Abstract
A principle that regulates detailed architecture in the brain is that active terminals have a competitive advantage over less active terminals in establishing synaptic connections. This principle is known to apply to fibers within a single neuronal population competing for a common target domain. Here we uncover an additional rule that applies when two neuronal populations compete for two contiguous territories. The cerebellar Purkinje cell dendrites have two different synaptic domains with spines innervated by two separate excitatory inputs, parallel fibers (PFs) and climbing fibers (CFs). Glutamate δ-2 receptors are normally present only on the PF spines where they are important for their innervation. After block of activity by tetrodotoxin, numerous new spines form in the CF domain and become innervated mainly by PFs; all spines, including those still innervated by the CFs, bear δ-2 receptors. Thus, in the absence of activity, PFs gain a competitive advantage over CFs. The entire dendritic arbor becomes a uniform territory with the molecular cues associated with the PFs. To access their proper territory and maintain synaptic contacts, CFs must be active and locally repress the cues of the competitor afferents.
During development, electrical activity has a role in establishing (1, 2) and maintaining (3) the highly refined and specific pattern of connections that leads to the final architecture of the nervous system. Two different developmental processes may be distinguished. One process aims at eliminating redundant synapses for the refinement of initially imprecise connections, and the more active and synchronous terminals have a competitive advantage (1, 2, 4, 5, §). This Hebbian principle has been shown to operate widely in the competition for a common target domain between fibers belonging to the same neuronal population. Molecular cues for this activity-dependent competition between homologous fibers have been recently studied in the visual system (6, 7). A second process is in the selection of two separate but contiguous domains by two different neuronal populations. The role and the mechanisms of activity in this competition between heterologous afferent fibers are unknown. Our article addresses this issue in the mature cerebellum.
Purkinje cells (PCs) of the cerebellar cortex provide a useful model to analyze this issue, because two different types of excitatory afferents connect with two well defined dendritic domains (Fig. 1 a and b; ref. 8). Each climbing fiber (CF), a terminal branch of an inferior olivary axon, makes excitatory contact with the entire extent of the proximal domain of a single PC via spines that are arranged in a series of clusters in relation to presynaptic varicosity of the CF. Numerous PFs, the axons of the granule cells, form synapses with the spines of the distal compartment. Both inputs are characterized by the constitutive presence in their presynaptic terminals of well known growth-associated proteins such as GAP-43, Krox-24, and MARCKS, and a high degree of structural plasticity is present in the CF terminal arbor (9). In addition, both types of synapses show long-term depression, which is considered important for motor learning (10, 11).
Figure 1.
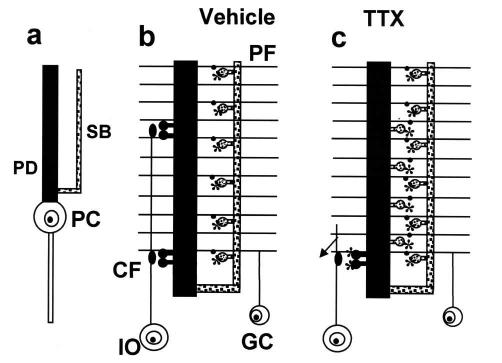
Main synaptic changes induced in PCs by blocking electrical activity. (a) PC dendritic tree with the proximal dendrite (PD) and the spiny branchlet (SB) domains. (b) Excitatory connections under control conditions with sparse clustered spines of the proximal dendrites making contact with CF varicosities and densely packed spiny branchlet spines, with glutamate δ-2 receptors (asterisks) making contact with PF varicosities. (c) Excitatory connections after TTX treatment, showing loss of spines connected to CFs from the proximal dendrite compartment and the appearance of numerous δ-2 receptors containing spines connected to PFs and CFs. Note the atrophy of the CF arbor. GC, granule cells; IO, inferior olive.
