Abstract
Hepatozoon spp. are protozoan parasites that infect a wide range of domestic and wild animals. The infection occurs by ingestion of an infected tick. This study was carried out to detect and characterize Hepatozoon spp. in ticks collected from captive lions (Panthera leo) in Thailand based on the partial 18S rRNA gene sequence. A total of 30 ticks were collected and identified as Rhipicephalus sanguineus. The collected ticks were separated into 10 tick pools by sex and life stages. Of the 10 tick pools examined, only one (10%) was found to be infected with the Hepatozoon species. Sequencing and phylogenetic analysis showed a clustering of the partial 18S rRNA gene sequence like that of H. felis from the GenBank database. This is the first report of H. felis in R. sanguineus ticks collected from captive lions in Thailand. Our results indicated that R. sanguineus may be a possible vector of feline Hepatozoon in Thailand.
Keywords: Hepatozoon felis, Rhipicephalus sanguineus, Ribosomal RNA, Lion, Thailand
Introduction
Hepatozoon is a genus of apicomplexan parasites that have been described in a wide variety of mammals, birds, reptiles and amphibians infected by ingestion of a tick containing mature oocysts (Smith 1996; Baneth et al. 2003). The infection presents as subclinical symptoms with low levels of parasitemia or clinical signs such as emaciation, weight loss, lethargy, fever, anemia and lymphadenopathy with high parasitemia (Baneth and Weigler 1997; Baneth et al. 2003). Several tick species can act as potential vectors (Giannelli et al. 2013). The brown dog tick, Rhipicephalus sanguineus, is the main vector of H. canis in domestic dogs (Baneth et al. 2003).
Studies on Hepatatozoon infections in wild animals, especially wild canids and felids, have been conducted in several countries (Averbeck et al. 1990; Metzger et al. 2008; André et al. 2010; Kubo et al. 2010; Salakij et al. 2010; Pawar et al. 2012; Farkas et al. 2014; Hodžic et al. 2015). These studies have reported host species and tick vectors characterized the parasite species. Molecular methods such as polymerase chain reaction (PCR) are used as epidemiology and diagnostic tools for the detection and identification of the Hepatozoon species (Criado-Fornelio et al. 2003; Rubini et al. 2005; Li et al. 2008). The 18S ribosomal RNA (18S rRNA) gene has been widely used as a marker for detection and genetic characterization of parasites (Inokuma et al. 2002; Criado-Fornelio et al. 2003; Metzger et al. 2008; Kubo et al. 2010; Pawar et al. 2012).
In Thailand, although Hepatozoon infections have been reported in domestic and wild animals and tick vectors (Jittapalapong et al. 2006; Salakij et al. 2008, 2010; Sumrandee et al. 2015), no studies have recorded the presence of Hepatozoon species in ticks collected from wild felid. Thus, this study was carried out to detect and characterize Hepatozoon spp. in ticks collected from captive lions in Thailand based on the partial 18S rRNA gene sequence.
Materials and methods
Ethics statement
General permission for sample collection from lions was obtained from the zoo owner. The lions were treated in accordance with veterinarians and CCAC (Canadian Council on Animal Care) guidelines on the care and use of wildlife.
Tick collection and identification
In September 2015, ticks were directly collected from three captive lions (n = 3) in a private zoo (the natural habitat in Africa) in Chonburi Province, Thailand. During the tick collection, lions were restrained in a squeeze cage without the use of any anesthetic agents. The ticks were preserved in 70% alcohol and sent to the Monitoring and Surveillance Center for Zoonotic Diseases in Wildlife and Exotic Animals, Faculty of Veterinary Science, Mahidol University for further analysis. All specimens were identified to the species level, and development stage and gender were determined with a stereomicroscope using standard taxonomic keys (Kohls 1957). The ticks were pooled by sex and life stages ranging from 1 to 5.
