Abstract
Developmental plasticity of cardiorespiratory physiology in response to chronic hypoxia is poorly understood in larval fishes, especially larval air‐breathing fishes, which eventually in their development can at least partially “escape” hypoxia through air breathing. Whether the development air breathing makes these larval fishes less or more developmentally plastic than strictly water breathing larval fishes remains unknown. Consequently, developmental plasticity of cardiorespiratory physiology was determined in two air‐breathing anabantid fishes (Betta splendens and Trichopodus trichopterus). Larvae of both species experienced an hypoxic exposure that mimicked their natural environmental conditions, namely chronic nocturnal hypoxia (12 h at 17 kPa or 14 kPa), with a daily return to diurnal normoxia. Chronic hypoxic exposures were made from hatching through 35 days postfertilization, and opercular and heart rates measured as development progressed. Opercular and heart rates in normoxia were not affected by chronic nocturnal hypoxic. However, routine oxygen consumption (~4 μmol·O2/g per hour in normoxia in larval Betta) was significantly elevated by chronic nocturnal hypoxia at 17 kPa but not by more severe (14 kPa) nocturnal hypoxia. Routine in Trichopodus (6–7 μmol·O2/g per hour), significantly higher than in Betta, was unaffected by either level of chronic hypoxia. P Crit, the PO2 at which decreases as ambient PO2 falls, was measured at 35 dpf, and decreased with increasing chronic hypoxia in Betta, indicating a large, relatively plastic hypoxic tolerance. However, in contrast, P Crit in Trichopodus increased as rearing conditions grew more hypoxic, suggesting that hypoxic acclimation led to lowered hypoxic resistance. Species‐specific differences in larval physiological developmental plasticity thus emerge between the relatively closely related Betta and Trichopodus. Hypoxic rearing increased hypoxic tolerance in Betta, which inhabits temporary ponds with nocturnal hypoxia. Trichopodus, inhabiting more permanent oxygenated bodies of water, showed few responses to hypoxia, reflecting a lower degree of developmental phenotypic plasticity.
Keywords: Circulation, hypoxia, larval fishes, phenotypic plasticity, respiration
Introduction
Acute hypoxic exposure in aquatic fishes triggers reflex responses aimed at maintaining homeostasis, including reflex branchial hyperventilation – for reviews see (Abdallah et al. 2015; Martin 2014; Milsom 2012; Perry 2011; Porteus et al. 2011). Frequently concurrent with hypoxia‐induced increases in gill ventilation is a reflex bradycardia, and increases in stroke volume and branchial vascular resistance (Farrell 2007; Gamperl and Driedzic 2009; Gamperl and Farrell 2004; Pelster 1999; Stecyk et al. 2008; Tota et al. 2011; Wilson et al. 2015). These physiological and behavioral responses to aquatic hypoxia in fishes – collectively representing the hypoxic ventilatory reflex – are often accompanied by numerous other additional physiological adjustments, including changes in hemoglobin oxygen binding affinity, blood O2 carrying capacity, stroke volume, and branchial vascular resistance. All these adjustments to hypoxia can contribute to enhanced O2 transfer and potentially lowered ventilatory convection requirement (Gamperl and Driedzic 2009; Perry et al. 2009). The branchial hyperventilation reflex of aquatic fishes minimizes reductions in arterial‐blood PO2 associated with aquatic hypoxia, but may also be metabolically expensive (Perry 2011; Perry et al. 2009). Indeed, as aquatic PO2 falls, the high cost of gill ventilation with water may become prohibitive, especially when combined with failure of adequate tissue oxygen transport associated with low arterial PO2 (Diaz and Breitburg 2009; Farrell and Richards 2009; Graham 1997; Randall et al. 1981).
Although air breathing as a response to aquatic hypoxia is an evolutionarily exotic solution to maintaining ventilation in hypoxic aquatic environments, air breathing has nonetheless independently evolved nearly 50 times in Teleost fishes (Graham 1997; Little 2009; Randall et al. 1981). Air breathing may be a response to nocturnal or seasonal hypoxia in so‐called “facultative” air‐breathing fishes, or may be required in “obligatory” air breathers – for reviews, see (Abdallah et al. 2015; Burggren 1982; Burggren and Johansen 1986; Johansen and Lenfant 1968; Martin 2014; Milsom 2012; Perry 2011; Porteus et al. 2011). The physiological and metabolic responses to hypoxia of adult air‐breathing fishes have been investigated in numerous species. However, natural selection acts very heavily on the embryos, larvae, and juveniles of aquatic and air‐breathing fishes alike, with high mortalities occurring in the earliest developmental stages (Browman 1989; Holzman et al. 2015; Mendez‐Sanchez and Burggren 2014). Thus, understanding the biology of these early stages has additional significance.
In air‐breathing fishes, an initial period of aquatic respiration using a combination of gills and an air‐breathing organ occurs prior to the functional development of both ventilated and perfused air‐breathing organ and the associated onset of air breathing. Yet, relatively few studies have investigated the morphological and physiological transitions to air breathing in larval air breathing fishes (Ahmad and Hasnain 2005; Blank and Burggren 2014; Brauner and Rombough 2012; Burggren 1979; Islam 2005; Liem 1981; Mendez‐Sanchez and Burggren 2014; Terjesen et al. 2001) or, indeed, any tropical species (Peck and Moyano 2016). Only a few of those studies have considered how hypoxia affects the aquatic larva during this morphological, behavioral, and physiological transition. What makes larval air breathing fishes particularly interesting in the study of hypoxia‐induced developmental plasticity is that at some point in their development such fishes have the ability to at least partially “escape” hypoxia and its consequences through the onset of air breathing. Whether this option of air breathing in later larval life makes early larvae prior to the onset of air breathing less or more developmentally plastic than strictly water breathing larval fishes is unknown.
The aim of this study, then, was to evaluate the effect of chronic hypoxia on larvae of the air‐breathing fishes the gourami (Trichopodus trichopterus) and the Siamese fighting fish (Betta splendens) prior to the onset of air breathing. Trichopodus and Betta are relatively closely related anabantids from the family Osphronemidae (Froese and Pauly 2016), both possessing a suprabranchial labyrinth organ that serves as the site of aerial respiration as juveniles and adults. Although both species use labyrinth organs for air breathing, these two air breathing fishes live and reproduce in distinctly different habitats. Betta breeds in temporal isolated ponds with standing waters located in flood plains such as rice paddies. These ponds are frequently hypoxic, even anoxic on the bottom, because of the high temperatures and high organic content (Froese and Pauly 2016; Monvises et al. 2009; Rainboth 1996). Betta is a bubble nest builder and males provide intense care for eggs and early larva (Monvises et al. 2009; Ruber et al. 2006). Young larval Betta are unable to escape aquatic hypoxia and may have a greater tolerance to aquatic hypoxia. In contrast to Betta, Trichopodus reproduces in lowland wetlands like marshes, swamps, and canals with seasonal floods that facilitate temporary lateral migrations from river mainstreams to seasonally flooded areas, with a return to the permanent water bodies as the dry season approaches (Froese and Pauly 2016; Rainboth 1996). Compared to Betta, Trichopodus larvae, and juveniles are more active swimmers based on our observations of larvae in holding tanks, potentially enabling migration back to rivers and other larger bodies of water from the increasingly hypoxic receding floodwater ponds.
Against this backdrop, we hypothesized that chronic environmental hypoxia will alter heart and opercular rate as well as and P Crit in the larvae of both species, as in many strictly aquatic larval fishes. Additionally, we hypothesized that these physiological responses would differ qualitatively and/or quantitatively between Trichopodus and Betta, based on the different habitats of these two species, based in part upon their differential adjustments in larval onset of air breathing in response to hypoxia (Mendez‐Sanchez and Burggren 2014). To our knowledge this is the first study to consider development of larval air‐breathing fishes reared in the more natural condition of nocturnal hypoxia and diurnal normoxia.