By blocking electrical activity in the mature cerebellar cortex with tetrodotoxin (TTX), we recently showed that spine density in the two dendritic domains of PCs is differently regulated by the activity of each of the two afferents. Spine density was unchanged in the branchlets, whereas in the proximal dendrites there was a remarkable increase. The increase in spine density in the proximal compartment was associated with a marked atrophy of the CF terminal arbors. In some cases, thin terminal fibers bearing small varicosities emerged from these arbors and extended toward the granule cell layer. These changes were reversible upon removal of the TTX block (12). These findings suggest that electrical activity may control the distribution of the afferent connections on the postsynaptic region of a given neuron. The present study was undertaken to investigate at the ultrastructural level the synaptic reorganization of the PC afferents after blocking activity.
Materials and Methods
Toxin Delivery.
We used adult Wistar albino rats (body weight 150–250 g, age 1.5–3 months; Charles River Breeding Laboratories). TTX [80 μM in 0.12 M phosphate buffer (PB), pH = 7.2; Sigma] was infused for 7 days into the dorsal vermal cortex by means of an osmotic minipump (Alzet 2002; Alza). Control animals were infused only with vehicle. All surgical procedures were performed under general anesthesia obtained by a mixture of ketamine (100 mg/kg; Ketalar, Parke–Davis) and xylazine (5 mg/kg; Rompum; Bayer, Wuppertal, Germany). The experimental plan was designed according to the guidelines of the Italian Law for Care and Use of Experimental Animals (DL 116/92) and approved by the Italian Ministry of Health.
Electron Microscopy.
For ultrastructural analysis, rats were transcardially perfused with 1,000 ml of Karnovsky fixative (1% paraformaldehyde/1% glutaraldehyde in 0.12 M PB). Vibratome slices of the cerebellum were embedded in Epon/Araldite resin (Fluka). Ultrathin sections were stained with uranyl acetate and lead citrate and examined in a Philips (Eindhoven, The Netherlands) 410 electron microscope. Proximal and distal dendrites were identified according to described morphological features (12–14). For serial reconstructions, a profile of a proximal dendrite was selected in the central section of each series and all spines in continuity with that profile were identified. Each spine was followed in successive sections in both directions until it disappeared (Fig. 2d i–viii). Each set of data was derived from two groups of rats, each consisting of three animals. Statistical evaluation was performed by using a Student's t test.
Figure 2.
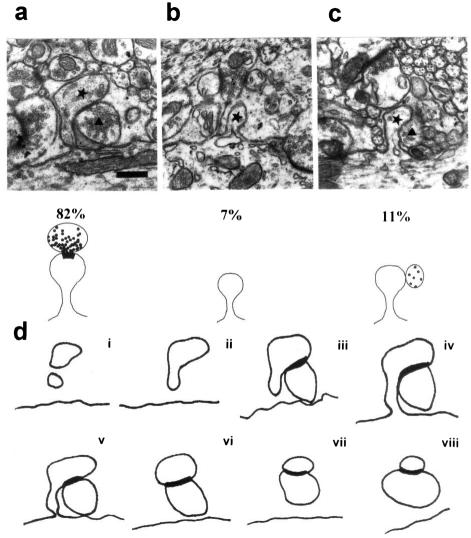
New dendritic protrusions attached to proximal dendrites after TTX treatment. (a) Electron micrograph of a dendritic spine and its presynaptic terminal traced in d (v). (b) A protrusion from the dendrite entirely surrounded by glial elements. (c) A dendritic protrusion in contact with a presynaptic terminal, but without synaptic specialization. The lower parts of a–c indicate the three types of dendritic protrusion observed and their relative frequencies. The ★ and the ▴ indicate, respectively, the spines and the presynaptic terminals with which they are in contact. (d) A series of tracings from successive 100-nm sections of a spine and a presynaptic terminal. The thick outline represents the extent of the PSD. [Bar = 0.42 μm.] For a higher magnification of a–c, see Fig. 7, which is published as supplemental data on the PNAS web site, www.pnas.org.
Postembedding Immunocytochemistry for γ-Aminobutyric Acid (GABA) Staining.
After blocking with 5% (vol/vol) normal goat serum (NGS), ultrathin sections were incubated with the primary rabbit GABA Ab (Sigma), diluted 1:10,000 in Tris-buffered saline and 0.1% Triton X-100 (TBST), overnight at room temperature (RT). After rinsing, sections were incubated with goat anti-rabbit IgG coupled to 10 nm of colloidal gold particles for 1 h at RT, followed by rinsing and uranyl acetate/lead citrate staining.
