Abstract
Haemonchus contortus (H. contortus) remains important nematode that infecting sheep all over the world. Truthful diagnosis of haemonchosis needs reliable Enzyme linked immune sorbent assay test as well as the immuno-reactive protein profile of different prepared H. contortus antigens; larval (L), excretory secretory product (ESP) and adult somatic H. contortus (AS). The current study fulfilled that L antigen is the talented antigen for such serological diagnosis. Immunodominant band at molecular weight 57 kDa were answerable for highest specificity and accuracy of positive predictive value of this antigen. Moreover, the highest apparent prevalence value was 92 and 75% obtained by L and ESP antigens, respectively. The results of cross reactivity among AS, Monezia expansa (M. expansa) and Fasciola spp. revealed that AS antigen appeared major cross reactivity with other cestode and trematode. Best dilution of serum was (1:800) to rise above this phenomenon.
Keywords: Haemonchus contortus, Monezia expansa, Fasciola spp, ELISA, Cross reactivity
Introduction
Haemonchus contortus is one of the most pathogenic gastrointestinal nematodes that affecting sheep. All larval, immature and mature worm stages are blood sucking thus, this abomasal nematode causing severe anemia which may be fatal particularly to young animals. So, haemonchosis is considered as life threatening disease disturbing sheep population (Kandil et al. 2015; Besier et al. 2016). Economically, H. contortus and/or other gastrointestinal nematodes cost the livestock sheep industry. The diagnosis of haemonchosis is usually based on clinical signs observation and microscopic examination of fecal samples (Meshgi and Hosseini 2007). The low sensitivity of fecal examination and unapparent clinical signs leads to limitation of accurate diagnosis of the disease. Therefore, reliable serological diagnosis assay is needed for more accurate diagnosis. ELISA enables detection of infection as well as sero-epidemiological studies in massive intensive breeding of sheep livestock (Li et al. 2007; Prasad et al. 2008; Demeler et al. 2012). The detection of anti-H. contortus antibodies in sheep sera using a dependable serological assay as ELISA circumvent the limitation of traditional methods depending on the use of specifically immunogenic antigens (Lone et al. 2012; Kandil et al. 2015). Many different antigens prepared from H. contortus nematode are used in serological diagnosis of haemonchosis with different impact on their sensitivity and specificity (Kandil et al. 2015). Therefore, our study aimed to characterize and evaluate the different prepared H. contortus antigens; L, ESP and AS antigens in serodiagnosis of haemonchosis in sheep.
Materials and methods
Collection of worms and serum samples
Adult worms of H. contortus, M. expansa and Fasciola spp. were collected from abomasa, intestine and liver of slaughtered sheep, respectively at El-Bassatin, El-warrak and El-Monieb abattoirs in Egypt. Worm recovery was carried out according to standard procedures described by MAFF (1986). Three hundred and twenty serum samples were collected by centrifugation (2000 rpm/15 min) blood of slaughtered sheep. All samples were divided and labeled as random, infected and non-infected sheep according to their post-mortem inspection.
Preparation of antigens and hyper immune sera
Adult somatic antigens of H. contortus, M. expansa and Fasciola spp. were prepared from collected adult worms according to Johnson et al. (2004). In addition, ESP and L antigens of H. contortus were prepared according to Prasad et al. (2008) and Tariq et al. (2008), respectively. Total protein content was estimated for all antigens according to Lowry et al. (1951).
Eighteen healthy white New Zealand males’ rabbits weighing 1.5–2 kg. B. WT. each, were grouped and immunized (3 rabbits for each antigen) with all different prepared antigens (L, ESP, AS, M. expansa and Fasciola spp.) and keep one group as control. Hyper immune sera were prepared according to Fagbemi et al. (1995) in order to obtain their homologous mono-specific hyper immune sera.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting technique
Fifty µg of the three prepared H. contortus antigens; L, ESP, and AS were electrophoresed using 10% gel under reducing conditions according to Laemmli (1970). After that, the protein bands were transferred from SDS-PAGE gel to a nitrocellulose sheet according to Towbin et al. (1979).
