Abstract
Immune-neuroendocrine phenotypes (INPs) stand for population subgroups differing in immune-neuroendocrine interactions. While mammalian INPs have been characterized thoroughly in rats and humans, avian INPs were only recently described in Coturnix coturnix (quail). To assess the scope of this biological phenomenon, herein we characterized INPs in Gallus gallus (a domestic hen strain submitted to a very long history of strong selective breeding pressure) and evaluated whether a social chronic stress challenge modulates the individuals’ interplay affecting the INP subsets and distribution. Evaluating plasmatic basal corticosterone, interferon-γ and interleukin-4 concentrations, innate/acquired leukocyte ratio, PHA-P skin-swelling and induced antibody responses, two opposite INP profiles were found: LEWIS-like (15% of the population) and FISCHER-like (16%) hens. After chronic stress, an increment of about 12% in each polarized INP frequency was found at expenses of a reduction in the number of birds with intermediate responses. Results show that polarized INPs are also a phenomenon occurring in hens. The observed inter-individual variation suggest that, even after a considerable selection process, the population is still well prepared to deal with a variety of immune-neuroendocrine challenges. Stress promoted disruptive effects, leading to a more balanced INPs distribution, which represents a new substrate for challenging situations.
Introduction
Interactions among nervous, endocrine and immune systems have been vastly characterized in the last decades in vertebrates as well as in invertebrates1–6. In vertebrates, particularly in birds and mammals, physiological communicational paths and nexus at molecular, cellular and organismal levels evidence the interactive nature of what has been called immune-neuroendocrine (INE) system2, 6–11. This system nucleates integrated physiological responses in the organism, covering a wide spectrum of possibilities ranging from local inflammation to systemic stress responses. A concept that emerged in this area in relation to population studies is the existence of immune neuroendocrine phenotypes (INPs) that represent individuals neuro-hormonally and immunologically similar and different from another group within the same animal population10, 12–14. Each of the mentioned similar groups share common characteristics referred to: (i) neuroendocrine mediator concentrations, (ii) activity and expression of hormonal receptors and (iii) cytokine levels belonging to pro- or anti-inflammatory profiles.
The LEWIS/FISCHER paradigm is a well characterized and central model for INE as well as for classical immunological studies12, 15–17. In LEWIS rats with strong Th1-pro-inflammatory responses rich in mediators such as interferon (IFN)-γ and interleukin (IL)-12, basal levels of Corticosterone (CORT) are low. Instead, FISCHER rats show a low to moderate Th1-pro-inflammatory profile that correlates with high basal CORT levels and other associated complementary INE parameters. Broadening the scope of INPs in mammals, Elenkov et al. reported the existence of these phenotypes in humans in 200813. They identified two subgroups in healthy human individuals with relatively low and high epinephrine outputs that had opposite innate cytokine profiles, providing a clear link with the LEWIS/FISCHER paradigm. In 2015 we described for the first time the existence of INPs in an avian species (Coturnix coturnix), thus extending the scope to which this notion may be considered and at the same time expanding the evolutionary interpretation of the phenomenon10, 14.
Animal husbandry and production in human related environments usually implies suboptimal group sizes, altered or forced social structures, manipulation routines, and other diverse situations reported to be stressful as well as important health challenges. Laboratory animals and domesticated poultry species are far from being an exception18–21. The stated situations require a series of responses from the INE mega-system in order to cope with the challenges pursuing the ultimate goal of maintaining homeostasis22, 23. Animals with opposite INPs have divergent stress responses as reported in studies with LEWIS and FISCHER rats submitted to different stressors12, 13, 15–17. Physiological insights of their INE interactions have shown different secretory activity of their hypothalamic cells. This congenital difference configures a number of parameters and biochemical responses, which together characterize the depicted INPs.
