Supplemental Digital Content is available in the text.
Keywords: fluid therapy, goal-directed, hemodynamic, meta-analysis, resuscitation
Abstract
Objective:
Dynamic tests of fluid responsiveness have been developed and investigated in clinical trials of goal-directed therapy. The impact of this approach on clinically relevant outcomes is unknown. We performed a systematic review and meta-analysis to evaluate whether fluid therapy guided by dynamic assessment of fluid responsiveness compared with standard care improves clinically relevant outcomes in adults admitted to the ICU.
Data Sources:
Randomized controlled trials from MEDLINE, EMBASE, CENTRAL, clinicaltrials.gov, and the International Clinical Trials Registry Platform from inception to December 2016, conference proceedings, and reference lists of relevant articles.
Study Selection:
Two reviewers independently identified randomized controlled trials comparing dynamic assessment of fluid responsiveness with standard care for acute volume resuscitation in adults admitted to the ICU.
Data Extraction:
Two reviewers independently abstracted trial-level data including population characteristics, interventions, clinical outcomes, and source of funding. Our primary outcome was mortality at longest duration of follow-up. Our secondary outcomes were ICU and hospital length of stay, duration of mechanical ventilation, and frequency of renal complications. The internal validity of trials was assessed in duplicate using the Cochrane Collaboration’s Risk of Bias tool.
Data Synthesis:
We included 13 trials enrolling 1,652 patients. Methods used to assess fluid responsiveness included stroke volume variation (nine trials), pulse pressure variation (one trial), and stroke volume change with passive leg raise/fluid challenge (three trials). In 12 trials reporting mortality, the risk ratio for death associated with dynamic assessment of fluid responsiveness was 0.59 (95% CI, 0.42–0.83; I2 = 0%; n = 1,586). The absolute risk reduction in mortality associated with dynamic assessment of fluid responsiveness was –2.9% (95% CI, –5.6% to –0.2%). Dynamic assessment of fluid responsiveness was associated with reduced duration of ICU length of stay (weighted mean difference, –1.16 d [95% CI, –1.97 to –0.36]; I2 = 74%; n = 394, six trials) and mechanical ventilation (weighted mean difference, –2.98 hr [95% CI, –5.08 to –0.89]; I2 = 34%; n = 334, five trials). Three trials were adjudicated at unclear risk of bias; the remaining trials were at high risk of bias.
Conclusions:
In adult patients admitted to intensive care who required acute volume resuscitation, goal-directed therapy guided by assessment of fluid responsiveness appears to be associated with reduced mortality, ICU length of stay, and duration of mechanical ventilation. High-quality clinical trials in both medical and surgical ICU populations are warranted to inform routine care.
Fluid resuscitation has the potential to restore tissue perfusion to vital end organs in critically ill patients with hypotension and shock (1). It has been proposed that an increase in stroke volume (SV) by 10–15% after a fluid challenge (250–500 cc) defines “fluid responsiveness” (2). Despite the daily and ubiquitous use of fluids in ICUs, as few as 40% of critically ill patients are fluid responsive (3). Fluid overload has been shown to be an independent predictor of mortality in the critically ill, including patients with septic shock, acute respiratory distress syndrome, and those undergoing major surgery (4–7). When an indication for fluid loading exists, separating patients who may respond (increase SV) from those that are fluid nonresponsive may then identify those who can benefit from fluid therapy while avoiding unnecessary fluids and their adverse effects in the critically ill (4).
Static indices such as the central venous pressure (CVP) consistently fail to predict fluid responsiveness, calling to question their utility in goal-directed therapy algorithms (8, 9). Although three large trials of goal-directed therapy with CVP-based fluid loading have failed to show clinical benefit compared with standard care, a growing body of inquiry into goal-directed therapy incorporating dynamic assessment of fluid responsiveness (FT-DYN) has emerged (10–13). This approach assesses changes in SV or surrogate dynamic variables (e.g., SV variation [SVV], pulse pressure variation [PPV]) during alterations in cardiac preload provoked by ventilation, passive leg raise, or fluid challenge (14). Although these methods appear to accurately predict fluid responsiveness, the clinical impact of FT-DYN remains unclear. Our objective was to determine whether, among adult patients admitted to the ICU, fluid therapy for acute volume resuscitation based on FT-DYN impacts clinically relevant outcomes compared with standard care.
MATERIALS AND METHODS
Using a prespecified published protocol (15), we performed a systematic review congruent with the Methodological Expectations of Cochrane Intervention Reviews (16) and reported outcomes according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (17).