Material and Methods
Rearing and maintenance
Large numbers of eggs are produced at each breeding in both Betta (500 eggs) and Trichopodus (1000–2000 eggs). Eggs hatch within 24–48 h and become free‐swimming in 3–4 days at 27°C (Mendez‐Sanchez and Burggren 2014; Pollak et al. 1981). Larval Trichopodus and Betta were maintained from hatching to 35 days postfertilization (dpf). Details of rearing protocol, water quality, and larvae maintenance have been previously described in detail (Mendez‐Sanchez and Burggren 2014).
All larvae for a single experiment were taken from the same clutch to avoid intraclutch effects. Day of fertilization was designated 0 dpf. For each subsequent 24 h cycle a unit of 1 day was added. Larvae were reared from hatching for 48 h in normoxia. Thereafter, larvae were transferred into different floating containers (250 mL) in 40 L aquaria and raised in either continuous normoxia (20 kPa) or in intermittent nocturnal hypoxia (17 and 14 kPa) until 35 dpf (Trichopodus) or 38 dpf (Betta). Oxygen levels of the water in the aquaria and in the containers containing the larvae were regulated by directing into the water a stream of either room air (control, 20 kPa) or a mixture of room air and nitrogen gas, creating hypoxia at levels of either 17 kPa or 14 kPa. Gas flows were regulated with flowmeters set to deliver the appropriate gas mixture.
The containers were sealed with Plexiglass covers with exhaust valves preventing atmospheric air from leaking into the containers. Each rearing container was filled with water to 80% of its capacity, with the remaining 20% receiving the gas emerging up from the water in the container. This configuration ensured that the gas and water phases of the containers were in PO2 equilibrium. Water PO2 was monitored daily using an optical oximeter probe ProODO (YSI Incorporated). PO2 of the gas phase was measured and monitored daily with a ProOx 110 oxygen sensor (Biospherix, Ltd).
Previous experiments with Betta and Trichopodus have revealed that even relatively mild hypoxia when delivered continuously produces very high larval mortality (Mendez‐Sanchez and Burggren 2014). Thus, the current experiments employed a far more natural regime of intermittent nocturnal hypoxia exposure synchronized with the experimental light:dark cycle. This protocol mimics the dial cycle of hypoxia in the tropical habitats were these fishes evolved. Specifically, all larval populations of both species were exposed to 12 h of normoxia (PO2 = 20 kPa) during the day. The control population also experienced only normoxia at night. Those designated as the mild hypoxia (17 kPa) or more severe hypoxia (14 kPa) populations were additionally exposed to their specified level of hypoxia for 12 h during the night. Each of the three PO2 populations was created in triplicate, with 150 larvae placed in three 0.25 L containers, each containing 50 larvae. Larvae for a particular physiological experiment were randomly sampled from one of the three containers. Once measurements were made, they were subsequently euthanized by submersion in a diluted solution of buffered MS222 (250 mg/L) until opercular movements stopped.
Larvae of the three populations were sampled for physiological variables at 5 day intervals until 35 dpf. Each larva was fasted for 12 h prior to any measurement. Because of the smaller clutch size of Betta, samples of this species were only taken from 20 to 35 dpf to ensure sufficient individuals for each measurement. The body mass and length of the larvae of both species are shown in Table 1.
Table 1.
Mean ± standard error. N = 5 for each mean. The values correspond to the larvae used in the experimental measurements
| Species | DPF | REARING PO2 (kPa) | |||||
|---|---|---|---|---|---|---|---|
| 14 | 17 | 20 | |||||
| Wet mass average (mg) | Length average (mm) | Wet mass average (mg) | Length average (mm) | Wet mass average (mg) | Length average (mm) | ||
| Betta splendens | 20 | 1.80 ± 0.24 | 5.88 ± 0.74 | 1.90 ± 0.24 | 5.47 ± 0.74 | 2.27 ± 0.24 | 5.62 ± 0.74 |
| 25 | 2.50 ± 0.24 | 6.31 ± 0.74 | 5.10 ± 0.21 | 6.96 ± 0.64 | 1.10 ± 0.24 | 5.06 ± 0.74 | |
| 30 | 2.90 ± 0.24 | 6.80 ± 0.74 | 6.13 ± 0.24 | 7.53 ± 0.74 | 5.40 ± 0.24 | 6.79 ± 0.74 | |
| 35 | 3.60 ± 0.24 | 6.98 ± 0.74 | 15.20 ± 0.24 | 10.49 ± 0.74 | 5.50 ± 0.24 | 7.31 ± 0.74 | |
| 38 | 7.09 ± 0.14 | 8.26 ± 0.43 | 7.80 ± 0.13 | 8.62 ± 0.41 | 10.49 ± 0.14 | 8.94 ± 0.43 | |
| Trichopodus trichopterus | 5 | 0.26 ± 0.08 | 3.61 ± 0.42 | 0.26 ± 0.08 | 3.61 ± 0.42 | 0.26 ± 0.08 | 3.61 ± 0.42 |
| 10 | 0.40 ± 0.12 | 3.37 ± 0.67 | 0.27 ± 0.10 | 3.55 ± 0.55 | 0.23 ± 0.10 | 3.21 ± 0.55 | |
| 15 | 0.57 ± 0.10 | 4.29 ± 0.55 | 0.50 ± 0.10 | 3.81 ± 0.55 | 0.50 ± 0.10 | 4.36 ± 0.55 | |
| 20 | 1.13 ± 0.10 | 5.89 ± 0.55 | 0.80 ± 0.10 | 5.41 ± 0.55 | 1.00 ± 0.10 | 5.52 ± 0.55 | |
| 25 | 0.93 ± 0.10 | 5.38 ± 0.55 | 0.97 ± 0.10 | 5.20 ± 0.55 | 1.33 ± 0.10 | 6.07 ± 0.55 | |
| 30 | 1.67 ± 0.10 | 6.02 ± 0.55 | 1.43 ± 0.10 | 5.94 ± 0.55 | 2.70 ± 0.10 | 6.93 ± 0.55 | |
| 35 | 5.11 ± 0.06 | 7.95 ± 0.33 | 3.35 ± 0.07 | 7.46 ± 0.39 | 3.61 ± 0.06 | 7.88 ± 0.33 | |
| 37 | 6.05 ± 0.07 | 8.14 ± 0.39 | 3.95 ± 0.05 | 7.73 ± 0.30 | 4.36 ± 0.05 | 7.71 ± 0.30 | |
Opercular rate and heart rate
Opercular rate (f Op, opercular beats min−1) and heart rate (f H , beats min−1) were measured at 27°C every 5 days from 5 to 35 dpf. In larvae of both Betta and Trichogaster the heart could be directly observed through the transparent body wall at these early ages. Larvae were gently placed in a 4.5 mL transparent flow‐through chamber for observation of opercular and heart rates. The chamber had water pumped at a rate of ~mL/min from the experimental rearing aquarium at the corresponding PO2. Pilot studies revealed that larvae returned to resting values of heart rate and gill ventilation with 10–15 min of gentle handling. Nonetheless, larvae were allowed to acclimate to the chambers for 1 h before measurements were begun. f Op and f H was recorded for 60 sec using a digital microscope (Celestron 44302‐A) at a magnification of 150x.
A 10 sec section of each video was analyzed with Tracker 4.72, an open source physics video analyzer (Brown, 2017). This software was used to automatically and simultaneously track heartbeat and operculum movements using changes in luminance (brightness in an image = the “black‐and‐white” or achromatic portion of the image) occurring through time in a selected area of the video. An example of the traces of f Op and f H obtained using Tracker 4.72 is shown in figure.
