Abstract
Objectives
Baicalein is a Chinese traditional medicine that inhibits tumor migration and growth. Pancreatic neuroendocrine tumors (pNETs) have a high incidence in China, but there are still no effective treatments. The aim of our study was to investigate whether baicalein could inhibit pNETs.
Methods
In vitro, we used BON1—a cell line of pNETs—to analyze the apoptosis and migration and invasion after baicalein treatment via flow cytometry and Western blot. In vivo, we used a xenograft tumors model to evaluate the size of tumors after baicalein treatment. Western blot was used to analyze the expression of apoptosis and migration-related protein.
Results
In vitro, the Cell Counting Kit 8 assay showed that baicalein decreased BON1 viability, and flow cytometry demonstrated that baicalein induced BON1 apoptosis and protein changes. In addition, baicalein inhibited BON1 migration and invasion as shown via a Transwell assay. In vivo, baicalein inhibited tumor growth and migration and also increased apoptosis-related protein expression.
Conclusions
Baicalein could increase caspase-3 and Bax expression and decrease survivin and Bcl-2 to induce apoptosis. It inhibits migration and invasion by decreasing expression of vascular endothelial growth factor and matrix metalloproteinases 2 and 9.
Key Words: apoptosis, baicalein, BON1, migration, pancreatic neuroendocrine tumors, survivin
Pancreatic neuroendocrine tumors (pNETs) are a group of rare neoplasms originating from the endocrine pancreas with increased incidence and high risk. In the United States, there are 1000 cases reported per year.1 In a retrospective review of patients with pNETs in China and the United States, insulinomas are the most common tumors in Chinese patients relative to American patients. Nonfunctional tumors are significantly larger in Chinese patients than in US patients and are mostly located in the head/neck.2 However, pNETs still have long survival times and a much better prognosis than exocrine adenocarcinomas of the pancreas.1,3 CgA is a marker of pNET, and it is increased in the serum of pNETs patients4 and associated with tumor differentiation, disease progression, and treatment efficiency.5 Surgical resection and streptozocin-based chemotherapy are the most effective strategies for pNETs. However, these conventional therapeutic methods are also quite toxic with many adverse effects.
Survivin is a unique member of the inhibitor of apoptosis protein family that plays a critical role in regulating apoptosis and cell division.6 Its high expression has been shown in many tumors, with low levels in most normal tissues.7,8 Survivin blocks apoptosis by inhibiting the effector caspases9; therefore, survivin activity is strongly activated in tumor cells. Moreover, high levels of survivin are associated with cancer progress, drug resistance, and patientsurvival.10 Survivin is overexpressed in pNETs, and it can be used as a prognosis marker.11 Thus, survivin is an attractive molecular target for cancer gene therapy.
Baicalein (5,6,7-trihydroxy-2-phenyl-4H-1-benzopyran-4-one,C15H10O5) is a traditional Chinese herbal medicine that is extracted from the roots of Scutellaria baicalensis. Baicalein has been reported to have potent antitumor, anti-inflammation, and antioxidation properties. Growing evidence has shown its antitumor activity in a variety of human cancers by inducing cancer cell apoptosis12–14 and suppressing migration and invasion.15–18 Baicalein can also be used as chemotherapy without severe adverse effects because it does not cause chromosomal alterations or mutagenesis.19 Baicalein is also affordable and is a promising medicine for cancer treatment. However, to the best of our knowledge, there are no reports about the effects of baicalein on pNETs. Therefore, we tested the effects of treatment with baicalein on pNETs and investigated the underlying mechanism. Our results demonstrated that baicalein could induce pNET cell apoptosis, which will inhibit its migration, invasion, and tumor growth.
MATERIALS AND METHODS
Cell Culture
BON1 cells were preserved in our laboratory and grown in Dulbecco modified Eagle medium (Hyclone, Tauranga, New Zealand) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, Mass), 100 U/mL penicillin, and 100 μg/mL streptomycin (Hyclone) in a 5% CO2 humidified incubator at 37°C.
Reagents
Baicalein (purity 99.8%) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Monoclonal antibodies to survivin, caspase-3, vascular endothelial growth factor (VEGF), matrix metalloproteinase 2 (MMP-2), MMP-9, Bax and Bcl-2 were purchased from Abcam Inc (Cambridge, Mass).