Enzyme linked immuno sorbant assay (ELISA)
ELISA was applied in order to evaluate the diagnostic value of different prepared H. contortus antigens in detection of anti-H. contortus IGg in sera of naturally infected and non infected sheep. As well as, sensitivity, specificity, negative and positive predictive values and apparent prevalence were calculated for each antigen according to Schallig et al. (1995). In addition, the cross reactivity with other cestode which is M. expansa and nematode spp represented by Fasciola spp. was estimated in comparison to AS antigen. Briefly, the optimal concentration of antigens and dilutions of tested sera and conjugate were estimated by checker-board titrations. The well were coated with 100 μl of each diluted antigen at the concentration of 2 μg/well in carbonate-bicarbonate buffer, pH 9.6 and incubated overnight at 4 °C. After blocking with 200 μl/well of 2% dry skimmed milk in PBS, pH 7.2. A 100 μl diluted sera of infected and non infected samples (1:200) in PBS-T and double fold serial dilution hyper immune sera raised against AS, M. expansa and Fasciola spp. (1:50–1:3200) was incubated at 37 °C/2 h. One hundred μl/well of appropriate conjugate diluted at 1:1000 were added and incubated for 1 h at 37 °C. After washing, 100 μl/well of substrate solution (O, phenylenediamine, one tablet (20 mg/ml) dissolved in 50 ml substrate buffer, pH 5 and 25 μl 30% H2O2) were added to all wells and the plates were incubated at 37 °C for 10–20 min. Finally, the optical densities (OD) were read at wave length of 450 nm with an ELISA reader (BIO-TEK, INC., ELx, 800UV). Positive values were assigned as those values with absorbance reading greater than the cut-off value which was calculated from mean OD value of the negative control sera plus three fold standard deviations. Data analysis parameters were calculated for each antigen as following according to Tabouret et al. (2001).
Statistical analysis
OD data were expressed as arithmetic mean with standard deviation. The apparent prevalence parameter was analyzed using Chi square test by statistical computer package for social science (SPSS) version 15.
