013). Guidance for prescribing of dabigatran (Pradaxa) rivaroxaban (Xarelto) and apixaban (Eliquis) in patients with non‐valvular AF [online]. Available at http://www.cumbria.nhs.uk/ProfessionalZone/MedicinesManagement/Guidelines/Prescribing‐Guidance‐for‐NOACs.pdf (last accessed 20 April 2016).
- 14. National Institute for Health and Clinical Excellence (2012). Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation. NICE technology appraisal guidance 256 [online]. Available at https://www.nice.org.uk/guidance/ta256 (last accessed 20 April 2016).
- 15. National Institute for Health and Clinical Excellence (2012). Dabigatran etexilate for the prevention of venous thromboembolism after hip or knee replacement surgery in adults. NICE technology appraisal guidance 157 [online]. Available at https://www.nice.org.uk/guidance/ta157 (last accessed 20 April 2016).
- 16. National Institute for Health and Clinical Excellence (2015). Apixaban for the treatment and secondary prevention of deep vein thrombosis and/or pulmonary embolism. NICE technology appraisal guidance 341 [online]. Available at https://www.nice.org.uk/guidance/ta341 (last accessed 20 April 2016).
- 17. Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44: 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. García Rodríguez LA, Pérez Gutthann S. Use of the UK general practice research database for pharmacoepidemiology. Br J Clin Pharmacol 1998; 45: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wood L, Martinez C. The general practice research database. Drug Saf 2004; 27: 871–881. [DOI] [PubMed] [Google Scholar]
- 20. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jick SS, Kaye JA, Vasilakis‐Scaramozza C, Rodríguez LAG, Ruigómez A, Meier CR, et al. Validity of the general practice research database. Pharmacotherapy 2003; 23: 686–689. [DOI] [PubMed] [Google Scholar]
- 22. Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the general practice research database: a systematic review. Br J Gen Pract 2010; 60: e128–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001; 285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 24. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro heart survey on atrial fibrillation. Chest 2010; 137: 263–272. [DOI] [PubMed] [Google Scholar]
- 25. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest 2010; 138: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 26. Agence nationale de sécurité du médicament et des produits de santé (2014). Les anticoagulants en France en 2014: état des lieux, synthèse et surveillance. Available at http://ansm.sante.fr/var/ansm_site/storage/original/application/26ed375830c56499badf0014eb3bb81b.pdf (last accessed 20 April 2016).
- 27. Schuh T, Reichardt B, Finsterer J, Stöllberger C. Age‐dependency of prescribing patterns of oral anticoagulant drugs in Austria during 2011–2014. J Thromb Thrombolysis 2016; 42: 447–451. [DOI] [PubMed] [Google Scholar]
- 28. Weitz JI, Semchuk W, Turpie AG, Fisher WD, Kong C, Ciaccia A, et al. Trends in prescribing oral anticoagulants in Canada, 2008–2014. Clin Ther 2015; 37: 2506–14 e4. [DOI] [PubMed] [Google Scholar]
- 29. Choudhry NK, Soumerai SB, Normand S‐LT, Ross‐Degnan D, Laupacis A, Anderson GM. Warfarin prescribing in atrial fibrillation: the impact of physician, patient, and hospital characteristics. Am J Med 2006; 119: 607–615. [DOI] [PubMed] [Google Scholar]
- 30. Srivastava A, Hudson M, Hamoud I, Cavalcante J, Pai C, Kaatz S. Examining warfarin underutilization rates in patients with atrial fibrillation: detailed chart review essential to capture contraindications to warfarin therapy. Thromb J 2008; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heeringa J, van der Kuip DAM, Hofman A, Kors JA, van Herpen G, Stricker BHC, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006; 27: 949–953. [DOI] [PubMed] [Google Scholar]
- 32. Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation – quality and cost implications. Am J Med 2014; 127: 1075–82.e1. [DOI] [PubMed] [Google Scholar]