Cell Viability Assay
Cell viability assay was measured with Cell Counting Kit 8 (CCK-8; Beyotime Biotechnology, Inc, Beijing, China). Briefly, cells were seeded in a 96-well microplate, and baicalein was added at different concentrations. Twenty-four hours after stimulation, the cell viability was measured by CCK-8 according to the manufacturer’s instructions.
Apoptosis Assays
Apoptotic cells were determined with an annexin V–fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Biosciences, San Jose, Calif) according to the manufacturer’s instructions. Briefly, cells were incubated with baicalein for 24 and 48 hours. Apoptotic cells were analyzed with flow cytometry.
Cell Migration Assay
Briefly, approximately 1 × 105 cells were seeded onto a Matrigel-coated polycarbonate membrane insert in Transwell chambers (Costar, Cambridge, Mass) and maintained in serum-free media. Meanwhile, different concentrations of baicalein were added to the treatment groups; untreated cells served as controls. Twenty-four hours after stimulation at 37°C, migrated cells were fixed by 4% paraformaldehyde and stained with 0.1% crystal violet. Five fields of view were collected for each chamber at random, and the number of cells was counted under a microscope.
Western Blot
Cells were lysed by radio immunoprecipitation assay, and the lysate was centrifuged at 12,000g at 4°C for 20 minutes. Protein concentration was quantified using the BCA protein assay kit (Qiagen, Valencia, Calif). Protein was separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. After blocking with 5% nonfat milk at room temperature for 1 hour, the membrane was incubated with primary antibodies diluted in blocking solution overnight at 4°C. After washing the blot in tris-buffered saline with Tween 3 times, secondary antibodies conjugated with horseradish peroxidase (Abcam) were incubated for 2 hours at room temperature. After 3 rounds of washing in TBST, the protein bands were detected via an enhanced chemiluminescence detection system.
Animals and Xenografts
Female nude mice were purchased from Nanjing Medical University Animal Center. Animal care followed Nanjing Medical University guidelines, and research was approved by Nanjing Medical University Animal Center. For tumor implantation, 1 × 106 BON1 cells were administrated into the head of the pancreas. Baicalein treatment began 2 weeks later, and the treatment group received baicalein (10 mg/kg per day) intrahepatically for 7 weeks.20 After 9 weeks, the mice were killed, and the primary tumors were collected. Tumor volume was then calculated as length × width2 / 2.21
Statistical Analysis
All data are expressed as the mean ± SEM. Statistical analysis was performed with GraphPad Prism Vision 5.0 (GraphPad Software, La Jolla, Calif). The significance of differences between experimental groups and controls were assessed using Student’s t-test and 1-way analysis of variance. P < 0.05 indicates a statistically significant difference.
RESULTS
Baicalein Inhibited BON1 Viability and Down-regulated Expression of Survivin in BON1 Cells
To determine the effect of baicalein on BON1 cells, cells were incubated with various concentrations (50–150 μM) of baicalein for 24 hours, and cell viability was measured by CCK-8. Figure 1A shows that cell viability was inhibited by baicalein in a concentration-dependent manner. To exclude the cytotoxicity of high-dose baicalein, we used H9 human T lymphocytes as controls. The H9 cell viability was measured with CCK-8 via the same treatment as BON1. Low concentrations of baicalein did not affect the viability of H9, but high concentrations (125–150 μM) of baicalein inhibited the viability of H9 cells (Fig. 1B). At 100 μM, the viability of BON1 was clearly inhibited, but there was no effect on H9 cells. To understand whether baicalein could depress the expression of survivin on BON1, we stimulated BON1 with 100 μM baicalein for 12, 24, and 48 hours. Next, a Western blot was used to analyze the survivin expression, and the results confirmed that the expression of survivin was depressed in a time-dependent manner (Fig. 1C). Quantitative analysis showed that the level of survivin was decreased significantly 12 hours after stimulation; the survivin was almost completely inhibited 24 and 48 hours after stimulation (Fig. 1C).
FIGURE 1.