Research Question and Eligibility Criteria
We posed the following research question: “In adult patients admitted to the ICU requiring acute volume resuscitation, does goal-directed fluid therapy guided by FT-DYN compared with standard care result in improved clinically relevant outcomes?” Our primary outcome was the incidence of mortality at the longest duration of reported follow-up. Secondary outcomes were ICU and hospital length of stay, duration of mechanical ventilation, and frequency of renal complications. Additional physiologically relevant secondary outcomes included serum lactate at end intervention and quantities of IV fluids administered. We included trials satisfying the following criteria: prospective randomized controlled trials (RCTs) in human subjects, adult patients (≥ 18 yr old), greater than or equal to 80% of patients admitted to the ICU, patients having indication for acute volume resuscitation (e.g., hypovolemia, hypotension, shock), allocation to resuscitation based on a goal-directed therapy algorithm that included fluid therapy guided by FT-DYN (14), and comparator group receiving standard care (empiric fluid therapy based on weight, static variables, or the clinical examination) (18). Observational trials were not included to reduce the potential for performance and selection bias. We excluded trials evaluating electrical bioimpedance techniques due to published concerns regarding their accuracy in predicting fluid responsiveness (19). We also excluded quasi-randomized, cross-over, or cluster randomized trials and trials in which control groups received fluid therapy guided by FT-DYN.
Search Strategy and Study Selection
We searched MEDLINE, EMBASE, and CENTRAL (The Cochrane Library—Wiley) from inception to December 2016 using the Cochrane Highly Sensitive Search Strategy as a search model (20). A library sciences specialist designed the original search query for MEDLINE and translated to other databases where required (Supplementary File 1, Supplemental Digital Content 1, http://links.lww.com/CCM/C687—legend, Supplemental Digital Content 16, http://links.lww.com/CCM/C702). Selected search terms included “fluid responsiveness, fluid therapy, goal-directed therapy, hemodynamic therapy, dynamic, cardiac volume, cardiac output, SV, pulse pressure, systolic pressure, pleth variability, and vena cava” with filters for randomized trials. To identify planned or ongoing trials, we searched clinicaltrials.gov and the World Health Organization’s International Clinical Trials Registry Platform (inception to December 2016) and the previous 3 years of conference proceedings of the Society of Critical Care Medicine and the European Society of Intensive Care Medicine ending in December 2016. Reference lists of review articles were hand searched for relevant citations. A two-step process was used for study selection. Two reviewers (J.M.B., J.A.F.) independently screened titles and abstracts of search results to determine whether each citation met the inclusion criteria. The full texts of citations classified as “include” or “unclear” were reviewed independently with reference to the predetermined inclusion and exclusion criteria. Consensus was achieved through discussion in cases of disagreement.
Data Abstraction and Quality Assessment
Two reviewers (J.M.B., J.A.F.) independently abstracted trial-level data using a standardized and prepiloted electronic spreadsheet (Microsoft Excel, V 14.4.1; Microsoft, Redmond, WA). Abstracted descriptive study characteristics included trial demographics, number of sites, country, funding source, patient population (e.g., surgery, trauma, sepsis), baseline illness severity scores, monitoring technology used, goal-directed therapy targets, type of protocolized fluid, timing of intervention, clinical outcomes, and follow-up duration. Corresponding authors were contacted to obtain missing data related to trial demographics, methods and outcomes where required.
We evaluated internal validity using the Cochrane Collaboration’s Risk of Bias tool (20), which provides specific criteria for appraisal of risk according to the following domains: randomization and allocation of participants; blinding of participants, personnel, and outcome assessors; incomplete or selective reporting; and other relevant sources of bias.
Data Analysis
Data from included trials were analyzed using Cochrane Review Manager (RevMan version 5.3.5, 2014; The Cochrane Collaboration, Copenhagen, Denmark). Pooled dichotomous data were represented as risk ratios (RRs) with 95% CIs using Mantel-Haenszel statistics. Mortality was assessed at the longest duration of follow-up. Summary estimates of continuous data were expressed as a weighted mean difference (WMD) with 95% CI using inverse variance. We used random effect models for all analyses and explored statistical heterogeneity using the I2 test with 95% uncertainty intervals. Summary effect measures were based on intention-to-treat data where available. In one trial that included more than one interventional arm evaluating FT-DYN (21), the interventions were pooled into a single group for the main outcome measures. Publication bias was assessed for the primary outcome using the trim and fill method (22). In the presence of plot asymmetry assumed to be publication bias, this modeling method imputes missing outcome data from small to moderate sized trials to create a symmetrical plot. The modeled summary effect estimate is then recalculated and compared with the original effect estimate (20, 22). Prespecified subgroup analysis for the primary outcome was planned to investigate the potential differential impact of patient population (medical, surgical, or cardiac surgical ICU), fluid responsiveness criteria (SVV, PPV, threshold increase in SV), type of constituent fluid administered (crystalloid, colloid, variable), timing and duration of intervention, risk of bias (low, unclear, high), and source of funding (industry, nonindustry).
