Abstract
Objectives
We previously described adoptive immunotherapy (AIT) with cytotoxic T lymphocytes (CTLs) stimulated by the mucin 1 (MUC1)–expressing human pancreatic cancer cell line YPK-1 (MUC1-CTLs) and demonstrated that MUC1-CTLs might prevent liver metastasis. In the present study, we combined gemcitabine (GEM) and AIT for the treatment of pancreatic cancer.
Methods
A total of 43 patients who underwent radical pancreatectomy received treatment with MUC1-CTLs and GEM. After surgery, MUC1-CTLs were induced and administered intravenously 3 times, and GEM administered according to the standard regimen for 6 months. The patients whose relative dose intensity of GEM was 50% or more and who received 2 or more MUC1-CTL treatments were used as the adequate treatment group (n = 21).
Results
In the adequate treatment group, disease-free survival was 15.8 months, and overall survival was 24.7 months. Liver metastasis was found only in 7 patients (33%), and local recurrence occurred in 4 patients (19%). The independent prognostic factor of long-term disease-free survival on multivariate analysis was the average number of CTLs administered (P = 0.0133).
Conclusions
The combination therapy with AIT and GEM prevented liver metastasis and local recurrence. Moreover, the disease free-survival was improved in patients who received sufficient CTLs.
Key Words: adoptive immunotherapy, pancreatic cancer, MUC1
Pancreatic cancer has a poor prognosis owing to the inability to detect the tumor at an early stage, its high potential for early dissemination, and its relatively poor sensitivity to chemotherapy or radiation therapy. The ratio of overall mortality to incidence is almost 98%. Only a minority of patients present with localized disease that allows for tumor resection with curative intent. However, even after microscopically pathologically complete removal of the tumor (R0), the vast majority of patients relapse within 2 years, leading to a 5-year survival rate of less than 25%.1
In 2005, a large phase III study, Charité Onkologie 001 (CONKO-001), was presented at the American Society of Clinical Oncology Annual Meeting by Oettle et al.2 They compared a gemcitabine (GEM) therapy group with a surgery-only group after macroscopically curative resection of pancreatic cancer, and disease-free survival (DFS) was significantly longer in the GEM group than in the observation group. However, overall survival (OS) did not differ significantly between the GEM and surgery-only groups. Hence, novel therapies are needed.
Immunotherapy has an advantage over radiation and chemotherapies because it can act specifically against the tumor without damaging normal tissue. Mucin 1 (MUC1) is overexpressed in an incompletely glycosylated form in various human cancers.3 We previously reported that MUC1 was expressed in cancer cells from all pancreatic ductal adenocarcinomas and liver metastases, as determined by immunohistochemistry. In contrast, MUC1 was not expressed in specimens from normal pancreas, chronic pancreatitis, or ductal hyperplasia of the pancreas.4 We also found that MUC1 is a tumor-associated antigen that is overexpressed in invasive ductal carcinomas of the pancreas and that MUC1-specific cytotoxic T lymphocytes (CTLs) recognize MUC1 molecules in a human leukocyte antigen (HLA)–unrestricted manner.4
Based on these reports, we developed an adoptive immunity therapy that targets MUC1 antigen. We previously described adoptive immunotherapy (AIT) with CTLs stimulated by a MUC1-expressing human pancreatic cancer cell line YPK-1 (MUC1-CTLs) for unresectable pancreatic cancer.5 Mucin 1-CTLs monotherapy against unresectable pancreatic cancer did not improve prognosis significantly but suppressed development of liver metastasis, and no adverse event was observed. Thus, we performed adjuvant therapy in the postsurgical treatment of pancreatic cancer.5 Although this treatment was safe and decreased the incidence of liver recurrence (only 5.0%), local recurrence remained high (65%) and the 3-year survival was only 19.4%.5
Gemcitabine, which is a standard chemotherapeutic agent for pancreatic cancer,6 is not immunosuppressive and may enhance responses to specific vaccines or immunotherapy administered to activate or support immune responses directed toward driving effector immunity to cancer cells.6,7 To create a more effective therapy for pancreatic cancer, we combined AIT with MUC1-CTLs plus GEM.
Here, we present a preliminary study of AIT plus GEM as adjuvant therapy for resectable pancreatic cancer. We evaluated DFS and OS retrospectively and also compared this study with other previous adjuvant therapy reports.
MATERIALS AND METHODS
Patients
From 2007 to 2013, a total of 44 patients with pancreatic cancer were enrolled in this therapy, and leukapheresis was performed preoperatively. One patient was excluded because dissemination was found during surgery. The remaining 43 patients were treated with the combination of MUC1-CTLs and the standard chemotherapeutic agent, GEM (Table 1). This treatment was approved as “advanced health care” by the Japanese Ministry of Health, Labor, and Welfare (acceptance number: Senn-121-1) and was provided for all patients who could pay the treatment cost; therefore, this study was not registered in the Clinical Trials Registry. This study was approved by the Research Ethics Committee of Yamaguchi University Graduate School of Medicine (IRB number: 1999-05-12) and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before administering this therapy.
TABLE 1.
Patient Characteristics and Treatment Adequacy
Eligibility Criteria
Patients who had been preoperatively diagnosed with resectable pancreatic cancer by clinical and pathological findings were eligible for the study. The criteria for operability were diagnosed by computed tomography and endoscopic ultrasonography, and inclusion criteria are as follows: no tumor involvement of the celiac axis or the superior mesenteric artery; no extra-regional metastasis except for paraaortic lymph node involvement at a level as low as the left renal vein; and no distant metastasis. The location of the primary tumor did not matter. In brief, further eligibility included the following: age, 20 years and older; and adequate hepatic, renal, and bone marrow function (serum creatinine level, <2.0 mg/dL; bilirubin level, <3.0 g/dL; platelet count, ≥75,000/mL; total white blood cell count, ≥3000/mL and ≤15,000/mL). All patients had to have an Eastern Cooperative Oncology Group performance status of 0 to 2 at the time of initial consultation.
