Abstract
Anti-cardiac troponin antibodies have been studied in different types of clinical diseases and in healthy populations. A systematic review of published data on anti-troponin antibodies was carried out (search performed on PubMed, ISI Web of Knowledge and Scopus databases). From title and abstract analysis, thirty-three articles were included that met the pre-specified criteria; after full-text analysis, nine articles were excluded. Most studies assessed anti-troponin I antibodies. The prevalence of anti-cardiac troponin antibodies in healthy individuals ranged from 0.0% to 20.0%. The prevalence of anti-troponin I autoantibodies in dilated cardiomyopathy (DCM) ranged from 7.0% to 22.2%. Other conditions under study were myocardial infarction, ischemic cardiomyopathy (ICM), peripartum cardiomyopathy (PPCM), Chagas disease, Emery-Dreifuss muscular dystrophy (EDMD) and renal transplantation. In the different patient populations studied, anti-cardiac troponin antibodies have been shown to be either positively or negatively associated with prognostic and clinical features. In what concerns a possible value as biomarkers, these assays have not emerged up to the present moment as important aids for practical clinical decisions in cardiac or other types of patients. In what concerns pathophysiology, anti-cardiac troponin autoantibodies may play a role in different diseases. It can be speculated that these antibodies could be involved in perpetuating some degree of cardiac injury after an event, such as myocardial infarction or PPCM.
Keywords: Anti-cardiac troponin antibodies, systematic review, cardiomyopathy, troponin
The major function of the heart is its contractile function, and cardiac muscle contains sarcomeres, which include different types of molecules, of which actin and myosin are essential for strength generation during contraction. Other molecules, such as cardiac troponins, play a role of regulation concerning cardiac muscle contraction. Cardiac troponins I and T have been shown to be different molecules than the corresponding ones in skeletal muscle, and have gained importance as cardiac biomarkers (1,2).
Research has shown that antibodies against cardiac troponins exist in different settings, and questions about their role in the cardiovascular pathological continuum have emerged over the last years. The possible role of cardiac troponin autoantibodies in disease processes [and particularly in dilated cardiomyopathy (DCM) and ischemic cardiomyopathy (ICM)] has gained interest (3-6).
Animal models in mice have demonstrated that anti-cardiac troponin I autoantibodies are capable of inducing heart dilatation and dysfunction, apparently through interactions with the calcium balance in cardiomyocytes (7). Okazaki et al. showed that in mice antibodies to cardiac troponin I stained the surface of cardiomyocytes, implying the presence of troponin I on the cell surface (differing from cardiac troponin T) (7). Göser et al. immunized mice with cardiac troponin I, leading to severe inflammation of the myocardium, cardiomegaly, fibrosis and 30% mortality over 270 days (8). However, in a study carried out in cultured neonatal rat ventricular myocytes, anti-cardiac troponin I autoantibodies obtained from human patients were unable to bind to cardiomyocytes or to influence calcium transients (9). Halley et al. described a heightened cellular interleukin-10 response of peripheral blood mononuclear cells to cardiac troponin I in a subset of patients with idiopathic DCM, in association with reduced systemic levels of C-reactive protein and a lower prevalence of advanced diastolic dysfunction (10).
Published data concerning anti-cardiac troponin antibodies has not yet provided a clear overall picture. For instance, conflicting data have been published concerning the impact on prognosis in heart disease (4). Leuschner et al. showed that the absence of autoantibodies against cardiac troponin I predicted an improvement of left ventricular function after an acute myocardial infarction (MI) (11). Doesch et al., however, described a beneficial effect of anti-troponin I autoantibodies in the setting of DCM (improved survival), but not in patients with ICM (12). Thus, although having been described over 20 years ago (initially as a source of potentially false-negative immunoassay results), the specific role of these autoantibodies, if any, remains elusive (13,14).
Autoantibodies can play different roles in autoimmune diseases. They can be useful for diagnostic purposes, as markers of disease activity and in some cases for establishing prognosis (15,16). B lymphocytes, responding to discrete changes in the balance between activation and inhibition signals, play a pivotal role in the production of autoantibodies (17,18). Activation of lymphocyte clones reactive against “self” antigens is an important step in the autoimmune response, and the ensuing collaboration between B and T lymphocytes seems critical in several disease entities (17-20). In addition to their role in the production of autoantibodies, B lymphocytes can present small fragments of peptides to T lymphocytes, and in an appropriate context lead to activation of these clones reactive against “self” antigens (21). Several factors can be involved in the modulation of the autoimmune response, including hormonal, immunological, genetic (both MHC and non-MHC genes) and environmental (namely smoking, diet and the presence of infectious agents such as the Epstein-Barr virus and Cytomegalovirus) (22). Also, the age and sex of the individual in question are relevant considerations (22). Interestingly, the CD4/CD8 ratio can influence the immune response (namely the response to certain infectious agents), being itself influenced by the hormonal environment (22). Also involved in altered lymphocyte response (and more broadly immune response) are different receptors and proteins which are able to influence immunological mechanisms (23-26). In addition to these mechanisms, the cytokine milieu (and specifically imbalances in their expression) is also of relevance in the regulation of inflammatory and autoimmune mechanisms (27).
However, the production of autoantibodies implies that the immune system fails to recognize some antigens as “self”. The induction of thymic tolerance is indispensable for the self-regulation of the immune system and the induction of self-antigen tolerance. This complex regulation is achieved by the elimination of high affinity self-reactive T cells and by the stimulation of T lymphocyte clones acting as inhibitors of the autoimmune response (namely regulatory T lymphocytes). This control is genetically driven, usually in polyclonal form (28), but as previously mentioned may also be influenced by different factors (22). The loss of this mechanism of tolerance (which can lead to the so-called ‘friendly fire’ and the development of autoimmune disease) can affect different individuals in the general population (29).
Given the present data, addressing the importance of anti-cardiac troponin autoantibodies is of relevance. In the present report, we aimed to systematically review the data concerning the prevalence of anti-cardiac troponin autoantibodies (both against cardiac troponin I and T) in different human clinical contexts (including apparently healthy individuals), and its possible clinical significance.
Methods
Search strategy
The study started with a search on three databases, Medline (PubMed), ISI Web of Knowledge and Scopus, using the queries “anti-troponin AND antibody”, “antitroponin AND antibody”, “anti troponin AND antibody”, “anti-troponin AND antibodies”, “antitroponin AND antibodies”, “anti troponin AND antibodies”. The search took place between June and July 2016, and no articles were excluded based on publication date. The aim of our search was to identify studies evaluating the presence of anti-troponin antibodies in different clinical contexts. The queries resulted in 523 articles on the PubMed database, 884 on ISI Web of Knowledge and 876 on Scopus. Additional studies were found after searching the references of previous review articles and other relevant sources, including articles related to the topic in question as well as articles citing the selected articles (allowing the inclusion of a paper published in 2016 with publication date 2017).
Inclusion criteria
Only human studies were included, and both observational and interventional studies were considered within the scope of this review. Both anti-troponin I and T autoantibodies were considered relevant for the purposes of this review.
Exclusion criteria
Articles written in languages other than English, as well as mechanistic and animal studies, were excluded. Case reports and studies containing less than ten subjects were also excluded.
Articles concerning mainly the characterization of anti-cardiac troponin autoantibodies (rather than focusing on assessing their presence on a given context), although of relevance, were considered outside the scope of this review, which aimed at addressing the prevalence and significance of anti-cardiac troponin autoantibodies.
Summary measure
We aimed at presenting an overview of studies assessing anti-cardiac troponin autoantibodies in different clinical contexts (qualitative synthesis). The primary summary measure (quantitative synthesis) in this analysis was the determination of the number of individuals with detectable anti-troponin antibodies. The number of participants in some studies was calculated from the published value corresponding to the percentage. In order to be included in the quantitative synthesis the article had to specifically define the number of individuals with detectable (i.e., above a pre-defined threshold) anti-troponin autoantibodies.