Results
Protein profile of different H. contortus antigens using SDS-PAGE and western blotting technique
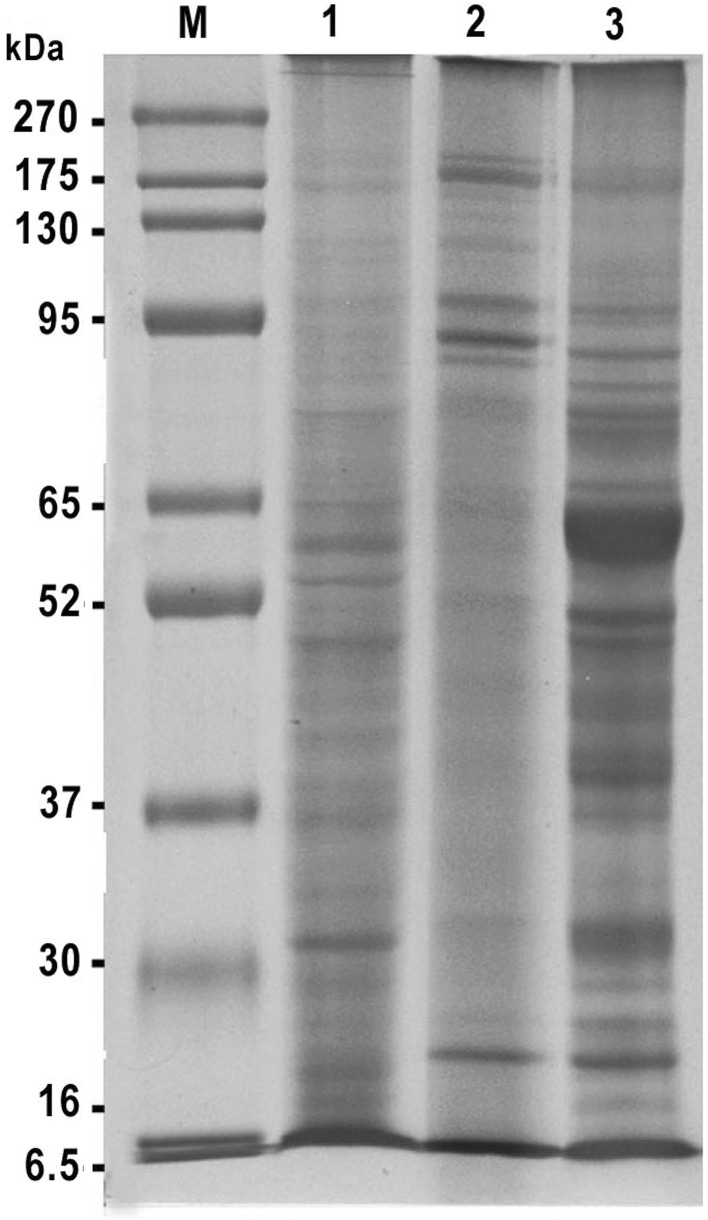
Electrophoretic protein profile of different H. contortus prepared antigens (L, ESP and AS) demonstrated variable protein bands with different molecular weights (Fig. 1). The L antigen showed 23 bands which detected at molecular weights 204, 173, 122, 115, 101, 92, 81, 73, 59, 54, 50, 47, 44, 43, 40, 38, 36, 33, 31, 28, 24, 22, and 18 kDa. While, the ESP antigen demonstrated 15 protein bands at molecular weights 212, 188, 162, 122, 102, 91, 85, 79, 75, 58, 49, 44, 38, 31, and 22 kDa. Moreover, the protein bands of AS antigen were 22 protein fractions at molecular weights 267, 195, 178, 135, 112, 100, 87, 80, 73, 70, 61, 56, 49, 47, 43, 38, 37, 31, 28, 24, 22, and 19 kDa. Common protein bands at molecular weights 22 and 38 kDa were detected among the three H. contortus prepared antigens. Also, the bands at molecular weights 22, 24, 28, 31, 43 and 47 kDa appeared in both L and AS antigens. In addition to the band at 49 kDa was dominant between AS and ESP antigens. Also, the band at 44 kDa was dominant between L and ESP antigens (Fig. 1).
Fig. 1.
Electrophoretic profile of different prepared Haemonchus contortus antigens (L, ESP and AS). M BLUltra Prestained protein ladder, genedirex, Lane 1 larval of H. contortus antigen, Lane 2 excretory secretory product of H. contortus antigen, Lane 3 adult somatic of H. contortus antigen
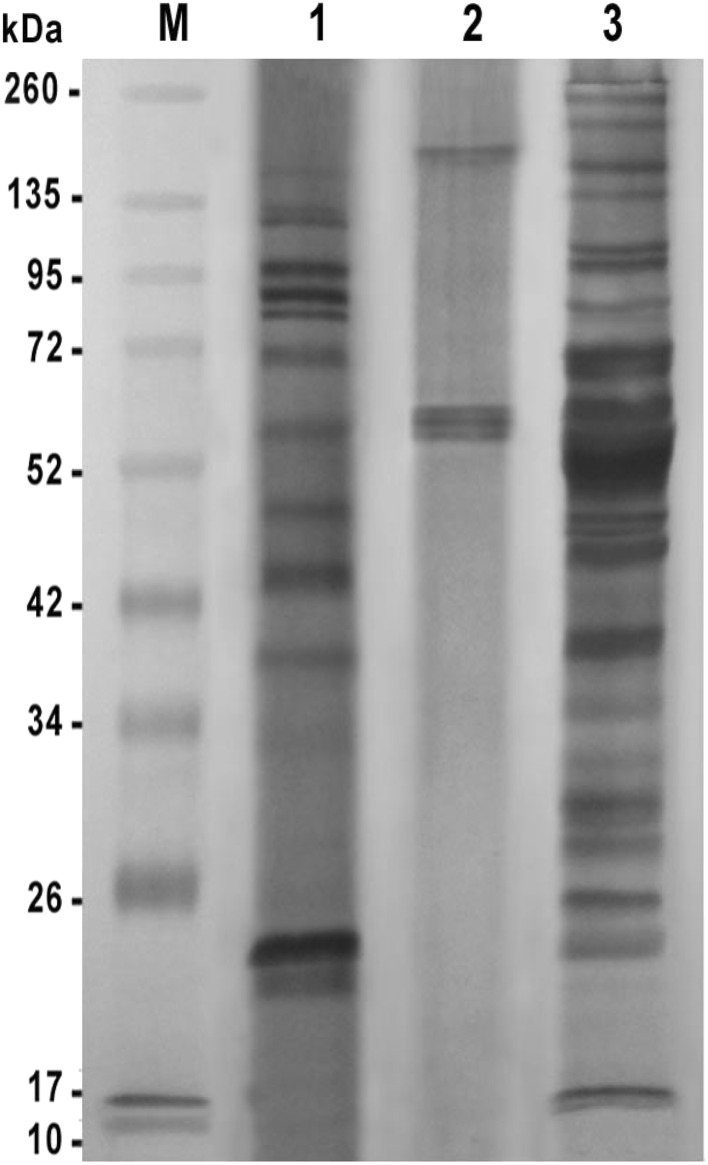
Immunogenic reactive bands profile of different H. contortus prepared antigens appeared when reacted with their homologous hyper immune sera (Fig. 2). The L antigen detected 13 reactive bands at molecular weights 170, 130, 124, 98, 88, 82, 70, 57, 49, 44, 39, 23, and 22 kDa. However, the ESP antigen gave only 4 bands at molecular weights 171, 61, 59, 57 kDa. The AS antigen showed 21 reactive bands at molecular weights 252, 222, 174, 138, 105, 98, 82, 69, 59, 51, 47, 46, 45, 38, 34, 32, 29, 28, 25, 22, and 17 kDa. The bands at molecular weights 98, 82 and 22 kDa were common between L and AS antigens. Also, the band at molecular weight 57 and 59 kDa were immune-dominant in ESP, Land ESP, AS antigens, respectively.