DNA extraction and 18S rRNA amplification
Genomic DNA was extracted from individual adult fully engorged females, pools of adult females, and a pool of adult males using a QIAamp DNA Mini Kit (QIAGEN, Germany) according to the manufacturer’s protocol. PCR amplification of the 18S rRNA gene with a 737-base pair (bp) fragment was performed using a primer pair consisting of Hep-001F (5′-CCTGGCTATACATGAGCAAAATCTCAACTT-3′) for the forward primer and Hep-737R (5′-CCAACTGTCCCTATCAATCATTAAAGC-3′) for the reverse primer (Kledmanee et al. 2009). The PCR cycle was performed in a C1000™ Thermal Cycler (Bio-Rad) with a total reaction volume of 25 μl containing 12.5 μl of QIAGEN Multiplex PCR Master Mix (QIAGEN, Germany), 10.3 μl of nuclease free water, 0.1 μl of each primer (100 μM), and 2 μl of DNA template. The PCR cycling conditions were an initial denaturation at 95 °C for 15 min, followed by 35 cycles of 94 °C for 45 s, 65 °C for 45 s, and 72 °C for 90 s, with a final extension at 72 °C for 10 min. Amplified PCR products were separated in 2% agarose gel electrophoresis, and the GeneRuler 100 bp DNA Ladder (Thermo Scientific) was used as a size marker to visualize the size of DNA fragments presented in the samples.
DNA cloning
The pGEM®-T Easy Vector System (Promega) was used for cloning the PCR product. The PCR product was first inserted in the vector according to the protocol for ligation. Then, the created ligation vector was transformed into E. coli competent cells. The competent cells were cultured on an LB plate with ampicillin/IPTG/X-Gal. The white colonies were selected for screening the 737-bp fragment insertion by PCR. The colony carrying the fragment of interest was used for LB/ampicillin culture. Next, an E. coli overnight culture was performed to extract the plasmids (the ligation vectors) using the QIAprep Spin Miniprep Kit according to the manufacturer’s protocol.
DNA sequencing and phylogenetic analysis
DNA sequencing of the PCR product was performed by Solgent Co. Ltd. (Daejeon, South Korea) using an ABI 3730 XL DNA analyzer. The DNA sequence was carried out with the T7 universal primer. The nucleotide sequence result was compared with available sequences in the GenBank database using the Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple alignments of all relative nucleotide sequences were performed using the BioEdit tool. Phylogenetic tree of partial 18S rRNA gene (566 bp) was constructed using neighbor-joining and the maximum likelihood method with bootstrapping (1000 replications) by MEGA 6 software (Tamura et al. 2013). The best nucleotide substitution model was a Tamura 3-parameter (T92). The sequence was compared to published sequences from the GenBank database under the following accession numbers: AB771562, AB771545, GQ377217, GQ377218, HQ829443, HQ829444, HQ829439, HQ829440, HQ829442, HQ829445, HQ829446, AY620232, AY628681, KC138533, GQ377216, AY461375, GU376455, AY731062, HQ829437 and EU041718.
Results
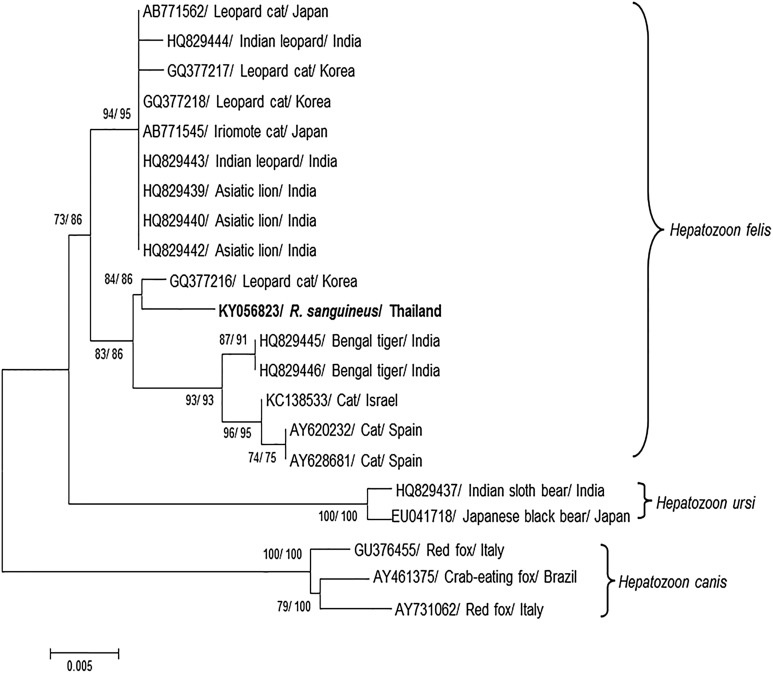
A total of 30 ticks, including 20 adult females, 6 engorged adult females, and 4 adult males were collected from infested lions. All collected ticks were identified as the Rhipicephalus sanguineus species. One (10%) out of 10 tick pools was positive for the 18S rRNA gene fragment of the Hepatozoon species. This pool came from engorged adult females. PCR samples from the positive R. sanguineus ticks were sequenced. The nucleotide sequence was compared with available sequences in the GenBank database by BLAST. The result showed high sequence identity (99%) with the 18S rRNA gene of H. felis isolated from a leopard cat (Prionailurus bengalensis) in Korea (GQ377216). Additionally, the nucleotide sequence presented close identity (98%) to H. felis isolated from Bengal tigers (Panthera tigris) in India (HQ829445 and HQ829446), a domestic cat (Felis catus) in Israel (KC138533), and domestic cats in Spain (AY620232 and AY628681). Phylogenetic relationships among the Hepatozoon species can be divided into three groups (Fig. 1). H. felis (KY056823) isolated from ticks in this study was included in the group with other isolated H. felis; this group was clearly separated from other Hepatozoon species.