Research has centred its attention mostly on responses ranging from physiological to cellular or molecular aspects1, 10, 24–27. However, the analyses of the information considering eco-immunological backgrounds could lead to additional interpretations. As recently pointed out by Ashley and Demas10, much work is still needed to improve our understanding of the constraints and advantages created by the tripartite neuro-endocrine-immune interactions. In a previous study by Nazar et al., (2015b) we evaluated in laying hens the effects of a chronic social disruption, induced by changing the appearance of some of the penmates, on immune and stress response variables. Only hens housed in socially disrupted pens showed leukocyte changes after treatment that were consistent with a chronic stress. Hens in control non-disrupted pens remained unchanged and therefore stable throughout the whole study. Herein, we focused on the assessment of individual INE responses within the socially disrupted pens both prior and after chronic social stress disruptions. This study allowed us to answer three main questions: (1) Are the INPs described for Coturnix coturnix 14 also observed and similarly distributed in Gallus gallus (an avian species that has been submitted to a much longer history of intensive domestication process and directed selective breeding28–33)? And if so, (2) do the INPs remain within similar proportions (stable) prior and after the chronic stress disruption? And (3) are the same individuals conforming each of the INPs prior and after the chronic stress disruption?
Considering the theoretical framework described, we hypothesize that INE arrangements as those previously described in Hommo sapiens sapiens, Rattus norvegicus and Coturnix coturnix will generalize to the avian model Gallus gallus. On the other hand, we also expect these arrangements to be modulated by induced chronic stress, influencing the individual and population levels.
Material and Methods
Animals and rearing conditions
Newly hatched one-day-old Hy-line brown female chicks were obtained from Avigán Terralta hatchery and transported to the experimental poultry facility at the Neiker-Tecnalia research centre (Vitoria-Gasteiz, Spain). Immediately upon arrival, 300 chicks were randomly assigned to one of 12 pens and housed in groups of 10 or 40 birds, 6 pens per each group size. Birds were kept at the same density (8 birds/m2), management and housing/environmental conditions described elsewhere34. At two days of age, birds were individually identified with two white laminated paper tags placed on each wing side35. Before the laying period started, pens were also provided with proportional nest space. Birds were maintained in the described conditions and were stressed by an induced modification of the social group structure (see below) by altering the birds´ appearance (changing the colour of the head feathers, see below).
Basal situation: all group with homogeneous appearance
Upon arrival, all birds within half of the pens were either maintained (100% unmarked) or their physical appearance was modified (100% marked) by placing a black mark with a non-toxic dye on the back of the head34, 36. Birds remained with the same initially assigned appearance until 34 wk. of age.
Chronic social stress disruption: sequential changing of some group members’ appearance
Groups were altered by sequentially changing the appearance of 30, 50 and 70% of individuals when adults at 34, 38 and 44 wk. of age (1st, 2nd and 3rd change, respectively). These changes were accomplished by either randomly marking the birds’ head (for the initially unmarked homogeneous groups) or unmarking them (for the initially marked homogenous groups). The manipulation used is known to engender chronic stress responses in the group members36, 37 and thus representing a social stressor: a pre-established social dynamic mismatch and at the same time the reestablishment and adaptation to the new social structure34, 38. Corticosterone and haematological indicator (heterophil/lymphocyte ratio) were assessed to ensure the applied protocol was indeed inducing a chronic stress reaction (see Supplementary Fig. S1).
INPs determination
INP characterizations through analyses of INE variables were performed within the same ontogenetic phase (fully grown and mature hens). Measurements were taken at 29 wk. of age (basal - prior to the beginning of social stress disruption) and at 46 wk. of age (post-chronic social stress disruption −2 wk. after the last alteration was applied). Six randomly chosen birds within each pen (treatment replica) were designated to analyse INE variables in order to determine their basal and post-chronic social stress INPs (same animals were analysed prior and after chronic social stress disruption). A total of 72 birds were studied.
Based on previous reports of INPs in mammals (Rattus norvegicus 12, 15 and Homo sapiens sapiens 13), and our previous data in birds (Coturnix coturnix 14), the following INE variables were evaluated: plasma CORT as representative of the hypothalamus-pituitary-adrenal axis; skin-swelling response to Phytohemagglutinin-p (PHA-P), antibody response against sheep red blood cells (SRBC) and leukocyte populations as INE effectors; and two different INE interplay mediators that favour opposite milieu polarization: IFN-γ (pro-inflammatory) and IL-4 (anti-inflammatory). These two mediators have also been described as the hallmarks of pro- and anti-inflammatory INE responses in Gallus gallus 39–41.