Freeze Substitution and Lowicryl Embedding.
After perfusion, cerebellar slices (500 μm) were cut by a Vibratome (St. Louis, MO) and slammed to a polished copper block cooled with liquid N2 (MM80 E cryofixation apparatus; Reichert). Small blocks were trimmed from the cryofixed tissue and transferred to a freeze-substitution apparatus (CS Auto; Leica, Deerfield, IL) for dehydration in methanol at −80°C for 36 h and embedding in methanol/Lowicryl HM20 (Chemische Werke Lowi, Waldkraiburg, Germany; ref. 15). Ultrathin sections (90–110 nm) from Lowicryl-embedded blocks were dipped for 2 sec into a saturated solution of NaOH in ethanol (100 g of NaOH in 700 ml of absolute ethanol). The concentration of the primary Ab (rabbit anti-δ-2 Ab, kindly provided by M. Watanabe, Hokkaido University, Sapporo, Japan) was 1.25 mg/ml of TBST containing 2% human serum albumin (HSA; Sigma). At such Ab concentration, there was no labeling in mitochondria (16). Ultrathin sections then were incubated for 1 h with goat anti-rabbit IgG [(1:20 in TBST with 2% HSA and 5 mM polyethylene glycol-PEG (Sigma)] coupled to 10 nm of colloidal gold particles.
Results
As a first step, we determined whether the new spines that appear on the proximal dendritic domain of the PCs after block of activity have synapses, and identified the types of afferents involved. We reconstructed 55 proximal dendritic spines in 5 series of consecutive ultrathin sections from rats treated for 7 days with TTX. Forty-five spines showed a clear postsynaptic density (PSD) and were in contact with a presynaptic profile (Fig. 2a). Six spines exhibited a distinct head but with no ultrastructural evidence of a synapse (Fig. 2c), although three spines came into close apposition with a presynaptic terminal. The remaining four spines were small protrusions surrounded by glia (Fig. 2b). Thus, all proximal dendritic spines bearing a PSD in the proximal dendrites were innervated. A small number of spines with no PSD were free, and they may still have been in the process of developing synaptic contacts.
The next step was to identify the nature of the presynaptic elements making contact with the new spines of the PC proximal dendrites. In control rats, the two types of excitatory boutons synapsing with PC dendritic spines (Fig. 1b) are identified by their particular morphology, in addition to their lack of GABA immunoreactivity (13, 14). Small clusters of vesicles abutting the synaptic contact (Fig. 3a) characterize PF terminals. In contrast, CF boutons contain a high density of vesicles and some dense core vesicles (Fig. 3b). A third type of input to the PC dendrites is made onto the smooth surface of the trunks by the terminals of inhibitory interneurons and of PC recurrent collaterals that are additionally identified by GABA Ab labeling (Fig. 3c/c1).
Figure 3.