Fig. 1.
Phylogenetic analysis of the Hepatozoon partial 18S rRNA gene sequence. Hepatozoon felis (KY056823) was amplified from R. sanguineus ticks infested on captive lions in Thailand and analyzed for comparison with other Hepatozoon spp. from the GenBank database. The numbers on branches indicate percent bootstrap support of the neighbor-joining and the maximum likelihood based on 1000 bootstrap replications
Discussion
This study is the first to report on R. sanguineus infected with the feline Hepatozoon species in Thailand using molecular diagnostic assay. The results suggest ticks as a possible vector of H. felis in captive lions in Thailand. Our results support the recent studies of Aktas (2014) and Maia et al. (2014), which detected H. felis DNA in R. sanguineus in Turkey and Portugal, respectively. However, the vectors of H. felis remain unknown (Lloret et al. 2015). The specific DNA of the Hepatozoon species in our ticks may be derived from host blood meal consumed from infected lions. Unfortunately, our study did not include the collection of the larval or nymphal stage of this tick. Hence, no vector potential of this tick was discovered. Further studies on intrastadial transmission of ticks should be performed to determine their potential as a vector. Several tick genera have been reported to infest lions, such as Amblyomma, Haemaphysalis, Hyalomma, Ixodes and Rhipicephalus (Horak et al. 2000). In the present study, the infestation of R. sanguineus on lions is in accordance with previous research studies (Fyumagwa et al. 2008; Mbaya et al. 2008). In Thailand, R. sanguineus has been reported as common ectoparasite of domestic dogs (Changbunjong et al. 2009). However, this tick very seldom feeds on species other than domestic dogs (Horak et al. 2010). Besides Hepatozoon, R. sanguineus has also been reported as an important vector of many pathogens such as Ehrlichia canis, Anaplasma platys, Babesia gibsoni, B. canis vogeli, Leishmania infantum, Mycoplasma haemocanis and Rickettsia rickettsii (Dantas-Torres and Otranto 2015).
Feline hepatozoonosis caused by H. felis has been described in domestic cats and wild felids in several countries (Metzger et al. 2008; Kubo et al. 2010; Pawar et al. 2012; Kelly et al. 2014; Lloret et al. 2015; Tateno et al. 2015). This Hepatozoon has been found to be common in lions in Africa (Kelly et al. 2014). However, H. felis has never been found in domestic dogs, cats and also wildlife hosts in Thailand. Several tick species have been reported to harbor H. felis, including Haemaphysalis longicornis, H. hystricis, H. megaspinosa, H. campanulata, Amblyomma testudinarium and Ixodes tanuki collected from wildcats in Japan (Tateno et al. 2015) and R. sanguineus collected from domestic cats in Portugal (Maia et al. 2014). In Thailand, a Hepatozoon species that is closely related to H. felis was found in Dermacentor astrosignatus and D. auratus ticks collected from wild boars (Sumrandee et al. 2015). In the present study, the prevalence of H. felis in R. sanguineus ticks was as high as 10% whereas a rate of 0.5% was reported in Portugal (Maia et al. 2014).