- 33. Olesen JB, Sørensen R, Hansen ML, Lamberts M, Weeke P, Mikkelsen AP, et al. Non‐vitamin K antagonist oral anticoagulation agents in anticoagulant naïve atrial fibrillation patients: Danish nationwide descriptive data 2011–2013. Europace 2015; 17: 187–193. [DOI] [PubMed] [Google Scholar]
- 34. Schumock GT, Walton SM, Park HY, Nutescu EA, Blackburn JC, Finley JM, et al. Factors that influence prescribing decisions. Ann Pharmacother 2004; 38: 557–562. [DOI] [PubMed] [Google Scholar]
- 35. Baker D, Wilsmore B, Narasimhan S. The adoption of direct oral anticoagulants for stroke prevention in atrial fibrillation. Heart Lung Circ 2016; 24: S394. [DOI] [PubMed] [Google Scholar]
- 36. Staerk L, Fosbøl EL, Gadsbøll K, Sindet‐Pedersen C, Pallisgaard JL, Lamberts M, et al. Non‐vitamin K antagonist oral anticoagulation usage according to age among patients with atrial fibrillation: temporal trends 2011–2015 in Denmark. Sci Rep 2016; 6: 31477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. National Health Services: East and North Hertfordshire Clinical Commissioning Group (2016). Guidelines for oral anticoagulation of patients with non‐valvular atrial fibrillation (AF) to prevent stroke [online]. Available at http://www.enhertsccg.nhs.uk/sites/default/files/content_files/Prescribing/Local_Decisions/Cardiovascular_system/Anticoagulants/Atrial%20Fibrillation%20Oral%20Anticoagulation%20Guidelines%20long%20v6%20ENHCCG.pdf (last accessed 20 April 2016).
- 38. National Health Services: Nottinghamshire Area Prescribing Committee (2016). Atrial fibrillation (non‐valvular): prescriber decision support on anticoagulation [online]. Available at http://www.nottsapc.nhs.uk/media/1043/anticoagulants‐in‐af.pdf (last accessed 20 April 2016).
- 39. National Health Services: Forth Valley (2014). Rivaroxaban as treatment for deep vein thrombosis and pulmonary embolism in adults [online]. Available at http://www.carronbank.co.uk/Clinical_Guidance/rivaroxaban-in-dvt-pe.pdf (last accessed 20 April 2016).
- 40. National Health Services: Wiltshire Clinical Commissioning Group (2014). Direct oral anticoagulants (DOACs) for DVT and PE in adults. Available at http://www.gwh.nhs.uk/media/236108/doacs‐for‐dvt‐pe‐august‐2016‐v‐9.pdf (last accessed 20 April 2016).
- 41. Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol 2013; 29: S24–S33. [DOI] [PubMed] [Google Scholar]
- 42. Baik SH, Hernandez I, Zhang Y. Evaluating the initiation of novel oral anticoagulants in Medicare beneficiaries. J Manag Care Spec Pharm 2016; 22: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hamilton M, Kawabata H, Liu X, Brixner D, Biskupiak J. Utilization patterns of anticoagulants in non‐valvular atrial fibrillation after the entry of novel oral anticoagulants in the United States. Circulation 2016; 126 (Suppl. 21): A9664. [Google Scholar]
- 44. Pan X, Kawabata H, Hamilton M, Liu X. Patient characteristics associated with the initiation of novel oral anticoagulants versus warfarin in patients with atrial fibrillation. Eur Heart J 2014; 34 (Suppl. 1): 543. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Distribution of first‐time oral anticoagulant users in the UK Clinical Practice Research Datalink, from 2009 to 2015
Figure S2 Rates of new users of novel oral anticoagulants with an indication for atrial fibrillation (left) and venous thromboembolism (right) in the UK Clinical Practice Research Datalink, from 2009 to 2015
Table S1 Temporal trends in the rate of new users of novel oral anticoagulants in the UK Clinical Practice Research Datalink, from 2009 to 2015
Table S2 Baseline characteristics of patients newly prescribed novel oral anticoagulants in the UK Clinical Practice Research Datalink in 2015
Table S3 Odds ratios (95% confidence intervals) for the association between patient characteristics and the initiation of novel oral anticoagulants in the UK Clinical Practice Research Datalink in 2015, stratified by oral anticoagulant indication