Fig. 2.
Reactivity of different prepared Haemonchus contortus antigens against their homologous hyper immune sera (L, ESP and AS). M Prestained molecular weight protein ladder, Fermentas, Lane 1 larval of H. contortus antigen, Lane 2 excretory secretory product of H. contortus antigen, Lane 3 adult somatic of H. contortus antigen
Immunogenic reactivity of different H. contortus antigens using indirect ELISA
Immunogenic reactivity of three prepared antigens (L, ESP and AS) were evaluated by ELISA using the collected sheep’s sera. ELISA results indicated that OD values of detecting H. contortus antibodies were highly varied among different serum samples in their reactivity with different H. contortus prepared antigens. The apparent prevalence of H. contortus infection among sheep’s sera differed according to the used antigen. The highest value was 92 and 75% obtained by L and ESP antigens, respectively and statistical analysis clarified that the higher percentage of L antigen was insignificantly versus ESP antigen (χ2 = 1.731, p < 0.188). However, AS antigen achieved 35.6% and the statistical analysis elucidated that lower percentage of AS antigen was significantly versus ESP antigen (χ2 = 13.703, p < 0.001) and also versus L antigen (χ2 = 24.500, p < 0.001). The maximum values of specificity and positive predictive value 100% were recorded by L antigen (Table 1). The antigens of AS and ESP were gained 61.5, 50 and 42.85%, 60% for specificity and positive predictive value, respectively. Otherwise, the lowest negative predictive value 20% obtained by L antigen followed with ESP antigen which achieved 30%. Nevertheless, the sensitivity of ELISA was 71.4, 46.15 and 55.55% when AS, ESP and L antigens were used, respectively (Table 1).
Table 1.