RESULTS
Of 19,664 citations retrieved, we included 13 unique trials enrolling 1,652 patients (Fig. 1 and Table 1) (21, 23–34). All included trials were published articles in English-language peer-reviewed journals. Trials included a median of 65 patients (interquartile range, 61–109). The mean age of patients was 68 ± 11 years. Illness severity scores were not reported in the majority of trials. The ICU population was characterized by nine trials that enrolled patients undergoing high risk intra-abdominal surgery (21, 23, 26, 27, 29, 30, 32–34), three trials of cardiac surgery patients (24, 25, 31), and one trial of patients with septic shock (28). Ten of 13 included trials prohibited the enrollment of patients with dysrhythmias or atrial fibrillation (21, 23–26, 29, 31–34). Seven trials mandated a lower limit of tidal volume of 8 cc/kg during mechanical ventilation (23–26, 29, 30, 33).
Figure 1.
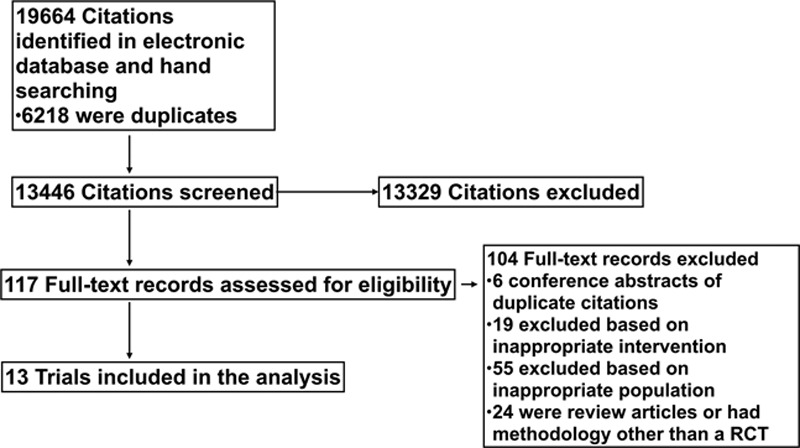
Preferred reporting items for systematic reviews and meta-analysis flow diagram. RCT = randomized controlled trial.
TABLE 1.
Characteristics of Included Trials
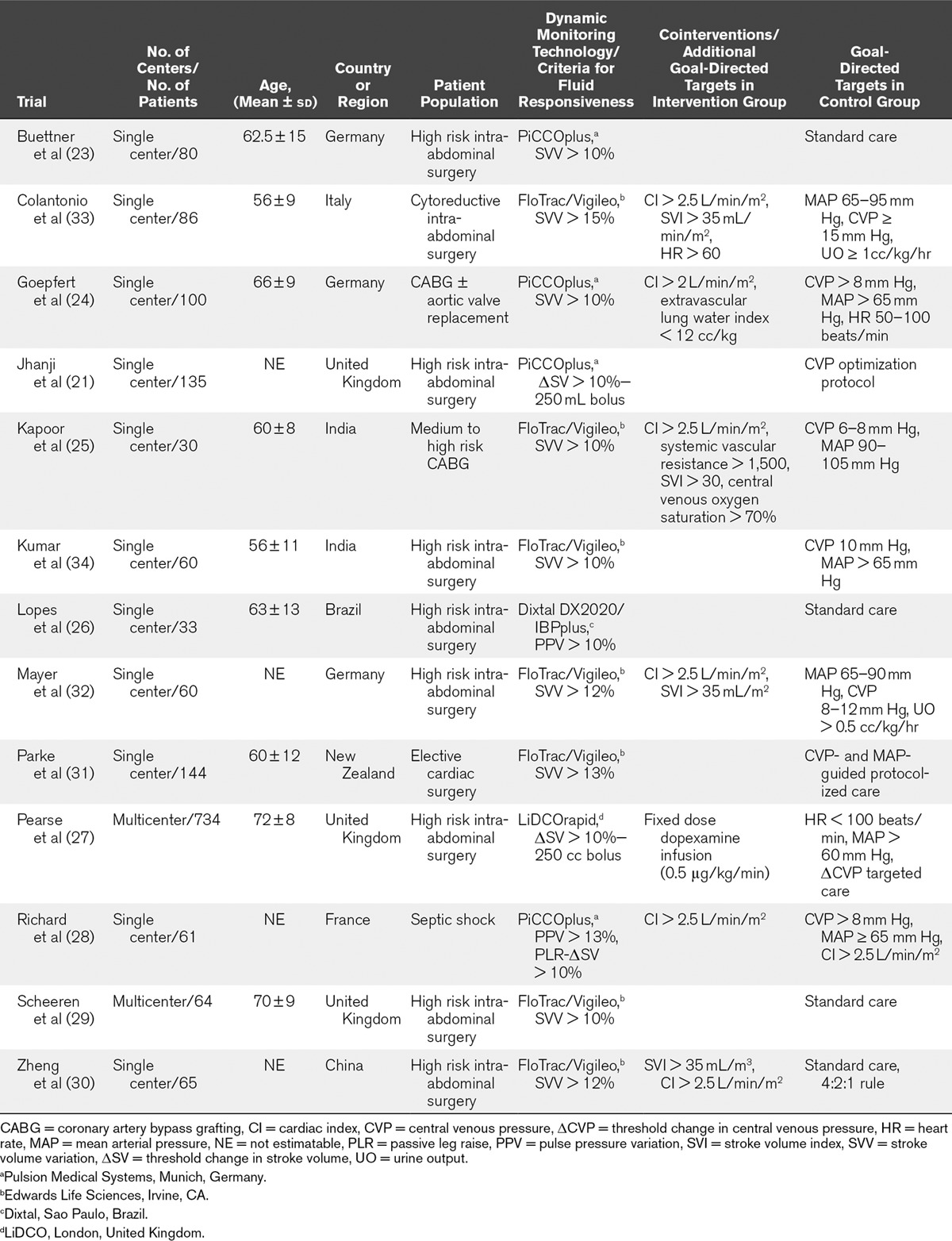
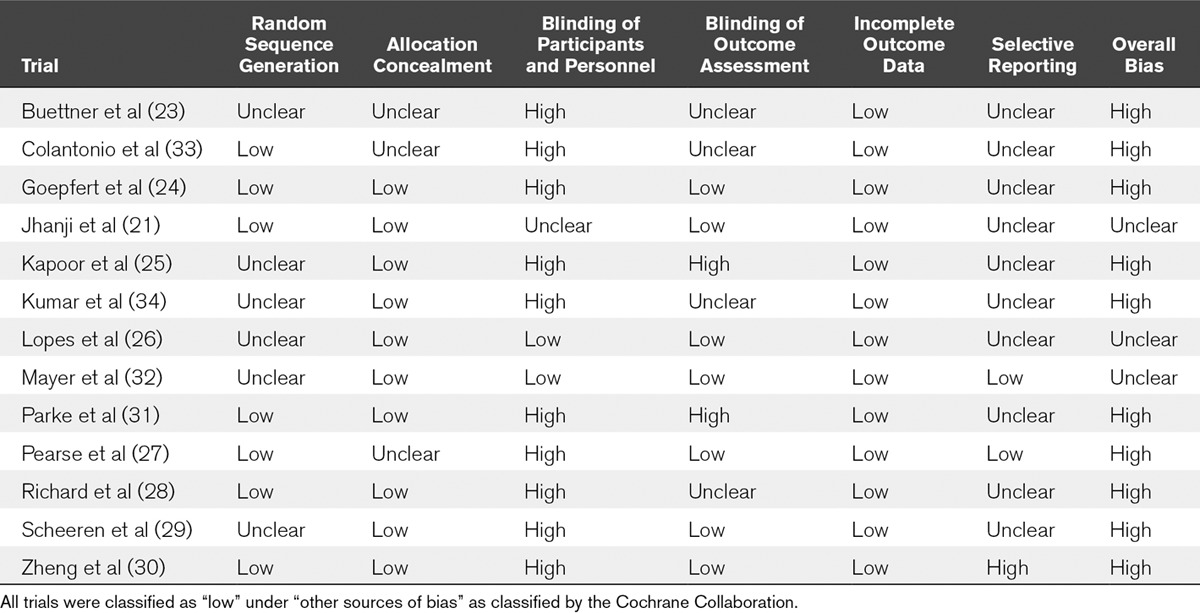