Oxygen consumption
Every 5 days following fertilization, larvae from each treatment group were assessed for routine mass‐specific O2 consumption (, μmol·O2/g per hour) in normoxia (PO2 = 20 kPa) at 28°C, using intermittent closed respirometry via a Loligo Systems respirometry system (Tjele, Denmark) – see also (Lefevre et al. 2016). All measurements were made on 12 h fasting larvae during daylight hours in respirometers initially filled with normoxic water (PO2 = 20 kPa). Larvae were placed in a 2.0 mL borosilicate glass microrespirometer chamber and allowed to acclimate for 1 h in the respirometer while water was gently refreshed from the outer reservoir using a peristaltic pump. Four chambers were used simultaneously. One chamber for each contained only aerated water and served as blank sample to determine the effects of possible microbial respiration. The designated blank chamber was rotated through all four chambers during the course of four runs. Inevitably, microbial oxygen consumption was below the system's detection level (accurate to<<0.0005 μmol·O2/g per hour). For determination of routine , larvae were placed in aerated water, and the decline in PO2 (typically 1–3 kPa) in each respirometer containing a fish and the blank was measured for 2–6 h, depending on the larva's O2 consumption. PO2 decline was measured using temperature‐compensated fiber‐optic planar sensors attached to an OXY‐4 meter (PreSens). The signals from these probes were displayed on a computer with the automated data acquisition system DAQ‐M. The AutoResp software (Loligo Systems ApS) calculated mass‐specific oxygen consumption from the rate of O2 decline in each chamber over time, the elapsed time, the chamber volume, and the fish mass. This was designated routine metabolic rate (RMR) since although there was little or no effect of specific dynamic action associated with feeding given the fasting nature of the fish, the activity level of the fishes could not be directly observed in the individual respirometers. However, separate observations of larval Betta indicated that locomotor movement was infrequent for the first 35 dpf, suggest that our measured RMR was close to standard metabolic rate, at least for this species.
Critical oxygen tension (P Crit, in kPa) is the oxygen partial pressure (PO2) at which routine can no longer be maintained at normoxic levels as PO2 further decreases. P Crit was measured using the same experimental design and apparatus as for measurement of routine but using closed respirometry as opposed to intermittent flow‐through respirometry. PO2 in the respirometers was allowed to fall to close to 0 kPa. A two‐phase linear regression model (Mueller et al. 2011; Yeager and Ulstch 1989) was applied to the PO2 versus data. To avoid the confounding effects of intraclutch variation, P Crit was only measured in six groups originally obtained from the same egg clutch. P Crit was determined on the first day at which air breathing was observed (~35 pdf).
Statistical analysis
MANOVA comparisons were performed on P Crit , f H, and f Op using PO2, age and, in some cases, species, as factors. MANCOVA was also performed on to correct for the effect of age, using days of postfertilization as a covariate. To compare the effect of PO2 on the relationship between f H and f Op, linear comparison of slopes and intersections were also utilized.
Treatment groups were considered significantly different at P < 0.05. All data are expressed as mean ± 1 standard error (SE). N values are indicated for individual means, and total numbers of larvae used are indicated in the statistical descriptions.
All larval fishes used in this research were treated according to U.S. federal regulations and guidelines. This study was approved and monitored by the University of North Texas Animal Care and Use Committee (Project number 1111‐16).
Results
Opercular ventilation rate
Normoxic development: Larval Betta reared and measured in normoxia were rapidly and extensively ventilating their gills at 5 dpf, the first day of measurement, and had completely consumed their egg yolk. f Op in larval Betta did not significantly vary through development (F = 0.4, df = 2, 63, and P > 0.05), averaging 77 ± 3 opercular beats/min over the developmental span monitored (Fig. 1A). In contrast to Betta, larval Trichopodus reared and measured in normoxia had not yet established opercular beating on dpf 5 (Fig. 1B). However, once opercular movements began much later at 10 dpf, the overall f Op average value of 173 ± 6 beats/min was higher in Trichopodus than in Betta at any given developmental stage.
Chronic hypoxia: Rearing in chronic hypoxia (combined aquatic and aerial) did not induce any significant changes in gill ventilation measured in normoxia in larval Betta (F = 7, df = 6, 63, and P > 0.05) (Fig. 1A). Although in the normoxic population of Trichopodus there was no opercular beating at 5 dpf, the dpf 5 Trichopodus chronically exposed to mild hypoxia (PO2 = 17 kPa of) were actually exhibiting opercular beating (Fig. 1B). Surprisingly, however, the population reared in an even lower level of oxygen (14 kPa) was not yet ventilating their gills at dpf 5. From 10 dpf onwards all three populations of Trichopodus were actively ventilating their gills, but there were no significant variation associated with level of hypoxia (F = 0.6, df = 2, 60, and P > 0.05) (Fig. 1B).
Figure 1.

Comparison of opercular rate (f Op) from 5 days through 35 days postfertilization in (A) Betta splendens and (B) Trichopodus trichogaster reared in three different levels of PO 2. Means ± SE are presented. N = 9. The horizontal lines represent the average fH for all ages.
Heart rate
Normoxic development: Heart rate in larval Betta reared and measured in normoxia showed no significant changes as a function of development (F = 0.4, df = 2, 63, and P > 0.05) (Fig. 2A), with overall heart rate averaging 156 ± 4 beats/min. This value was ˜25% lower than for Trichopodus at all comparable stages of development, where overall f H across the entire monitored period was 212 ± 3 beats/min. Unlike for Betta, however, f H in Trichopodus decreased slowly and significantly with development (F = 29.1, df = 2, 60, and P < 0.0001) (Fig. 2B).
Hypoxic development: Heart rate in larvae of both Betta and Trichopoduschronically reared in hypoxia showed no significant changes as a function of rearing oxygen level (F = 0.4, df = 2, 63, and P > 0.05) and (F = 0.6, df = 2, 60, and P > 0.05) (Fig. 2).
Figure 2.
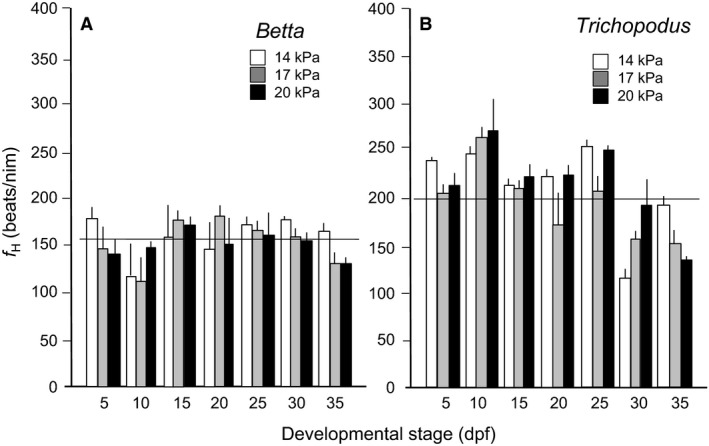
A comparison of heart rate (fH) in larval Betta splendens (A) and Trichopodus trichogaster (B) through 35 days postfertilization in three levels of PO 2. Means ± SE are presented. N = 9. The horizontal lines represent the average fH for all ages.
Heart beat‐opercular rate relationship
Interactions between heart rate and opercular rate are often taken as an indication of neural cardiorespiratory coordination in fishes (Dick et al. 2014; Schulz et al. 2013; Taylor 1985; Taylor et al. 2006). Representative traces of simultaneously recorded opercular rate and heart rate in a dpf 35 Betta splendens are shown in Figure 3. In part because of considerable variations in duration of the opercular cycle within individual fish, the interaction of f H and f Op showed no differences across ages in either Betta or Trichopodus, with the sole exception of dpf 5 Trichopodus. Consequently, the effect of this factor was not considered from the perspective of their interaction, and data for the f H :f Op relationship was determined from a pool of all individuals from 5 to 35 dpf. From this general population it was determined that the interaction of f H and f Op was significantly affected by hypoxia rearing level in both Betta and Trichopodus. Larval Betta reared in normoxia varied significantly from the line of identity (1:1 ratio), showing an approximate timing of 3 heartbeats per 1 opercular beat (Fig. 4A). However, rearing in either level of chronic hypoxia resulted in patterns of f H:f Op ratio of ~2:1, which were significantly different from each other, from the 1:1 ratio, and from the line describing the normoxic ratio (F slopes = 2.57; df = 3,7; P < 0.05 and F intercepts = 14.02; df = 3,7; P < 0.001). Essentially, hypoxic treatments increased f Op compared with the normoxic larva with the same f H. Larval Betta also tended to have a f H:f Op with a slope more similar to the 1:1 ratio than to the normoxic slope. For larval Trichopodus the f H:f Op relationship was statistically identical to a 1:1 ratio (Fig. 4B), and showed no significant differences between larvae reared in normoxia and either level of hypoxia larvae (F slopes = 1.62; df = 3,7; P > 0.05 and F intercepts = 0.83; df = 3,7; P > 0.05).
