Abstract
Pancreatic cancer (PC), one of the most lethal malignancies, accounts for 8% to 10% of digestive system cancers, and the incidence is increasing. Surgery, chemotherapy, and radiotherapy have been the main treatment methods but are not very effective. Cryosurgery was first used in 1984 for treatment of locally advanced PC and has since become a considerable treatment for most cases of unresectable PC. During the past decade, cryosurgery has been applied in some hospitals in China, and the newly developed technique of computed tomography– and/or ultrasound-guided percutaneous cryosurgery has shown better results than chemotherapy in cases of unresectable locally advanced PC, with the 1-year survival rate reported to be more than 50%. To develop standardized criteria for the application of cryosurgery in PC, the International Society of Cryosurgery and Asian Society of Cryosurgery assembled experts from Austria, Japan, and China to discuss treatment methods and arrive at a consensus on the indications, contraindications, and preferred techniques of PC cryosurgery.
Key Words: consensus, cryosurgery, guidelines, pancreatic cancer
Pancreatic cancer (PC), one of the most lethal cancers, accounts for 8% to 10% of all digestive system cancers and shows an increasing incidence. According to the American Cancer Society, more than 48,960 new cases of PC were diagnosed in 2015 in the United States, and 83% of these patients failed to survive, making PC the fourth most common cause of cancer deaths.1 The overall survival rates in stages II, III, and IV disease are only 5% to 7%, 3%, and 1%, respectively.2 However, to this knotty disease, there has been almost no improvement in survival rates in recent years, but the incidence keeps going up.3–5 Radical surgical resection is considered the only curative option currently, but unfortunately, more than four-fifths of cases are diagnosed too late to meet the operation indications.6 Chemotherapy and radiotherapy do benefit those unresectable cases in some degree, but they also have shown limitations in prolonging survival and preventing severe adverse effects.7,8 Results showed as presented during the annual meeting (2015) of the American Society of Clinical Oncology, that no matter whether chemoradiotherapy or chemotherapy only was used, the overall survival in unresectable PC is no more than 12 months, and improvement in the toxicity profile of current regimens is needed.9
Cryosurgery had been used for primary and secondary malignant diseases early in the 20th century.10–13 It is recognized as a therapeutic option for various cancers,14,15 and it has been suggested in clinical guidelines for non–small cell lung cancer, hepatobiliary cancer, renal cell carcinoma, and prostate cancer by the National Comprehensive Cancer Network,16–19 as well as for PC, especially those in advanced stage, cryosurgery is generally considered to be an effective palliative treatment.
Professor Korpan20 first reported the application of cryosurgery for locally advanced PC with a liquid nitrogen probe in 1984.21 Several years later, in 1991, Patiutko et al22 performed cryosurgery for locally advanced PC. In 2002, Kovach et al23 reported good pain control, without any serious complications in 9 patients treated with operative cryosurgery, and in 2007, Korpan24 described local cryonecrosis and cryoapoptosis of cryoablation for the first time. He found that ultrastructural changes in the exocrine pancreatic cells were increased, which was noticed in the first signs of dystrophic processes of cells. In the last decade, hospitals in China have begun to widely apply this technique in the treatment of PC and have contributed to most cryosurgeries in the world. According to our previous systemic review,25 besides the advantages of minimal invasion (percutaneous approach) and being potentially safe with less pain to the patients, cryosurgery, especially percutaneous cryosurgery, is also safe enough to be conducted in patients with PC and shows superiority in improving survival in advanced PC when performed alone or combined with 125-iodine (125I) seed implantation, immunotherapy, or various other treatments. Median overall survival in patients with locally advanced PC treated with cryosurgery combined with 125I seed implantation showed 2.6 months longer survival compared with patients who underwent gemcitabine (16.2 vs 13.6 months).26,27 Similar results were also reported in pancreas-originated liver metastases. Bala et al28 recruited 123 consecutive patients with liver metastases to compare the efficacy of cryosurgery and conventional surgery, and they found 3, 5, and 10 years’ survival rates in the cryosurgery group were 60%, 44%, and 19% compared with 51%, 36%, and 8% in the conventional surgery group. Recurrence in the liver was observed in 86% of patients in the cryosurgery group, whereas 95% of the patients in the conventional surgery group had a recurrence. Although there was no significant difference between the 2 groups, data in the cryosurgery group obviously presented a trend in obtaining better results and a preferable pain control.