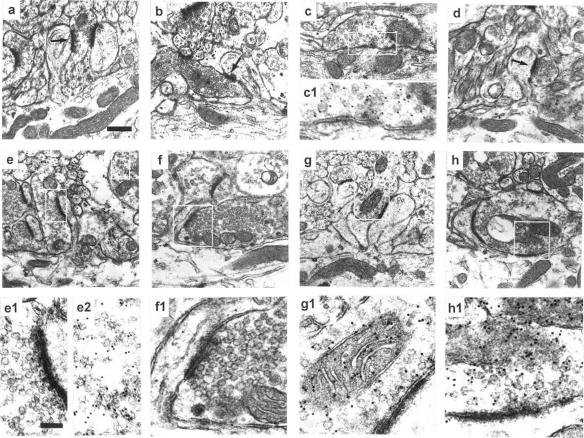
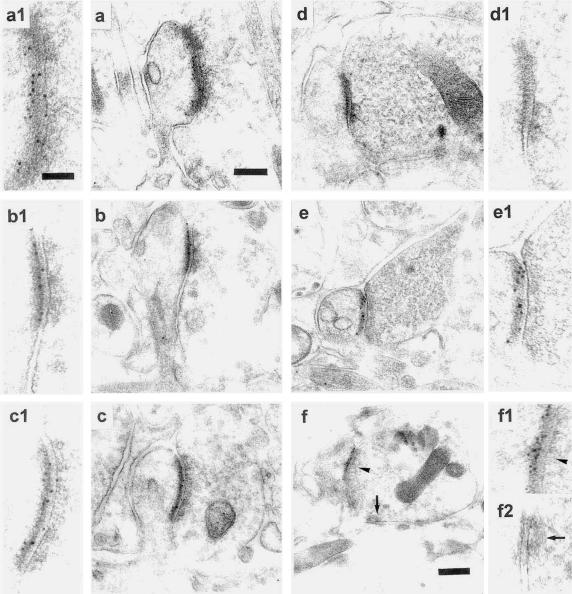
PC dendritic spines and their innervation. (a, b, and c/c1) Synaptic profiles of the three types of synapses of the PC dendrites in a vehicle-treated cerebellum labeled with GABA Ab. PF synapses on branchlet spines (a), CF synapses on spines of the proximal dendrites (b), and synapses made by the inhibitory neuron on the smooth dendritic shaft (c/c1). Note GABA Ab labeling is present only in the third type of synapse. (d) PF synapse made onto branchlet spines under TTX. (e–h) Synapses made on spines of the proximal dendrites after TTX treatment. Spines contacted by terminals of a PF (e/e1), a CF (f/f1), and an inhibitory neuron (g/g1 and h). In h, the inhibitory terminal makes a synapse also with the dendritic shaft. Note that in e1, the lack of GABA immunoreactivity is accompanied by reactivity in another element (e2). (e1–h1) Higher magnification of the boxes outlined in the above-located pictures to show more clearly that the GABA labeling is absent in the terminals of e1 and f1 and present in those of g1 and h1. [Bar = 0.35 μm (for a–h) and 0.13 μm (for c1 and e1–h1).] For a higher magnification of a, b, and d, see Fig. 8, which is published as supplemental data on the PNAS web site.
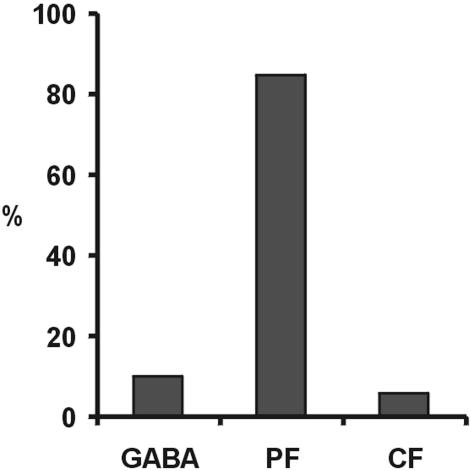
In our TTX-treated cerebella, we selected under the electron microscope those spines in continuity with a proximal dendritic profile (mean diameter 3.2 mm ± 1.09 SD) and having a PSD. As shown in Fig. 4, of 123 spines, 12 (9.8%) had terminals labeled by GABA Ab (Fig. 3 g/g1 and h). Of these terminals, eight made a synapse onto the smooth dendritic surface in addition to the spine synapse (Fig. 3h/h1). A second group of 104 spines (84.5% of the total) were in contact with profiles typical of PF boutons (Fig. 3e/e1). The remaining seven spines (5.7%) were contacted by a typical CF profile (Fig. 3f/f1). In a similar way, in TTX-treated cerebella, we identified under the electron microscope 82 spines of spiny branchlets (mean diameter 1.52 mm ± 0.4 SD), each bearing a PSD. All these spines formed synapses with typical PF boutons (Fig. 3d) none of which were labeled by GABA Ab. Identical results were obtained on 143 branchlet spines identified in vehicle-treated cerebella (Fig. 3a). In conclusion, after TTX treatment, a large majority of the new spines that appear on the proximal dendritic compartment are innervated by PFs, whereas the remainders are innervated by inhibitory neurons or remain in contact with CFs.