Figure 3.

Representative traces taken from software analysis of video images of Betta splendens (35 days postfertilization) raised in hypoxia (17 kPa). The dashed vertical lines toward the right of the panels illustrate the ~2:1 ratio of heart beat to opercular movement in this trace. See text for additional details.
Figure 4.

The heart rate:opercular rate relationship (f H: f Op) in larvae of (A) Betta splendens and (B) Trichopodus trichopterus. Data from 5 dpf to 35 dpf were pooled, since preliminary analysis revealed no significant effects of development. The lines show the data for three groups reared in different levels of PO 2. n = 20 for each PO2 group of each species. In all cases r > 0.9 and P < 0.05 indicating significance of relationship. See text for statistical analysis of differences between experimental groups.
Mass‐specific O2 consumption
Normoxic development: Larval Betta showed a complex pattern of developmental change in routine oxygen consumption () under normoxic conditions, with significantly higher values at early stages and declining values later in larval development (F = 74, df = 4, 228, and P < 0.0001) (Fig. 5A). Trichopodus similarly presented higher routine during earliest stages of development (F = 137, df = 6, 167, and P < 0.0001) (Fig. 6A), but quickly settled into a relatively stable routine ˜9.03 μmol O2/g per hour throughout the rest of development (Fig. 6A). This level of was approximately 60% higher than in Betta over comparable developmental stages.
Hypoxic development. The effects of hypoxic rearing were complex in Betta, as were the changes during its normoxic development. Mild chronic hypoxic exposure (17 kPa) actually stimulated routine above control (normoxic) levels. However, more severe chronic hypoxic rearing (14 kPa) in Betta depressed routine at all developmental stages (F = 73.9, df = 2, 228, and P < 0.0001) (Fig. 5A). Analyzing the effect of hypoxia on corrected for age (i.e., using age as a covariate) in Betta revealed a significant effect of hypoxia in the mildly hypoxic 17 kPa group (F = 18.2, df = 2, 227, and P < 0.0001) (Fig. 5B). In stark contrast to Betta, however, in Trichopodus neither level of chronic hypoxia induced significant changes in , confirmed by analyzing the effect of hypoxia corrected for age (F = 0.2, df = 2, 167, and P > 0.05) (Fig. 6B).
Figure 5.

Routine oxygen consumption () of larval Betta splendens. (A) from 20 through 20–35 dpf reared in three levels of PO2.(B) corrected for aged differences in the three larval populations. Means ± SE are presented. n = 9. An * indicates a significant difference from control (20 kPa).
Figure 6.

Routine oxygen consumption of larval Trichopodus trichopterus. (A) through 35 dpf reared in three levels of PO2. The horizontal line indicates the average value across all developmental stages. (B) corrected for aged differences in the three larval populations. Means ± SE are presented. n = 9. An * indicates a significant difference from control.
Critical oxygen partial pressure (P Crit)
An example of the raw data for the calculation of P Crit is shown in Figure 7. Betta and Trichopodus showed two different, opposing patterns for the effects of rearing oxygen level on P Crit measured on dpf 35 (Fig. 8). In larval Betta, P Crit was positively correlated with rearing PO2 (F = 5.5, df = 2, 21, and P < 0.05), with P Crit at 14 kPa 37% lower than the value in the normoxic population. In contrast, in Trichopodus larvae the P Crit actually increased as rearing conditions grew more hypoxic (F = 17, df = 2, 19, and P < 0.0001), with chronic rearing at a PO2 of 17 and 14 kPa PO2 increasing P Crit to higher PO2s by 24% and 70%, respectively.
Figure 7.

Representative traces of routine with declining PO 2 in four larval Trichopodus (35 dpf) reared in chronic intermittent hypoxia (14 kPa). Different symbols represents a different larva, and each individual symbol represents a single measurement of at the indicated PO 2.
Figure 8.

Comparison of P Crit for larval Betta and Trichopodus reared in different levels of PO2 at 35 dpf. Boxes enclose statistically identical means (P > 0.05). n values for each group are in parentheses.
Discussion
Development in Normoxia
The ventilatory frequency (f Op) of larval Betta and Trichopodus in normoxia was ~80 and ~175 beats/min, respectively. Although these two values cover a fairly wide range of opercular rates, they fall within the wide range of values for larvae of other aquatic freshwater teleost fishes at similar temperatures or when corrected for different temperatures using a Q10 of 2 – e.g., (Burggren et al. 2016; Holeton 1971; Lerner et al. 2007; Vosyliene et al. 2005). These gill ventilation rates of larvae are considerably higher than ventilation rates in the much larger adults of the same species of either strictly aquatic or air breathing fishes – for example, (Cerezo et al. 2006; Kalinin et al. 2000; McKenzie et al. 2007; Porteus et al. 2011; Richards and Haswell 2011), as would be expected from scaling effects on physiological processes.
Heart rate in larval Betta and Trichopodus was ~160 and ~210 beats/min, respectively. Relatively few measurements of heart rate exist for the larvae of either air breathing or strictly aquatic species, but the rates recorded in this study are within the range of those recorded at similar temperatures (27–28̊°C) in another larval air‐breathing fish, the tropical gar Atractosteus tropicus (Burggren et al. 2016) and in unrestrained, larval zebrafish (Danio rerio), for example, (Barrionuevo and Burggren 1999; Jacob et al. 2002; Kopp et al. 2014; Miller et al. 2014; Parker et al. 2014; Rider et al. 2012; Rombough 2007; Steele et al. 2011; Velasco‐Santamaria et al. 2011). In this respect, the earliest strictly aquatic stages of these air‐breathing fishes physiologically resemble those of aquatic freshwater fishes, generally.
Both opercular rate and heart rates typically change over early development in vertebrates, showing complex patterns of change that are often characterized by an initial increase during the early phases of organogenesis followed by declines predicted from allometric scaling (Burggren and Warburton 2005; Burggren 2005). Yet, in this study neither opercular nor heart rates showed any major changes during development in either Betta or Trichopodus. This may suggest a lack of neural and hormonal control of both parameters during the first 35 days of postfertilization (McKenzie et al. 2007; Taylor et al. 2010) prior to the onset of air breathing in these species (Mendez‐Sanchez and Burggren 2014). This would be a much later development of respiratory reflexes than in the air‐breathing tropical gar, Atractosteus tropicus, but this species starts air breathing far earlier at around 4–5 dpf at similar temperatures (Burggren et al. 2016). In any event, challenge by stressors (e.g., temperature, oxygen, activity) will be required to confirm the specific timing of onset of cardiorespiratory regulation in Betta and Trichopodus.
Coupling of cardiac and respiratory activity has long been appreciated, and gives insights into the maturity and complexity of physiological regulatory systems (Dick et al. 2014; Schulz et al. 2013; Taylor 1985; Taylor et al. 2006). The f H:f Op relationship for larval Betta was approximately 3 heartbeats for every 1 opercular beat. The f H:f Op ratio in larval Trichopodus was much lower at ~1:1. It has been speculated that a coordination between ventilation and heart rate in normoxia may improve gas transport across the gills by matching convective delivery of oxygen to the gills with the ability of the perfusing blood to remove it (Rombough 1998; Smatresk et al. 1986). This would potentially making Trichopodus more efficient in extracting O2 from the water. In the adults of the facultative air‐breathing fish, Lepisosteus osseus (Rahn et al. 1971), the coupling between heart rate and gill ventilation ratio in normoxia was also ~1:1 (Smatresk et al. 1986). For adults of another facultative air‐breather Hoplerythrinus unitaeniatus (Oliveira et al. 2004), the mean ventilatory frequency was slightly more than twice the heart frequency, a typical relation for a strictly water breathing fish (McKenzie et al. 2007). For the hypoxia tolerant adult water breather Piaractus mesopotamicus, f H:f Op approximated 3:1 (Leite et al. 2007; Taylor et al. 2009). Clearly, larval Betta showed less opercular movements per heart beat than the other two species of facultative air breathers employing aquatic respiration. This could be related to the low level of physical activity we have observed in larval Betta. Thus, in normoxic conditions the high f H: f Op ratio could allow adequate tissue O2 delivery. Also, during early developmental stages skin breathing is likely also directly involved in providing gas exchange (see (Blank and Burggren 2014; Feder and Burggren 1985; Liem 1981; Wells and Pinder 1996b), deemphasizing cardiorespiratory coupling in early developmental stages. However, a definitive answer on the importance cardiorespiratory coupling in developing, as opposed to adult, fishes will have to await additional data.