Quality assessment of studies and data extraction
Study quality and eligibility were individually assessed by four investigators. Different opinions regarding the relevance of articles were solved by consensus between the authors. In the case of more than one report from the same research group, data from each report were assessed separately, whenever the authors did not indicate that the same cohort was being studied.
Results
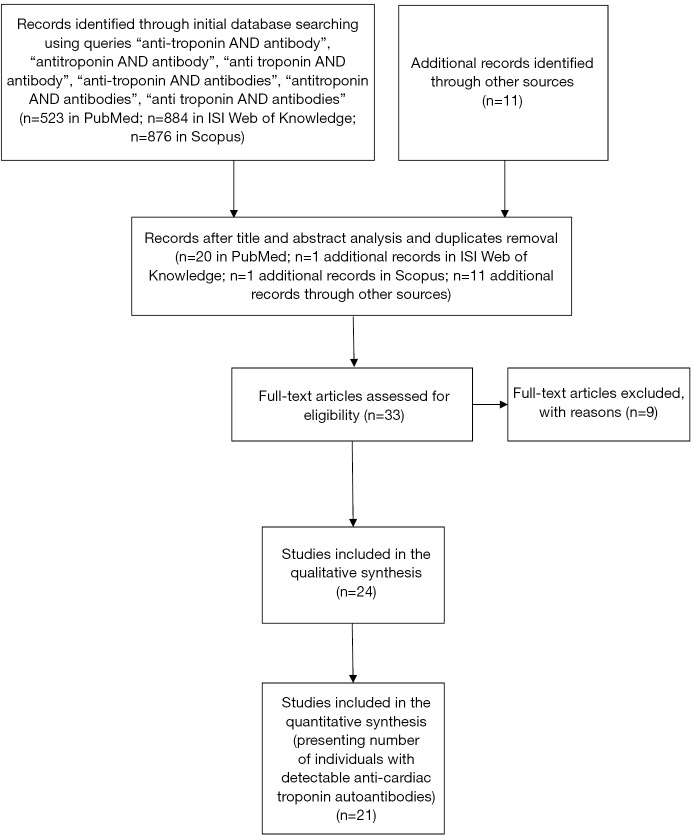
From title and abstract analysis, thirty-three articles were included that met the pre-specified criteria, and this set of articles was analyzed by the authors. After full-text analysis, nine articles were excluded. Of these, six did not specify that anti-cardiac troponin was assessed (30-35), one did not specify that anti-troponin autoantibodies were assessed (referring only anti-heart autoantibodies) (36), and two were mainly concerned with the characterization of anti-troponin autoantibodies (37,38). A flowchart showing the literature search method, as well as the resulting number of articles selected, is displayed in Figure 1.
Figure 1.
Flowchart showing literature search method. n, number of articles.
The articles included in the qualitative synthesis were published between 2006 and 2016, and comprised a total of 10,229 individuals. Table 1 presents an overview of the articles included in the qualitative synthesis.
Table 1. Overview of trials meeting the pre-specified criteria, included in the qualitative synthesis.
| Study [year] | n | Male/female ratio | Mean age (years) | Clinical context | Anti-cardiac troponin autoantibody assessed | Analytical parameters assessed |
|---|---|---|---|---|---|---|
| Fan et al. (39) [2017] | 491 ST elevation MI patients + 166 healthy controls | 426/65 (patients); 144/22 (controls) | 57.31±10.61 (patients); 58.60±10.64 (controls) | ST-elevation MI | Anti-TnI | Anti-beta1-adrenoceptor autoantibodies; CK; CK-MB isoenzyme; hemoglobin; TC; HDLc; LDLc; TG |
| Haghikia et al. (40) [2015] | 70 PPCM patients + 50 healthy controls | – (all females) | 34±6 (test population) | PPCM | Anti-TnI | Anti-cardiac sarcomeric myosin autoantibodies; AST; ALT; creatinine; CK; prolactin CRP; hemoglobin; full blood count; cardiac troponin T; NT-proBNP; thyroid-stimulating hormone; TC |
| Nunes et al. (41) [2015] | 48 renal transplant recipients | 30/18 | 52.9±12.6 | Renal transplant | Anti-TnI | – |
| Savukoski et al. (42) [2014] | 510 suspected MI | 300/210 | – (data divided according to autoantibody status) | Suspected MI | Anti-TnI | – |
| Nunes et al. (43) [2013] | 131 Chagas disease patients + 45 controls (30 healthy + 15 ICM patients) | 68/63 (test population) | 49±13 (test population) | Chagas disease (different clinical forms) | Anti-TnT | Anti-myosin autoantibodies; anti-trypanosoma cruzi antibodies |
| Erer et al. (44) [2013] | 50 VH/NC (24 normal EF/26 reduced EF) + 23 healthy controls | 10/14 (test population normal EF); 21/5 (test population reduced EF); 10/13 (controls) | 36±16.8 (test population normal EF); 37±16.2 (test population reduced EF); 34±10.0 (controls) | VH/NC (normal vs. reduced EF) | Anti-TnI Anti-TnT |
Cardiac troponin I; cardiac troponin T |
| Tang et al. (45) [2012] | 121 acute MI patients + 210 healthy controls | 77/44 (test population); 126/84 (controls) | 67 (median; test population); 52 (controls) | Acute MI | Anti-TnI | – |
| Doesch et al. (12) [2011] | 249 DCM patients + 141 ICM patients | 190/59 (DCM); 128/13 (ICM) | 51.9±11.7 (DCM); 59.4±8.8 (ICM) | DCM and ICM | Anti-TnI | Hemoglobin; creatinine; CRP; sodium; NT-proBNP; osteopontin |
| Matsumori et al. (46) [2011] | 1,315 HF patients + 1,115 healthy controls | – | – | HF | Anti-TnI | – |
| Lappé et al. (47) [2011] | 44 DCM + 35 healthy controls | 29/15 (DCM); 16/19 (controls) | 52±13 (DCM); 47±14 (healthy controls) | DCM | Anti-TnI | Cardiac troponin I, BNP; high-sensitivity CRP; myeloperoxidase; creatinine |
| Düngen et al. (48) [2010] | 138 HF patients + 300 healthy controls | 66/72 (test population); 150/150 (controls) | 73.0±5.6 (test population); 40 (median; controls) | HF (elderly) | Anti-TnI | Creatinine; LDLc; cardiac troponin I; NT-proBNP |
| Adamczyk et al. (49) [2010] | 345 normal blood donors | – (data divided according to autoantibody status) | – (data divided according to autoantibody status) | Samples from blood donors | Anti-TnI Anti-TnT |
– |
| Lindahl et al. (50) [2010] | 957 non-ST elevation ACS | 688/269 | – (data divided according to autoantibody status) | Non-ST elevation ACS | Anti-TnI | Creatinine; high-sensitivity CRP; cardiac troponin I; NT-proBNP |
| Pettersson et al. (51) [2009] | 81 non-ST elevation ACS | 59/22 | 64.3±9.7 | Non-ST elevation ACS | Anti-TnI | CK-MB isoenzyme; high-sensitivity CRP; NT-proBNP; cardiac troponin I; cardiac troponin T |
| Adamczyk et al. (52) [2009] | 467 healthy blood donors | 219/248 | 39.7 | Samples from blood donors | Anti-TnT | – |
| Adamczyk et al. (14) [2009] | 173 cardiac troponin I positive + 200 BNP positive + 264 Chagas disease patients + 200 hepatitis C virus positive + 50 hepatitis B virus positive + 136 systemic lupus erythematosus patients + 137 rheumatoid factor positive + 750 healthy blood donors | – (data divided according to subject groups) | 71.0 (cardiac troponin I positive); 79.0 (BNP positive); 41.9 (Chagas disease); 42.8 (hepatitis C virus positive); – (hepatitis B virus positive); 55.3 (systemic lupus erythematosus); 53.9 (rheumatoid factor positive); 37.8 (healthy controls) | Samples from populations tested positive for cardiac troponin I, BNP, Chagas disease, hepatitis C virus, hepatitis B virus, systemic lupus erythematosus, rheumatoid factor. Samples from healthy blood donors | Anti-TnI | Anti-double stranded DNA; anti-hepatitis B virus antibodies; anti-hepatitis C virus antibodies; hepatitis C virus viral load; BNP; cardiac troponin I; rheumatoid factor |
| Doesch et al. (53) [2009] | 27 DCM | 24/3 | 57.0±9.7 | DCM (submitted to immunoadsorption) | Anti-TnI | Creatinine; urea; CRP; sodium; potassium; calcium; HDLc; LDLc; TG; TC hemoglobin; hematocrit; glucose; NT-proBNP; total immunoglobulin G |
| Niebroj-Dobosz et al. (54) [2008] | 10 EDMD patients + 10 healthy controls | 6/4 (test population) | – | EDMD (autosomal dominant and X-linked) | Anti-TnI | – |