Treatment Protocol
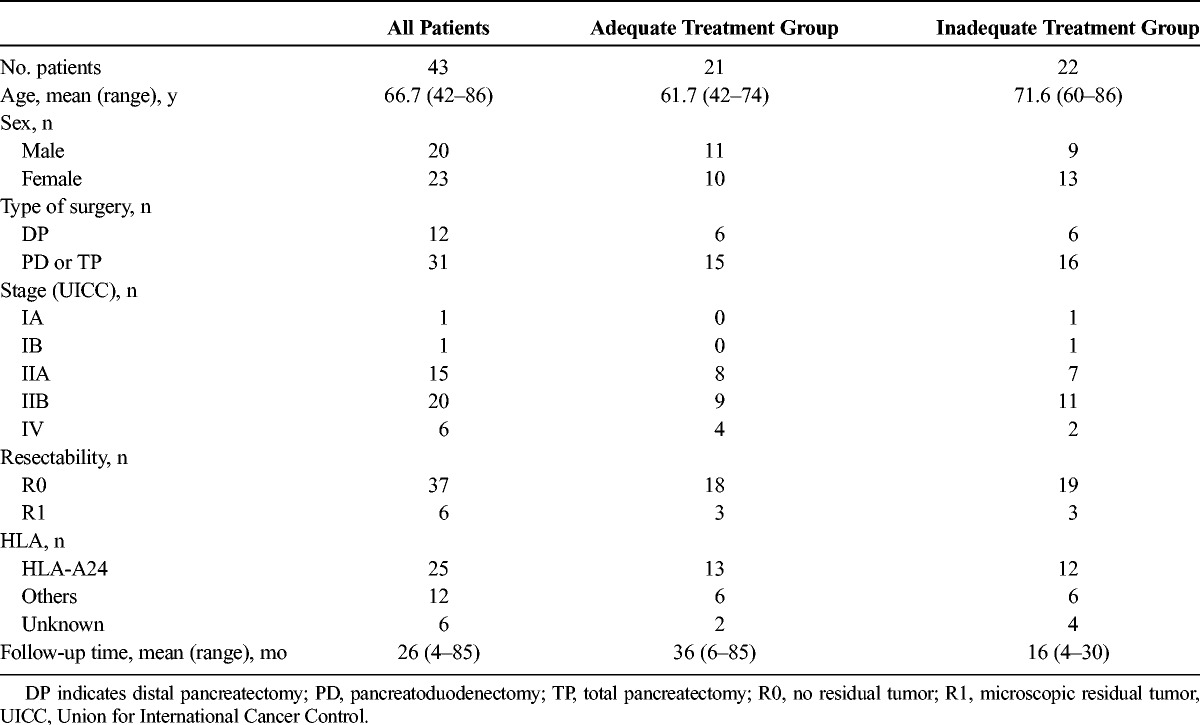
Cytotoxic T lymphocytes were induced before surgery and administered intravenously within a week after surgery. The initial treatment with intravenous GEM (1000 mg2/m) was administered on postoperative day 28. Gemcitabine was administered on days 1, 8, and 15, every 4 weeks for 6 cycles (Fig. 1). If patients developed leukocyte counts of less than 2000/mm3 or more than 12,000/mm3, or platelet counts of less than 75,000/mm3 during chemotherapy, GEM administration was stopped until recovery. When patients developed grade 4 leukopenia or neutropenia, febrile neutropenia or infection with grade 3 leukopenia or neutropenia, a platelet count of less than 25,000/mm3, or nonhematological toxic effects of grade 3 or greater, the dose of GEM was reduced from 1000 mg/m2 to 700 mg/m2. For the second and third CTL treatments, CTLs were re-induced and administered in about 1-month intervals in parallel with GEM. Recurrence was evaluated by computed tomography and/or magnetic resonance imaging every 3 months, or autopsy.
FIGURE 1.
Treatment regimen of postoperative AIT with MUC1-CTL plus GEM for pancreatic cancer. POD indicates postoperative day.
CTL Induction
The human pancreatic cancer cell line used for CTL induction was YPK-1, which was established in our department and expresses HLA-A24. YPK-1 cells were maintained in Dulbecco modified Eagle medium (Nissui Pharmaceutical Co, Ltd, Tokyo, Japan) containing 10% fetal bovine serum (Sigma-Aldrich, St Louis, Mo) in 5% CO2.
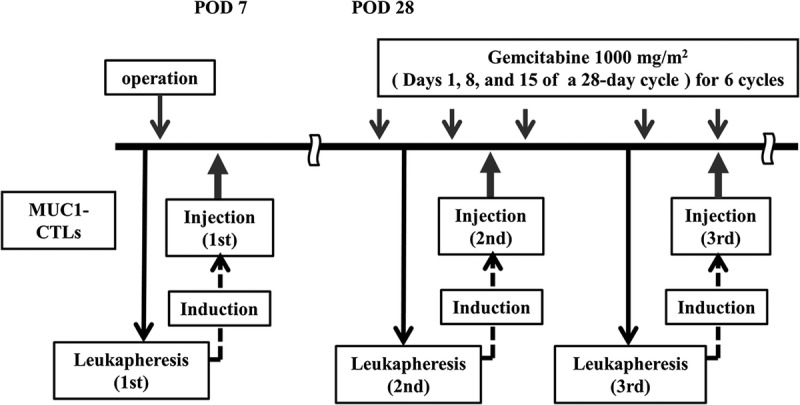
Peripheral blood mononuclear cells (PBMCs) were harvested with the COBE Spectra Apheresis System (COBE BCT, Inc, Lakewood, Colo). Peripheral blood mononuclear cells from 3000 mL of blood were enriched by density gradient centrifugation with Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden). The PBMCs were cultured in serum-free medium (A1M-V) (Gibco, Paisley, Scotland) with the MUC1-expressing pancreatic cancer cell line YPK-1 (HLA-A*24:02) inactivated with 0.2 mg/mL mitomycin C (Kyowa Hakko Kogyo Co, Ltd, Tokyo, Japan). The PBMC to YPK-1 cell ratio was 1000:1. After 3 days of culture, the cells were cultured with 10 Japan reference unit/mL interleukin (IL)-2 (Shionogi Pharmaceutical Co, Tokyo, Japan) in a CO2 incubator for 7 days. Cultures were checked for bacterial contamination. Induced CTLs were washed 3 times with saline, suspended in 100 mL saline, and administered intravenously (Fig. 2).