To propel a wider and further discussion on application of cryosurgery, the International Society of Cryosurgery and the Asian Society of Cryosurgery assembled professionals and experts from China, Austria, and Japan for discussions on the indications, contraindications, and techniques of cryosurgery in PC, with the aim of developing a standardized approach for clinical application. The preliminary consensus statement is presented in this article.
INDICATIONS AND CONTRAINDICATIONS
Indications
Pathologically (or cytologically) and radiologically diagnosed locally advanced or metastatic pancreatic ductal cancer or resectable PC, but patient unwilling to undergo surgery
Eastern Cooperative Oncology Group (ECOG) Performance Status of 2 or less
White blood cell count of more than 3.0 × 109/L, platelets of more than 80 × 109/L, hemoglobin of more than 100 g/L, creatinine clearance of more than 50 mL−1 · min−1, serum aspartate transaminase and alanine transaminase levels less than 3 times the upper limit, and serum bilirubin of less than 20 μmol/L
Informed consent for the procedure
Satisfactory immune index when consider combining with immotherapy29
Contraindications
ECOG Performance Status of greater than 2
Coagulation disorder or obvious bleeding tendency
Severe cardiopulmonary dysfunction
Liver and kidney dysfunction
Local adhesions as a result of previous surgery
Coexisting pancreatitis
Bile duct or digestive tract obstruction
Ascites or intestinal tympanites, with increased intra-abdominal pressure
Hypersensitivity to physical, chemical, or biological factors
CLINICAL PATHWAY
A clinical pathway for PC is released for possible guidance in applying cryosurgery alone or with other therapeutic methods. As a proven effective ablative approach, cryosurgery could be performed depending on patients’ preference and related practice principles (Fig. 1).
FIGURE 1.
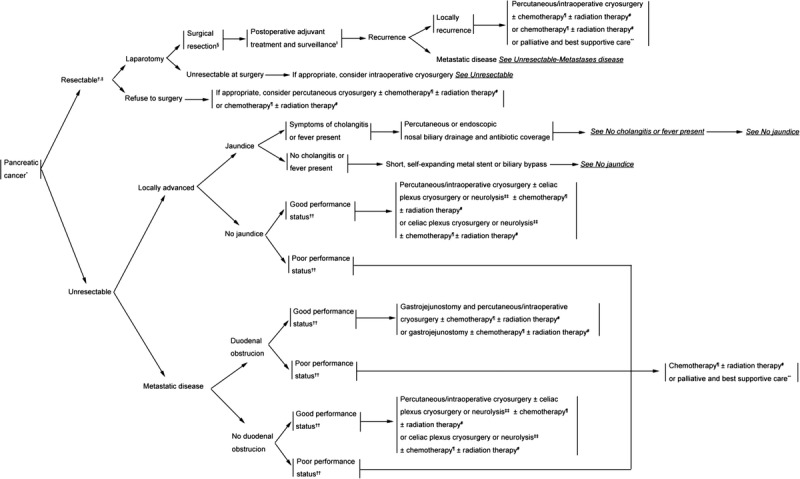
Clinical pathway. *Pathologically or cytologically or imaging diagnosed PC. †See principles of diagnosis, imaging, and staging in NCCN Guidelines: Pancreatic Adenocarcinoma.30 ‡See criteria defining resectability status in NCCN Guidelines: Pancreatic Adenocarcinoma.30 §See principles of surgical techniques in NCCN Guidelines: Pancreatic Adenocarcinoma.30 ∥Patient informed consent belong to the patient’s or his/her authorized person’s will. ¶See principles of chemotherapy in NCCN Guidelines: Pancreatic Adenocarcinoma.30 #See principles of radiation therapy in NCCN Guidelines: Pancreatic Adenocarcinoma.30 **See principles of palliation and supportive care in NCCN Guidelines: Pancreatic Adenocarcinoma.30 ††Defined as ECOG 0 to 1 with good performance status. ‡‡When patient has indication of pain.