| Leuschner et al. (11) [2008] | 272 DCM patients + 185 ICM patients + 108 acute MI patients + 10 healthy athletes | – (DCM and ICM); 88/20 (acute MI); 10/0 (athletes) | – (DCM and ICM); 60.1±11.3 (acute MI); 52 (median; athletes) | DCM and ICM; acute MI; athletes | Anti-TnI Anti-TnT |
Cardiac troponin T; NT-proBNP; LDLc; TC; glycated hemoglobin; (data available for acute MI group) |
| Baba (55) [2008] | 104 DCM patients + 10 ICM patients | 81/23 (DCM); 2/8 (ICM) | – (DCM); 56±10 (ICM) | DCM | Anti-TnI | Anti-Na+K+ATPase autoantibodies; anti-beta 1 adrenoceptor autoantibodies; anti-muscarinic M2 acetylcholine receptor autoantibodies; ANP; norepinephrine |
| Landsbergeret al. (56) [2008] | 98 DCM patients + 49 ICM patients + 98 normal left ventricular function controls | 76/22 (DCM); 43/6 (ICM); 80/18 (controls) | 52±11 (DCM); 61±11 (ICM); 55±11 (controls) | DCM and ICM | Anti-TnI | Anti-beta 1 adrenoceptor autoantibodies; anti-Kv channel-interacting protein 2.6 autoantibodies; viral genomes (DCM group) |
| Miettinen et al. (57) [2008] | 95 DCM patients | 72/23 | 56 (median) | DCM | Anti-TnI | Aldosterone; epinephrine; norepinephrine; high-sensitivity CRP; interleukin-6; interleukin-10; cardiac troponin I; NT-proANP; NT-proBNP; renin; tumor necrosis factor-α |
| Shmilovich et al. (9) [2007] | 32 DCM patients + 33 ICM patients + 42 healthy controls | 22/10 (DCM); 27/6 (ICM); 13/29 (controls) | 63.3±2.3 (DCM); 71.2±1.8 (ICM); 33.7 (controls) | DCM and ICM | Anti-TnI | Hemoglobin |
| Niebroj-Dobosz et al. (58) [2006] | 14 EDMD patients + 10 DCM patients + 10 healthy controls | – | – | EDMD (autosomal dominant and X-linked) | Anti-TnI | Anti-tropomyosin autoantibodies; Anti-actin autoantibodies; CK; CK-MB isoenzyme (test population) |
ACS, acute coronary syndrome; ALT, alanine aminotransferase; ANP, atrial natriuretic peptide; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; CK, creatine kinase; CRP, C-reactive protein; DCM, dilated cardiomyopathy; EDMD, Emery-Dreifuss muscular dystrophy; EF, ejection fraction; HDLc, high-density lipoprotein cholesterol; HF, heart failure; ICM, ischemic cardiomyopathy; LDLc, low-density lipoprotein cholesterol; MI, myocardial infarction; n, number of individuals; NT-proANP, N-terminal pro-hormone of atrial natriuretic peptide; NT-proBNP, N-terminal pro-hormone of brain natriuretic peptide; PPCM, peripartum cardiomyopathy; TC, total cholesterol; TG, triglycerides; VH/NC, ventricular hypertrabeculation/noncompaction.
The articles included in the quantitative synthesis were also published between 2006 and 2016, and comprised a total of 7,406 individuals. Table 2 presents an overview of the articles included in the quantitative synthesis.
Table 2. Overview of studies presenting number of individuals with presence of anti-cardiac troponin autoantibodies.
| Study [year] | Proportion (and percentage) of individuals with detectable anti-cardiac troponin autoantibodies, assessed at different time points according to different studies |
|---|---|
| Fan et al. (39) [2017] | 94/491 (19.1%) ST-elevation MI patients |
| Haghikia et al. (40) [2015] | 18/70 (25.7%) PPCM patients |
| 3/50 (6.0%) healthy controls | |
| Nunes et al. (41) [2015]* | 18/48 (37.5%) renal transplant recipients |
| Savuskoski et al. (42) [2014] | 37/510 (7.3%) suspected MI patients (old assay) |
| 47/510 (9.2%) suspected MI patients (new assay) | |
| Nunes et al. (43) [2013] | 78/131 (59.5%) Chagas disease patients |
| 0/15 (0.0%) ICM patients | |
| Tang et al. (45) [2012]** | 18/121 (14.9%) AMI patients (assessed by ELISA) |
| 13/121 (10.7%) AMI patients (assessed by WB) | |
| 1/210 (0.5%) healthy controls (assessed by ELISA) | |
| 0/210 (0.0%) healthy controls (assessed by WB) | |
| Doesch et al. (12) [2011] | 43/249 (17.3%) DCM patients |
| 30/141 (21.3%) ICM patients | |
| Lappé et al. (47) [2011] | 6/44 (13.6%) DCM patients |
| 7/35 (20.0%) healthy controls | |
| Düngen et al. (48) [2010] | 12/138 (8.7%) HF patients at baseline |
| 20/138 (14.5%) HF patients at follow-up (median 85 days) | |
| 28/300 (9.3%) healthy controls | |
| Adamczyk et al. (49) [2010] | 33/345 (9.6%) normal blood donors (anti-TnI autoantibodies) |
| 28/345 (8.1%) normal blood donors (anti-TnT autoantibodies) | |
| Lindahl et al. (50) [2010] | 7/957 (0.7%) non-ST elevation ACS patients only at baseline |
| 42/957 (4.4%) non-ST elevation ACS patients only at 6 months | |
| 62/957 (6.5%) non-ST elevation ACS patients at baseline and 6 months | |
| 111/957 (11.6%) non-ST elevation ACS patients considering any time point | |
| Pettersson et al. (51) [2009] | 9/81 (11.1%) non-ST elevation ACS patients at baseline |
| 12/81 (14.8%) non-ST elevation ACS patients at 12 months | |
| 14/81 (17.3%) non-ST elevation ACS patients considering any time point | |
| Adamczyk et al. (52) [2009] | 46/467 (9.9%) healthy blood donors |
| Adamczyk et al. (14) [2009] | 18 /173 (10.4%) cardiac troponin I positive individuals |
| 21/200 (10.5%) brain natriuretic peptide positive individuals | |
| 31/264 (11.7%) Chagas disease patients | |
| 27/200 (13.5%) hepatitis C virus positive individuals | |
| 7/50 (14.0%) hepatitis B virus positive individuals | |
| 13/136 (9.6%) systemic lupus erythematosus individuals | |
| 28/137 (20.4%) rheumatoid factor positive individuals | |
| 95/750 (12.7%) healthy blood donors | |
| Doesch et al. (53) [2009] | 6/27 (22.2%) DCM at baseline |
| 1/27 (3.7%) DCM after immunoadsorption | |
| 4/27 (14.8%) DCM at 6 months | |
| Niebroj-Dobosz et al. (54) [2008] | 10/10 (100.0%) EDMD patients at baseline |
| 10/10 (100.0%) EDMD patients at follow-up (1–6 years after diagnosis) | |
| Leuschner et al. (11) [2008] | 19/272 (7.0%) DCM patients (anti-TnI autoantibodies) |
| 5/272 (1.8%) DCM (anti-TnT autoantibodies) | |
| 17/185 (9.2%) ICM patients (anti-TnI autoantibodies) | |
| 1/185 (0.5%) ICM patients (anti-TnT autoantibodies) | |
| 10/108 (9.3%) AMI patients at baseline | |
| 10/108 (9.3%) AMI patients at follow-up (6–9 months) | |
| 0/10 (0.0%) healthy athletes (assessed at multiple timepoints) | |
| Landsberger et al. (56) [2008] | 20/98 (20.4%) DCM patients |
| 9/49 (18.4%) ICM patients | |
| 4/98 (4.1%) controls with normal left ventricular function | |
| Miettinen et al. (57) [2008] | 9/95 (9.5%) DCM patients at baseline |
| 7/95 (7.4%) DCM patients at follow-up (median 4.1 years) | |
| 15/95 (15.8%) DCM patients at any time point | |
| Shmilovich et al. (9) [2007] | 5/32 (15.6%) DCM patients |
| 6/33 (18.2%) ICM patients | |