Criteria used for determining fluid responsiveness included assessment of SVV with threshold greater than 10–15% in nine trials (23–25, 29–34), assessment of PPV with threshold greater than 10% in one trial (26), and the use of passive leg raise or mini fluid challenge with threshold of an increase in SV of 10% in three trials (21, 27, 28). Cointerventions and additional goal-directed therapy targets are itemized in Table 1. In control groups, patients were managed with traditional goal-directed therapy algorithms targeting static variables including the CVP in nine trials (21, 24, 25, 27, 28, 31–34), weight-based fluid loading in one trial (30), and standard care per treatment team in three trials (23, 26, 29). The protocolized fluid was colloid of any type in five trials (21, 25, 27, 32, 33), hydroxyethyl starch in three trials (24, 26, 29), and either crystalloid or physician choice in five trials (23, 28, 30, 31, 34). The intervention was delivered in the operating room (OR) prior to ICU admission in six trials (23, 26, 29, 32–34), the OR and ICU in four trials (24, 25, 27, 30), and the ICU only in three trials (21, 28, 31). The duration of the intervention ranged from 5 to 82 hours. Three trials were appraised as having unclear risk of bias; the remaining trials were at high risk of bias due to lack of blinding (Table 2). Five trials received funding from industry manufacturers of the device used in the intervention (21, 24, 29, 31, 32).