Larval Trichopodus had a routine of ~9.03 μmol O2/g per hour, which was considerably higher than larval Betta, 4.8 ± 0.34 μmol O2/g per hour. In fact, the routine of larval gourami is in the range of the larvae of active strictly aquatic fishes. The mean routine of larval Betta was between reported values of larval air breathers and water breathers (Bagatto et al. 2001; Barrionuevo et al. 2010; Gore and Burggren 2012; Graham 1997; Lucas et al. 2014; Peters 1978; Rombough 1998; Wells and Pinder 1996a). Simple observation of larval Trichopodus raised in normoxia reveals them to be active swimmers with a high oxygen demand, compared with larval Betta which are much more likely to rest on the substrate (Mendez‐Sanchez and Burggren 2014),
Trichopodus and Betta occupy considerably different positions on the behavioral and locomotor gradient of fish lifestyles (Dwyer et al. 2014; Stoffels 2015). The slower lifestyle of Betta, which is an ambush predator or “saltatory” forager, has mostly benthic habits, yet is capable of rapid starts and turns for high‐acceleration prey capture. Consequently, low metabolic rates and high PCrit values characterize the metabolism of Betta.
The P Crit of larval Betta was 7.2 ± 0.4 kPa. This compares with 4.6–8.1 kPa for larval Hoplosternum littorale (Sloman et al. 2009), 9.3 ± 1.0 kPa for adults of the facultative air‐breathing Amia calva (Porteus et al. 2014), and 2–6 kPa for adults of Mogurnda adspersa, Melanotaenia fluviatilis, and Hypseleotris sp. exposed to natural hypoxia episodes as a consequence of droughts (Stoffels 2015). P crit for larvae of the aquatic Danio rerio are in the range of 7.3–9.9 kPa (Barrionuevo and Burggren 1999; Barrionuevo et al. 2010). P Crit has been considered to be an indicator of hypoxia tolerance (Chapman et al. 2002; Mandic et al. 2013). Again, this characteristic for Betta is in the middle of a range of aquatic and facultative air‐breathing fishes, pointing to its facultative air‐breathing habit. An intermediate P Crit makes easier the possibility to adjust its respiratory performance to either a water or air‐breathing strategy (Robertson et al. 2014).
Once again in contrast to Betta, Trichopodus fits with a faster lifestyle – an active cruising and pursuit predator with endurance swimming and sprints for sustained chases, patrolling, drift feeding, searching, etc. These traits are reflected in this species higher metabolic rates and low PCrit, which are characteristic of more rapidly swimming fishes.
Development in chronic intermittent hypoxia
Different fish species employ different approaches for dealing with aquatic hypoxia. Hypoxia resistance, the ability to actively maintain O2 extraction and thus routine metabolic rate even as O2 levels fall, allows animals to exploit environments with variable O2 levels (Mandic et al. 2013). The hyperventilation reflex is critical for this strategy. However, some species exhibit hypoxic tolerance (distinct from resistance), whereby as environmental PO2 falls, the fish no longer actively maintains normal rates of aerobic O2 consumption. This requires the ability to tolerate increasing levels of tissue hypoxia (Perry 2011), and is often correlated with lower critical PO2 (Chapman et al. 2002; Mandic et al. 2013). Betta and Trichopodus both responded physiologically to rearing in chronic intermittent hypoxia, but with highly species‐specific differences, as will now be considered.
Betta
In Betta reared under mild hypoxia (PO2 = 17 kPa), routine was elevated at most stages of development (Fig. 5), showing that these larvae can regulate and increase aquatic oxygen consumption, even at an early larval stage. In this respect, larval Betta appears to be like the larvae of active aquatic species such as Danio rerio, whose oxygen uptake appears to be enhanced by mild hypoxia (Barrionuevo and Burggren 1999; Barrionuevo et al. 2010). However, routine in Betta was reduced compared to normoxia under more severe oxygen stress (14 kPa) (Fig. 6). Physiological adjustments to oxygen extraction from hypoxic water to maintain homeostasis are expensive (Perry 2011; Perry et al. 2009) and the cost cannot easily be sustained in more severe hypoxia. In any event, the low routine presented by larval Betta at 14 kPa also reflects their limited ability to maintain O2 uptake at this level of hypoxia, which is reflected in low survival at this PO2 in both continuous and intermittent hypoxia (Mendez‐Sanchez and Burggren 2014).
Larval Betta chronically reared under hypoxic conditions showed a P Crit that was 30% lower than when reared in normoxia. A lower P Critreflects the ability of larval Betta to continue extracting O2 at progressively lower oxygen levels, essentially making them more resistant to hypoxia and correlating with that fact that this species has evolved in more hypoxic waters than Trichopodus (Froese and Pauly 2016; Monvises et al. 2009; Rainboth 1996).
Facultative air breathers evolved the air‐breathing habit in response to unpredictable environmental conditions, especially with respect to ambient temperature and oxygen (Brauner et al. 2004; Graham 1997; Randall et al. 1981). To be a facultative air breather implies the ability to adjust physiological variables in the face of unpredictable environment situations. Such ability was evident in larval Betta when examining the f H:f Op relationship and how this variable was affected by hypoxic rearing. Chronic hypoxia induced higher levels of f Op at the same f H, and the relationship between these two variables changed from 3:1 in normoxia to 2:1 in hypoxia. This relative increase in perfusion compared to ventilation may also improve gas transport across the gills (Smatresk et al. 1986), potentially making this species more efficient in extracting O2 from the water under hypoxic conditions.
Trichopodus
Larval Trichopodus, unlike larval Betta, showed little to no physiological response to chronic aquatic hypoxia, a lack of physiological plasticity potentially making this species less tolerant to hypoxia (Chapman et al. 2002; Mandic et al. 2013; Perry 2011). This characteristic may also explain the low overall survival of larval Trichopodus in hypoxia (Mendez‐Sanchez and Burggren 2014). As an obligate air breather, the only mechanism that larval Trichopodus have to escape aquatic hypoxia is to resort to air‐breathing (Graham 1997). Rearing in chronic hypoxia had no significant effect on the routine of larval Trichopodusbut did increase P Crit. Thus, Trichopodus showed less hypoxia tolerance when reared in a more hypoxic environment. The inability of larval Trichopodus to maintain aquatic oxygen consumption is likely correlated with fact that the juvenile and adult Trichopodus are obligate air breathers (Graham 1997).
Collectively, then, the physiological responses to hypoxia exhibited by Betta can be considered more plastic than Trichopodus, allowing enhanced physiological compensation to environmental hypoxia (West‐Eberhard 2005). Larval Betta was able to adjust , PCrit, f H, and f Op in response to hypoxia to increase its ability to withstand hypoxia. Facultative air breathers might be expected to be more plastic in their responses, given that they have two options for environmentally derived oxygen – air or water – and to some extent with enhanced plasticity they could emphasize one respiratory approach over another depending upon environmental conditions (Graham 1997). This larval developmental phenotypic plasticity induced by hypoxia in Betta similarly occurs in the fully aquatic Danio rerio, in which its increased hypoxia tolerance (lower PCrit) was associated with the induction of HIF‐1 during critical developmental windows (Robertson et al. 2014).