PREOPERATIVE PREPARATION
Laboratory investigations: routine examination of blood, urine, and stool; liver and kidney function tests; coagulation function; tumor markers; immunological function; blood type; infection screen; electrocardiogram; and lung function tests to be assessed within 2 weeks before operation
Imaging examination: abdominal enhanced computed tomography (CT), positron emission tomography/positron emission tomography–CT, or ultrasonography (US) within 2 to 4 weeks before operation
Pathological examination: percutaneous fine-needle aspiration biopsy31
Perform clinical staging according to the National Comprehensive Cancer Network Guidelines for Pancreatic Adenocarcinoma (version I.2015)
Treatment plan: plan the position of the patient during the procedure, the puncture site, and the probe route according to the tumor site, size, number, and shape, as well as its positional relationship with the stomach, duodenum, and abdominal vessels
Equipment: cryosurgery system, cryoprobes, CT, ultrasound, electrocardiogram monitor, oxygen breathing apparatus, resuscitation cart, and other rescue-related devices
Medication: anesthetic agents and analgesic drugs, antitussives, hemostatic drugs, and so on
Patient preparation: (a) obtain informed consent from the patient or an authorized representative, (b) fast the patient for at least 6 hours preoperatively, (c) mark the puncture point on the skin, (d) educate the patient regarding the procedure
PROCEDURE
Percutaneous Cryoablation
This is applicable in all PC patients satisfying the indications for cryosurgery.
Transabdominal puncture
Used for tumors in the pancreatic head and body. There are 3 possible approach routes, depending on the site of the tumor: (a) for tumors of more than 5 cm: the cryoprobe can be passed between the stomach and intestines, using pressure by the US cryoprobe to push the organs aside; (b) penetration through the left lobe of liver; (c) penetration through the stomach.
Transdorsal puncture
Used for tumors in the pancreatic body and tail. The skin is punctured between T12 and L1 on the left side of the back, 3 to 7 cm away from the spine. The cryoablation cycle is initiated when the cryoprobe tip is in the tumor, 0.5 cm from its distal edge.
Intraoperative Cryoablation
This is applicable for tumors recognized as unresectable in patients who are willing to accept cryosurgery and are planned for surgery for gastrojejunostomy or other reasons.
CRYOABLATION TECHNIQUE
Position the patient face up or face down on the CT docking table, depending on whether transabdominal or transdorsal cryoablation is to be undertaken. Obtain color US images and CT images by double-row helical CT prior to the procedure to design the number, angle, and depth of insertion of the cryoprobes. Administer local anesthesia with 1% lidocaine; if necessary, combine with basic systemic anesthesia with midazolam 0.01 mg/kg, propofol 20 mL/h, and fentanyl 3 to 5 μg/kg.
Under CT and US monitoring, insert the cryoprobe (1.47, 1.7, 2, or 3 mm in diameter), and advance it through the center of the mass until the tip is 0.5 cm away from the distal inner margin of the tumor; use 1 to 2 cryoprobe(s) for masses of less than 2-cm diameter, 2 to 4 cryoprobes for masses of 2- to 4-cm diameter, and 4 to 8 cryoprobes for masses of more than 4-cm diameter.23,32 The ideal distance between the tips of any 2 cryoprobes is 2 cm. More than 1 session may be necessary, for example, when a residual tumor remains after the first session or when dealing with large masses. All metastatic tumors whose diameters are of more than 3 cm should be frozen simultaneously.
First, 100% quantity of argon is input to freeze down to −160°C ± 10°C (cooling rate >200°C/min) for 5 to 10 minutes, then slowly warm up to 15°C ± 5°C by helium transmission (warming rate >80°C/min) for 5 to 10 minutes. The cycle is then repeated.33
For masses in the pancreatic body and tail, an ice ball that is 0.5 cm larger than the mass will help achieve complete ablation, whereas for pancreatic head masses, isovolumetric ablation with 10% quantity of argon is much safer.34 Use CT to carefully monitor the ice ball coverage and the positional relationship between the ice ball and adjacent organs, with special care taken to avoid damage to the stomach or duodenum.