| 0/42 (0.0%) healthy controls | |
| Niebroj-Dobosz et al. (58) [2006] | 14/14 (100.0%) EDMD patients |
| 10/10 (100.0%) DCM patients | |
| 0/10 (0.0%) healthy controls |
*, considered if titre ≥1:40; **, western blot analysis performed on subgroup of individuals (those positive for anti-cardiac troponin I with ELISA and 19 additional negative healthy controls). ACS, acute coronary syndrome; AMI, acute myocardial infarction; DCM, dilated cardiomyopathy; EDMD, Emery-Dreifuss muscular dystrophy; ELISA, enzyme-linked immunosorbent assay; HF, heart failure; ICM, ischemic cardiomyopathy; MI, myocardial infarction; PPCM, peripartum cardiomyopathy; WB, western blot.
Most studies assessed anti-troponin I antibodies (9,12,14,39-42,45-48,50,51,53-58), whereas anti-troponin T antibodies were assessed in only 16% (n=1,636) of individuals (11,43,44,49,52) (including 993 individuals where both types of autoantibodies were evaluated) (11,44,49).
Over half of the studies included assessed individuals with cardiovascular diseases (9,11,12,14,39,40,44-48,50,51,53,55-57), especially DCM. The prevalence of anti-troponin I autoantibodies in DCM ranged from 7.0% (11) to 22.2% (in patients prior to immunoadsorption, notably decreased to 3.7% after immunoadsorption) (53). Only one study presented data concerning anti-troponin T autoantibodies in DCM (11). Importantly, a total of 3,661 healthy (or presumably healthy) individuals were also assessed, though mainly as control groups (9,11,14,39,40,43-49,52,54,56,58).
Healthy volunteers
The current literature included a total of 3,661 healthy (or presumably healthy) individuals. Of these, 2,317 were included in the quantitative synthesis (Table 2) (9,11,14,40,45,47-49,52,56,58). The prevalence of anti-troponin antibodies in healthy individuals ranged from 0.0% to 20.0% for anti-troponin I (9,11,14,45,47,58) and from 0.0% to 9.9% for anti-troponin T (11,49,52).
Emery-Dreifuss muscular dystrophy (EDMD)
Two studies assessed anti-cardiac troponin I autoantibodies in the context of EDMD (54,58). In these studies, anti-cardiac troponin I autoantibodies were present in all patients with EDMD (X-linked and autosomal dominant-type). The investigators reported on a significant difference between the levels of anti-cardiac troponin I autoantibodies among both forms of the disease, these being significantly higher in the X-linked type (54). Interestingly, although persistently detectable, at follow-up the levels of these autoantibodies were decreasing in the X-linked type, whereas they were increasing in the autosomal dominant-type. In neither study, however, were levels of anti-cardiac troponin I autoantibodies significantly correlated with cardiovascular symptoms.
Myocardial infarction
Leuschner et al. studied 108 patients with acute MI (both with or without ST segment elevation) (11). Ten patients had anti-cardiac troponin I with IgG titres ≥1:160. Patients without anti-cardiac troponin I autoantibodies showed a significant increase in left ventricular ejection fraction 6–9 months after the event, in contrast to those where these autoantibodies were detected (11). Interestingly, in this study, all patients positive for anti-cardiac troponin I autoantibodies presented positivity at baseline (while none of the remaining patients developed autoantibodies throughout the follow-up).
In the context of non-ST elevation acute coronary syndromes (ACS), the presence of anti-cardiac troponin I autoantibodies was associated with higher troponin I release (50,51). Lindahl et al. studied 957 patients with non-ST elevation ACS, with outcomes assessed through 5 years. In this study, despite being associated (as previously described) with chronically elevated troponin concentrations, anti-cardiac troponin autoantibodies were not independently associated with death and MI during follow-up (50).
In a recent study, Fan et al. studied both anti-beta1 adrenoceptor and anti-cardiac troponin I autoantibodies in patients with ST-elevation acute MI (39). Both types of autoantibodies were independent predictors of left ventricular remodeling, whereas only the first type of antibody was an independent predictor of major adverse cardiovascular events (39). Savukoski et al. reported that the presence of anti-cardiac troponin I autoantibodies was not correlated with 12-month outcome (studying a population of 510 individuals presenting with suspected MI, of which 167 had the diagnosis confirmed) (42).
Heart failure, including DCM and ICM
Several studies assessed individuals with DCM and ICM. On the first published report concerning this clinical setting, Shmilovich et al. studied anti-cardiac troponin I autoantibodies in patients with idiopathic DCM and with ICM (9). IgG antibodies were detected more frequently in both groups of patients, when compared to controls (9) (Table 2).
On a seminal study, Leuschner et al. measured both anti-cardiac troponin I and T autoantibodies in 272 patients with DCM and 185 with ICM, with 7.0% and 9.2% of patients having anti-troponin I IgG antibody titre ≥1:160, respectively (11). Anti-troponin T autoantibodies were found much more rarely (11).
Baba studied 104 patients with DCM, and found that cardio-depressant autoantibodies (defined as those able to lead to a significant depression of left ventricular ejection fraction) were similarly found in patients with and without antibodies against troponin I (55).
Landsberger et al. studied anti-cardiac troponin I autoantibodies in patients with DCM and ICM. In accordance with previous data, in both cases these antibodies were more frequently detected than in a control population (56) (Table 2).
Miettinen et al. studied 95 patients with idiopathic DCM (57). The presence of anti-cardiac troponin I autoantibodies was not associated with patients’ clinical status or outcome, in contrast to findings related to cardiac troponin I (57).
Düngen et al. studied anti-cardiac troponin I autoantibodies in elderly HF patients, as well as the effect of treatment with beta-blockers. At baseline levels of anti-cardiac troponin antibodies were not significantly different between HF patients and controls (0.56 vs. 0.53 relative value units, respectively), but increased after beta-blocker titration. The antibody values were not associated with the severity of HF, and there was no correlation between levels of these antibodies and cardiac troponin I (48).