FIGURE 2.
Induction of MUC1-CTLs. MUC1-CTLs were induced by coculture with YPK-1, a human pancreatic cancer cell line, and then with IL-2. MMC, mitomycin C; rhIL-2, recombinant human interleukin 2; JRU, Japan reference unit.
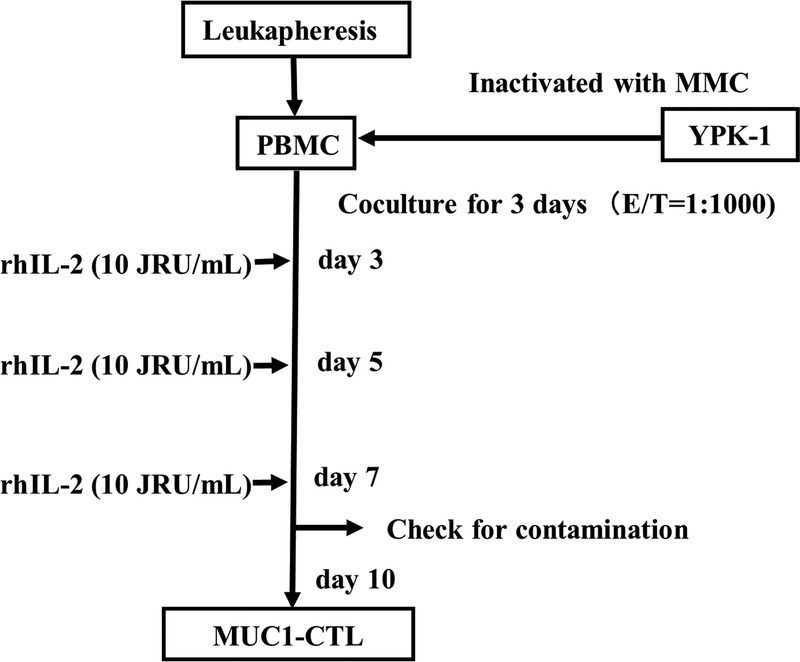
Flowchart of the Study Population and Selection of the Adequate Treatment Group
Because this study is a retrospective cohort study, dose reduction and discontinuation of GEM were not strictly determined. Therefore, the average relative dose intensity (RDI) of GEM in this study is lower than in previous reports,2,8 and not all patients received sufficient numbers of CTLs. Thus, it was difficult to assess the synergic effect of GEM and MUC1-CTL. We selected patients with an RDI of greater than or equal to 50% who received at least 2 MUC1-CTL treatments as the adequate treatment group (n = 21, Fig. 3). A summary of the adequate patient profiles is shown in Table 2. Other patients were classified as the inadequate treatment group (n = 22, Fig. 3).
FIGURE 3.
Flowchart of the study population.
TABLE 2.
Patient Demographics and Clinical Outcomes of the Adequate Treatment Group
Analysis of PBMCs and CTL Subsets
Lymphocyte subsets were analyzed with monoclonal antibodies against surface antigens of human lymphocytes. All monoclonal antibodies were purchased from Coulter Immunology (Hialeah, Fla). Fluorescein isothiocyanate–conjugated anti-CD3 (T3), −CD4 (T4), −CD20 (B1), −CD25 (IL-2R1), −CD56 (NKH-1), −HLA-DR (I2), and −CD11b (MO1) were used. Phycoerythrin-conjugated anti-CD8 (T8) and -TQ1 (cluster unknown) were also used. A 2-color analysis was performed with a combination of TQ1/CD4 (suppressor-inducer T and helper T-cell) and CD8/CD11b (cytotoxic T and suppressor T-cell). Samples were analyzed with an EPICS flow cytometer (Coulter Electronics, Inc, Hialeah, Fla) at a fluorescence excitation wavelength of 488 nm at 200 to 500 mW. For each sample, 5000 lymphocytes were analyzed.
Analysis of Prognostic Factors
We investigated whether parameters such as clinicopatohlogical status, biochemical examination, lymphocyte subset, tumor markers, RDI of GEM, and average number of administrated CTLs are biomarkers of therapeutic effect in the adequate treatment group. The cut-off value of each parameter was determined by using the receiver operating characteristic curve. Using these cutoff values, univariate and multivariate analyses were performed to determine the prognostic factors.
Statistical Analysis
Changes in surface markers were assessed by using the Student’s t-test for paired or unpaired means. A P value of less than 0.05 was considered significant. Values are presented as mean ± standard error (SE). A Kaplan-Meier analysis was used to estimate the cumulative survival. Univariate analysis was performed using the log-rank test. Significant univariate factors were included in a Cox proportional hazards regression model to determine multivariate significance. Statistical analysis was performed by using JMP version 9.0 (SAS Institute Japan, Tokyo, Japan).
RESULTS
Patient Characteristics
From 2007 to 2013, we postsurgically treated 43 patients with resectable pancreatic cancer. Patient characteristics and treatment are detailed in Table 1. Tumor, nodes and metastases stages according to the Union for International Cancer Control for these patients were between IA and IV, and their HLA-A phenotype varied.