TABLE 2.
Assessment of Risk of Bias of Included Trials
Primary Outcome
Data on mortality were available in 12 of 13 trials (21, 23–29, 31–34). Mortality was recorded at hospital discharge in seven trials (23–26, 29, 32, 34), 28–30 days in four trials (21, 27, 28, 33), and 90 days in one trial (31). Fluid therapy guided by FT-DYN was associated with decreased mortality compared with standard care (RR, 0.59; 95% CI, 0.42–0.83; I2 = 0%; n = 1,586) (Fig. 2). The absolute risk reduction in mortality associated with FT-DYN was –2.9% (95% CI, –5.6% to –0.2%). Although funnel plot asymmetry appeared to exist for this outcome, trim and fill analysis where we imputed potentially unpublished trials showing no benefit or perhaps even harm associated with FT-DYN did not statistically modify the summary statistic for mortality (Fig. S1, Supplemental Digital Content 2, http://links.lww.com/CCM/C688—legend, Supplemental Digital Content 16, http://links.lww.com/CCM/C702). Mortality associated with FT-DYN was evaluated according to prespecified clinical (patient population [medical, surgical, cardiac surgical ICU], fluid responsiveness criteria [SVV, PPV, or threshold increase in SV], type of fluid administered [colloid unspecified, hydroxyethyl starch, crystalloid, or physician choice], timing [OR, ICU, OR and ICU], and duration of intervention [< 6, 6–24, > 24 hr]) and methodologic subgroups (risk of bias and source of funding). Summary effect estimates of mortality across clinical subgroups were consistent with varying confidence limits (Supplementary Table 1, Supplemental Digital Content 3, http://links.lww.com/CCM/C689; Fig. S2, Supplemental Digital Content 4, http://links.lww.com/CCM/C690; Fig. S3, Supplemental Digital Content 5, http://links.lww.com/CCM/C691; Fig. S4, Supplemental Digital Content 6, http://links.lww.com/CCM/C692; Fig. S5, Supplemental Digital Content 7, http://links.lww.com/CCM/C693—legend, Supplemental Digital Content 16, http://links.lww.com/CCM/C702; Fig. S6, Supplemental Digital Content 8, http://links.lww.com/CCM/C694; Fig. S7, Supplemental Digital Content 9, http://links.lww.com/CCM/C695; and Fig. S8, Supplemental Digital Content 10, http://links.lww.com/CCM/C696). The effect of funding source or adjudicated risk of bias was not statistically significant across subgroups.
Figure 2.
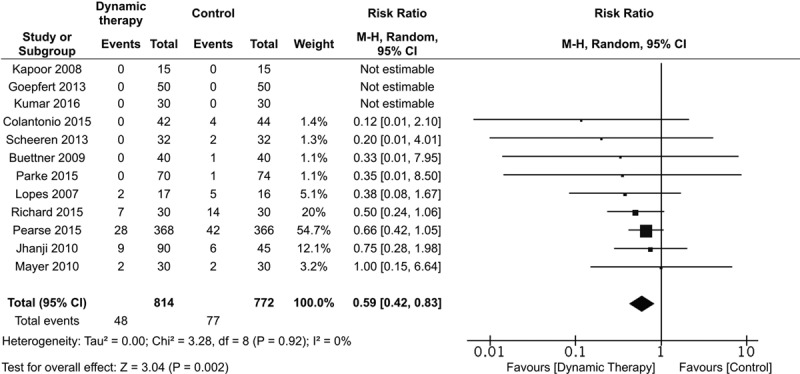
Effect of goal-directed fluid therapy guided by dynamic assessment of fluid responsiveness on mortality.
Secondary Outcomes
Compared with usual care, FT-DYN was associated with reduced ICU length of stay (WMD, –1.16 d; 95% CI, –1.97 to –0.36; I2 = 74%; n = 394) in six trials with extractable data (23–25, 29, 32, 34), with statistical heterogeneity attributable to varying treatment effects all favoring the intervention. Among four trials examining ICU length of stay but lacking measures of variance suitable for meta-analysis, two trials reported reduced ICU length of stay in patients receiving FT-DYN (26, 30), and two trials found no difference (28, 31). FT-DYN was also associated with a reduction in duration of mechanical ventilation (WMD, –2.98 hr (95% CI, –5.08 to –0.89); I2 = 34%; n = 334, five trials) (23–25, 29, 32). There was no difference in hospital length of stay in four trials reporting data suitable for meta-analysis (WMD, –0.65 d; 95% CI, –3.25 to 1.94; I2 = 86%; n = 270) (23–25, 34), with heterogeneity driven by a small trial reporting a shorter median but wide range of hospital length of stay in patients receiving FT-DYN (23). Of seven additional trials reporting hospital length of stay but lacking measures of variance suitable for meta-analysis, five trials reported shorter hospital length of stay in patients receiving FT-DYN (26, 27, 30, 32, 33), whereas two trials found no significant difference (21, 31).