Doesch et al. [2009] studied anti-troponin I antibodies in 27 DCM patients, as well as the effects of immunoadsorption (53). Mean left ventricular ejection fraction was not significantly improved overall, but an increase in exercise capacity was seen after immunoadsorption (53). In this study the prevalence of anti-troponin autoantibodies changed during follow-up. After immunoadsorption the number of individuals with autoantibodies decreased (from 6/27 at baseline to 1/27), though 6 months after this therapeutic intervention the number increased again (to 5/27).
In another study, Doesch et al. [2011] studied the prognostic impact of anti-troponin I antibodies in HF patients (12). The authors reported superior survival in patients with DCM when compared to ICM, and the presence of autoantibodies in plasma was associated with an improved survival in patients with chronic DCM (though not in ICM) (12).
Matsumori et al. studied anti-cardiac troponin I autoantibodies in HF patients, including patients with myocarditis with or without HCV infection (46). Elevated antibody titres were detected in those with myocarditis, and were even higher in HCV-infected patients (46).
Peripartum cardiomyopathy (PPCM)
Haghikia et al. studied a group of 70 patients with PPCM. The presence of autoantibodies to either cardiac sarcomeric myosin or troponin I was associated with a significantly lower baseline left ventricular ejection fraction and lower rate of full cardiac recovery at follow-up (6±2 months) (40). In this study, patients with anti-cardiac troponin I antibodies also had more frequent pericardial effusion (40).
Chagas disease
Two studies presented data concerning anti-cardiac troponin autoantibodies in Chagas disease (14,43). In one study, Nunes et al. assessed the possible impact of anti-troponin T autoantibodies in patients with different clinical forms of Chagas disease (43). The investigators showed that in the chronic phase of Chagas disease, total IgG anti-troponin T was correlated with left ventricular end-systolic dimension and had a negative correlation with left ventricular ejection fraction. However, similarly high levels of anti-troponin T antibodies were seen when patients with cardiac disease were compared to indeterminate forms of the disease (43).
In another study (14), Adamczyk et al. presented data from samples from clinically diagnosed Chagas disease subjects. In this latter study, 11.7% of individuals presented anti-cardiac troponin I autoantibodies, a value not significantly different from the positive rate in healthy controls (see Table 2 for details).
Left ventricular hypertrabeculation/non-compaction (VH/NC)
Erer et al. assessed anti-troponin autoantibodies (IgM and IgG for troponin T, only IgM for troponin I) in patients with VH/NC (44). The authors reported a correlation between anti-troponin I autoantibodies and troponin I, whereas there was no significant correlation between anti-troponin T autoantibodies and troponin T. Contrary to anti-troponin T autoantibodies, which were elevated only in patients with systolic dysfunction, levels of anti-troponin I autoantibodies were elevated both in the presence and absence of systolic dysfunction. There was no significant correlation between autoantibody levels and left ventricular ejection fraction, number of segments with hypertrabeculation/non-compaction, end-diastolic or end-systolic volume.
Renal transplantation
One study presented data regarding the prevalence of anti-troponin I antibodies in a group of 48 renal transplant patients under immunosuppressive therapy (41). The presence of an anti-troponin I antibody titre ≥1:80 was not associated with the presence of clinical cardiac disease, but was associated with statin therapy status, being less frequent in patients under statin therapy.
Discussion
We have reviewed the available evidence concerning anti-cardiac troponin autoantibodies in different clinical scenarios. A plethora of conditions has been presented, ranging from healthy (or presumably healthy) subjects to different degrees of cardiovascular and non-cardiovascular pathology. In this review, the prevalence of anti-cardiac troponin antibodies ranged from 0.0% to 100% (in the different groups under study—see Table 2).
The role of autoimmunity in heart disease has attracted attention (3,4). Theoretically, autoantibodies against different cardiac antigens could be involved in different dimensions of physiopathology, such as having a causative role in disease processes, lead to aggravation of concomitant or pre-existent cardiovascular disease, act as an indicator of disease (not exclusively cardiac disease) or be an epiphenomenon without a causal relationship to cardiac disease.
Research has shown that autoantibodies against different cardiac antigens (such as beta-1 adrenoceptors) can have an important role in the pathophysiology of heart diseases, namely HF (4,56). In a recent study in the setting of ST-elevation acute MI, beta-1 adrenoceptor autoantibodies were associated with adverse outcomes (being an independent predictor of major adverse cardiovascular events) (39). Evermore sensitive assays for cardiac troponins have demonstrated that these biomarkers may be detectable in several clinical conditions, as well as in a significant proportion of healthy subjects (59,60). Anti-cardiac troponin autoantibodies have also been described in different settings (namely in healthy subjects and after MI) (6). Although the possible role of autoimmunity mediated by autoantibodies against cardiac troponin has raised interest, this phenomenon has still not been fully elucidated (3,4).
Anti-cardiac troponin antibodies were reported in some healthy individuals. In light of this data, it appears that the presence of these antibodies per se does not necessarily imply the presence of clinical disease. The mechanisms by which healthy individuals develop anti-troponin autoantibodies, however, are not yet fully characterized. Although subclinical cardiovascular disease could be present in at least some individuals (11,14), in some studies the prevalence of autoantibodies did not differ from that of the cardiac disease group (47,48). Moreover, the mechanisms explaining the wide range of positivity (0.0% to 20.0%) should also be addressed (some of which are detailed below). As new high-sensitivity assays reveal that in different physiological activities increased levels of cardiac troponins (and other cardiac biomarkers) may be detectable in apparently healthy individuals (61,62), it would be interesting to explore if there is an association between these two phenomena. The long-term meaning of the presence of anti-cardiac troponin autoantibodies (and their prevalence, throughout time, in a given healthy population) is still not ascertained, and future studies should address this relevant issue.
One issue worth considering would be the possibility of a given type of heart disease (with myocardial injury and possible release of self-antigens) initiating (or modulating) further pathological phenomena. Regardless of the origin of the myocardial insult, it may initiate a series of autoimmune-like immunological mechanisms mediated either by humoral or cellular immunity, and thus perpetuate myocardial injury. Although this notion seems plausible, the immunological mechanisms involved in the induction, modulation or severity of heart disease are not yet known (3). As previously described, in an animal model the administration of anti-cardiac troponin I antibodies induced inflammation of the myocardium, dilation of cardiac chambers with fibrosis and a higher mortality (8). In addition, the inoculation of T lymphocytes specific for cardiac troponin I in another animal model induced inflammation, fibrosis and impairment of cardiac contractility (63). In situations of myocardial ischemia the release of cardiac molecules (self-antigens) may occur and these, if recognized by the immune system, may initiate mechanisms of autoimmunity. The presence of these autoantibodies could induce direct cell injury and apoptosis, as well as having a cytotoxic action by means of complement activation (5,7).
In EDMD (54,58), anti-troponin I autoantibodies were present in all patients studied, however their levels were not significantly correlated with cardiovascular symptoms. Cardiac involvement in EDMD can have different manifestations (54). Whereas in some cases it presents primarily as a cardiac conduction defect, in others functional impairment of the ventricular myocardium is predominant (64). Although only a small number of patients is represented in the current literature, given the reported prevalence for anti-cardiac troponin autoantibodies, the role for these antibodies in EDMD (as well as the mechanisms behind the different kinetics observed in one of the studies, regarding different forms of this entity) deserves further clarification.