Surgical Treatments
Resection was performed in the absence of hematogenous metastases, peritoneal dissemination, gross retroperitoneal tumor infiltration, and complex vascular infiltration. A limited invasion of the portal or superior mesenteric veins was regarded as an indication for portal vein resection. All patients received pancreatoduodenectomy, pylorus preserving pancreatoduodenectomy, or distal pancreatectomy with extended retroperitoneal lymphadenectomy. Five of the 11 patients who underwent resection of the portal vein showed histological invasion. Thirty-seven patients underwent curative resection (R0). Six patients underwent noncurative resection in which microscopic involvement of the resection margin (R1) was found. All patients had histologically confirmed invasive ductal carcinoma. The histological classifications were as follows: well-differentiated adenocarcinoma (n = 4), moderately differentiated adenocarcinoma (n = 31), poorly differentiated adenocarcinoma (n = 6), adenosquamous carcinoma (n = 1), and anaplastic carcinoma (n = 1).
CTL Treatments
Thirty-two patients received 3 CTL treatments; 6 patients received 2 treatments; and 3 patients received 1 treatment. The total number of CTLs administered was between 2.0 × 108 and 1.7 × 109. The CTL treatment was completed within 2 months after surgery for 5 patients, within 3 months after surgery for 10 patients, within 4 months after surgery for 22 patients, and between 4 and 15 months after surgery for 7 patients.
GEM Treatments
For administration of GEM, 4 patients (10.3%) discontinued treatment within 1 cycle, and 15 patients (38.5%) completed the scheduled treatment. The reasons for withdrawal from treatment included adverse events (23 patients), surgical complications (1 patient), and patient preference (4 patients). The dose of GEM was decreased in 18 patients because of hematological toxicity. The median number of cycles was 3, and the median number of GEM doses was 8. The median dose intensity of GEM was 377 mg/m2 per week, and the median RDI was 50.8%. The median (range) time from surgery to the start of chemotherapy was 36 days (22–183 d).
Clinical Outcomes
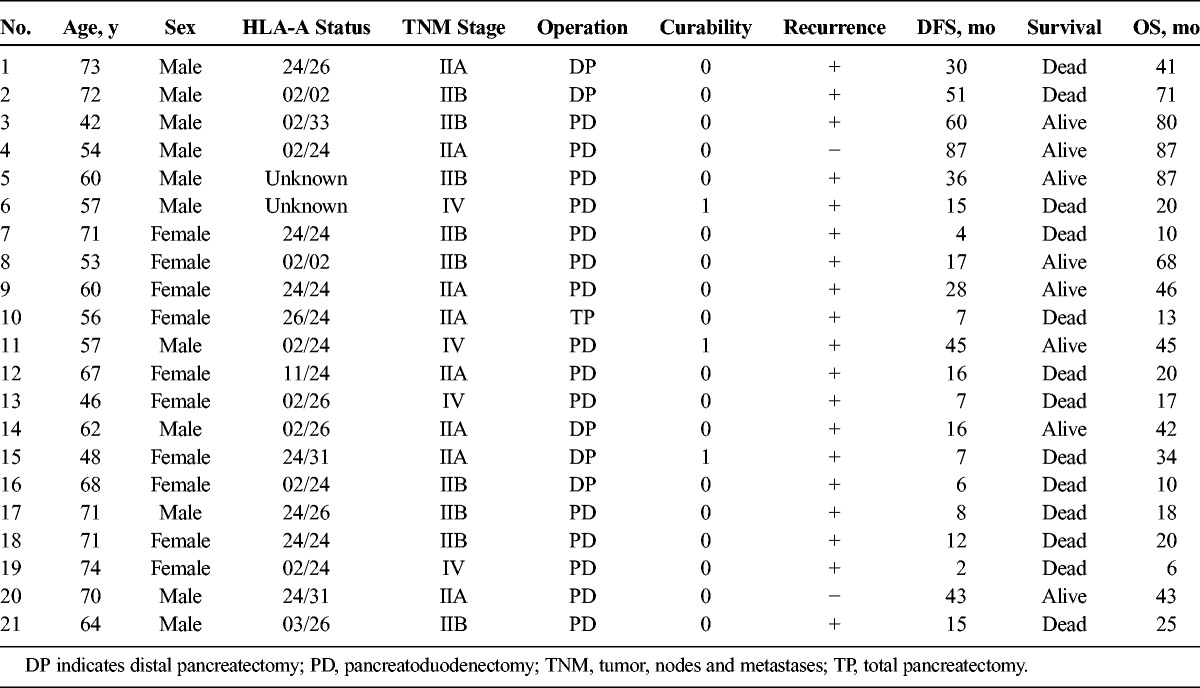
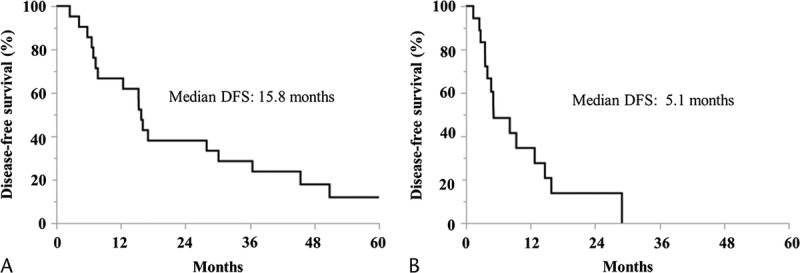
The median DFS and OS of the adequate treatment group were 15.8 months and 24.7 months, respectively, and the median DFS and OS of the inadequate treatment group were 5.1 months and 14.7 months, respectively (Figs. 4 and 5).
FIGURE 4.
Kaplan-Meier curves for DFS. A, The adequate treatment group. B, The inadequate treatment group.
FIGURE 5.