The phylogenetic analysis, neighbor-joining, and the maximum likelihood method revealed highly similar results. H. felis (KY056823) was included in the group with other isolated H. felis. It was found to be closely related to H. felis isolated from a leopard cat in Korea (Kubo et al. 2010), Bengal tigers in India (Pawar et al. 2012), a domestic cat in Israel (Baneth et al. 2013), and domestic cats in Spain (Criado-Fornelio et al. 2006). A group of H. felis was clearly separated from H. canis isolated in domestic dogs and wild canids and from H. ursi isolated in various bears. Our analyses confirm and support the results of previous studies showing cluster groups of the Hepatozoon species based on phylogenetic analysis of the 18S rRNA gene (Pawar et al. 2011, 2012).
Although blood parasite infections in many wildlife species are typically asymptomatic, they can present clinical signs under certain circumstances such as unnatural hosts, stress, habitat degradation, climate fluctuation, or immunosuppression (Williams et al. 2014). However, the captive lions in our study showed no signs of hepatozoonosis. The lack of apparent clinical signs is in accordance with studies on bobcats and ocelots in the United States (Mercer et al. 1988) and in a leopard cat in Thailand (Salakij et al. 2010). Many parasites can be spread from wildlife to domestic animals (Otranto et al. 2015). In this study, the captive lions were infested with R. sanguineus, which may be shared with domestic dogs and cats. Hence, close contact between wildlife and domestic animals may lead to the transmission of parasite pathogens and infectious diseases.
In conclusion, this study is the first to detect and characterize H. felis in R. sanguineus ticks collected from captive lions in Thailand. Further studies should be conducted with a large number of ticks sampled across the country to confirm the prevalence of H. felis. Furthermore, additional studies of Hepatozoon in association with the tick vector will be helpful to protect and conserve wildlife species.
Acknowledgements
This research is supported by Faculty of Veterinary Science, Mahidol University. We would like to thank the staff of the Department of National Parks, Wildlife and Plant Conservation for providing specimens.
Compliance with ethical standards
Conflict of interest
We declare that we have no conflict of interest.
References
- Aktas M. A survey of ixodid tick species and molecular identification of tick-borne pathogens. Vet Parasitol. 2014;200:276–283. doi: 10.1016/j.vetpar.2013.12.008. [DOI] [PubMed] [Google Scholar]
- André MR, Adania CH, Teixeira RH, Vargas GH, Falcade M, Sousa L, Salles AR, Allegretti SM, Felippe PA, Machado RZ. Molecular detection of Hepatozoon spp. in Brazilian and exotic wild carnivores. Vet Parasitol. 2010;173:134–138. doi: 10.1016/j.vetpar.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Averbeck GA, Bjork KE, Packer C, Herbst L. Prevalence of hematozoans in lions (Panthera leo) and cheetah (Acinonyx jubatus) in Serengeti National Park and Ngorongoro Crater, Tanzania. J Wildl Dis. 1990;26:392–394. doi: 10.7589/0090-3558-26.3.392. [DOI] [PubMed] [Google Scholar]
- Baneth G, Weigler B. Retrospective case-control study of hepatozoonosis in dogs in Israel. J Vet Intern Med. 1997;11:365–370. doi: 10.1111/j.1939-1676.1997.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Baneth G, Mathew JS, Shkap V, Macintire DK, Barta JR, Ewing SA. Canine hepatozoonosis: two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol. 2003;19:27–31. doi: 10.1016/S1471-4922(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Baneth G, Sheiner A, Eyal O, Hahn S, Beaufils JP, Anug Y, Talmi-Frank D. Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood form morphology, and possible transplacental transmission. Parasit Vectors. 2013;6:102. doi: 10.1186/1756-3305-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changbunjong T, Buddhirongawatr R, Suwanpakdee S, Siengsanan J, Yongyuttawichai P, Cheewajorn K, Jangjaras J, Sangloung C, Ratanakorn P. A survey of ectoparasitic arthropods on domestic animals in Tak Province, Thailand. Southeast Asian J Trop Med Public Health. 2009;40:435–442. [PubMed] [Google Scholar]