In light of the data presented in the setting of MI it can be said that anti-cardiac troponin I antibodies have not been shown, up to the present moment, to be associated to an adverse clinical prognosis in this context, although they have been shown to be associated with lack of recovery in left ventricular function, chronically elevated cardiac troponin concentrations and in certain subgroups with adverse left ventricular remodeling. Given these data, and the fact that there are still gaps in our knowledge, especially concerning remodelling mechanisms (6,39), a reappraisal of anti-cardiac troponin autoantibodies in this setting seems important.
In HF patients, anti-cardiac troponin antibodies were found to be more frequently seen than in controls in some but not all reports. A clear association between the presence of these antibodies and functional status was not apparent, and there was even a description of improved survival in patients with chronic DCM in association with the presence of cardiac troponin I autoantibodies (12). Interestingly, when studying myocarditis patients, Matsumori et al. compared the levels of anti-cardiac troponin I autoantibodies between those who satisfied Dallas criteria (n=88) and those who did not (n=1,227) (46). Although levels tended to be higher among those who satisfied the Dallas criteria, they did not reach significance. However, an additional analysis showed that individuals who satisfied Dallas criteria and had hepatitis C virus infection (n=5) had significantly higher autoantibody levels than those who did not have this viral infection (P<0.05) (46). As suggested by the authors, these findings highlight the possible interplay between infectious, inflammatory and immunological phenomena.
In PPCM (40), the presence of autoantibodies to cardiac troponin I was associated with a significantly lower baseline left ventricular ejection fraction and lower rate of full cardiac recovery at follow-up. Though a single study assessed this entity, data seems to point towards the importance of immunological mechanisms in this setting, thus warranting further studies.
Previous research showed that high-sensitivity cardiac troponin T was significantly higher in patients with Chagas cardiomyopathy when compared to a control population, and that troponin T value was correlated with the severity of the cardiomyopathy (65). Given the pathobiology of Chagas disease and the possible importance of cardiac troponin as a biomarker, it seems important to address the role of cardiac troponin autoantibodies in this disease. In a study of Chagas disease patients, similarly high levels of anti-cardiac troponin T autoantibodies were seen when patients with cardiac disease were compared to indeterminate forms of the disease (43). In another study (14), the prevalence of patients with anti-cardiac troponin I autoantibodies was not significantly different from that of healthy controls (11.7% vs. 12.7%, respectively). At the present moment, in Chagas disease, data seems to be insufficient to suggest a role for anti-cardiac troponin antibodies in the assessment of these patients (acting as biomarkers).
Only one study assessed patients with VH/NC (44), and given the results (vide supra) the role of anti-cardiac troponin autoantibodies in this pathology is yet to be fully characterized.
In renal transplant patients (under immunosuppressive therapy) (41), the presence of an anti-troponin I antibody titre ≥1:80 was not associated with clinical cardiac disease, but was associated with statin therapy status. This find is particularly peculiar, and although possible immunomodulatory statin effects may play a role in the mechanism behind these findings, other explanations (such as chance or a selection bias, as acknowledged by the authors) warrant further consideration. An additional case of renal transplant was previously described in which a partial reversion of DCM was seen in association with immunosuppressive therapy, in the presence of a high value for anti-troponin I antibodies (66).
Two concepts deserve further consideration. One relates to the interplay between different cell and immunoglobulin subtypes. Understanding the complex interplay between IgM and IgG formation, as well as the relative significance between different cell types and cytokines, seems pivotal in order to adequately define the pathways by which immunological mechanisms may relate to cardiac disease (67-69). Only with a profound knowledge of these phenomena can adequate strategies of risk prediction (and possible therapeutic interventions) be properly developed (70).
A second pitfall relates to why certain individuals develop autoantibodies. As previously described, several mechanisms could be involved, namely genetic, environmental, hormonal and immunological. Studying the mechanisms which predispose to the formation of anti-cardiac troponin autoantibodies per se seems of relevance (15,22,71).
Study limitations
The heterogeneity of the data presented in this review is associated with several limitations in its interpretation. Firstly, different methods of assessing anti-cardiac troponin autoantibodies were used in different studies [although enzyme-linked immunosorbent assays (ELISA) methods were used in many, other methods such as chemiluminescent microplate assays were also present]. These may lead to discrepancies in the number of individuals with positive autoantibodies, as was shown in a study which compared ELISA with western blotting (45). This possible source of bias was also highlighted when comparing indirect and direct methods of assessing these autoantibodies (52), and in a comparison between two immunoassays (42). The fact that different cut-offs were used to define positivity for autoantibodies (such as a titre of 1:160 in the study by Leuschner et al. vs. a titre of 1:320 in the study by Niebroj-Dobosz et al.) is a factor which must be taken into consideration when assessing results (11,54). In fact, when considering a titre of 1:160 as a cut-off value, Leuschner et al. described a total of 7.0% and 9.2% of DCM and ICM (respectively) as being anti-cardiac troponin I autoantibody positive (as illustrated in Table 2), whereas if a titer of ≥1:40 was considered [as in other studies (41)] a total of 27.9% and 27.6% of DCM and ICM patients (respectively) would have been considered anti-cardiac troponin I autoantibody positive (11).
Furthermore, the assessments were made at different time points. Whereas in some studies autoantibodies were assessed after an acute MI, in others they were assessed after years of cardiovascular disease (such as DCM). As was described in several studies, levels of autoantibodies can vary over time (50,51). In addition, different clinical contexts were assessed, and should be taken into consideration (72). As put forward by Doesch et al., different risk factors play a role in distinct cardiac disorders (namely DCM and ICM) (12), a fact that has been previously highlighted (73). Also, the fact that medication could affect its values is worth considering in future research (41,48). As such, only with large patient groups with similar characteristics can solid conclusions be reached on such a complex issue.
Conclusions
Anti-cardiac troponin autoantibodies are present in a relevant proportion of individuals with cardiovascular disease, and are also detectable in other pathologies and in up to 20% of healthy individuals.
In the different patient populations studied, anti-cardiac troponin antibodies have been shown to be either positively or negatively associated with prognostic and clinical features. These assays have not emerged, up to the present moment, as important practical aids for clinical decisions in cardiac or other types of patients.