Kaplan-Meier curves for OS. A, The adequate treatment group. B, The inadequate treatment group.
Safety
During the entire treatment period, no adverse event was observed arising from MUC1-CTLs administration such as rash, fever, or autoimmune reactions.
Further Analysis in the Adequate Treatment Group
We have further analyzed in patients whose RDI of GEM was greater than or equal to 50% and who received at least 2 MUC1-CTL treatments as the adequate treatment group (Fig. 3). Average RDI of the adequate treatment group was 72%, and the average number of MUC1-CTL treatments was 2.7. A summary of patient profiles in the adequate treatment group is shown in Table 2.
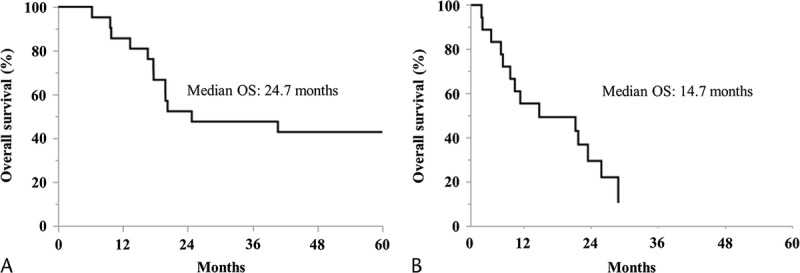
The major grades 3 and 4 adverse events, according to the Common Terminology Criteria for Adverse Events, are summarized in Table 3. The most common grade 3 or 4 hematologic adverse event was leukocytopenia (35%). Nonhematologic adverse events of grade 3 or 4 were not seen. No AIT-related adverse events such as rash, fever, chills, or injection site reaction were observed. There was no clinical or radiological evidence of autoimmune reactions in any of the patients.
TABLE 3.
Grades 3 and 4 Adverse Events in the Adequate Treatment Group (n = 21)
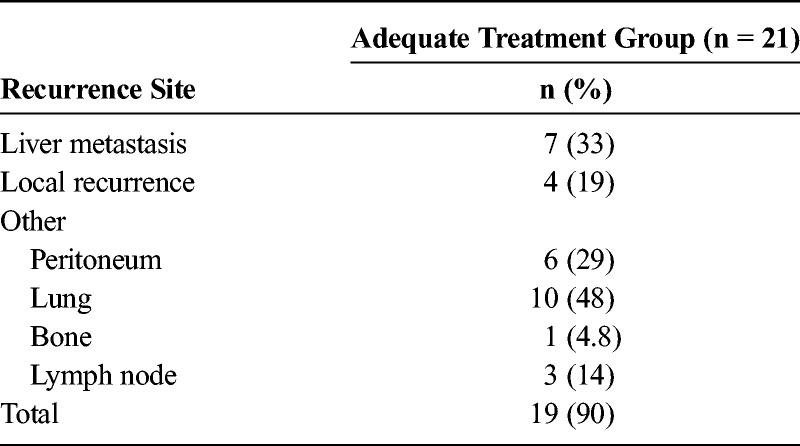
The 1-, 2-, 3-, and 5-year survival rates of the adequate treatment group were 86.3%, 54.6%, 49.6%, and 44.1%, respectively. Liver metastasis was found in 7 patients (33%), and local recurrence was found in 4 patients (19%) (Table 4).
TABLE 4.
Recurrence Sites in the Adequate Treatment Group
There was no significant difference in OS between the R0 group and R1 group [median survival time; 32.7 mo vs 19.8 mo (P = 0.15)].
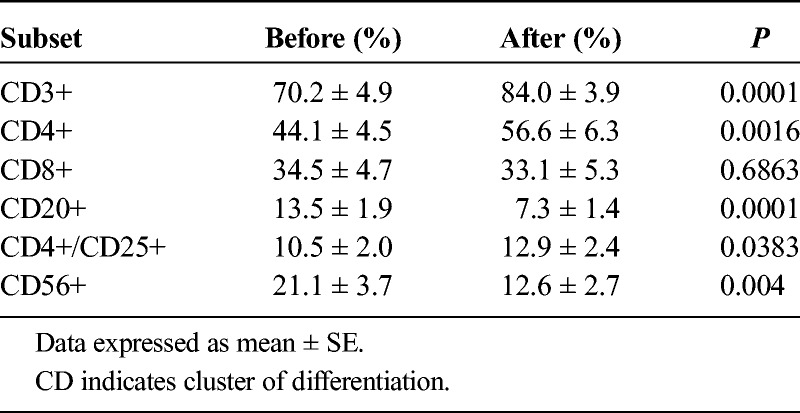
Changes in lymphocyte subsets before and after the culture of PBMCs are detailed in Table 5. The proportions of CD3+ and CD4+ cells increased significantly after treatment (P < 0.05), whereas CD20+, CD56+, and CD4+/CD25+ cells decreased significantly (P < 0.05).
TABLE 5.
Lymphocyte Subset Changes Before and After the Culture of MUC1-CTLs in the Adequate Treatment Group
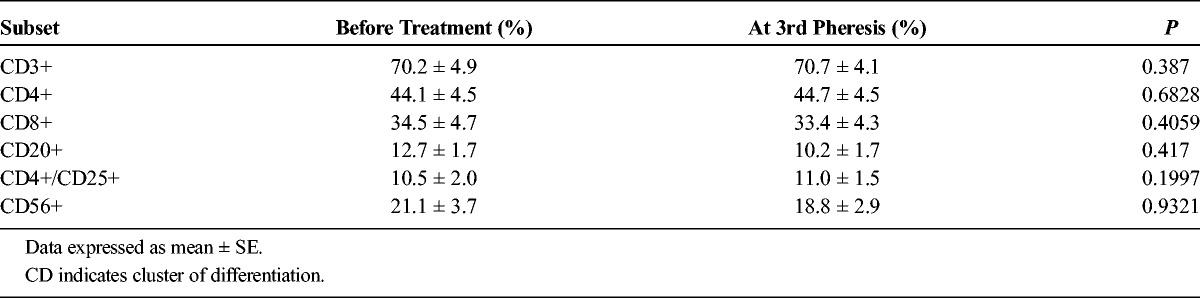
Changes of lymphocyte subsets of PBMCs before treatment and at the third pheresis are detailed in Table 6. None the subsets changed significantly.