Baicalein inhibits survivin expression on BON1 cells. A, Cell Counting Kit 8 assay was used to detect the toxicity of baicalein in BON1 cells; 50 to 150 μM baicalein reduced the cell viability. ***P < 0.001 compared with the control group. B, Cell Counting Kit 8 assay was used to detect the toxicity of baicalein on H9 cells; 125 and 150 μM baicalein reduced the cell viability (%). **P < 0.01, ***P < 0.001 compared with the control group. C, Western blotting of survivin levels 12, 24, and 48 hours after baicalein treatment on BON1. (***P < 0.001). Con indicates control.
Baicalein Promoted BON1 Cell Apoptosis
To determine the effect of baicalein-induced apoptosis in BON1 cells, BON1 cells were stimulated by 100 μM baicalein for 3, 6, 12, 24, and 48 hours. These were then stained with annexin V–FITC/propidium iodide and determined by flow cytometry (Fig. 2A). The analysis was repeated twice, and the result showed that the apoptotic rates were gradually increased with time (Fig. 2A). Next, to confirm the proapoptotic role of baicalein in pNETs, we analyzed the expression of caspase-3 after baicalein stimulation. The Western blot result demonstrated that caspase-3 levels increased with stimulation time (Fig. 2B).
FIGURE 2.
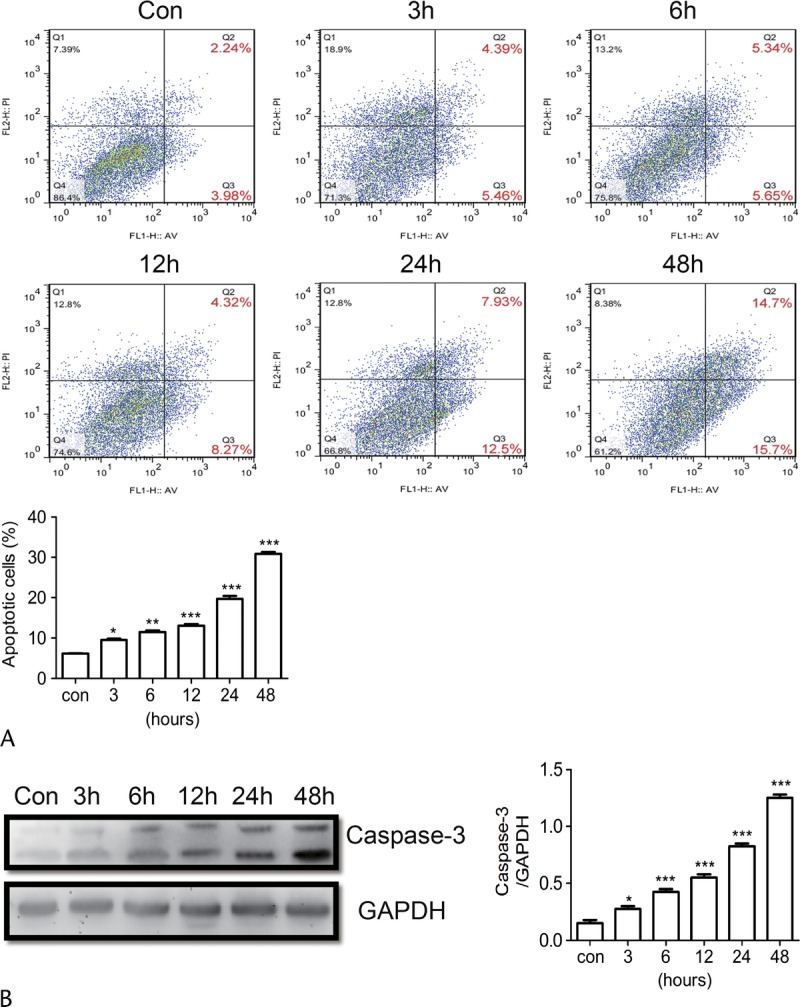
Baicalein promotes BON1 apoptosis. A, BON1 was stimulated with 100 μM Baicalein for different time, and cells were stained with annexin V–FITC and propidium iodide then analyzed by flow cytometry. B, The expression of activated caspase-3 was detected with Western blot, and the results are also presented. *P < 0.05, **P < 0.01, ***P < 0.001. Con indicates control.