POSTOPERATIVE MANAGEMENT
Closely observe the patient in the intensive care unit for at least 6 hours after the procedure, and the patient should fast for 24 hours. Pressure exerted by an abdominal corset can aid in achieving hemostasis; use antibiotic (cephalosporin for 3–5 days) and metronidazole for preventing infection, and antacids, imidazole, and omeprazole for suppressing gastric acidity; pancreatic secretion can be inhibited with octreotide acetate.
COMPLICATIONS AND THEIR MANAGEMENT
Acute Pancreatitis
Withhold oral feeds; prevent shock and improve microcirculation; provide pain relief with analgesics and antispasmodics; inhibit pancreatic enzyme secretion (with the use of parenteral nutrition, continuous aspiration of gastric secretions, pancreatic enzyme inhibitors, somatostatin analogs, aprotinin, and so on); and administer antibiotics.
Pancreatic Fistula
Inhibit pancreatic secretion, accumulate pancreatic secretions, and drain pancreatic secretions using measures such as percutaneous catheter drainage, surgical drainage, and endoscopic drainage.
Gallbladder Fistula
Provide drainage, with stenting if appropriate; stop oral feeds and provide parenteral nutrition instead; administer effective antibiotics; monitor the patient’s condition; and offer surgical repair if necessary.
Intra-abdominal Bleeding
Achieve hemostasis; provide blood transfusion and proper antishock treatment. Identify the cause of the bleeding, and consider surgery when necessary.
Jaundice
Protect liver function; consider catheter drainage via endoscopic retrograde cholangiopancreatography if necessary.
Serum Amylase Elevation
In the absence of symptoms of acute pancreatitis, no special treatment is required; however, observe the patient closely.
Thoracic and Abdominal Cavity Effusion
Perform pleural and peritoneal drainage and detect amylase activity. If a pancreatic fistula has formed, manage according to the standard guidelines for pancreatic fistula treatment.
Fever
Consider the possibilities of pancreatitis, biliary infection, and pyrexia due to absorption of toxins from the necrosed tumor tissue. For low-grade fever, physical cooling process may help; for high-grade fever, perform further examination and investigations to identify the cause.
Lower-Extremity Edema
Conduct a detailed examination to identify the cause, and provide targeted management.
FOLLOW-UP AND EVALUATION
Ice Ball Coverage Rate
Ice ball coverage rate = maximum cross-sectional size of the ice ball/maximum cross-sectional size of the tumor. Perform CT scan immediately after cryosurgery. Complete cryoablation can be considered to have been achieved if the ice ball coverage rate is 100%.35
CT Scan
Obtain CT scan every 2 to 6 months after cryosurgery. (1) CT value: a decrease of more than 30 to 50 HU implies inactivation of cancer cells. Effective rate of tumor inactivation = (number of masses that show decrease of >30 HU in CT value) / (number of masses cryoablated) × 100%; (2) enhanced CT: no enhancement in local mass indicates effective cryoablation36; (3) functional CT: reduction of tumor blood flow and blood volume indicates effective ablation37; (4) RECIST (Response Evaluation Criteria in Solid Tumors) analysis38: (a) complete response: all masses disappear, or decrease in total maximum diameter of masses by more than 75%; (b) partial response: decrease in total maximum diameter of masses by more than 30%; (c) stable disease: decrease in total maximum diameter of masses by not more than 30% or increase by not more than 20%; (d) progressive disease: increase in total maximum diameter of masses by greater than 20%.
Serum CA 19-9 and Carcinoembryonic Antigen
Recheck every 2 to 6 months after the procedure.
Clinical Benefit Response
Assess clinical benefit response.39 Check if, in the absence of clinical deterioration, any of the following is present and is sustained for more than 4 weeks: (1) analgesics dosage reduced by more than 50%, (2) cancer pain score relieved by more than 50%, (3) weight gain of more than 2 kg, and (4) ECOG Performance Status improved by 20 or greater.
Survival
Follow up patients monthly. Analyze median progression-free survival time, medium survival time, 6-month survival rate, and 12-month survival rate.
COMBINATION WITH OTHER THERAPIES
Cryoablation Combined With Surgery
Cryoablation combined with surgery can be applied in patients with locally advanced disease and unresectable cancer, without metastases, in whom gastroduodenostomy or other laparotomic procedure needs to be performed. Intraoperatively, first confirm that the PC is unresectable. Identify the main pancreatic duct before introducing the cryoprobe. Using CT and/or US guidance, ensure that the superior mesenteric vein, portal vein, and splenic vein are not injured. Ablate after the cryoprobe has entered the target.