- Criado-Fornelio A, Martinez-Marcos A, Buling-Saraña A, Barba-Carretero JC. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Vet Parasitol. 2003;113:189–201. doi: 10.1016/S0304-4017(03)00078-5. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A, Ruas JL, Casado N, Farias NA, Soares MP, Müller G, Brumt JG, Berne ME, Buling-Saraña A, Barba-Carretero JC. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J Parasitol. 2006;92:93–99. doi: 10.1645/GE-464R.1. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9–13. doi: 10.1016/j.vetpar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Farkas R, Solymosi N, Takács N, Hornyák Á, Hornok S, Nachum-Biala Y, Baneth G. First molecular evidence of Hepatozoon canis infection in red foxes and golden jackals from Hungary. Parasit Vectors. 2014;7:303. doi: 10.1186/1756-3305-7-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyumagwa RD, Simmler P, Willi B, Meli ML, Sutter A, Hoare R, Dasen G, Hofmann-Lehmann R, Lutz H. Molecular detection of haemotropic Mycoplasma species in Rhipicephalus sanguineus tick species collected on lions (Panithera leo) from Ngorongoro Crator, Tanzania. S Afr J Wildl Res. 2008;38:117–122. doi: 10.3957/0379-4369-38.2.117. [DOI] [Google Scholar]
- Giannelli A, Ramos RA, Dantas-Torres F, Mencke N, Baneth G, Otranto D. Experimental evidence against transmission of Hepatozoon canis by Ixodes ricinus. Ticks Tick Borne Dis. 2013;4:391–394. doi: 10.1016/j.ttbdis.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Hodžić A, Alić A, Fuehrer HP, Harl J, Wille-Piazzai W, Duscher GG. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasit Vectors. 2015;8:88. doi: 10.1186/s13071-015-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak IG, Braack LE, Fourie LJ, Walker JB. Parasites of domestic and wild animals in South Africa. XXXVIII. Ixodid ticks collected from 23 wild carnivore species. Onderstepoort J Vet Res. 2000;67:239–250. [PubMed] [Google Scholar]
- Horak IG, Heyne H, Donkin EF. Parasites of domestic and wild animals in South Africa. XLVIII. Ticks (Acari: Ixodidae) infesting domestic cats and wild felids in southern Africa. Onderstepoort J Vet Res. 2010;77:E1–E7. doi: 10.4102/ojvr.v77i1.3. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Okuda M, Ohno K, Shimoda K, Onishi T. Analysis of the 18S rRNA gene sequence of a Hepatozoon detected in two Japanese dogs. Vet Parasitol. 2002;106:265–271. doi: 10.1016/S0304-4017(02)00065-1. [DOI] [PubMed] [Google Scholar]
- Jittapalapong S, Rungphisutthipongse O, Maruyama S, Schaefer JJ, Stich RW. Detection of Hepatozoon canis in stray dogs and cats in Bangkok, Thailand. Ann N Y Acad Sci. 2006;1081:479–488. doi: 10.1196/annals.1373.071. [DOI] [PubMed] [Google Scholar]
- Kelly P, Marabini L, Dutlow K, Zhang J, Loftis A, Wang C. Molecular detection of tick-borne pathogens in captive wild felids, Zimbabwe. Parasit Vectors. 2014;7:514. doi: 10.1186/s13071-014-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kledmanee K, Suwanpakdee S, Krajangwong S, Chatsiriwech J, Suksai P, Suwannachat P, Sariya L, Buddhirongawatr R, Charoonrat P, Chaichoun K. Development of multiplex polymerase chain reaction for detection of Ehrlichia canis, Babesia spp. and Hepatozoon canis in canine blood. Southeast Asian J Trop Med Public Health. 2009;40:35. [PubMed] [Google Scholar]
- Kohls GM. Acarina: Ixodoidea. Insects of Micronesia. 1957;3:85–104. [Google Scholar]