TABLE 6.
Lymphocyte Subset Changes of PBMCs Before and After Treatment in Adequate Treatment Group
The parameters investigated as possible prognostic factors are shown in Table 6. When the average number of administered CTLs was at least 1.3 × 109, there was a significant difference in both the univariate and multivariate analysis (Table 7). The DFS of the patients who received an average of at least 1.3 × 109 CTLs was significantly improved (Fig. 6).
TABLE 7.
Prognostic Factors of Long DFS in the Adequate Treatment Group
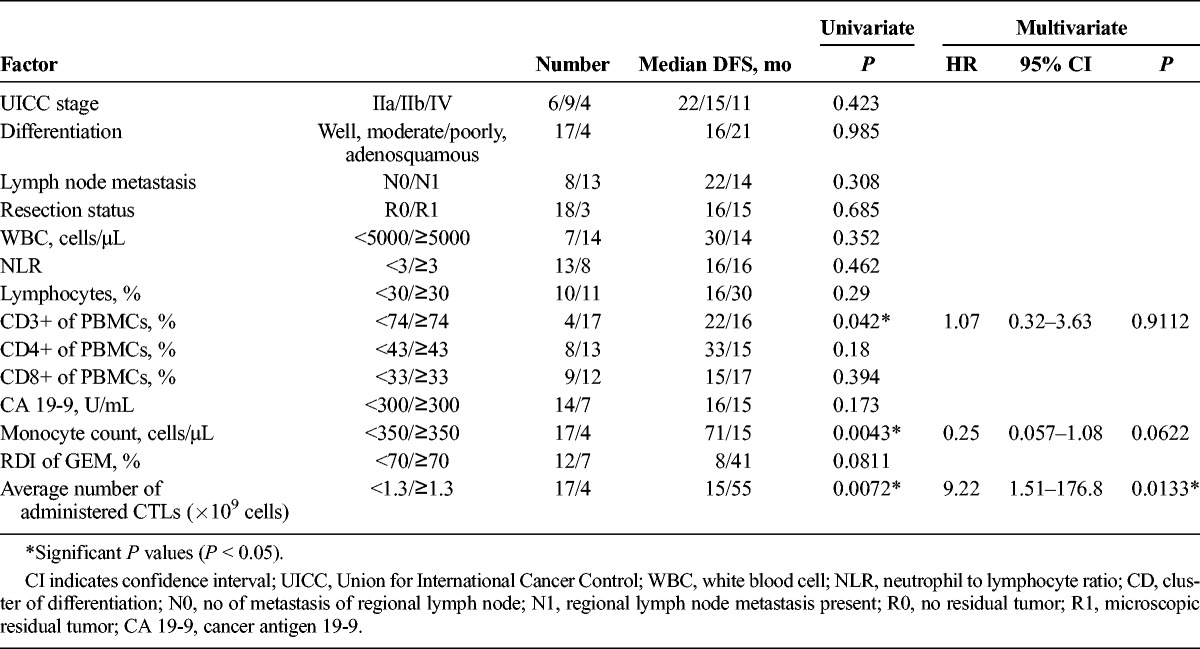
FIGURE 6.
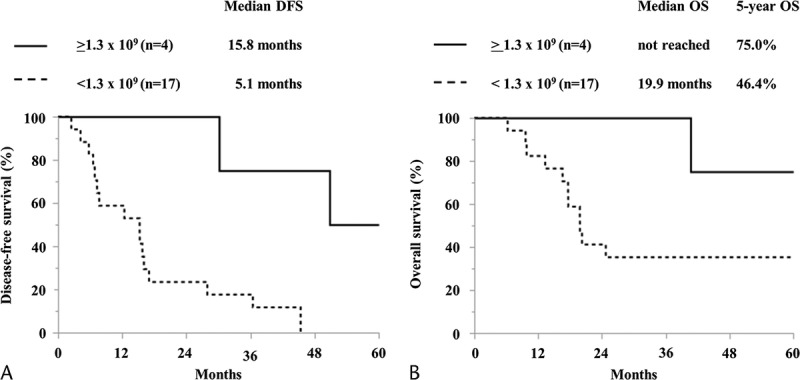
Kaplan-Meier curves compared by average number of administered CTLs. A, DFS. B, OS.
DISCUSSION
In the present study, a combination of systemic GEM administration and AIT with MUC1-CTL was performed in patients with advanced pancreatic cancer as adjuvant therapy after radical pancreatectomy. This therapy was performed safely, and there were no adverse events specific to the immunotherapy. As compared with previous clinical trials with adjuvant setting GEM,2,8 adverse events such as myelosuppression or autoimmune disease were not increased.