Respiratory developmental plasticity and physiological heterokairy
Interestingly, in larval Trichopodus (but not Betta) chronic rearing in mild hypoxia actually advanced the onset of opercular beating to before 5 dpf, compared to 10 dpf in the normoxic population. This response, apparently adaptive in that it would allow earlier access to aquatic oxygen, provides another example of heterokairy, along with the advancement of the onset of air breathing (Mendez‐Sanchez and Burggren 2014). Heterokairy, a form of developmental phenotypic plasticity within populations or individuals, is the change in the timing of the onset of development, especially of physiological regulatory systems and their components, at the individual or population level (Spicer and Burggren 2003; Spicer et al. 2011). Such examples have mostly been shown in invertebrates, so the observation of this phenomenon in a vertebrate is noteworthy.
In conclusion, these physiological data indicate that larval Betta is in many ways better adapted to aquatic survival than larval Trichopodus. This correlates well with the more hypoxic habitats typically inhabited by larval Betta.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgments
We appreciate constructive criticism of this study by Drs. Ed Dzialowski, Dane Crossley II, Ione von Herbing, and Pamela Padilla.
Mendez‐Sanchez J. F., Burggren W. W.. Cardiorespiratory physiological phenotypic plasticity in developing air‐breathing anabantid fishes (Betta splendens and Trichopodus trichopterus), Physiol Rep, 5 (15), 2017, e13359, https://doi.org/10.14814/phy2.13359
Funding Information
Support for this study was provided by NSF operating grant IOS‐1025823 and IOS‐ 1543301 to Warren Burggren. The Collaboration Network in Comparative Ecophysiology of Vertebrates UAEM‐PRODEP 11067 also provided support.
References
- Abdallah, S. J. , Thomas B. S., and Jonz M. G.. 2015. Aquatic surface respiration and swimming behaviour in adult and developing zebrafish exposed to hypoxia. J. Exp. Biol. 218:1777–1786. [DOI] [PubMed] [Google Scholar]
- Ahmad, R. , and Hasnain A. U.. 2005. Ontogenetic changes and developmental adjustments in lactate dehydrogenase isozymes of an obligate air‐breathing fish Channa punctatus during deprivation of air access. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 140:271–278. [DOI] [PubMed] [Google Scholar]
- Bagatto, B. , Pelster B., and Burggren W. W.. 2001. Growth and metabolism of larval zebrafish: effects of swim training. J. Exp. Biol. 204:4335–4343. [DOI] [PubMed] [Google Scholar]
- Barrionuevo, W. R. , and Burggren W. W.. 1999. O2 consumption and heart rate in developing zebrafish (Danio rerio): influence of temperature and ambient O2 . Am. J. Physiol. 276:R505–R513. [DOI] [PubMed] [Google Scholar]
- Barrionuevo, W. R. , Fernandes M. N., and Rocha O.. 2010. Aerobic and anaerobic metabolism for the zebrafish, Danio rerio, reared under normoxic and hypoxic conditions and exposed to acute hypoxia during development. Brazilian journal of biology 70: 425–434. [DOI] [PubMed] [Google Scholar]
- Blank, T. , and Burggren W. W.. 2014. Hypoxia‐induced developmental plasticity of the gills and air‐breathing organ of the air‐breathing fish blue gourami (Trichopodus trichopterus). J. Fish Biol. 84:808–826. [DOI] [PubMed] [Google Scholar]
- Brauner, C. J. , and Rombough P. J.. 2012. Ontogeny and paleophysiology of the gill: new insights from larval and air‐breathing fish. Respir. Physiol. Neurobiol. 184:293–300. [DOI] [PubMed] [Google Scholar]
- Brauner, C. J. , Matey V., Wilson J. M., Bernier N. J., and Val A. L.. 2004. Transition in organ function during the evolution of air‐breathing; insights from Arapaima gigas, an obligate air‐breathing teleost from the Amazon. J. Exp. Biol. 207:1433–1438. [DOI] [PubMed] [Google Scholar]
- Brown, D. 2017. Tracker Video Analysis and Modeling Tool for Physics Education. [online] Physlets.org. Available at http://physlets.org/tracker/ (accessed July 24, 2017). [Google Scholar]
- Browman, H. I. 1989. Embryology, ethology and ecology of ontogenetic critical periods in fish. Brain Behav. Evol. 34:5–12. [DOI] [PubMed] [Google Scholar]
- Burggren, W. W. 1979. Bimodal gas exchange during variation in environmental oxygen and carbon dioxide in the air breathing fish Trichogaster trichopterus . J. Exp. Biol. 82:197–214. [Google Scholar]
- Burggren, W. W. 1982. ‘Air Gulping’ improves blood oxygen transport during aquatic hypoxia in the goldfish Carassius auratus . Physiol. Zool. 55:327–334. [Google Scholar]
- Burggren, W. W. 2005. Developing animals flout prominent assumptions of ecological physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 141:430–439. [DOI] [PubMed] [Google Scholar]
- Burggren, W. W. , and Johansen K.. 1986. Circulation and respiration in lungfishes (Dipnoi). J. Morphol. Suppl. 1:217–236. [Google Scholar]
- Burggren, W. , and Warburton S.. 2005. Comparative developmental physiology:an interdisciplinary convergence. Annu. Rev. Physiol. 67:203–223. [DOI] [PubMed] [Google Scholar]
- Burggren, W. W. , Bautista G. M., Coop S. C., Couturier G. M., Delgadillo S. P., Garcia R. M., et al. 2016. Developmental cardiorespiratory physiology of the air‐breathing tropical gar, Atractosteus tropicus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311:R689–R701. [DOI] [PubMed] [Google Scholar]
- Cerezo, J. , Martínez F. J., and García B.. 2006. Oxygen consumption and ventilatory frequency responses to gradual hypoxia in common dentex (Dentex dentex): basis for suitable oxygen level estimations. Aquaculture 256:542–551. [Google Scholar]
- Chapman, L. J. , Chapman C. A., Nordlie F. G., and Rosenberger A. E.. 2002. Physiological refugia: swamps, hypoxia tolerance and maintenance of fish diversity in the Lake Victoria region. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 133:421–437. [DOI] [PubMed] [Google Scholar]
- Diaz, R. J. , and Breitburg D. L.. 2009. The hypoxic environment Pp. 2–25 in Richards J. G., Farrell A. P. and Brauner C. J., eds. Fish physiology: hypoxia. Acadaemic Press, San Diego, CA. [Google Scholar]
- Dick, T. E. , Hsieh Y. H., Dhingra R. R., Baekey D. M., Galan R. F., Wehrwein E., et al. 2014. Cardiorespiratory coupling: common rhythms in cardiac, sympathetic, and respiratory activities. Prog. Brain Res. 209:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer, G. K. , Stoffels R. J., and Pridmore P. A.. 2014. Morphology, metabolism and behaviour: responses of three fishes with different lifestyles to acute hypoxia. Freshw. Biol. 59:819–831. [Google Scholar]
- Farrell, A. P. 2007. Tribute to P. L. Lutz: a message from the heart–why hypoxic bradycardia in fishes? J. Exp. Biol. 210:1715–1725. [DOI] [PubMed] [Google Scholar]
- Farrell, A. P. , and Richards J. G.. 2009. Defining hypoxia: an integrative synthesis of the responses of fish to hypoxia Pp. 487–503. in Richards J. F., Farrell A. P., , Brauner C. J., eds. Fish physiology: hypoxia. Academic Press, San Diego, CA. [Google Scholar]
- Feder, M. E. , and Burggren W. W.. 1985. Cutaneous gas exchange in vertebrates: design, patterns, control and implications. Biol. Rev. Camb. Philos. Soc. 60:1–45. [DOI] [PubMed] [Google Scholar]
- Froese, R. , and Pauly D.. 2016. FishBase.