Figure 4.
Percent distribution of the type of innervation of the proximal dendritic spines after TTX treatment. The three columns represent the complement innervated by GABAergic terminals, by the PFs, and by the CFs, respectively.
The next step was to find a possible mechanism for this competitive advantage. Under control conditions, glutamate δ-2 receptors are abundant in branchlet spines (Fig. 1b *), although they are not expressed (17) or show low expression (16) in proximal dendritic spines. These receptors have no channel function (18, 19), although PC degeneration in lurcher mice is caused by a mutation in the δ-2 receptor gene that leads to Na+ leakage into PCs (20). On the other hand, in δ-2-deficient mice, the number of PF to PC synapses is reduced to nearly half, although spine density is normal. Therefore, this receptor is involved in the stabilization and strengthening of this specific synaptic connection (21, 22).
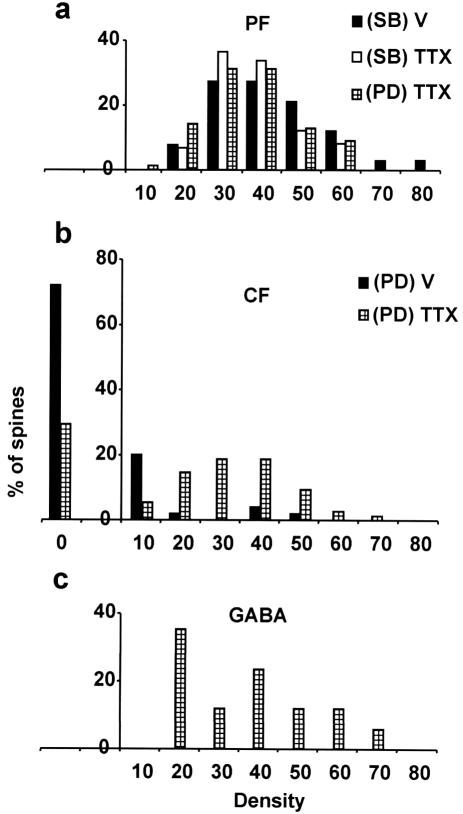
We evaluated quantitatively (Table 1) the localization of δ-2 receptors by postembedding immunogold labeling of ultrathin sections cut from slices prepared with the freeze-substitution/Lowicryl-embedding technique (15). All branchlet spines, both in vehicle- (Fig. 5a/a1) and TTX-treated (Fig. 5b/b1) cerebella, were contacted by PFs and had a relatively high density of immunogold particles, with no difference in their mean values (P > 0.05). The proximal compartment spines innervated by the PFs (Fig. 5c/c1) had a high gold particle density value that was slightly smaller (P < 0.05) than that found in the branchlet spines in the vehicle-treated cerebellum but was not significantly different from that found in the branchlet spines in the TTX-treated cerebella (P > 0.05). Fig. 6a shows that the density distribution of gold particles appears similar in all PF-innervated spines. For CF-innervated spines, we randomly selected a number of spines whether they were seen emerging from a dendrite or not, because spines contacted by CFs were never present on the branchlets. As shown in Table 1 and Fig. 6b, the average gold particle density on CF-innervated spines in the vehicle-treated cerebella was very low, with 72% of spines being free of labeling (Fig. 5d/d1). In contrast, after TTX treatment (Fig. 5e/e1), only 29.3% of the proximal dendritic spines were unlabeled and the mean particle density value was significantly higher (P ≤ 3.1 ⋅ 10−10), although it appeared lower than that of the branchlet spines (P ≤ 2 ⋅ 10−4). The δ-2 expression in the inactive CF synapses suggests that the lack of δ-2 expression that normally occurs in these CF-innervated spines results from an activity-driven mechanism and selective local repression. This interpretation is supported by the fact that δ-2 receptors are present on the new spines (R.C., L.M., and P.S., unpublished data) that appear in the proximal dendrites after selective CF deletion (23, 24). Therefore, lack of CF-induced repression switches the postsynaptic membrane to become receptive to the competitive input. In 53 spines innervated by a CF after TTX treatment and showing δ-2 expression (Fig. 6), we never observed the coexistence of both PF and CF innervation. This fact implies that a PF contact is not necessary for δ-2 expression in these CF synapses, which is in line with the demonstration that in the agranular cerebellum there are free spines bearing δ-2 receptors (25).