- Kubo M, Jeong A, Kim SI, Kim YJ, Lee H, Kimura J, Agatsuma T, Sakai H, Yanai T. The first report of Hepatozoon species infection in leopard cats (Prionailurus bengalensis) in Korea. J Parasitol. 2010;96:437–439. doi: 10.1645/GE-2270.1. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang C, Allen KE, Little SE, Ahluwalia SK, Gao D, Macintire DK, Blagburn BL, Kaltenboeck B. Diagnosis of canine Hepatozoon spp. infection by quantitative PCR. Vet Parasitol. 2008;157:50–58. doi: 10.1016/j.vetpar.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Lloret A, Addie DD, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, Hartmann K, Horzinek MC, Hosie MJ, Lutz H, Marsilio F, Pennisi MG, Radford AD, Thiry E, Truyen U, Möstl K. Hepatozoonosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2015;17:642–644. doi: 10.1177/1098612X15589879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia C, Ferreira A, Nunes M, Vieira ML, Campino L, Cardoso L. Molecular detection of bacterial and parasitic pathogens in hard ticks from Portugal. Ticks Tick Borne Dis. 2014;5:409–414. doi: 10.1016/j.ttbdis.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Mbaya AW, Aliyu MM, Nwosu CO, Ibrahim UI. Captive wild animals as potential reservoirs of haemo and ectoparasitic infections of man and domestic animals in the arid region of Northeastern Nigeria. Veterinarski Arhiv. 2008;78:429–440. [Google Scholar]
- Mercer SH, Jones LP, Rappole JH, Twedt D, Lack LL, Craig TM. Hepatozoon sp. in wild carnivores in Texas. J Wildl Dis. 1988;24:574–576. doi: 10.7589/0090-3558-24.3.574. [DOI] [PubMed] [Google Scholar]
- Metzger B, dos Santos Paduan K, Rubini AS, de Oliveira TG, Pereira C, O’Dwyer LH. The first report of Hepatozoon sp. (Apicomplexa: Hepatozoidae) in neotropical felids from Brazil. Vet Parasitol. 2008;152:28–33. doi: 10.1016/j.vetpar.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Otranto D, Cantacessi C, Pfeffer M, Dantas-Torres F, Brianti E, Deplazes P, Genchi C, Guberti V, Capelli G. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part I: protozoa and tick-borne agents. Vet Parasitol. 2015;213:12–23. doi: 10.1016/j.vetpar.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Pawar RM, Poornachandar A, Arun AS, Manikandan S, Shivaji S. Molecular prevalence and characterization of Hepatozoon ursi infection in Indian sloth bears (Melursus ursinus) Vet Parasitol. 2011;182:329–332. doi: 10.1016/j.vetpar.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Pawar RM, Poornachandar A, Srinivas P, Rao KR, Lakshmikantan U, Shivaji S. Molecular characterization of Hepatozoon spp. infection in endangered Indian wild felids and canids. Vet Parasitol. 2012;186:475–479. doi: 10.1016/j.vetpar.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Rubini AS, dos Santos Paduan K, Cavalcante GG, Ribolla PE, O’Dwyer LH. Molecular identification and characterization of canine Hepatozoon species from Brazil. Parasitol Res. 2005;97:91–93. doi: 10.1007/s00436-005-1383-x. [DOI] [PubMed] [Google Scholar]
- Salakij C, Salakij J, Narkkong NA, Sirinarumitr T, Pattanarangsan R. Hematologic, cytochemical, ultrastructural, and molecular findings of Hepatozoon-infected flat-headed cats (Prionailurus planiceps) Vet Clin Pathol. 2008;37:31–41. doi: 10.1111/j.1939-165X.2008.00011.x. [DOI] [PubMed] [Google Scholar]
- Salakij C, Sirinarumitr T, Tongthainun D. Molecular characterization of Hepatozoon species in a Leopard Cat (Prionailurus bengalensis) from Thailand. Vet Clin Pathol. 2010;39:199–202. doi: 10.1111/j.1939-165X.2010.00216.x. [DOI] [PubMed] [Google Scholar]
- Smith TG. The genus Hepatozoon (Apicomplexa: Adeleina) J Parasitol. 1996;82:565–585. doi: 10.2307/3283781. [DOI] [PubMed] [Google Scholar]
- Sumrandee C, Baimai V, Trinachartvanit W, Ahantarig A. Hepatozoon and Theileria species detected in ticks collected from mammals and snakes in Thailand. Ticks Tick Borne Dis. 2015;6:309–315. doi: 10.1016/j.ttbdis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno M, Sunahara A, Nakanishi N, Izawa M, Matsuo T, Setoguchi A, Endo Y. Molecular survey of arthropod-borne pathogens in ticks obtained from Japanese wildcats. Ticks Tick Borne Dis. 2015;6:281–289. doi: 10.1016/j.ttbdis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Williams BM, Berentsen A, Shock BC, Teixiera M, Dunbar MR, Becker MS, Yabsley MJ. Prevalence and diversity of Babesia, Hepatozoon, Ehrlichia, and Bartonella in wild and domestic carnivores from Zambia, Africa. Parasitol Res. 2014;113:911–918. doi: 10.1007/s00436-013-3722-7. [DOI] [PubMed] [Google Scholar]