In what concerns pathophysiology, anti-cardiac troponin antibodies may play a role in different diseases. It can be speculated that these antibodies could be involved in perpetuating some degree of cardiac injury after an acute event, such as a myocardial infarction or PPCM. A possible role for immune modulation in this setting cannot be ruled out at the present stage, and should be the subject of future studies.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cummins B, Auckland ML, Cummins P. Cardiac-specific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction. Am Heart J 1987;113:1333-44. 10.1016/0002-8703(87)90645-4 [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012;33:2551-67. 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 3.Popp RL. Troponin: messenger or actor? J Am Coll Cardiol 2013;61:611-4. 10.1016/j.jacc.2012.11.024 [DOI] [PubMed] [Google Scholar]
- 4.Kaya Z, Leib C, Katus HA. Autoantibodies in heart failure and cardiac dysfunction. Circ Res 2012;110:145-58. 10.1161/CIRCRESAHA.111.243360 [DOI] [PubMed] [Google Scholar]
- 5.Nussinovitch U, Shoenfeld Y. Anti-troponin autoantibodies and the cardiovascular system. Heart 2010;96:1518-24. 10.1136/hrt.2010.195255 [DOI] [PubMed] [Google Scholar]
- 6.O’Donohoe TJ, Ketheesan N, Schrale RG. Anti-troponin antibodies following myocardial infarction. J Cardiol 2017;69:38-45. 10.1016/j.jjcc.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 7.Okazaki T, Tanaka Y, Nishio R, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med 2003;9:1477-83. 10.1038/nm955 [DOI] [PubMed] [Google Scholar]
- 8.Göser S, Andrassy M, Buss SJ, et al. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation 2006;114:1693-702. 10.1161/CIRCULATIONAHA.106.635664 [DOI] [PubMed] [Google Scholar]
- 9.Shmilovich H, Danon A, Binah O, et al. Autoantibodies to cardiac troponin I in patients with idiopathic dilated and ischemic cardiomyopathy. Int J Cardiol 2007;117:198-203. 10.1016/j.ijcard.2006.04.077 [DOI] [PubMed] [Google Scholar]
- 10.Halley CM, Lappe JM, Cotleur AC, et al. Antiinflammatory autoimmune cellular responses to cardiac troponin I in idiopathic dilated cardiomyopathy. J Card Fail 2011;17:359-65. 10.1016/j.cardfail.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leuschner F, Li J, Goser S, et al. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur Heart J 2008;29:1949-55. 10.1093/eurheartj/ehn268 [DOI] [PubMed] [Google Scholar]
- 12.Doesch AO, Mueller S, Nelles M, et al. Impact of troponin I-autoantibodies in chronic dilated and ischemic cardiomyopathy. Basic Res Cardiol 2011;106:25-35. 10.1007/s00395-010-0126-z [DOI] [PubMed] [Google Scholar]
- 13.Bohner J, von Pape KW, Hannes W, et al. False-negative immunoassay results for cardiac troponin I probably due to circulating troponin I autoantibodies. Clin Chem 1996;42:2046. [PubMed] [Google Scholar]
- 14.Adamczyk M, Brashear RJ, Mattingly PG. Circulating cardiac troponin-I autoantibodies in human plasma and serum. Ann N Y Acad Sci 2009;1173:67-74. 10.1111/j.1749-6632.2009.04617.x [DOI] [PubMed] [Google Scholar]
- 15.Shoenfeld Y, Blank M, Abu-Shakra M, et al. The mosaic of autoimmunity: prediction, autoantibodies, and therapy in autoimmune diseases--2008. Isr Med Assoc J 2008;10:13-9. [PubMed] [Google Scholar]
- 16.Fritzler MJ. Challenges to the use of autoantibodies as predictors of disease onset, diagnosis and outcomes. Autoimmun Rev 2008;7:616-20. 10.1016/j.autrev.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Zenewicz LA, Abraham C, Flavell RA, et al. Unraveling the genetics of autoimmunity. Cell 2010;140:791-7. 10.1016/j.cell.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson SW, Kolhatkar NS, Rawlings DJ. B cells take the front seat: dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr Opin Immunol 2015;33:70-7. 10.1016/j.coi.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 2006;6:205-17. 10.1038/nri1786 [DOI] [PubMed] [Google Scholar]
- 20.Wellmann U, Letz M, Herrmann M, et al. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A 2005;102:9258-63. 10.1073/pnas.0500132102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noorchashm H, Lieu YK, Noorchashm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol 1999;163:743-50. [PubMed] [Google Scholar]
- 22.Perricone C, Agmon-Levin N, Ceccarelli F, et al. Genetics and autoantibodies. Immunol Res 2013;56:206-19. 10.1007/s12026-013-8396-9 [DOI] [PubMed] [Google Scholar]
- 23.Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med 2011;208:2033-42. 10.1084/jem.20110200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamagna C, Hu Y, DeFranco AL, et al. B cell-specific loss of Lyn kinase leads to autoimmunity. J Immunol 2014;192:919-28. 10.4049/jimmunol.1301979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyogoku C, Langefeld CD, Ortmann WA, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet 2004;75:504-7. 10.1086/423790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozyrev SV, Abelson AK, Wojcik J, et al. Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nat Genet 2008;40:211-6. 10.1038/ng.79 [DOI] [PubMed] [Google Scholar]
- 27.Sweet RA, Lee SK, Vinuesa CG. Developing connections amongst key cytokines and dysregulated germinal centers in autoimmunity. Curr Opin Immunol 2012;24:658-64. 10.1016/j.coi.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 28.Abramson J, Husebye ES. Autoimmune regulator and self-tolerance - molecular and clinical aspects. Immunol Rev 2016;271:127-40. 10.1111/imr.12419 [DOI] [PubMed] [Google Scholar]
- 29.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun 2009;33:197-207. 10.1016/j.jaut.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruchala M, Kosowicz J, Baumann-Antczak A, et al. The prevalence of autoantibodies to: myosin, troponin, tropomyosin and myoglobin in patients with circulating triiodothyronine and thyroxine autoantibodies (THAA). Neuro Endocrinol Lett 2007;28:259-66. [PubMed] [Google Scholar]
- 31.Zamanou A, Tsirogianni A, Terzoglou C, et al. Anti-smooth muscle antibodies (ASMAs) and anti-cytoskeleton antibodies (ACTAs) in liver diseases: a comparison of classical indirect immunofluorescence with ELISA. J Clin Lab Anal 2002;16:194-201. 10.1002/jcla.10040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haralambous S, Blackwell C, Mappouras DG, et al. Increased natural autoantibody activity to cytoskeleton proteins in sera from patients with necrobiosis lipoidica, with or without insulin-dependent diabetes mellitus. Autoimmunity 1995;20:267-75. 10.3109/08916939508995704 [DOI] [PubMed] [Google Scholar]
- 33.Takaya M, Kawahara S, Namba T, et al. Antibodies against myofibrillar proteins in myasthenia gravis patients. Tokai J Exp Clin Med 1992;17:35-9. [PubMed] [Google Scholar]
- 34.Dighiero G, Lymberi P, Monot C, et al. Sera with high levels of anti-smooth muscle and anti-mitochondrial antibodies frequently bind to cytoskeleton proteins. Clin Exp Immunol 1990;82:52-6. 10.1111/j.1365-2249.1990.tb05402.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga K, Abe S, Hashimoto H, et al. Western-blotting method for detecting antibodies against human muscle contractile proteins in myositis. J Immunol Methods 1987;105:15-21. 10.1016/0022-1759(87)90409-1 [DOI] [PubMed] [Google Scholar]
- 36.Caforio AL, Brucato A, Doria A, et al. Anti-heart and anti-intercalated disk autoantibodies: evidence for autoimmunity in idiopathic recurrent acute pericarditis. Heart 2010;96:779-84. 10.1136/hrt.2009.187138 [DOI] [PubMed] [Google Scholar]
- 37.Eriksson S, Halenius H, Pulkki K, et al. Negative interference in cardiac troponin I immunoassays by circulating troponin autoantibodies. Clin Chem 2005;51:839-47. 10.1373/clinchem.2004.040063 [DOI] [PubMed] [Google Scholar]