Baicalein Inhibited BON1 Cell Invasion
A Transwell assay was used to evaluate the invasion ability of BON1 cells after baicalein treatment. Our results showed that invasion ability of BON1 was inhibited by baicalein in a time-dependent manner. Twenty-four hours after treatment by baicalein, invasion of 50% BON1 was inhibited; 48 hours after treatment, most cell invasion was inhibited (Fig. 3A). To further understand the mechanism of baicalein inhibition on BON1 cells, a Western blot was used to examine the variation of protein expression. The levels of Bcl-2 VEGF, MMP-2, and MMP-9 all decreased with time after baicalein treatment. However, the level of Bax was increased after baicalein treatment (Fig. 3B). These results indicated that baicalein inhibited BON1 invasion by down-regulating the expression of VEGF, MMP-2, and MMP-9, which confirmed that baicalein induced BON1 apoptosis via Bcl-2 and Bax pathways.
FIGURE 3.
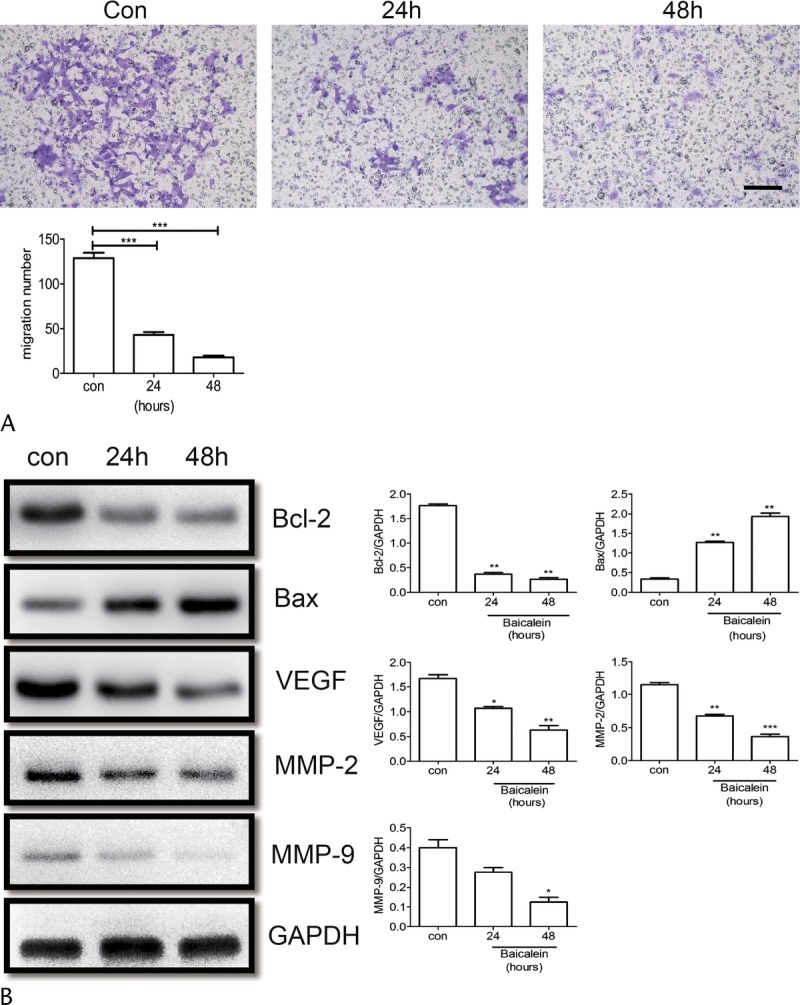
Baicalein inhibited BON1 migration and invasion. A, Migration activity of BON1 treated with 100 μM baicalein for 24 and 48 hours was measured by Transwell assay. Cell migration number is presented. B, The protein levels of Bax, Bcl-2, VEGF, MMP-2, and MMP-9 were analyzed by Western blot, and quantification assay of Western blot results is also presented. *P < 0.05, **P < 0.01, ***P < 0.001. Con indicates control.