Sampling procedure
The complete sampling procedure both in basal and post-chronic social stress disruption took 3 days, within a period of one week. To ensure a reliable CORT value, the extraction procedure took no longer than 80 s from the moment the animal was initially captured. On day 1, the brachial vein of the bird’s left wing was punctured in order to obtain 1 ml of EDTA-anticoagulated blood for smears and for quantifying basal concentration of plasmatic CORT, IFN-γ and IL-4. Plasma was obtained by blood centrifugation at 2,500 g during 15 min and it was immediately stored at −20 °C until further analyses. At the same time PHA-P skin-swelling response was induced (see below). Immediately after, birds were intraperitoneally injected with 0.5 ml of a 10% SRBC suspension in order to induce a humoral immune response (see below). One week later blood was from the right brachial vein for SRBC primary antibody response assessment.
Determinations
Skin-swelling response to PHA-P
To determine birds in-vivo general proinflammatory potentiall42, the responses to PHA-P, a lectin from Phaseolus vulgaris (Sigma Chemical, St. Louis, MO), was measured in the wing web of each bird following the methods described elsewhere42–45. Briefly, on day 1, a 0.05 ml intradermal injection of a solution of 1 mg/ml PHA-P in phosphate saline buffer (PBS) was injected in the wing web of each bird. The dermal swelling response was measured as the percentage increase in wing web thickness at the injection site 24 h post-PHA-P injection (day 2). Measurements were recorded to the nearest 0.01 mm using a mechanic digital micrometre.
Innate/acquired (INN/ACQ) ratio
Leukocyte counts were performed in blood smears stained with May-Grünwald Giemsa. Differential counts of 100 white cells per blood smear were made46, 47. The INN/ACQ cell ratio was calculated using the following formula: INN/ACQ = (number of basophils + number of heterophils + number of monocytes)/(number of eosinophils + number of lymphocytes).
Antibody response against SRBC
To evaluate the induced humoral immune response, the antibody titer was assessed with a micro agglutination assay43, 48, 49. Briefly, one week after the intraperitoneal administration of the SRBC suspension we took blood samples and 20 μl of complement-inactivated plasma (through heating to 56 °C) was serially diluted in 20 μl of PBS. Next, 20 μl of a 2% suspension of SRBC in PBS was added to all wells. Microplates were incubated at 40 °C for 1 h, and hemagglutination of the plasma samples was compared to the blanks (PBS only) and negative controls (wells with no SRBC suspension). Antibody titters were reported as the Log2 of the highest dilution yielding significant agglutination.
Plasma CORT, IFN-γ and IL-4 determinations
Plasma CORT (pg/ml) was quantified using a validated specific CORT ELISA kit (ENZO Life Sciences - ADI-901-097)50. IFN-γ and IL-4 (pg/ml) were quantified using validated specie-specific ELISA kits (Uscn Life Science Inc., IFN-γ: E90049Ga; IL-4: E90077Ga). The reactivity with CORT was 100% with a sensitivity of 27.0 pg/ml detecting concentration ranging from 32 to 20.000 pg/ml. The cross reactivity of related steroids compound was: deoxycorticosterone 28.6%, progesterone 1.7%, testosterone 0.13%, tetrahydrocorticosterone 0.28%, aldosterone 0.18%, cortisol 0.046, pregnenolone and cortisone <0.03%. The reactivity with IL-4 was 100% with a sensitivity of 15.63 pg/ml detecting concentration ranging from 16 to 1.000 pg/ml. The reactivity with IFN-γ was 100% with a sensitivity of 15.63 pg/ml detecting concentration ranging from 16 to 1.000 pg/ml. No significant cross-reactivity or interference between the analysed cytokines and their analogues was observed. Intra- and inter-assay variability for each target molecule were 8.4% and 8.2% (corticosterone), 9% and 11% (IL-4 and IFN-γ). Determinations were done following the procedure specified by the manufacturer. All samples for each target molecule were assessed the same day and by duplicate.
Statistical analyses
Correlation analysis between birds sampling order and every variable analysed were performed in order to ensure there was no influence of this procedure on the obtained results. An ANOVA on previous and post-chronic social stress data that included birds’ appearance (marked vs. unmarked) and GS (10 vs. 40) as a factors was also performed to rule out potential influences of those variables or their interactions on the results of the INE studied variables.