- 38.Eriksson S, Junikka M, Laitinen P, et al. Negative interference in cardiac troponin I immunoassays from a frequently occurring serum and plasma component. Clin Chem 2003;49:1095-104. 10.1373/49.7.1095 [DOI] [PubMed] [Google Scholar]
- 39.Fan Y, Chen Y, Wan Z, et al. The prognostic value of autoantibodies against beta1-adrenoceptor and cardiac troponin-I for clinical outcomes in STEMI. J Cardiovasc Med (Hagerstown) 2017;18:34-41. 10.2459/JCM.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 40.Haghikia A, Kaya Z, Schwab J, et al. Evidence of autoantibodies against cardiac troponin I and sarcomeric myosin in peripartum cardiomyopathy. Basic Res Cardiol 2015;110:60. 10.1007/s00395-015-0517-2 [DOI] [PubMed] [Google Scholar]
- 41.Nunes JP, Sampaio S, Cerqueira A, et al. Anti-troponin I antibodies in renal transplant patients. Rev Port Cardiol 2015;34:85-9. 10.1016/j.repc.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 42.Savukoski T, Ilva T, Lund J, et al. Autoantibody prevalence with an improved immunoassay for detecting cardiac troponin-specific autoantibodies. Clin Chem Lab Med 2014;52:273-9. 10.1515/cclm-2013-0310 [DOI] [PubMed] [Google Scholar]
- 43.Nunes DF, Guedes PM, Andrade Cde M, et al. Troponin T autoantibodies correlate with chronic cardiomyopathy in human Chagas disease. Trop Med Int Health 2013;18:1180-92. 10.1111/tmi.12169 [DOI] [PubMed] [Google Scholar]
- 44.Erer HB, Guvenc TS, Kemik AS, et al. Troponin and anti-troponin autoantibody levels in patients with ventricular non-compaction. PLoS One 2013;8:e57648. 10.1371/journal.pone.0057648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang G, Wu Y, Zhao W, et al. Multiple immunoassay systems are negatively interfered by circulating cardiac troponin I autoantibodies. Clin Exp Med 2012;12:47-53. 10.1007/s10238-011-0141-x [DOI] [PubMed] [Google Scholar]
- 46.Matsumori A, Shimada T, Hattori H, et al. Autoantibodies against cardiac troponin I in patients presenting with myocarditis. CVD Prevention Control 2011;6:41-6. 10.1016/j.cvdpc.2011.02.004 [DOI] [Google Scholar]
- 47.Lappé JM, Pelfrey CM, Cotleur A, et al. Cellular proliferative response to cardiac troponin-I in patients with idiopathic dilated cardiomyopathy. Clin Transl Sci 2011;4:317-22. 10.1111/j.1752-8062.2011.00313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Düngen HD, Platzeck M, Vollert J, et al. Autoantibodies against cardiac troponin I in patients with congestive heart failure. Eur J Heart Fail 2010;12:668-75. 10.1093/eurjhf/hfq088 [DOI] [PubMed] [Google Scholar]
- 49.Adamczyk M, Brashear RJ, Mattingly PG. Coprevalence of autoantibodies to cardiac troponin I and T in normal blood donors. Clin Chem 2010;56:676-7. 10.1373/clinchem.2009.138099 [DOI] [PubMed] [Google Scholar]
- 50.Lindahl B, Venge P, Eggers KM, et al. Autoantibodies to cardiac troponin in acute coronary syndromes. Clin Chim Acta 2010;411:1793-8. 10.1016/j.cca.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 51.Pettersson K, Eriksson S, Wittfooth S, et al. Autoantibodies to cardiac troponin associate with higher initial concentrations and longer release of troponin I in acute coronary syndrome patients. Clin Chem 2009;55:938-45. 10.1373/clinchem.2008.115469 [DOI] [PubMed] [Google Scholar]
- 52.Adamczyk M, Brashear RJ, Mattingly PG. Prevalence of autoantibodies to cardiac troponin T in healthy blood donors. Clin Chem 2009;55:1592-3. 10.1373/clinchem.2009.125781 [DOI] [PubMed] [Google Scholar]
- 53.Doesch AO, Konstandin M, Celik S, et al. Effects of protein A immunoadsorption in patients with advanced chronic dilated cardiomyopathy. J Clin Apher 2009;24:141-9. [DOI] [PubMed] [Google Scholar]
- 54.Niebroj-Dobosz I, Marchel M, Madej A, et al. Circulating autoantibodies to troponin I in Emery-Dreifuss muscular dystrophy. Acta Myol 2008;27:1-6. [PMC free article] [PubMed] [Google Scholar]
- 55.Baba A. Autoantigen estimation and simple screening assay against cardiodepressant autoantibodies in patients with dilated cardiomyopathy. Ther Apher Dial 2008;12:109-16. 10.1111/j.1744-9987.2008.00555.x [DOI] [PubMed] [Google Scholar]
- 56.Landsberger M, Staudt A, Choudhury S, et al. Potential role of antibodies against cardiac Kv channel-interacting protein 2 in dilated cardiomyopathy. Am Heart J 2008;156:92-9. e2. [DOI] [PubMed]
- 57.Miettinen KH, Eriksson S, Magga J, et al. Clinical significance of troponin I efflux and troponin autoantibodies in patients with dilated cardiomyopathy. J Card Fail 2008;14:481-8. 10.1016/j.cardfail.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 58.Niebroj-Dobosz I, Dorobek M, Marchel M, et al. Evidence for autoimmunity to heart-specific antigens in patients with Emery-Dreifuss muscular dystrophy. Acta Myol 2006;25:68-72. [PubMed] [Google Scholar]
- 59.Eggers KM, Lindahl B. Application of Cardiac Troponin in Cardiovascular Diseases Other Than Acute Coronary Syndrome. Clin Chem 2017;63:223-35. 10.1373/clinchem.2016.261495 [DOI] [PubMed] [Google Scholar]
- 60.Zeller T, Tunstall-Pedoe H, Saarela O, et al. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J 2014;35:271-81. 10.1093/eurheartj/eht406 [DOI] [PubMed] [Google Scholar]
- 61.Vilela EM, Bastos JC, Rodrigues RP, et al. High-sensitivity troponin after running--a systematic review. The Netherlands journal of medicine 2014;72:5-9. [PubMed] [Google Scholar]
- 62.Vilela EM, Bettencourt-Silva R, Nunes JP, et al. BNP and NT-proBNP elevation after running--a systematic review. Acta Cardiol 2015;70:501-9. 10.1080/AC.70.5.3110509 [DOI] [PubMed] [Google Scholar]
- 63.Kaya Z, Goser S, Buss SJ, et al. Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation 2008;118:2063-72. 10.1161/CIRCULATIONAHA.108.788711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emery AE. Emery-Dreifuss syndrome. J Med Genet 1989;26:637-41. 10.1136/jmg.26.10.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saravia SG, Haberland A, Bartel S, et al. Cardiac troponin T measured with a highly sensitive assay for diagnosis and monitoring of heart injury in chronic Chagas disease. Arch Pathol Lab Med 2011;135:243-8. [DOI] [PubMed] [Google Scholar]
- 66.Nunes JP, do Sameiro Faria M, Sampaio S, et al. Partially reversible cardiomyopathy after renal transplant associated with anti-troponin I antibodies. Cardiology 2013;126:173-4. 10.1159/000353262 [DOI] [PubMed] [Google Scholar]
- 67.Kuan AP, Zuckier L, Liao L, et al. Immunoglobulin isotype determines pathogenicity in antibody-mediated myocarditis in naive mice. Circ Res 2000;86:281-5. 10.1161/01.RES.86.3.281 [DOI] [PubMed] [Google Scholar]
- 68.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med 2006;203:305-10. 10.1084/jem.20052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng X, Liao YH, Ge H, et al. TH1/TH2 functional imbalance after acute myocardial infarction: coronary arterial inflammation or myocardial inflammation. J Clin Immunol 2005;25:246-53. 10.1007/s10875-005-4088-0 [DOI] [PubMed] [Google Scholar]
- 70.Kaya Z, Katus HA, Rose NR. Cardiac troponins and autoimmunity: their role in the pathogenesis of myocarditis and of heart failure. Clin Immunol 2010;134:80-8. 10.1016/j.clim.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shoenfeld Y, Gilburd B, Abu-Shakra M, et al. The mosaic of autoimmunity: genetic factors involved in autoimmune diseases--2008. Isr Med Assoc J 2008;10:3-7. [PubMed] [Google Scholar]
- 72.Morais J. Autoantibodies to cardiac troponin in patients after renal transplant: a new target for statins? Rev Port Cardiol 2015;34:91-3. 10.1016/j.repc.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 73.Merlo M, Cannatá A, Vitagliano A, et al. Clinical management of dilated cardiomyopathy: current knowledge and future perspectives. Expert Rev Cardiovasc Ther 2016;14:137-40. 10.1586/14779072.2016.1125292 [DOI] [PubMed] [Google Scholar]