The Anti-pNET Effect of Baicalein In Vivo
To determine whether baicalein could inhibit pNET in vivo, mice treated with baicalein at10 mg/kg per day for 7 weeks had decreased tumor growth (Fig. 4A). The tumor volume of the control group (mean ± SEM) was 173.7 ± 5.426 mm3, and that of the baicalein-treated group was 24.83 ± 3.005mm3. The tumor volumes of baicalein treatment decreased significantly (P < 0.001). Western blot analysis demonstrated that Bcl-2 was inhibited, and Bax increased. The expression of survivin was obviously down-regulated (P < 0.05) relative to the untreated group. This indicated that baicalein promoted pNET apoptosis in vivo. We also used Western blotting to analyze protein expression of MMP-2 and MMP-9 in mice following baicalein treatment. Our results showed that baicalein decreased MMP-2 and MMP-9. In summary, these data suggest that baicalein is a potential tool to induce pNET apoptosis and inhibit migration and invasion (Fig. 4C).
FIGURE 4.
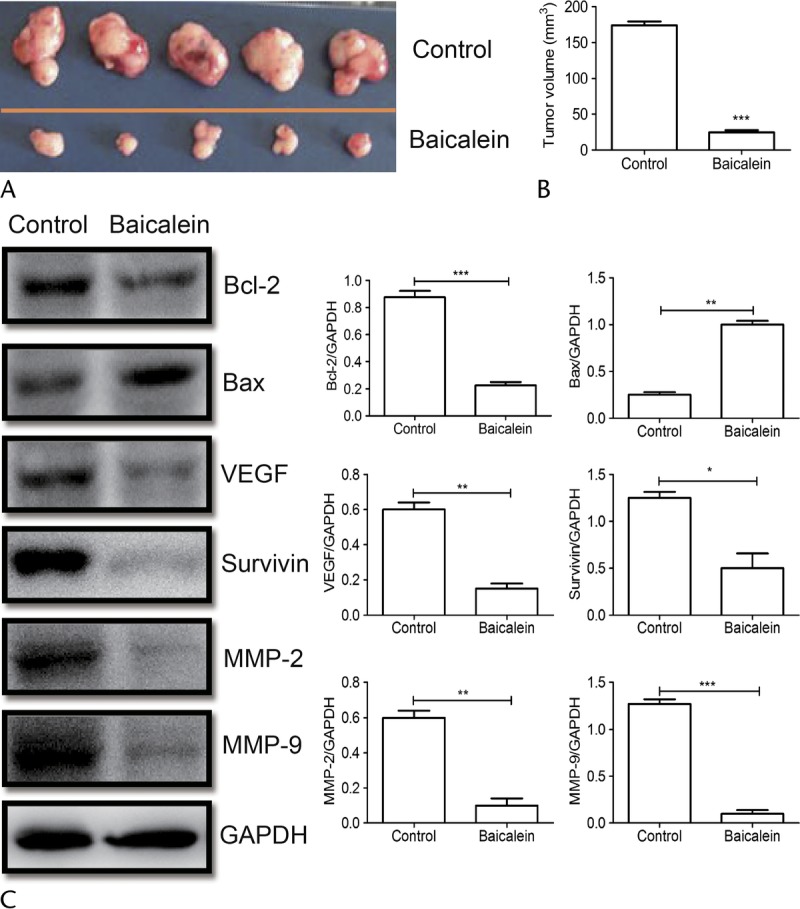
Baicalein abolished tumorigenesis of pNETs in mice. A, BON1 xenograft tumors from the treatment experiment. Mice were killed 7 weeks after baicalein treatment. B, The tumor volumes were calculated according to the following formula: volume = length × width2 / 2. C, The protein obtained from primary tumors in mice with or without baicalein treatment. The levels of Bax, Bcl-2, VEGF, survivin, MMP-2, and MMP-9 were analyzed by Western blot, and quantification assay of Western blot results is also presented. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Recently, baicalein was shown to be a bioactive flavonoid from Scutellaria radix that can induce tumor cell apoptosis and inhibit its migration.22–24 Various studies demonstrated that 15 to 50 μM baicalein could promote pancreatic cancer cell apoptosis via down-regulation of the antiapoptotic protein Mcl-1, and 60 to 80 μM baicalein could inhibit cell growth by attenuating CDC2 kinase activity.12,14 Baicalein was shown to retard tumor cell migration by inhibiting MMP-2 and MMP-9 activity.16,25 However, no studies have yet investigated the effects of baicalein on pNETs. In our study, we explored the mechanisms of baicalein in inhibiting pNETs. To the best of our knowledge, this is the first study on the function and mechanism of action of baicalein in pNETs.