Table 1.
Mean densities of glutamate δ-2 receptors (expressed in immunogold particles per micrometer of PSD) in vehicle- and TTX-treated cerebella
| Vehicle
|
TTX
|
|||||
|---|---|---|---|---|---|---|
| (SB) PF | (PD) CF | (SB) PF | (PD) PF | (PD) CF | GABA-spine | GABA-shaft |
| 36.9 ± 13.8 | 3.4 ± 9.9 | 33.5 ± 12.9 | 32.3 ± 11.4 | 20.5 ± 17.9 | 34.5 ± 16.2 | 0.4 ± 1.0 |
| (n = 66) | (n = 50) | (n = 76) | (n = 77) | (n = 75) | (n = 15) | (n = 15) |
| 19.7 ± 6.3 | 0 | |||||
| (n = 53) (17) | (n = 19) (17) | |||||
| 26.1 ± 0.9 | 1.5 ± 0.4 | |||||
| (n = 109) (16) | (n = 28) (16) | |||||
Note the similar values for the branchlet spines in both vehicle- and TTX-treated animals and the much higher values for proximal dendrite spines in TTX-treated than in the vehicle-treated animals, whatever type of presynaptic element is involved. Some previously published data are shown in the two lower rows for comparison (16, 17). PD, proximal dendrites; SB, spiny branchlets; GABA-spine, postsynaptic membrane of spine attached to GABAergic terminal; GABA-shaft, postsynaptic membrane of dendritic shaft attached to GABAergic terminal.
Figure 5.
Immunogold-labeled δ-2 receptors in spine synapses in vehicle- and TTX-treated cerebella. Dense labeling in a branchlet spine innervated by a PF varicosity (a/a1), and no labeling in CFs (d/d1) of vehicle-treated cerebella. Dense labeling under TTX in a branchlet spine (b/b1), in spines emerging from a proximal dendrite and innervated by a PF (c/c1) and a CF (e/e1). (f/f1) Under TTX, an inhibitory terminal having two synaptic contacts, respectively, with a spine that is densely δ-2-labeled and with a dendritic shaft that is unlabeled. a1–f2 are enlarged views of the synaptic areas. [Bar = 0.19 μm (for a–e), 0.26 μm (for f), and 0.095 μm (for a1–f2).]
Figure 6.
Density distribution of δ-2 receptors expressed as number of immunogold particles per micrometer of PSD in spines in vehicle- (V) and TTX-treated rats. (a) PF-innervated spines on spiny branchlets (SB) and on proximal dendrites (PD). (b) CF-innervated spines of the proximal dendrites. (c) Spines innervated by inhibitory interneurons.
Under TTX treatment, we have observed GABAergic terminals that form synapses with both the dendritic shaft and a spine (Fig. 3h). The δ-2 receptor/immunogold particle density in these “aberrant double synapses” is reported in Table 1. In the spine synapses formed with GABAergic terminals, δ-2 gold labeling was comparable to that of other synapses after TTX treatment (P > 0.05; Fig. 5f/f1), except those made with CFs, whose value was significantly smaller (P < 0.02). However, in the shaft synapses of GABAergic terminals, the average δ-2-gold particle density was negligible. These results demonstrate that in the absence of activity all types of PC spines bearing a PSD, even those abnormally innervated by GABAergic neurons, express δ-2 receptors.
Discussion
The organization of topographic maps in the nervous system requires the segregation of afferent inputs based on a competition between neurons belonging to the same neuronal population (homologous competition). The best known models of such segregation are the visual pathway (1, 2, 6, 7, §) and the neuromuscular junction (4, 5). Activity-driven segregation is assumed to be the result of competition between coinnervating nerve terminals for target-derived trophic factors. Among coinnervating terminals, those with a highly correlated activity are stabilized, whereas those with a weakly correlated activity are reduced or eliminated (26–29).