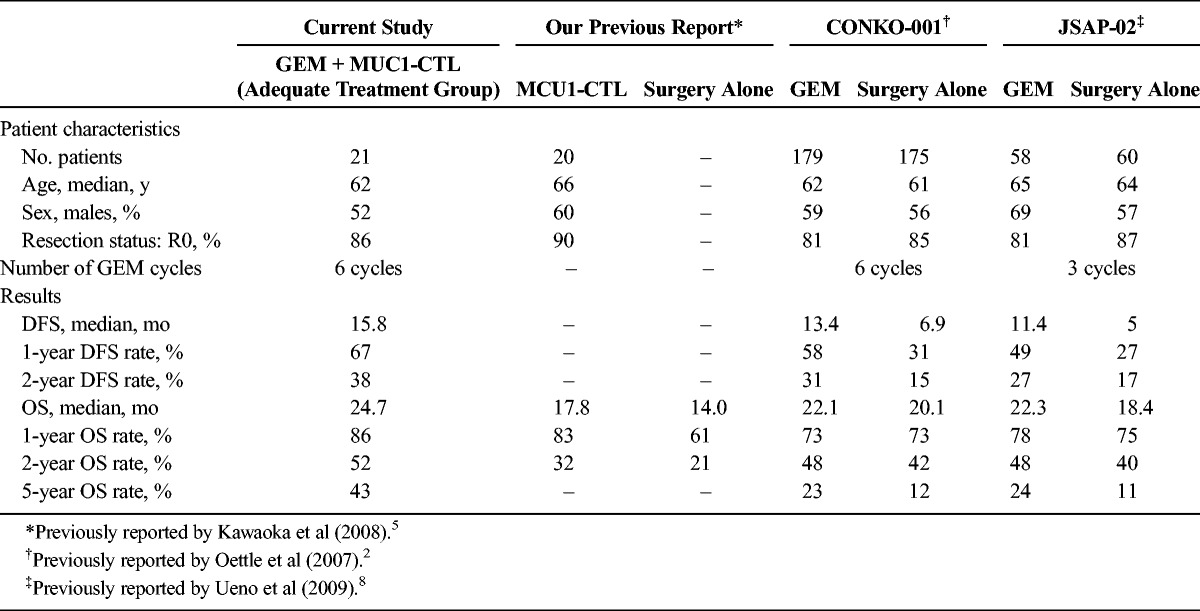
Next, we analyzed patients whose RDI of GEM was greater than or equal to 50% and who received at least 2 treatments with MUC1-CTL as the adequate treatment group. The average RDI of GEM in the adequate treatment group is still lower than in previous clinical trials2,8; thus, there might be some problems in the management of adverse effects of GEM in the present study. Although the number of cases in the adequate treatment group of present study was very small and, thus, exact comparison is difficult, the prognosis of these patients seemed to be better than in previous clinical trials (Table 8).2,8 That is, DFS of the adequate treatment group was better than the qualified analysis group in CONKO-001 (median DFS of 15.8 mo vs 13.7 mo, respectively), which excluded the cases that did not reflect the true therapeutic potential, such as cases with an administration frequency of less than 1 cycle. In recent years, a European group (European Study Group for Pancreatic Cancer-4) reported the favorable results of large clinical trials of adjuvant therapy for pancreatic cancer.9 This result showed that the adjuvant combination of GEM and capecitabine significantly improved prognosis than GEM monotherapy, but adverse events of grades 3 and 4 were increased in the combination group. Disease-free survival of the adequate treatment group of our study was not inferior to combination group of European Study Group for Pancreatic Cancer-4 without increasing adverse events. This result might be influenced by MUC1-CTLs because an average administration of at least 1.3 × 109 CTLs was the only independent factor of long DFS in both univariate and multivariate analysis (Table 5). As we reported previously, median survival time of adjuvant AIT with MUC1-CTLs was better than of surgery alone,5 and MUC1-CTLs may achieve enhanced effects in combination with standard adjuvant GEM in the present study.
TABLE 8.
Comparison Between the Current Study and Previous Report
The effects of GEM on immunity were reported: GEM does not inhibit the activity of lymphokine-activated killer cells or CTLs,10 GEM suppresses regulatory T-cell induction,7 and GEM directly inhibits myeloid-derived suppressor cells (MDSCs) and augments expansion of T-cells.11 Hence, GEM therapy is not immunosuppressive and may enhance the response to specific vaccines or AIT to activate or support immune responses directed toward driving effector immunity to cancer cells.12 Thus, we expected the synergistic effect of GEM and immunotherapy.
We have also reported that MUC1-CTL monotherapy after radical pancreatectomy could be expected to suppress liver metastasis,5 but local recurrence after MUC1-CTL monotherapy was high (65.0%).5 In the present study, we observed 33% of liver recurrence rate during the OS, while liver recurrence rate is typically about 60%.13,14 It has been reported that administrated lymphokine-activated killer cells localized primarily in the lungs after 2 hours after injection and then redistributed to the liver and spleen 24 hours after injection.15,16 In the present study, lymphocytes are considered to take a similar migration, which might be involved in liver metastasis inhibition.
The local recurrence in the present study was lower than in our previous report5 (19% vs 65%). The mechanism of local recurrence inhibition by GEM is not clear, but R1 patients who received adjuvant chemotherapy with GEM after surgery showed DFS comparable with R0 cases. Thus, GEM is suggested to be involved in local control.2 Hence, we speculated that MUC1-CTLs prevent hepatic metastasis and GEM possibly reduces local recurrence, as well as MUC1-CTLs and GEM have the synergic effect.
Inflammation, immune exhaustion, and immunosuppression might be involved in disturbance of the effect of CTLs. Inflammation was previously shown to be strongly related to the development and progress of pancreatic cancer.17 Regulatory T-cells increase and immunization are suppressed in pancreatic cancer patients.18 Although MDSCs are not found in the pancreas of a healthy person, they are easily detected in the stroma of pancreatic ductal adenocarcinoma, comprising approximately 67% of the infiltrating leukocytes.19 Myeloid-derived suppressor cells are also found in the blood and bone marrow of pancreatic ductal adenocarcinoma patients and are significantly higher in cases of metastatic disease as compared with patients with local tumors,19–21 which supports a previous report that the MDSC count correlates with disease stage.22 Exhaustion of CD8+ T-cells in solid tumors has also been reported.23 Because of these immunosuppressive mechanisms and immune exhaustion in pancreatic cancer patients, immunotherapy would not be expected to exhibit a sufficient effect. Examination of immune checkpoints is important, and it is necessary to plan to check these factors when performing similar trials in the future.