We used flow cytometry and Western blot to demonstrate that baicalein potently induced BON1 cell apoptosis as a model of pNET. The Transwell assay proved that baicalein decreased BON1 migration and invasion. To further confirm the antitumor effects of baicalein, we used an animal xenograft model, which also showed that baicalein could inhibit tumor growth in vivo. In summary, baicalein is a potential medicine for pNETs.
Matrix metalloproteinases are unregulated in some malignant tumors and play critical roles in tumor cell metastasis and invasion.26,27 Matrix metalloproteinases 2 and 9 have important roles in pNET metastasis.28,29 Consistent with these findings, our results also showed that the antimetastatic properties of baicalein were due to lower MMP-2 and MMP-9 expression levels.
The activation of caspase-3 plays a significant role in cell apoptosis including tumor cells. However, most tumor cells can block apoptosis, and thus understanding the mechanisms of apoptosis is critical for the prevention and treatment of various types of cancers. Generally, apoptosis is mediated via an extrinsic pathway (death receptor–mediated pathway) and an intrinsic pathway (mitochondrial-mediated pathway). Bcl-2 is encoded by the Bcl-2 gene and exerts antiapoptosis effects. Bax—the Bcl-2-associated X protein—belongs to the Bcl-2 gene family and promotes apoptosis.30 In our study, baicalein down-regulated the expression of Bcl-2 and up-regulated the Bax expression. This led to the cleavage of caspase-3 and caused BON1 cell apoptosis. Other research has shown that baicalein could not affect the expression of Bax and Bcl-2 in A549 cells in lung cancer strains. However, baicalein could increase the expression of tBID and release cytochrome c into cytosol. This led to apoptosis via a mitochondrial pathway in A549 cells.31
Survivin connects with caspase activation and cell apoptosis through a variety of stimuli.32 Survivin inhibition increases caspase-dependent apoptosis and shows significant antitumor activity in multiple tumor cells in vivo and in vitro.33 In our study, baicalein significantly inhibited the expression of survivin and decreased the expression of survivin-activated caspase-3.
Footnotes
This study was supported through grants from Nanjing Science and Technology Bureau Foundation (201503004, JA15) and Foundation of Standardized Diagnosis and Treatment of Key Diseases (2015, ZD15).
The authors declare no conflict of interest.
REFERENCES
- 1.Chen M, van Ness M, Guo Y, et al. Molecular pathology of pancreatic neuroendocrine tumors. J Gastrointest Oncol. 2012;3:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu LM, Tang L, Qiao XW, et al. Differences and similarities in the clinicopathological features of pancreatic neuroendocrine tumors in China and the United States: a multicenter study. Medicine (Baltimore). 2016;95:e2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–833. [DOI] [PubMed] [Google Scholar]
- 4.Panzuto F, Severi C, Cannizzaro R, et al. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. J Endocrinol Invest. 2004;27:6–11. [DOI] [PubMed] [Google Scholar]
- 5.Nikou GC, Marinou K, Thomakos P, et al. Chromogranin a levels in diagnosis, treatment and follow-up of 42 patients with non-functioning pancreatic endocrine tumours. Pancreatology. 2008;8:510–519. [DOI] [PubMed] [Google Scholar]
- 6.Malcles MH, Wang HW, Koumi A, et al. Characterisation of the anti-apoptotic function of survivin-DeltaEx3 during TNFalpha-mediated cell death. Br J Cancer. 2007;96:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kita A, Nakahara T, Takeuchi M, et al. [Survivin supressant: a promising target for cancer therapy and pharmacological profiles of YM155]. [Article in Japanese]. Nihon Yakurigaku Zasshi. 2010;136:198–203. [DOI] [PubMed] [Google Scholar]
- 8.Ryan BM, O'Donovan N, Duffy MJ. Survivin: a new target for anti-cancer therapy. Cancer Treat Rev. 2009;35:553–562. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S, Debatin KM. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337–346. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed MB, Shehata HH, Moussa M, et al. Prognostic significance of survivin and tumor necrosis factor-alpha in adult acute lymphoblastic leukemia. Clin Biochem. 2012;45:112–116. [DOI] [PubMed] [Google Scholar]