This article has addressed the mechanisms of competition between different neuronal populations (heterologous competition) for contiguous dendritic domains. We have shown that in the absence of activity, the proximal dendritic region of the PC, in addition to a reduced number of CF synaptic contacts (12), showed a high number of new spines mainly innervated by PFs (Fig. 1c), an input normally restricted to the branchlets. Our results show that an activity-independent mechanism provides a competitive advantage to one input, the PFs (Fig. 1c), whereas activity is necessary for the other input, the CFs, to displace the competitor afferents and to maintain their territory. In addition, the TTX-induced rewiring shows that the mature brain is able to undergo remarkable changes in its architecture in the absence of any neuronal lesion, a phenomenon so far associated primarily with critical periods during development (1, 2).
Our data suggest possible mechanisms that control competition between heterologous fibers in the adult cerebellar cortex. In the absence of synaptic activity, all PC spines generated in the proximal dendrites and bearing a PSD were innervated. Interestingly, all these spines showed a relatively high δ-2 receptor expression, even when they were innervated by GABAergic neurons or remained attached to silent CFs (Fig. 6). Thus, we suggest that δ-2 expression is an intrinsic property of all PC spines, independent from the afferent type. Because δ-2 receptors are involved in stabilizing and strengthening PF–PC synapses (21, 22), the competitive advantage of the PFs over the CFs in the absence of activity is attributable to the activity-independent expression of these receptors by the PCs. The low or absent expression of δ-2 receptors in primary dendritic spines under normal conditions is the result of a selective repression caused by activity at synapses other than that of PFs. Lack of such repression switches the postsynaptic membrane to become receptive to the competitive input.
The inhibition of expression of δ-2 receptors on the proximal dendritic spines in normal conditions might be caused by a specific molecule released by active CFs that would decluster δ-2 receptors. Declustering might also be the result of the high Ca2+ concentration in this dendritic domain when the CFs are active (8). It has been shown recently that δ-2 receptors are bound to spectrin, a membrane cytoskeletal protein, and that elevation of Ca2+ concentration dissociates the spectrin from these receptors (30).
In conclusion, we propose the following model for the mechanism of interaction between two neuronal populations that compete for two contiguous territories. Activity-independent mechanisms lead to the development of a uniform set of spines endowed with δ-2 receptors and compatible with PF innervation throughout the PC dendritic tree. During maturation, CFs stabilize their synapses in their own dendritic territory by an activity-dependent mechanism that suppresses the cues for the competitor afferents. An additional local action reduces the spine density on the large PC dendrites to match the specific function of the CFs; this low spine density allows a larger interspine interval to accommodate a large number of voltage-dependent Ca2+ channels that are responsible for the all-or-nothing Ca2+ current into the cell typical of the CF response (31). This activity-dependent competition then is maintained throughout life. The continued expression of GAP-43 and other growth-associated proteins in both PFs and CFs (9, 32) allows the two inputs to compete to maintain their territories. It is tempting to assume that the activity-dependent competition between these two excitatory inputs of the PC may have an important bearing on synaptic changes thought to underlie motor learning (5, 9).
Supplementary Material
Acknowledgments
We thank Dr. F. Rossi for constructive critical interactions, Dr. P. Somogyi for the help with the freeze-substitution technique, and Dr. M. Watanabe for kindly providing us with the δ-2 Ab. This work was supported by grants from the Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica, the Consiglio Nazionale delle Ricerche (Italy), and the Ministry of Health. R.R. was the recipient of an Ottolenghi Fellowship.
Abbreviations
- CF
climbing fiber
- PF
parallel fibers
- PC
Purkinje cell
- TTX
tetrodotoxin
- PSD
postsynaptic density
- GABA
γ-aminobutyric acid
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Stryker, M. & Strickland, S. I. (1984) Invest. Ophthalmol. Visual Sci., Suppl. 25, 278. (abstr.).