FT-DYN was not associated with significant difference in the frequency of renal complications (RR, 0.54; 95% CI, 0.28–1.04; I2 = 67%; n = 1,380, nine trials) (24–27, 29, 31–33). The frequency of renal complications was reported in a dichotomous manner with reference to AKIN in three trials (21, 24, 27), KDIGO in one trial (31), and RIFLE in one trial (29). Four trials defined renal complications as composite endpoints ranging from oliguria to the need for acute renal replacement therapy (25, 26, 32, 33). Requirement for renal replacement therapy was not reported as a distinct outcome. With a consistent summary estimate, heterogeneity was reduced (I2 = 35%) after excluding a single small trial that reported an increased frequency of renal complications in the intervention group (31). Forest plots for secondary outcomes are presented in Fig. S9 (Supplemental Digital Content 11, http://links.lww.com/CCM/C697), Fig. S10 (Supplemental Digital Content 12, http://links.lww.com/CCM/C698), Fig. S11 (Supplemental Digital Content 13, http://links.lww.com/CCM/C699), Fig. S12 (Supplemental Digital Content 14, http://links.lww.com/CCM/C700), and Fig. S13 (Supplemental Digital Content 15, http://links.lww.com/CCM/C701).
Outcome data pertaining to the differential quantities of fluid administered with appropriate measures of variance were reported in six trials for crystalloid (21, 23, 24, 26, 32, 33), seven trials for colloid (21, 23, 24, 26, 29, 32, 33), and seven trials for cumulative fluids at end intervention (23, 24, 26, 29, 32–34). Fluid quantity outcomes in each trial are presented in Figure 3; high statistical heterogeneity combined with opposing treatment effects precluded meta-analysis of crystalloid quantity and cumulative IV fluids administered. This heterogeneity was not well explained by clinical or methodologic subgroup analysis. Patients receiving FT-DYN received greater quantity of colloids with statistical heterogeneity attributed to varying treatment effects all favoring FT-DYN (WMD, 0.44 L; 95% CI, 0.24–0.64; I2 = 68%; n = 558, seven trials) (21, 23, 24, 26, 29, 32, 33). A significant reduction in serum lactate was observed at end intervention (–0.58 mmol/L, 95% CI, –1.05 to –0.11; I2 = 92%; n = 419, six trials) (23, 24, 26, 30, 33, 34), with statistical heterogeneity attributed to varying treatment effects all favoring FT-DYN.
Figure 3.
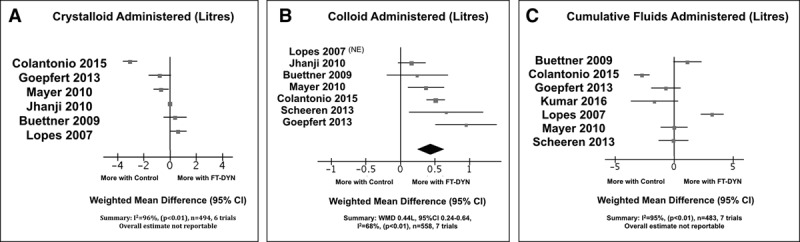
Effect of goal-directed fluid therapy based on dynamic assessment of fluid responsiveness (FT-DYN) compared with standard care for the following: quantity of crystalloid administered at end intervention (A); quantity of colloid administered at end intervention (B); and cumulative fluids administered at end intervention (C). NE = not estimable, WMD = weighted mean difference.
DISCUSSION
In our systematic review and meta-analysis, we observed a reduction in mortality among adults admitted to the ICU who received goal-directed therapy guided by FT-DYN for acute volume resuscitation compared with standard care. FT-DYN may also be associated with modest reductions in ICU length of stay, duration of mechanical ventilation, and serum lactate although data for these outcomes were limited.
In recent surveys, 89% of intensivists and up to 71% of anesthesiologists report titrating fluids to the CVP in clinical practice (35–37). Our analysis suggests that the widespread use of static measures to guide fluid therapy requires reexamination. Our findings are concordant with a recent consensus statement issued by an international panel of specialists in anesthesia and intensive care recommending that fluid therapy target fluid responsiveness in routine perioperative care (13). Conversely, the surviving sepsis guidelines, widely referenced by critical care practitioners, have historically supported static fluid therapy targets (38). More recently, these guidelines have issued a weak recommendation to incorporate dynamic rather than static variables to assess fluid responsiveness to guide initial resuscitation (39).
Our systematic review and meta-analysis builds on the findings of Benes et al (40) who found fluid therapy based on dynamic variables reduced perioperative morbidity compared with standard care in operative settings. Our review examined a broader population of patients receiving FT-DYN admitted to intensive care, and found a risk reduction in mortality. Although mechanisms through which FT-DYN may confer benefit in this population require further study, the differential mortality effects may be due to improved end-organ perfusion with FT-DYN, optimal timing of fluid bolus administration in relation to physiologic demand, or minimization of crystalloid volume (41, 42).