Cryoablation Combined With 125I
Cryoablation combined with 125I can be used for masses of irregular shape or adjacent to stomach, duodenum, bile duct, or other vital organs. For masses of less than 5-cm diameter, perform 125I seed implantation before cryosurgery, whereas for masses of more than 5-cm diameter, implant the 125I seeds in the margin of mass or beside the stomach, duodenum, pancreatic duct, or bile duct, following the treatment planning system plan, or add the seeds as supplement in or just beside the unablated mass. Recheck with CT scan 1 month later, and repeat cryoablation and/or 125I seed implantation if necessary.40
Cryoablation Combined With Interventional Embolization
Cryoablation combined with interventional embolization can be used for masses larger than 5 cm. Perform interventional embolization first. Cryoablate 2 weeks later if the mass shows obvious shrinkage or necrosis on CT; otherwise, repeat interventional embolization.
Cryoablation Combined With Immunotherapy
Cryoablation combined with immunotherapy can be used in all types of PC; it is especially useful in patients with metastases but with normal systemic immune function.41 Operational approaches: extract mononuclear cells from peripheral blood 2 days before cryosurgery, then separate them and resuspend in dentritic cells or cytokine-induced killer cells medium separately; coculture dentritic cells/cytokine-induced killer cells for 48 hours; intravenously inject to the patient every other day for a total of 5 times.
Cryoablation Combined With Celiac Plexus Block
Cryoablation combined with celiac plexus block42 can be useful in patients who suffer from moderate to severe pain. Perform the celiac plexus block during cryosurgery. When laparotomic cryoablation is performed, locate the aorta by its pulsation and pass the needle on either side of it to inject 20-mL absolute ethyl alcohol at the L1 level. When percutaneous cryoablation is performed, approach via diaphragm angle with guidance of ultrasound and CT scan.
Cryoablation Combined With Traditional Chinese Medicine
After relieving the tumor load by cryoablation, traditional Chinese medicine can be used for adjusting microenvironment of the tumor.
CONCLUSIONS
Cryosurgery is generally considered to be an effective palliative treatment for PC, especially for those at advanced stage. With guidance of CT and/or ultrasound, it has advantages of minimal invasion and improved targeting and is potentially safe with less invasiveness, less pain, and a lower level of complications compared with conventional resection. For some unresectable disease, cryosurgery can be offered to debulk the tumor and increase sensitivity to chemotherapy or radiotherapy43 or performed simultaneously with other modalities, such as chemotherapy, systemic radiotherapy, or partial irradiation by 125I seed implantation. Even though it has been observed to have some advantages as adjuvant therapy for PC, more advanced researches on cryosurgery are still needed for effect analysis and detailed mechanism explanation.
Randomized Clinical Trials
Nevertheless, cryosurgery has shown promising results in many clinical researches; multicenter randomized clinical trials are still needed for further proof of its benefits in each aspect of progression-free survival, overall survival, pain control, and so on, when performed alone or combined with other modalities.
Pain Management
It has been a concern and has been greatly discussed whether heat or cold ablation would play a better role in pain management. The effect of pain relief from cryosurgery has been demonstrated in treatment for various diseases including PC, liver cancer, sarcoma, head and neck tumor, and so on,42,44–46 and it led to a significantly lower dose of fentanyl (165.0 μg [radiofrequency {RF} group] vs 75.0 μg [cryoablation group], P < 0.001) and midazolam (2.9 mg [RF group] vs 1.6 mg [cryoablation group], P = 0.026) when compared with RFA.47 Irreversible electroporation (IRE), another rising physical technique of “heat ablation,” has recently been proposed to treat locally advanced PC. It takes effect with high-voltage current of ultrashort electrical pulses to create multiple microscopic holes within the cell membrane, resulting in irreversible cell damage in the end. This minimally invasive intervention also showed potential advantage in pain control after the procedure.48,49 A recent initial clinical study compared pain scores in 48 patients with PC in the IRE group and cryosurgery group and found that there was no significant difference in the mean pain score (4.95 [IRE] vs versus 4.85 [cryosurgery]) and 24-hour total hydromorphone use between them.50 In this sense, cryosurgery may be equal to IRE and better than RF on relieving pain, but a larger and multicenter analysis is needed.