References
- 1.Goodman C S, Shatz C J. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 2.Katz L C, Shatz C J. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 3.Chapman B. Science. 2000;287:2479–2482. doi: 10.1126/science.287.5462.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busetto G, Buffelli M, Tognana E, Bellico F, Cangiano A. J Neurosci. 2000;20:685–695. doi: 10.1523/JNEUROSCI.20-02-00685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtman J W, Colman H. Neuron. 2000;25:269–278. doi: 10.1016/s0896-6273(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 6.Corriveau R A, Shatz C J, Nedivi E. J Neurosci. 1999;19:7999–8008. doi: 10.1523/JNEUROSCI.19-18-07999.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huh G S, et al. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M. The Cerebellum and Neural Control. New York: Raven; 1984. [Google Scholar]
- 9.Strata P, Rossi F. Trends Neurosci. 1998;21:407–413. doi: 10.1016/s0166-2236(98)01305-8. [DOI] [PubMed] [Google Scholar]
- 10.Daniel H, Levenes C, Crepel F. Trends Neurosci. 1998;21:401–407. doi: 10.1016/s0166-2236(98)01304-6. [DOI] [PubMed] [Google Scholar]
- 11.Hansel C, Linden D J. Neuron. 2000;26:473–482. doi: 10.1016/s0896-6273(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 12.Bravin M, Morando L, Vercelli A, Rossi F, Strata P. Proc Natl Acad Sci USA. 1999;96:1704–1709. doi: 10.1073/pnas.96.4.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larramendi L M, Victor T. Brain Res. 1967;5:15–30. doi: 10.1016/0006-8993(67)90216-8. [DOI] [PubMed] [Google Scholar]
- 14.Palay S L, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Berlin: Springer; 1974. [Google Scholar]
- 15.Baude A, Nusser Z, Roberts J D, Mulvihill E, McIlhinney R A, Somogyi P. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H M, Wenthold R J, Petralia R S. J Neurosci. 1998;18:5517–5528. doi: 10.1523/JNEUROSCI.18-14-05517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landsend A S, Amiry-Moghaddam M, Matsubara A, Bergersen L, Usami S, Wenthold R J, Ottersen O P. J Neurosci. 1997;17:834–842. doi: 10.1523/JNEUROSCI.17-02-00834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araki K, Megure H, Kushiya E, Takayama C, Inoue Y, Mishina M. Biochem Biophys Res Commun. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- 19.Lomeli H, Sprengel R, Laurie D J, Kohr G, Herb A, Seeburg P H, Wisden W. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- 20.Zuo J, De Jager P L, Takashi K A, Jiang W, Linden D J, Heintz N. Nature (London) 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]
- 21.Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, et al. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 22.Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, Inoue Y, Watanabe M. J Neurosci. 1997;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotelo C, Hillman D E, Zamora A J, Llinás R. Brain Res. 1975;98:574–581. doi: 10.1016/0006-8993(75)90374-1. [DOI] [PubMed] [Google Scholar]
- 24.Rossi F, Van der Want J J L, Wiklund L, Strata P. J Comp Neurol. 1991;308:536–554. doi: 10.1002/cne.903080404. [DOI] [PubMed] [Google Scholar]
- 25.Takayama C, Nakagawa S, Watanabe M, Kurihara H, Mishina M, Inoue Y. Brain Res. 1997;745:231–242. doi: 10.1016/s0006-8993(96)01093-1. [DOI] [PubMed] [Google Scholar]
- 26.Cabelli R J, Hohn A, Shatz C J. Science. 1995;267:1662–1666. doi: 10.1126/science.7886458. [DOI] [PubMed] [Google Scholar]
- 27.Cellerino A, Maffei L. Prog Neurobiol. 1996;49:53–71. doi: 10.1016/0301-0082(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 28.Cabelli R J, Shelton D L, Segal R A, Shatz C J. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 29.Poo M. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 30.Hirai H, Matsuda S. Neurosci Res. 1999;34:281–287. doi: 10.1016/s0168-0102(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 31.Strata P, Morando L, Bravin M, Rossi F. Trends Neurosci. 2000;23:198. doi: 10.1016/s0166-2236(00)01571-x. [DOI] [PubMed] [Google Scholar]
- 32.Kruger L, Bendotti C, Rivolta R, Samanin R. J Comp Neurol. 1993;333:417–434. doi: 10.1002/cne.903330308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.