- Gamperl, A. K. , and Driedzic W. R.. 2009. Cardiovascular function and cardiac metabolism. Pp. 301–360 in Richards J. F. F. A., Brauner C. J., eds. Fish physiology: hypoxia. Academic Press, New York. [Google Scholar]
- Gamperl, A. K. , and Farrell A. P.. 2004. Cardiac plasticity in fishes: environmental influences and intraspecific differences. J. Exp. Biol. 207:2539–2550. [DOI] [PubMed] [Google Scholar]
- Gore, M. , and Burggren W. W.. 2012. Cardiac and metabolic physiology of early larval zebrafish (Danio rerio) reflects parental swimming stamina. Front. Physiol. 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, J. B. 1997. Air‐breathing fishes: evolution, diversity and adaptation. Academic Press, San Diego, CA. [Google Scholar]
- Holeton, G. F. 1971. Respiratory and circulatory responses of rainbow trout larvae to carbon monoxide and to hypoxia. J. Exp. Biol. 55:683–694. [DOI] [PubMed] [Google Scholar]
- Holzman, R. , China V., Yaniv S., and Zilka M.. 2015. Hydrodynamic constraints of suction feeding in low reynolds numbers, and the critical period of larval fishes. Integr. Comp. Biol. 55:48–61. [DOI] [PubMed] [Google Scholar]
- Islam, A. 2005. The gut of the juvenile African lungfish Protopterus annectens: a light and scanning electron microscope study. J. Morphol. 272:769–779. [DOI] [PubMed] [Google Scholar]
- Jacob, E. , Drexel M., Schwerte T., and Pelster B.. 2002. Influence of hypoxia and of hypoxemia on the development of cardiac activity in zebrafish larvae. Am. J. Physiol.‐ Regul. Integr. Comp. Physiol. 283:R911–R917. [DOI] [PubMed] [Google Scholar]
- Johansen, K. , and Lenfant C.. 1968. Respiration in the african lungfish Protopterus aethiopicus. II. Control of breathing. J. Exp. Biol. 49:453–468. [DOI] [PubMed] [Google Scholar]
- Kalinin, A. L. , Severi W., Guerra C. D., Costa M. J., and Rantin F. T.. 2000. Ventilatory flow relative to intrabuccal and intraopercular volumes in the serrasalmid fish Piaractus mesopotamicus during normoxia and exposed to graded hypoxia. Rev. Bras. Biol. 60:249–254. [DOI] [PubMed] [Google Scholar]
- Kopp, R. , Bauer I., Ramalingam A., Egg M., and Schwerte T.. 2014. Prolonged hypoxia increases survival even in Zebrafish (Danio rerio) showing cardiac arrhythmia. PLoS ONE 9:e89099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre, S. , Bayley M., and McKenzie D. J.. 2016. Measuring oxygen uptake in fishes with bimodal respiration. J. Fish Biol. 88:206–231. [DOI] [PubMed] [Google Scholar]
- Leite, C. A. , Florindo L. H., Kalinin A. L., Milsom W. K., and Rantin F. T.. 2007. Gill chemoreceptors and cardio‐respiratory reflexes in the neotropical teleost pacu, Piaractus mesopotamicus. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 193:1001–1011. [DOI] [PubMed] [Google Scholar]
- Lerner, D. T. , Bjornsson B. T., , and McCormick S. D.. 2007. Effects of aqueous exposure to polychlorinated biphenyls (Aroclor 1254) on physiology and behavior of smolt development of Atlantic salmon. Aquat. Toxicol.(Amsterdam, Netherlands) 81: 329–336. [DOI] [PubMed] [Google Scholar]
- Liem, K. F. 1981. Larvae of air‐breathing fishes as countercurrent flow devices in hypoxic environments. Science 211:1177–1189. [DOI] [PubMed] [Google Scholar]
- Little, C. H. 2009. The colonisation of land: origins and adaptations of terrestrial animals. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Lucas, J. , Schouman A., Lyphout L., Cousin X., and Lefrancois C.. 2014. Allometric relationship between body mass and aerobic metabolism in zebrafish Danio rerio. J. Fish Biol. 84:1171–1178. [DOI] [PubMed] [Google Scholar]
- Mandic, M. , Speers‐Roesch B., and Richards J. G.. 2013. Hypoxia tolerance in sculpins is associated with high anaerobic enzyme activity in brain but not in liver or muscle. Physiol. Biochem. Zool. 86:92–105. [DOI] [PubMed] [Google Scholar]
- Martin, K. L. 2014. Theme and variations: amphibious air‐breathing intertidal fishes. J. Fish Biol. 84:577–602. [DOI] [PubMed] [Google Scholar]
- McKenzie, D. J. , Campbell H. A., Taylor E. W., Micheli M., Rantin F. T., and Abe A. S.. 2007. The autonomic control and functional significance of the changes in heart rate associated with air breathing in the jeju, Hoplerythrinus unitaeniatus. J. Exp. Biol. 210:4224–4232. [DOI] [PubMed] [Google Scholar]
- Mendez‐Sanchez, J. F. , and Burggren W. W.. 2014. Environmental modulation of the onset of air breathing and survival of the Siamese fighting fish Betta splendens and the three spot gourami Trichopodus trichopterus . J. Fish Biol. 84:794–807. [DOI] [PubMed] [Google Scholar]
- Miller, S. , Pollack J., Bradshaw J., Kumai Y., and Perry S. F.. 2014. Cardiac responses to hypercapnia in larval zebrafish (Danio rerio): the links between CO2 chemoreception, catecholamines and carbonic anhydrase. J. Exp. Biol. 217:3569–3578. [DOI] [PubMed] [Google Scholar]
- Milsom, W. K. 2012. New insights into gill chemoreception: receptor distribution and roles in water and air breathing fish. Respir. Physiol. Neurobiol. 184:326–339. [DOI] [PubMed] [Google Scholar]
- Monvises, A. , Nuangsaeng B., Sriwattanarothai N., and Panijpan B.. 2009. The Siamese fighting fish: well‐known generally but little‐known scientifically. ScienceAsia 35:8–16. [Google Scholar]
- Mueller, C. A. , Joss J. M., and Seymour R. S.. 2011. The energy cost of embryonic development in fishes and amphibians, with emphasis on new data from the Australian lungfish, Neoceratodus forsteri . J. Comp. Physiol. [B] 181:43–52. [DOI] [PubMed] [Google Scholar]
- Oliveira, R. D. , Lopes J. M., Sanches J. R., Kalinin A. L., Glass M. L., and Rantin F. T.. 2004. Cardiorespiratory responses of the facultative air‐breathing fish jeju, Hoplerythrinus unitaeniatus (Teleostei, Erythrinidae), exposed to graded ambient hypoxia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 139:479–485. [DOI] [PubMed] [Google Scholar]
- Parker, T. , Libourel P. A., Hetheridge M. J., Cumming R. I., Sutcliffe T. P., Goonesinghe A. C., et al. 2014. A multi‐endpoint in vivo larval zebrafish (Danio rerio) model for the assessment of integrated cardiovascular function. J. Pharmacol. Toxicol. Methods 69:30–38. [DOI] [PubMed] [Google Scholar]
- Peck, M. A. , and Moyano M.. 2016. Measuring respiration rates in marine fish larvae: challenges and advances. J. Fish Biol. 88:173–205. [DOI] [PubMed] [Google Scholar]
- Pelster, B. 1999. Environmental influences on the development of the cardiac system in fish and amphibians. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 124:407–412. [DOI] [PubMed] [Google Scholar]
- Perry, S. F. . 2011. Hypoxia: Respiratory Responses to Hypoxia in Fishes Pp. 1751–1175X. In: Encyclopedia of fish physiology from genome to environment. Academic Press, New York. [Google Scholar]
- Perry, S. F. , Jonz M. G., and Gilmour K. M.. 2009. Oxygen sensing and the hypoxic ventilatory response Pp. 193–253 in Richards J. F., Farrell A. P. and Brauner C. J., eds. Fish physiology: hypoxia. Academic Press, New York. [Google Scholar]
- Peters, H. M. 1978. On the mechanism of air ventilation in Anabantoids (Pisces: Teleostei). Zoomorphologie 98:93–123. [Google Scholar]