Data analysis parameter of different Haemonchus contortus antigens using indirect ELISA
| Parameter | Types of antigens | |||||
|---|---|---|---|---|---|---|
| L | ESP | AS | ||||
| +ve | −ve | +ve | −ve | +ve | −ve | |
| NIS sera | 10/10 | 0/10 | 6/10 | 4/10 | 5/10 | 5/10 |
| NNIS Sera | 8/10 | 2/10 | 7/10 | 3/10 | 2/10 | 8/10 |
| Random Sera | 276/300 | 24/300 | 225/300 | 75/300 | 107/300 | 193/300 |
| Total | 294/320 | 26/320 | 238/320 | 82/320 | 114/320 | 206/320 |
| Sensitivity (%) | 55.55 | 46.15 | 71.4 | |||
| Specificity (%) | 100 | 42.85 | 61.5 | |||
| Negative predictive value (%) | 20 | 30 | 80 | |||
| Positive predictive value (%) | 100 | 60 | 50 | |||
| Apparent prevalence (%) | 92 | 75 | 35.6 | |||
NIS Naturally infected sera, NNIS Naturally non infected sera, L larval of H. contortus antigen, ESP excretory secretory product of H. contortus antigen, AS adult somatic of H. contortus antigen
Cross reactivity of different adult somatic H. contortus, M. expansa and Fasciola spp. antigens using indirect ELISA
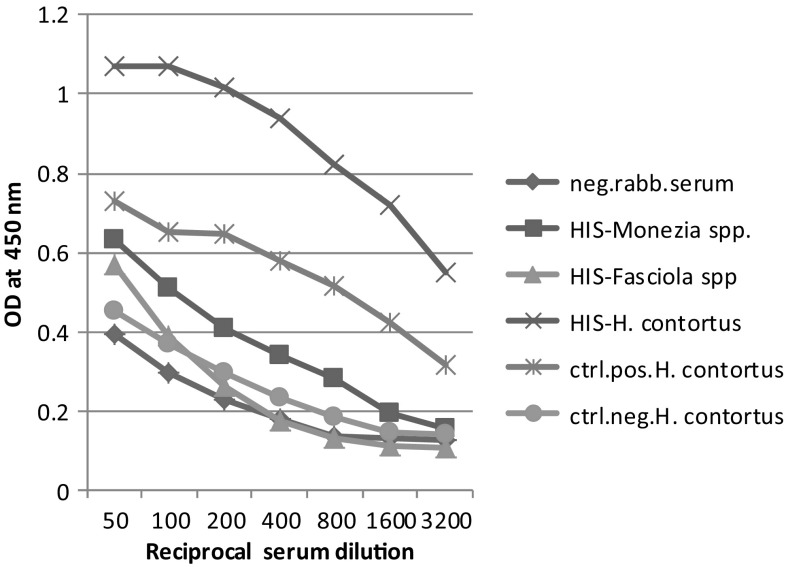
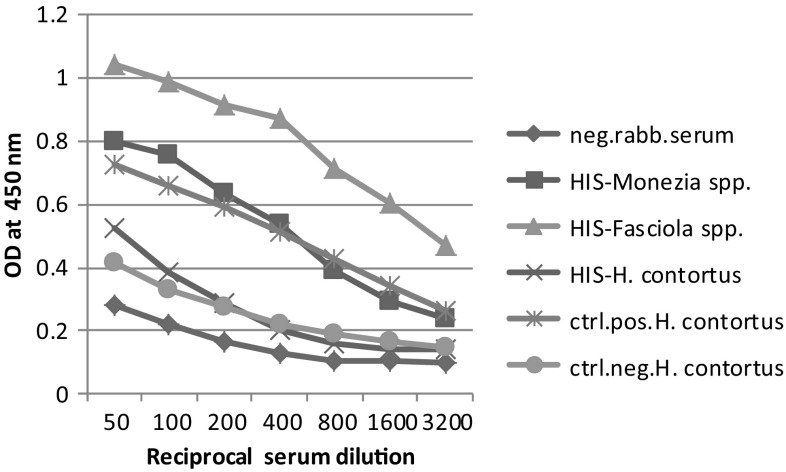
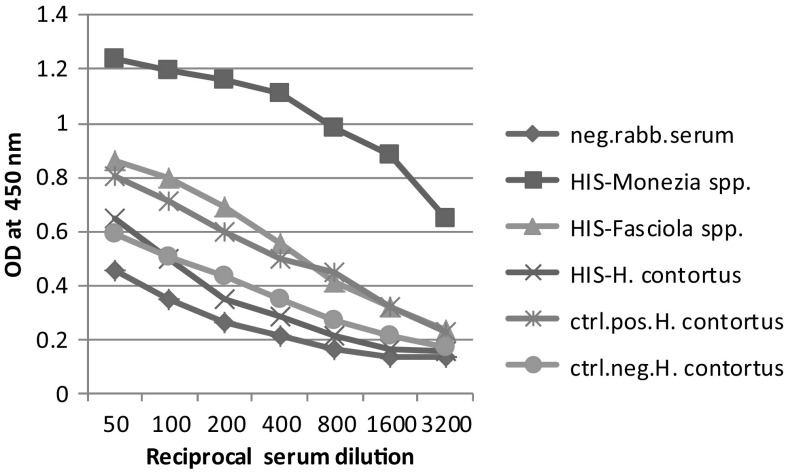
The obtained results in Figs. 3, 4 and 5 demonstrating the talent of the three antigens (H. contortus (AS), M. expansa and Fasciola spp.) in binding with their mono-specific antibodies of hyper immune sera raised against them as well as cross binding with each other revealed that there was cross-immune reactivity among the antigens and their sera still appeared even with rising serum dilutions. Moreover, these data showed comparable strong binding patterns with serially diluted sera with a common trait of high reactivity binding of antigens. The result showed that the highly diluted sera (1:3200) still specifically reacted with their respective antigens otherwise, at (1:800) serum dilution, the immunogenic potency of each antigen proved weakly with other heterologus sera.
Fig. 3.