Our systematic review has several strengths. Our clinical question was well defined and addressed a timely and relevant topic in critical care. Our systematic review protocol was prospectively registered. To reduce the potential for both selection and performance bias, we were careful to include only RCTs. We designed an exhaustive search strategy to capture multiple dynamic methods to assess fluid responsiveness, and we excluded trials that used bioreactance technology which is reported to have limited accuracy in the ICU setting (19). Our systematic review focused on clinically relevant patient outcomes and was congruent with recognized guidelines for systematic review methodology.
This systematic review has potential limitations. The majority of trials in this analysis involved a postsurgical ICU population. The performance of FT-DYN in general intensive care may differ from a surgical ICU setting and requires consideration of limitations of individual dynamic variables in predicting fluid responsiveness. Some of these limitations include the presence of dysrhythmias, right ventricular dysfunction, intra-abdominal hypertension, spontaneous respiratory effort, and the use of low tidal volume ventilation; limitations that may be mitigated by incorporating a passive leg raise into dynamic assessments. The majority of trials were at high risk of bias due to lack of blinding in the intervention arms, which may have contributed to performance bias. Outcome assessment was however blinded to treatment allocation in the majority of trials which would serve to reduce the potential for systematic error. Mortality reported in trials was generally low, which may limit the generalizability of findings to ICU populations with greater illness severity. Secondary outcomes were largely underreported. Funnel plot asymmetry indicates potential publication bias with the underreporting of small trials showing null effects or harm associated with the intervention; however, statistical imputation of these trials did not significantly alter the summary effect measure for mortality. Statistical heterogeneity among pooled secondary outcomes was moderate, but generally explained by excluding a single outlier (with preserved summary effect measures) or by varying magnitude of treatment effects all favoring FT-DYN. Clinical heterogeneity also existed in the population analyzed based on fluid responsiveness criteria, type of fluid administered, and timing and duration of interventions; however, these factors were addressed in a thorough and extensive subgroup analysis.
CONCLUSION
In adult patients admitted to intensive care and requiring acute volume resuscitation, goal-directed therapy guided by assessment of fluid responsiveness appears to be associated with reduced mortality, ICU length of stay, and duration of mechanical ventilation. High risk of bias due to lack of blinding limits the internal validity of published trials. High-quality clinical trials in both medical and surgical ICU populations are warranted to inform routine care.
Supplementary Material
Footnotes
All authors have significantly contributed to the conception, design, execution, and writing of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. Bednarczyk disclosed that Dr. Zarychanski receives salary support from the Canadian Institute of Health Research. Dr. Turgeon is the Canada Research Chair in Critical Care Neurology and Trauma. These entities have had no role in the design or conduct of the study or approval of the final report. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Glassford NJ, Eastwood GM, Bellomo R. Physiological changes after fluid bolus therapy in sepsis: A systematic review of contemporary data. Crit Care 2014; 18:696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marik P, Bellomo R. A rational approach to fluid therapy in sepsis. Br J Anaesth 2016; 116:339–349. [DOI] [PubMed] [Google Scholar]
- 3.Kanji HD, McCallum J, Sirounis D, et al. Limited echocardiography-guided therapy in subacute shock is associated with change in management and improved outcomes. J Crit Care 2014; 29:700–705. [DOI] [PubMed] [Google Scholar]
- 4.Boyd JH, Forbes J, Nakada TA, et al. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39:259–265. [DOI] [PubMed] [Google Scholar]
- 5.Micek ST, McEvoy C, McKenzie M, et al. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care 2013; 17:R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg AL, Dechert RE, Park PK, et al. ; NIH NHLBI ARDS Network: Review of a large clinical series: Association of cumulative fluid balance on outcome in acute lung injury: A retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med 2009; 24:35–46. [DOI] [PubMed] [Google Scholar]
- 7.Silva JM, Jr, de Oliveira AM, Nogueira FA, et al. The effect of excess fluid balance on the mortality rate of surgical patients: A multicenter prospective study. Crit Care 2013; 17:R288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care 2011; 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Anel R, Bunnell E, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med 2004; 32:691–699. [DOI] [PubMed] [Google Scholar]
- 10.Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouncey PR, Osborn TM, Power GS, et al. ; ProMISe Trial Investigators: Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372:1301–1311. [DOI] [PubMed] [Google Scholar]
- 12.Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371:1496–1506. [DOI] [PubMed] [Google Scholar]