- 11.Ekeblad S, Lejonklou MH, Stålberg P, et al. Prognostic relevance of survivin in pancreatic endocrine tumors. World J Surg. 2012;36:1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther. 2007;6:3039–3048. [DOI] [PubMed] [Google Scholar]
- 13.Jiang RH, Su WC, Liu HF, et al. Opposite expression of securin and γ-H2AX regulates baicalein-induced cancer cell death. J Cell Biochem. 2010;111:274–283. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Chen MC, Pham H, et al. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta. 2011;1813:1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rui X, Yan XI, Zhang K. Baicalein inhibits the migration and invasion of colorectal cancer cells via suppression of the AKT signaling pathway. Oncol Lett. 2016;11:685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu YW, Lin TH, Huang WS, et al. Baicalein inhibits the migration and invasive properties of human hepatoma cells. Toxicol Appl Pharmacol. 2011;255:316–326. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Zhuang M, Peng J, et al. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-β signaling pathway. Mol Med Rep. 2014;10:1999–2003. [DOI] [PubMed] [Google Scholar]
- 18.Wu B, Li J, Huang D, et al. Baicalein mediates inhibition of migration and invasiveness of skin carcinoma through Ezrin in A431 cells. BMC Cancer. 2011;11:527. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Mu J, Liu T, Jiang L, et al. The traditional Chinese medicine baicalein potently inhibits gastric cancer cells. J Cancer. 2016;7:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen K, Zhang S, Ji Y, et al. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One. 2013;8:e72927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholz A, Wagner K, Welzel M, et al. The oral multitarget tumour growth inhibitor, ZK 304709, inhibits growth of pancreatic neuroendocrine tumours in an orthotopic mouse model. Gut. 2009;58:261–270. [DOI] [PubMed] [Google Scholar]
- 22.Chen CH, Huang LL, Huang CC, et al. Baicalein, a novel apoptotic agent for hepatoma cell lines: a potential medicine for hepatoma. Nutr Cancer. 2000;38:287–295. [DOI] [PubMed] [Google Scholar]
- 23.Leung HW, Yang WH, Lai MY, et al. Inhibition of 12-lipoxygenase during baicalein-induced human lung nonsmall carcinoma H460 cell apoptosis. Food Chem Toxicol. 2007;45:403–411. [DOI] [PubMed] [Google Scholar]
- 24.Chang WH, Chen CH, Gau RJ, et al. Effect of baicalein on apoptosis of the human Hep G2 cell line was induced by mitochondrial dysfunction. Planta Med. 2002;68:302–306. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Ling Y, Chen Y, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42–48. [DOI] [PubMed] [Google Scholar]
- 26.Périgny M, Bairati I, Harvey I, et al. Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-11 in the prognosis of death by ovarian cancer. Am J Clin Pathol. 2008;129:226–231. [DOI] [PubMed] [Google Scholar]
- 27.Stack MS, Ellerbroek SM, Fishman DA. The role of proteolytic enzymes in the pathology of epithelial ovarian carcinoma. Int J Oncol. 1998;12:569–576. [DOI] [PubMed] [Google Scholar]
- 28.Shchors K, Nozawa H, Xu J, et al. Increased invasiveness of MMP-9–deficient tumors in two mouse models of neuroendocrine tumorigenesis. Oncogene. 2013;32:502–513. [DOI] [PubMed] [Google Scholar]
- 29.Roy R, Zurakowski D, Wischhusen J, et al. Urinary TIMP-1 and MMP-2 levels detect the presence of pancreatic malignancies. Br J Cancer. 2014;111:1772–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. [DOI] [PubMed] [Google Scholar]
- 31.Kim HJ, Park C, Han MH, et al. Baicalein induces caspase-dependent apoptosis associated with the generation of ROS and the activation of AMPK in human lung carcinoma A549 cells. Drug Dev Res. 2016;77:73–86. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y. A conserved tetrapeptide motif: potentiating apoptosis through IAP-binding. Cell Death Differ. 2002;9:93–95. [DOI] [PubMed] [Google Scholar]
- 33.Carrasco RA, Stamm NB, Marcusson E, et al. Antisense inhibition of survivin expression as a cancer therapeutic. Mol Cancer Ther. 2011;10:221–232. [DOI] [PubMed] [Google Scholar]