Cross-reactivity of adult H. contortus (AS) antigen against different prepared hyper immune sera. Neg. rabb. serum negative rabbit serum, HIS-Monezia spp: Hyper immune serum raised against M. expensa, HIS-Fasciola spp: Hyper immune serum raised against Fasciola spp, HIS-H. contortus: Hyper immune serum raised against H. contortus, ctrl.pos. H. contortus: control positive serum of adult H. contortus, ctrl.neg. H. contortus: control negative serum of adult H. contortus
Fig. 4.
Cross-reactivity of Fasciola spp. antigen against different prepared hyper immune sera. Neg. rabb. serum negative rabbit serum, HIS-Monezia spp: Hyper immune serum raised against M. expensa, HIS-Fasciola spp: Hyper immune serum raised against Fasciola spp, HIS-H. contortus: Hyper immune serum raised against H. contortus, ctrl.pos. H. contortus: control positive serum of adult H. contortus, ctrl.neg. H. contortus: control negative serum of adult H. contortus
Fig. 5.
Cross-reactivity of M. expansa antigen against different prepared hyper immune sera. Neg. rabb. serum negative rabbit serum, HIS-Monezia spp: Hyper immune serum raised against M. expensa, HIS-Fasciola spp: Hyper immune serum raised against Fasciola spp, HIS-H. contortus: Hyper immune serum raised against H. contortus, ctrl.pos. H. contortus: control positive serum of adult H. contortus, ctrl.neg. H. contortus: control negative serum of adult H. contortus
Discussion
Haemonchosis is considered as life threatening disease disturbing sheep population (Kandil et al. 2015). For serological diagnosis of H. contortus infection, various ELISA based methods have been described in order to detect the antibodies formed against H. contortus in sera of naturally and/or experimentally infected sheep (Li et al., 2007; Prasad et al. 2008; Lone et al. 2012; Arab et al. 2013). In this study, different prepared H. contortus antigens; L, ESP and AS were characterized and compared to precise the most reliable antigen that can be used in sero-diagnosis of haemonchosis in sheep.