Prognostic factors after radical pancreatectomy for pancreatic cancer were reported to include the clinical factors such as carbohydrate antigen (CA) 19-9, CEA,24 tumor size, tumor differentiation,25 lymph node metastasis,26 and the surgical margin27 as well as the immunological factors of neutrophil to lymphocyte ratio,28 CD4+ and CD8+ T-cell infiltration of cancer tissue,29 and circulating mesothelin.30 In the present study, the average number of administered CTLs was an independent prognostic factor for long-term DFS. This result suggests that a sufficient dose of CTLs improves prognosis and that the potential immunity of the host might be involved in cell proliferation. This result might indicate that introducing more CTLs, such as by using induced pluripotent stem cells, would enhance the effect of this therapy.31
On the other hand, as described above, MUC1-CTLs monotherapy for unresectable pancreatic cancer did not improve significantly.5 Thus, we combined MUC1 peptide pulsed dendritic cells with MUC1-CTLs, and this therapy improved prognosis with no severe adverse event.32 Then, we used MUC1-messenger RNA transfected dendritic cells and MUC1-CTLs with GEM for unresectable pancreatic cancer.33 This combination therapy showed no severe toxicities associated with immunotherapy and further improved the prognosis. Thus, adding MUC1-messenger RNA transfected dendritic cells to MUC1-CTLs plus GEM because adjuvant therapy for resectable pancreatic cancer might be expected to improve prognosis.
CONCLUSIONS
In conclusion, our cancer immunochemotherapy in combination with MUC1-CTL and GEM was safe, and effectiveness was suggested in the group with administration of sufficient dose of GEM and CTLs. Although this preliminary result must be verified by a much larger prospective randomized study, we believe that these findings will lead to a novel therapeutic strategy for pancreatic cancer.
ACKNOWLEDGMENT
The authors thank Ms Akiko Sano and Ms Kaori Kaneyasu for their excellent technical assistance with this work. This study was supported partially by a research program of the Project for Development of Innovative Research on Cancer Therapeutics, The Japan Agency for Medical Research and Development.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. [DOI] [PubMed] [Google Scholar]
- 3.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 4.Masaki Y, Oka M, Ogura Y, et al. Sialylated MUC1 mucin expression in normal pancreas, benign pancreatic lesions, and pancreatic ductal adenocarcinoma. Hepatogastroenterology. 1999;46:2240–2245. [PubMed] [Google Scholar]
- 5.Kawaoka T, Oka M, Takashima M, et al. Adoptive immunotherapy for pancreatic cancer: cytotoxic T lymphocytes stimulated by the MUC1-expressing human pancreatic cancer cell line YPK-1. Oncol Rep. 2008;20:155–163. [PubMed] [Google Scholar]
- 6.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. [DOI] [PubMed] [Google Scholar]
- 7.Kan S, Hazama S, Maeda K, et al. Suppressive effects of cyclophosphamide and gemcitabine on regulatory T-cell induction in vitro. Anticancer Res. 2012;32:5363–5369. [PubMed] [Google Scholar]
- 8.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 10.Alvino E, Fuggetta MP, Tricarico M, et al. 2′-2′-difluorodeoxycytidine: in vitro effects on cell-mediated immune response. Anticancer Res. 1998;18:3597–3602. [PubMed] [Google Scholar]
- 11.Le HK, Graham L, Cha E, et al. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9:900–909. [DOI] [PubMed] [Google Scholar]
- 12.Plate JM, Plate AE, Shott S, et al. Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother. 2005;54:915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. [DOI] [PubMed] [Google Scholar]
- 14.Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. [DOI] [PubMed] [Google Scholar]
- 15.Maghazachi AA, Herberman RB, Vujanovic NL, et al. In vivo distribution and tissue localization of highly purified rat lymphokine-activated killer (LAK) cells. Cell Immunol. 1988;115:179–194. [DOI] [PubMed] [Google Scholar]
- 16.Felgar RE, Hiserodt JC. In vivo migration and tissue localization of highly purified lymphokine-activated killer cells (A-LAK cells) in tumor-bearing rats. Cell Immunol. 1990;129:288–298. [DOI] [PubMed] [Google Scholar]
- 17.Evans A, Costello E. The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion. Front Physiol. 2012;3:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. [DOI] [PubMed] [Google Scholar]
- 19.Porembka MR, Mitchem JB, Belt BA, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61:1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basso D, Fogar P, Falconi M, et al. Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One. 2013;8:e54824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Distler M, Pilarsky E, Kersting S, et al. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas — a retrospective tumor marker prognostic study. Int J Surg. 2013;11:1067–1072. [DOI] [PubMed] [Google Scholar]
- 25.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. [DOI] [PubMed] [Google Scholar]
- 26.House MG, Gönen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. [DOI] [PubMed] [Google Scholar]
- 27.Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35:600–604. [DOI] [PubMed] [Google Scholar]
- 28.Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. [DOI] [PubMed] [Google Scholar]
- 29.Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. [DOI] [PubMed] [Google Scholar]
- 30.Johnston FM, Tan MC, Tan BR, Jr, et al. Circulating mesothelin protein and cellular antimesothelin immunity in patients with pancreatic cancer. Clin Cancer Res. 2009;15:6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vizcardo R, Masuda K, Yamada D, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12:31–36. [DOI] [PubMed] [Google Scholar]
- 32.Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28:379–387. [PubMed] [Google Scholar]
- 33.Shindo Y, Hazama S, Maeda Y, et al. Adoptive immunotherapy with MUC1-mRNA transfected dendritic cells and cytotoxic lymphocytes plus gemcitabine for unresectable pancreatic cancer. J Transl Med. 2014;12:175. [DOI] [PMC free article] [PubMed] [Google Scholar]