Optimize Process
To pursue an optimal operation standard, all suggestions in this article were raised based on a large amount of experiments and clinical experiences, for example, the temperature and duration of freeze and thaw, the number of probes and their distribution, preprocedure preparation, postprocedure nursing, and so on. However, this preliminary proposal is presented for further discussion and to provide some hints for future research.
Molecular Biological Mechanism
Our previous experiment illustrated that all cellular ultrastructure was destroyed, and only nuclei with broken crests and degranulated mitochondria and rough endoplasmic reticulum observable remained after 2 cycles of freeze-thaw. There was a significant increase of serum AMY level in reaction to cryoablation. For future studies, it is valuable to find out more about changes in the microenvironment with cryoablation intervention and whether it could introduce drugs into tumor cells and what the mechanisms are.
Immune Response
It has been proven that cryoablation could stimulate antigens on the surface of tumor cells, hence to activate and enhance host immune function, which is so-called cryoimmunity.51–53 For a better explanation of this new concept, much more work should be done, and this could probably provide a better understanding of the delayed necrosis of cells after cryoablation and its better use in combination with chemotherapy or gene-targeted therapy.24
Combination With Other Modalities
We found, for example, that previous application of chemical reagents, such as anhydrous alcohol injection, would increase the area of freezing within the tumor, which promotes ablative effect in large tumors when it is combined with transcatheter arterial chemoembolization. Similar questions are asked for further exploration to achieve optimal regimens for various diseases.
ACKNOWLEDGMENT
The authors thank Weijun Fan (Cancer Hospital, Sun-Yat Sen University), Yize Hu (Second Affiliated Hospital, Guangzhou Medical College), Zhiqiang Meng (Cancer Hospital, Fudan University), Ping Xue (Guangzhou Medical Collage Affiliated Second Hospital), Wuwei Yang (Beijing 307 Hospital of PLA), and Yanfang Zhang (Shenzhen People’s Hospital) for their helpful discussion, comments, and constant support and encouragement during this work.
Footnotes
L.H. and L.N. contributed equally to this work and share first authorship.
This work was supported by a grant from the National Clinical Key Specialty on Cancer, China (ZX001232).
The authors declare no conflict of interest.
REFERENCES
- 1.Cancer Facts & Figures 2015. American Cancer Society Web site. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html. Accessed February 26, 2017.
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 6.Brunner TB, Seufferlein T. Pancreatic cancer chemoradiotherapy. Best Pract Res Clin Gastroenterol. 2016;30:617–628. [DOI] [PubMed] [Google Scholar]
- 7.Rossi ML, Rehman AA, Gondi CS. Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. 2014;20:11142–11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peddi PF, Lubner S, McWilliams R, et al. Multi-institutional experience with FOLFIRINOX in pancreatic adenocarcinoma. JOP. 2012;13:497–501. [DOI] [PubMed] [Google Scholar]
- 9.Abrams MJ, Rakszawski K, Vasekar M, et al. Recent advances in pancreatic cancer: updates and insights from the 2015 annual meeting of the American Society of Clinical Oncology. Therap Adv Gastroenterol. 2016;9:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korpan NN, Hochwarter G, Sellner F. Cryosurgery of Pancreatic Cancer. In: XXX World Congress of the International College of Surgeons: Kyoto (Japan), November 25–26, 1996. Bologna, Italy: Monduzzi editore, International Proceedings Division; 1996:97. [Google Scholar]
- 11.Korpan NN. Cryosurgery: clinical observations on metastatic liver cancer. In: Advanced Course on Head and Neck Tumours. Chiesa F, Scully C, eds. Milan, Italy, LU: European School of Oncology; 1994:97. [Google Scholar]
- 12.Tanaka S. Cryosurgical treatment of advanced breast cancer. Skin Cancer. 1995;10:9–18. [Google Scholar]
- 13.Korpan NN. Hepatic cryosurgery for liver metastases. Long-term follow-up. Ann Surg. 1997;225:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korpan NN. (Ed). Basics of Cryosurgery. Vienna, Austria: Springer; 2001. [Google Scholar]
- 15.Xu K, Korpan NN, Niu L. (Eds). Modern Cryosurgery for Cancer. Singapore: World Scientific Publishing; 2012. [Google Scholar]
- 16.NCCN clinical practice guideline in oncology: hepatobiliary cancers. NCCN Web site. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#hepatobiliary. Accessed February 26, 2017. [DOI] [PMC free article] [PubMed]
- 17.NCCN clinical practice guideline in oncology: kidney cancer. NCCN Web site. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#kidney. Accessed February 26, 2017. [DOI] [PubMed]
- 18.NCCN clinical practice guideline in oncology: non–small cell lung cancer. NCCN Web site. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl. Accessed February 26, 2017.