Depending up on the indirect ELISA, as well as the immuno-reactive protein profile, the present data appeared that the L antigen yielded good results for detection of H. contortus infection in naturally infected and non-infected sheep sera. It achieved the highest specificity followed by AS antigens and this result go along with the presence of protein bands at the range 22–31 kDa which were reactive at performing immunogenic profile via western blotting technique. These results agree with the previous literature which stated that a 26 kDa antigen of adult worm is specific for diagnosis of H. contortus infection (Gomez et al. 1996; Molina et al. 1999; Arab et al. 2013; Kandil et al.2015) and deviate with the result of (Meshgi and Hosseini 2007). Although, L and AS antigens showed good specificity but the L antigen achieved the best results in detecting the anti-H. contortus antibodies in all collected sheep’s sera and recorded the highest apparent prevalence 92% followed by the ESP antigen which recorded 75%. These result may be attributed to that the AS antigen which prepared from the adult worm has the ability to escape host immunity by switching gene expression between variants stored within each genome during the total time of infection. As host immunity builds against a common variant, one or more newly expressed variants can rise. The host must then build another specific immune response against the new variants so this switching may help to avoid the immunological memory of a previously infected host (Frank 2002; Gasser et al. 2016). As well as, the immune evasion mechanisms, formation of circulating immune complexes and differences in status of host immune response may be responsible for variation in antibody level (Lone et al. 2012). So, the present study studied cross reactivity among adult H. contortus, M. expansa and Fasciola spp. and revealed that AS antigen showed prominent cross reactivity with other cestode and trematode represented by M. expansa and Fasciola spp. antigen and to overcome this phenomenon best dilution of serum was (1:800) and This result agrees with the recorded investigation of Molina et al. (1999) and Kandil et al. (2015).
The highest positive predictive value and apparent prevalence percentages achieved by L and ESP antigens harmonize with the emergence of an immunodominant band at 57 kDa which may be responsible for accurate diagnosis. But this result was in difference with the result of Meshgi and Hosseini (2007) who stated that two major peptide bands belong to intestine and uterus of adult H. contortus with 35 and 40 kDa, respectively were specific for such diagnosis. Our result may be discussed due to the persistence of the host immune response of latent infections which may be treated by anti-helminthic drugs so there is no worm detected at post-mortem inspection or may be probably due to cross reactivity of H. contortus with other trematode or cestode helminthes (Lone et al. 2012). So, the present study concluded that L antigen is the promising antigen for serological diagnosis of H. contortus infection in sheep as the immunodominant band at molecular weight 57 kDa responsible for specificity and accuracy of positive predictive value of this antigen.
Acknowledgements
This work was financially supported by co-project between Egypt and Bulgaria, which entitled ‘‘ Recent trails for diagnosis of haemonchosis’’ and funded from academy of scientific research in Egypt.