- 13.Navarro LH, Bloomstone JA, Auler JO, Jr, et al. Perioperative fluid therapy: A statement from the international Fluid Optimization Group. Perioper Med (Lond) 2015; 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerin L, Monnet X, Teboul JL. Monitoring volume and fluid responsiveness: From static to dynamic indicators. Best Pract Res Clin Anaesthesiol 2013; 27:177–185. [DOI] [PubMed] [Google Scholar]
- 15.Bednarczyk J, Fridfinnson J, Kumar A, et al. Incorporating fluid responsiveness into goal-directed therapy: Does it affect outcome? A systematic review and meta-analysis. PROSPERO International Prospective Register of Systematic Reviews. 2016. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016034089. Accessed April 1, 2017
- 16.Chandler J, Churchill R, Higgins J, et al. Methodological Expectations of Cochrane Intervention Reviews (MECIR) Methodological Standards for the Conduct of New Cochrane Intervention Reviews, Version 2.3. 2013London, Cochrane. [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, et al. PRISMA-P Group: Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevransky JE. Dynamic measures to determine volume responsiveness: Logical, biologically plausible, and unproven. Crit Care Med 2016; 44:1923–1926. [DOI] [PubMed] [Google Scholar]
- 19.Kupersztych-Hagege E, Teboul JL, Artigas A, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth 2013; 111:961–966. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 Edition. 2011London, The Cochrane Collaboration. [Google Scholar]
- 21.Jhanji S, Vivian-Smith A, Lucena-Amaro S, et al. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: A randomised controlled trial. Crit Care 2010; 14:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 23.Buettner M, Schummer W, Huettemann E, et al. Influence of systolic-pressure-variation-guided intraoperative fluid management on organ function and oxygen transport. Br J Anaesth 2008; 101:194–199. [DOI] [PubMed] [Google Scholar]
- 24.Goepfert MS, Richter HP, Zu Eulenburg C, et al. Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: A prospective, randomized controlled trial. Anesthesiology 2013; 119:824–836. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor PM, Kakani M, Chowdhury U, et al. Early goal-directed therapy in moderate to high-risk cardiac surgery patients. Ann Card Anaesth 2008; 11:27–34. [DOI] [PubMed] [Google Scholar]
- 26.Lopes MR, Oliveira MA, Pereira VO, et al. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: A pilot randomized controlled trial. Crit Care 2007; 11:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearse RM, Harrison DA, MacDonald N, et al. ; OPTIMISE Study Group: Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: A randomized clinical trial and systematic review. JAMA 2014; 311:2181–2190. [DOI] [PubMed] [Google Scholar]
- 28.Richard JC, Bayle F, Bourdin G, et al. Preload dependence indices to titrate volume expansion during septic shock: A randomized controlled trial. Crit Care 2015; 19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheeren TW, Wiesenack C, Gerlach H, et al. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: A prospective randomized multicentre study. J Clin Monit Comput 2013; 27:225–233. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H, Guo H, Ye JR, et al. Goal-directed fluid therapy in gastrointestinal surgery in older coronary heart disease patients: Randomized trial. World J Surg 2013; 37:2820–2829. [DOI] [PubMed] [Google Scholar]
- 31.Parke RL, McGuinness SP, Gilder E, et al. A randomised feasibility study to assess a novel strategy to rationalise fluid in patients after cardiac surgery. Br J Anaesth 2015; 115:45–52. [DOI] [PubMed] [Google Scholar]
- 32.Mayer J, Boldt J, Mengistu AM, et al. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: A randomized, controlled trial. Crit Care 2010; 14:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colantonio L, Claroni C, Fabrizi L, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg 2015; 19:722–729. [DOI] [PubMed] [Google Scholar]
- 34.Kumar L, Rajan S, Baalachandran R. Outcomes associated with stroke volume variation versus central venous pressure guided fluid replacements during major abdominal surgery. J Anaesthesiol Clin Pharmacol 2016; 32:182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIntyre LA, Hébert PC, Fergusson D, et al. ; Canadian Critical Care Trials Group: A survey of Canadian intensivists’ resuscitation practices in early septic shock. Crit Care 2007; 11:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cannesson M, Pestel G, Ricks C, et al. Hemodynamic monitoring and management in patients undergoing high risk surgery: A survey among North American and European anesthesiologists. Crit Care 2011; 15:R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller TE, Bunke M, Nisbet P, et al. Fluid resuscitation practice patterns in intensive care units of the USA: A cross-sectional survey of critical care physicians. Perioper Med (Lond) 2016; 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup: Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017; 43:304–377. [DOI] [PubMed] [Google Scholar]
- 40.Benes J, Giglio M, Brienza N, et al. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: A meta-analysis of randomized controlled trials. Crit Care 2014; 18:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kellum JA, Shaw AD. Assessing toxicity of intravenous crystalloids in critically ill patients. JAMA 2015; 314:1695–1697. [DOI] [PubMed] [Google Scholar]
- 42.Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med 2014; 42:1585–1591. [DOI] [PubMed] [Google Scholar]