- 19.NCCN clinical practice guideline in oncology: prostate cancer. NCCN Web site. Available at: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#prostate. Accessed February 26, 2017.
- 20.Korpan NN, Zemskov VS, Skiba VV. Cryosurgery for Liver and Pancreas Carcinoma. In: II National Conference on Mechanisms of Cryogenic Injury and Cryogenic Protection of Biological Objects: 1984. Kharkov, USSR: Institute for Problems of Cryobiology and Cryomedicine; 1984:65–69. [Google Scholar]
- 21.Zemskov VS, Mus’kin IuN, Korpan NN, et al. [Cryogenic action in abdominal surgery]. [Article in Russian]. Vestn Khir Im I I Grek. 1985;135:141–144. [PubMed] [Google Scholar]
- 22.Patiutko I, Barkanov AI, Kholikov TK, et al. [The combined treatment of locally disseminated pancreatic cancer using cryosurgery]. [Article in Russian]. Vopr Onkol. 1991;37:695–700. [PubMed] [Google Scholar]
- 23.Kovach SJ, Hendrickson RJ, Cappadona CR, et al. Cryoablation of unresectable pancreatic cancer. Surgery. 2002;131:463–464. [DOI] [PubMed] [Google Scholar]
- 24.Korpan NN. Cryosurgery: ultrastructural changes in pancreas tissue after low temperature exposure. Technol Cancer Res Treat. 2007;6:59–67. [DOI] [PubMed] [Google Scholar]
- 25.Luo XM, Niu LZ, Chen JB, et al. Advances in cryoablation for pancreatic cancer. World J Gastroenterol. 2016;22:790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. [DOI] [PubMed] [Google Scholar]
- 27.Xu KC, Niu LZ, Hu YZ, et al. A pilot study on combination of cryosurgery and (125)iodine seed implantation for treatment of locally advanced pancreatic cancer. World J Gastroenterol. 2008;14:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bala MM, Riemsma RP, Wolff R, et al. Cryotherapy for liver metastases. Cochrane Database Syst Rev. 2013:CD009058. [DOI] [PubMed] [Google Scholar]
- 29.Niu L, He L, Zhou L, et al. Percutaneous ultrasonography and computed tomography guided pancreatic cryoablation: feasibility and safety assessment. Cryobiology. 2012;65:301–307. [DOI] [PubMed] [Google Scholar]
- 30.NCCN clinical practice guideline in oncology: pancreatic adenocarcinoma. NCCN Web site. Available at: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed February 26, 2017.