Contributor Information
Omnia M. Kandil, Phone: +01005414113, Email: kandil_om@yahoo.com
Seham H. M. Hendawy, Email: shendawy2006@yahoo.com
Amira H. El Namaky, Email: amiraelnamaky@gmail.com
Margarita P. Gabrashanska, Email: m.gabrashanska@gmail.com
Veselin N. Nanev, Email: veselinnanev@gmail.com
References
- Arab RMH, Abu El Ezz NMT, Deghidy NS, Awed WSA, Hassan NMF. Protective value of Haemonchus contortus adult worm purified antigen against haemonchosis in sheep. Glob Vet. 2013;11(5):614–621. [Google Scholar]
- Besier RB, Kahn LP, Sargison ND, Van Wyk JA. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv Parasitol. 2016;93:95–143. doi: 10.1016/bs.apar.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Demeler J, Schein E, Von Samson-Himmelstjerna G. Advances in laboratory diagnosis of parasitic infections of sheep. Vet Parasitol. 2012;189:52–64. doi: 10.1016/j.vetpar.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Fagbemi BO, Obarisiagbon IO, Mbuh JV. Detection of circulating antigen in sera of F.gigantica infected cattle with antibodies reactive with a Fasciola specific 88 kDa antigen. Vet Parasitol. 1995;58:235–246. doi: 10.1016/0304-4017(94)00718-R. [DOI] [PubMed] [Google Scholar]
- Frank SA. Immunology and evolution of infectious disease. Princeton: Princeton University Press; 2002. [PubMed] [Google Scholar]
- Gasser RB, Schwarz EM, Korhonen PK, Young ND. Understanding H. contortus better through genomics and transcriptomics. Adv Parasitol. 2016;93:519–567. doi: 10.1016/bs.apar.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Gomez MMT, Cuquerella M, Alunda JM. Identification and partial purification of a 26 kDa antigen of adult H. contortus. Int J Parasitrol. 1996;26:311–318. doi: 10.1016/0020-7519(95)00127-1. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Behnke JM, Coles GC. Copro-antigen capture ELISA for the detection of Teladorsagia (Ostertagia) circumcincta in sheep: improvement of specificity by heat treatment. Parasitology. 2004;129:115–126. doi: 10.1017/S0031182004005256. [DOI] [PubMed] [Google Scholar]
- Kandil OM, Eid NA, Elakabawy LM, Abdelrahman KA, Helal MA. Immunodiagnostic potency of different H. contortus antigens for diagnosis of experimentally and naturally Haemonchosis in Egyptian sheep. Acta Parasitol Glob. 2015;6(3):238–247. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li X, Du A, Cai W, Hou Y, Pang L, Gao X. Evaluation of a recombinant excretory-secretory H. contortus protein for use in a diagnostic ELISA. Exp Parasitol. 2007;115:242–246. doi: 10.1016/j.exppara.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Lone BA, Chishti MZ, Ahmad F, Tak H, Hassan J. Immunodiagnosis of H. contortus infection in sheep by indirect enzyELISA. IJVR. 2012;13(1):49–53. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AB, Randall RJ. Protin measurement with the folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAFF (Ministry of Agriculture, Fisheries and Food) Manual of veterinary parasitological laboratory techniques. 3. London: HSMO Publications; 1986. p. 160. [Google Scholar]
- Meshgi B, Hosseini SH. Evaluation of different antigens in western blotting technique for diagnosis of sheep haemonchosis. Iran J Parasitol. 2007;2(4):12–16. [Google Scholar]
- Molina JM, Ruiz A, Rodriguez-Ponce E, Gutierrez AC, Gonzalez J, Hernandez S. Cross-reactive antigens of H. contortus adult worms in Teladorsagia circumcincta infected goats. Vet Res. 1999;30:93–399. [PubMed] [Google Scholar]
- Prasad A, Nasir A, Singh N. Detection of anti-H. contortus antibodies in sheep by dot-ELISA with immunoaffinity purified fraction of ES antigen during prepatency. IJEB. 2008;46:94–99. [PubMed] [Google Scholar]
- Schallig HD, Hornok S, Cornelissen JB. Comparison of two enzyme immunoassays for the detection of H. contortus infections in sheep. Vet Parasitol. 1995;57:329–338. doi: 10.1016/0304-4017(94)00693-7. [DOI] [PubMed] [Google Scholar]
- Tabouret G, Prevot F, Bergeaud JP, Dorchies Ph, Jacquiet P. Oestrus ovis (Diptera: Oestridae): Sheep humoral immune response to purified excreted/secreted salivary gland 28 KDa antigen complex from second and third instar larvae. Vet Parasitol. 2001;101:53–66. doi: 10.1016/S0304-4017(01)00501-5. [DOI] [PubMed] [Google Scholar]
- Tariq KA, Chishti MZ, Fayaz A, Shawl AS. Epidemiology of gastrointestinal nematodes of sheep managed under traditional husbandry system in Kashmir valley. Vet Parasitol. 2008;158:138–143. doi: 10.1016/j.vetpar.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staeheline T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Prot Nat Acad Sci. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]