- Pollak, E. I. , Thompson T., Stabler A. L., and Keener D.. 1981. Multiple matings in the blue gourami, Trichogaster trichopterus (Pisces, Belontiidae). Anim. Behav. 29:55–63. [Google Scholar]
- Porteus, C. , Hedrick M. S., Hicks J. W., Wang T., and Milsom W. K.. 2011. Time domains of the hypoxic ventilatory response in ectothermic vertebrates. J. Comp. Physiol. [B] 181:311–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus, C. S. , Abdallah S. J., Pollack J., Kumai Y., Kwong R. W., Yew H. M., et al. 2014. The role of hydrogen sulphide in the control of breathing in hypoxic zebrafish (Danio rerio). J. Physiol. 592:3075–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn, H. , Rahn K. B., Howell B. J., Gans C., and Tenney S. M.. 1971. Air breathing of the garfish (Lepisosteus osseus). Respir. Physiol. 11:285–307. [DOI] [PubMed] [Google Scholar]
- Rainboth, W. J. 1996. Fishes of the cambodian mekong Pp. 265 FAO species identification field guide for fishery purposes. FAO, Rome. [Google Scholar]
- Randall, D. J. , Burggren W. W., Farrell A. P., and Haswell M. S.. 1981. The evolution of air breathing in vertebrates. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Richards, J. G. , Haswell M. S. 2011. Physiological, behavioral and biochemical adaptations of intertidal fishes to hypoxia. J. Exp. Biol. 214:191–199. [DOI] [PubMed] [Google Scholar]
- Rider, S. A. , Tucker C. S., del‐Pozo J., Haswell M. S., Rose K. N., MacRae C. A., and Mullins JJ. 2012. Techniques for the in vivo assessment of cardio‐renal function in zebrafish (Danio rerio) larvae. J. Physiol. 590:1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, C. E. , Wright P. A., Koblitz L., and Bernier N. J.. 2014. Hypoxia‐inducible factor‐1 mediates adaptive developmental plasticity of hypoxia tolerance in zebrafish, Danio rerio. Proc. R. Soc. B 281:20140637 https://doi.org/10.1098/rspb.2014.0637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombough, P. J. 1998. Partitioning of oxygen uptake between the gills and skin in fish larvae: a novel method for estimating cutaneous oxygen uptake. J. Exp. Biol. 201:1763–1769. [DOI] [PubMed] [Google Scholar]
- Rombough, P. J. 2007. Ontogenetic changes in the toxicity and efficacy of the anaesthetic MS222 (tricaine methanesulfonate) in zebrafish (Danio rerio) larvae. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 148:463–469. [DOI] [PubMed] [Google Scholar]
- Ruber, L. , Britz R., and Zardoya R.. 2006. Molecular phylogenetics and evolutionary diversification of labyrinth fishes (Perciformes: Anabantoidei). Syst. Biol. 55:374–397. [DOI] [PubMed] [Google Scholar]
- Schulz, S. , Adochiei F. C., Edu I. R., Schroeder R., Costin H., Bar K. J., et al. 2013. Cardiovascular and cardiorespiratory coupling analyses: a review. Phil. Trans. A. Math. Phys. Eng. Sci. 371:20120191. [DOI] [PubMed] [Google Scholar]
- Sloman, K. A. , Sloman R. D., De Boeck G., Scott G. R., Iftikar F. I., Wood C. M., et al. 2009. The role of size in synchronous air breathing of Hoplosternum littorale. Physiol. Biochem. Zool. 82:625–634. [DOI] [PubMed] [Google Scholar]
- Smatresk, N. J. , Burleson M. L., and Azizi S. Q.. 1986. Chemoreflexive responses to hypoxia and NaCN in longnose gar: evidence for two chemoreceptor loci. Am. J. Physiol. 251:R116–R125. [DOI] [PubMed] [Google Scholar]
- Spicer, J. I. , and Burggren W. W.. 2003. Development of physiological regulatory systems: altering the timing of crucial events. Zoology 106:91–99. [DOI] [PubMed] [Google Scholar]
- Spicer, J. I. , Rundle S. D., and Tills O.. 2011. Studying the altered timing of physiological events during development: it's about time or is it? Respir. Physiol. Neurobiol. 178:3–12. [DOI] [PubMed] [Google Scholar]
- Stecyk, J. A. , Galli G. L., Shiels H. A., and Farrell A. P.. 2008. Cardiac survival in anoxia‐tolerant vertebrates: an electrophysiological perspective. Comp. Biochem. Physiol. Toxicol. Pharmacol. 148:339–354. [DOI] [PubMed] [Google Scholar]
- Steele, S. L. , Ekker M., and Perry S. F.. 2011. Interactive effects of development and hypoxia on catecholamine synthesis and cardiac function in zebrafish (Danio rerio). J. Comp. Physiol. [B] 181:527–538. [DOI] [PubMed] [Google Scholar]
- Stoffels, R. J. 2015. Physiological trade‐offs along a fast‐slow lifestyle continuum in fishes: what do they tell us about resistance and resilience to hypoxia? PLoS ONE 10:e0130303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, E. W. 1985. Control and co‐ordination of gill ventilation and perfusion. Symp. Soc. Exp. Biol. 39:123–161. [PubMed] [Google Scholar]
- Taylor, E. W. , Campbell H. A., Levings J. J., Young M. J., Butler P. J., and Egginton S.. 2006. Coupling of the respiratory rhythm in fish with activity in hypobranchial nerves and with heartbeat. Physiol. Biochem. Zool. 79:1000–1009. [DOI] [PubMed] [Google Scholar]
- Taylor, E. W. , Leite C. A., Florindo L. H., Belao T., and Rantin F. T.. 2009. The basis of vagal efferent control of heart rate in a neotropical fish, the pacu, Piaractus mesopotamicus. J. Exp. Biol. 212:906–913. [DOI] [PubMed] [Google Scholar]
- Taylor, E. W. , Leite C. A., McKenzie D. J., and Wang T.. 2010. Control of respiration in fish, amphibians and reptiles. Brazi. J. Med. Biol. Res. 43: 409–424. [DOI] [PubMed] [Google Scholar]
- Terjesen, B. F. , Chadwick T. D., Verreth J. A., Ronnestad I., and Wright P. A.. 2001. Pathways for urea production during early life of an air‐breathing teleost, the African catfish Clarias gariepinus Burchell. J. Exp. Biol. 204:2155–2165. [DOI] [PubMed] [Google Scholar]
- Tota, B. , Angelone T., Mancardi D., and Cerra M. C.. 2011. Hypoxia and anoxia tolerance of vertebrate hearts: an evolutionary perspective. Antioxid. Redox Signal. 14:851–862. [DOI] [PubMed] [Google Scholar]
- Velasco‐Santamaria, Y. M. , Handy R. D., and Sloman K. A.. 2011. Endosulfan affects health variables in adult zebrafish (Danio rerio) and induces alterations in larvae development. Comp. Biochem. Physiol. Toxicol. Pharmacol. 153:372–380. [DOI] [PubMed] [Google Scholar]
- Vosyliene, M. Z. , Kazlauskiene N., and Joksas K.. 2005. Toxic effects of crude oil combined with oil cleaner simple green on yolk‐sac larvae and adult rainbow trout Oncorhynchus mykiss. Environ. Sci. Pollut. Res. Int. 12:136–139. [DOI] [PubMed] [Google Scholar]
- Wells, P. , and Pinder A.. 1996a. The respiratory development of Atlantic salmon. I. Morphometry of gills, yolk sac and body surface. J. Exp. Biol. 199:2725–2736. [DOI] [PubMed] [Google Scholar]
- Wells, P. , and Pinder A.. 1996b. The respiratory development of Atlantic salmon. II. Partitioning of oxygen uptake among gills, yolk sac and body surfaces. J. Exp. Biol. 199:2737–2744. [DOI] [PubMed] [Google Scholar]
- West‐Eberhard, M. J. 2005. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102(Suppl 1):6543–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, C. M. , Cox G. K., and Farrell A. P.. 2015. The beat goes on: cardiac pacemaking in extreme conditions. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 186:52–60. [DOI] [PubMed] [Google Scholar]
- Yeager, D. P. , and Ulstch G. R.. 1989. Physiological regulation and conformation: a BASIC program for the determination of critical points. Physiol. Zool. 62:888–907. [Google Scholar]