- 31.Xu K, Zhou L, Liang B, et al. Safety and accuracy of percutaneous core needle biopsy in examining pancreatic neoplasms. Pancreas. 2012;41:649–651. [DOI] [PubMed] [Google Scholar]
- 32.Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu D, Niu L, Mu F, et al. The experimental study for efficacy and safety of pancreatic cryosurgery. Cryobiology. 2010;60:281–286. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Zhou L, Chen J, et al. Pancreatic head cryosurgery: safety and efficiency in vivo–a pilot study. Pancreas. 2012;41:1285–1291. [DOI] [PubMed] [Google Scholar]
- 35.Shyn PB, Oliva MR, Shah SH, et al. MRI contrast enhancement of malignant liver tumours following successful cryoablation. Eur Radiol. 2012;22:398–403. [DOI] [PubMed] [Google Scholar]
- 36.Kawamoto S, Permpongkosol S, Bluemke DA, et al. Sequential changes after radiofrequency ablation and cryoablation of renal neoplasms: role of CT and MR imaging. Radiographics. 2007;27:343–355. [DOI] [PubMed] [Google Scholar]
- 37.Henzler T, Shi J, Jafarov H, et al. Functional CT imaging techniques for the assessment of angiogenesis in lung cancer. Transl Lung Cancer Res. 2012;1:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiro J, Maintz D, Persigehl T. [Response criteria for malignant melanoma: RECIST and irRC]. [Article in German]. Radiologe. 2015;55:127–135. [DOI] [PubMed] [Google Scholar]
- 39.Bernhard J, Dietrich D, Glimelius B, et al. Clinical benefit response in pancreatic cancer trials revisited. Oncol Res Treat. 2014;37:42–48. [DOI] [PubMed] [Google Scholar]
- 40.Niu LZ, He LH, Zhou L, et al. [Percutaneous cryoablation and (125)I seed implantation combined with chemotherapy for advanced pancreatic cancer: report of 67 cases]. [Article in Chinese]. Zhonghua Zhong Liu Za Zhi. 2012;34:940–944. [DOI] [PubMed] [Google Scholar]
- 41.Niu L, Chen J, He L, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas. 2013;42:1143–1149. [DOI] [PubMed] [Google Scholar]
- 42.Niu L, Wang Y, Yao F, et al. Alleviating visceral cancer pain in patients with pancreatic cancer using cryoablation and celiac plexus block. Cryobiology. 2013;66:105–111. [DOI] [PubMed] [Google Scholar]
- 43.Homasson JP, Pecking A, Roden S, et al. Tumor fixation of bleomycin labeled with 57 cobalt before and after cryotherapy of bronchial carcinoma. Cryobiology. 1992;29:543–548. [DOI] [PubMed] [Google Scholar]
- 44.Xin'an L, Jianying Z, Lizhi N, et al. Alleviating the pain of unresectable hepatic tumors by percutaneous cryoablation: experience in 73 patients. Cryobiology. 2013;67:369–373. [PubMed] [Google Scholar]
- 45.Fan WZ, Niu LZ, Wang Y, et al. Initial experience: alleviation of pain with percutaneous CT-guided cryoablation for recurrent retroperitoneal soft-tissue sarcoma. J Vasc Interv Radiol. 2016;27:1798–1805. [DOI] [PubMed] [Google Scholar]
- 46.Guenette JP, Tuncali K, Himes N, et al. Percutaneous image-guided cryoablation of head and neck tumors for local control, preservation of functional status, and pain relief. AJR Am J Roentgenol. 2017;208:453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allaf ME, Varkarakis IM, Bhayani SB, et al. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation—initial observations. Radiology. 2005;237:366–370. [DOI] [PubMed] [Google Scholar]
- 48.Wagstaff PG, de Bruin DM, Zondervan PJ, et al. The efficacy and safety of irreversible electroporation for the ablation of renal masses: a prospective, human, in-vivo study protocol. BMC Cancer. 2015;15:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tasu JP, Vesselle G, Herpe G, et al. Irreversible electroporation for locally advanced pancreatic cancer. Diagn Interv Imaging. 2016;97:1297–1304. [DOI] [PubMed] [Google Scholar]
- 50.Li J, Sheng S, Zhang K, et al. Pain analysis in patients with pancreatic carcinoma: irreversible electroporation versus cryoablation. Biomed Res Int. 2016;2016:2543026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korpan NN. Cancer cryoimmunology: 30-year-long own experience. In: Presented at the 17th World Congress of the International Society of Cryosurgery: 2013. Bali, Indonesia: 2013. [Google Scholar]
- 52.Forest V, Peoc’h M, Ardiet C, et al. In vivo cryochemotherapy of a human lung cancer model. Cryobiology. 2005;51:92–101. [DOI] [PubMed] [Google Scholar]
- 53.Joosten JJ, Muijen GN, Wobbes T, et al. In vivo destruction of tumor tissue by cryoablation can induce inhibition of secondary tumor growth: an experimental study. Cryobiology. 2001;42:49–58. [DOI] [PubMed] [Google Scholar]