Basal analyses
First, a cluster analysis was performed in order to explore the degree of similarity among the birds in the study on the bases of the analysed variables (factors). Particularly, with the aim of identifying the best partition of the birds, described by the best orthogonal linear combinations of the factors according to the least-squares criterion, a K-means analysis was performed. The following 6 standardized variables were used in the analysis: plasma CORT, IFN-γ and IL-4, skin-swelling response to PHA-P, antibody response against SRBC and INN/ACQ ratio. Based on the theoretical framework previously exposed, the clustering was performed with a K value of 3 (2 subgroups with representatives of potential extreme INPs and 1 intermediate subgroup). Euclidean metrics were used for computing the distance between points and cluster centres. To graphically represent the results of the clustering in a multivariate manner, a Principal Component Analysis (PCA) was used with the 6 clustering variables as explanatory variables. Birds were labelled with their categorization according to the cluster they belong to. Lastly, to fully characterise each cluster, a means comparison was also done with a univariate general linear mixed (glm) model assuming Gaussian error distribution with an identity-link function for each response variable. Cluster was used as a fixed effect and pen identity was included as a random effect. Differences between the three clusters on basal CORT, IFN-γ and IL-4 levels, skin-swelling response to PHA-P, antibody response against SRBC and INN/ACQ ratio were studied.
Post-chronic social stress disruption analyses
Because it is expected that not all individuals will show the same stress response and therefore contributing differentially to the stress response at a population level, we used the following approaches:
K-means analyses was performed with post-chronic social stress data (K = 3, Euclidean metric). PCA bi-plot was again used as a graphical strategy. To characterize the identity of each clustered subgroup post-chronic social stress, univariate general linear mixed model was used assuming normal distribution of the data, the 3 cluster subgroups as a fixed factor and pen and each animal identification as random factors. Differences between the cluster subgroups on basal CORT, IFN-γ and IL-4 levels, skin-swelling response to PHA-P, antibody response against SRBC and INN/ACQ ratio were studied again.
Proportion tests were used to quantitatively characterize the change in the number of birds within each cluster obtained previous and post-chronic social stress disruption.
In every case, a p-value < 0.05 was considered to represent significant differences. All statistical analyses were performed through an ‘R’ (The R Foundation for Statistical Computing) user-friendly interface implemented in InfoStat51. Whenever significant main effects were detected, Fisher’s least significant difference (LSD) was used to compare the means. Cluster was performed using kmeans and models were fitted using the lme and glm R packages.
Results
No correlations were found between sampling order and the analysed variables in neither basal conditions nor post-stress (p > 0.65 in all cases). Furthermore, no significant effects were found on the influence of hens´ appearance (head feathers marked vs. unmarked), group size (10 vs. 40 individuals) and their interaction on the studied INE variables (p > 0.47 in all cases). Consequently, sampling order, hens’ appearance and group size were not included as influencing factors in the subsequent analysis.
Basal situation: all group with homogeneous appearance
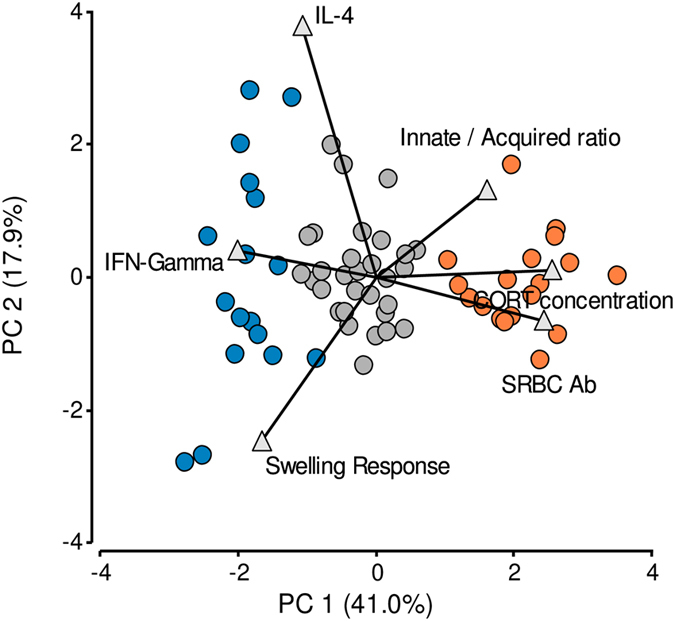
K-means analysis distributed the birds in 3 main dissimilar groups. The largest group consisted of 46 birds and the other 2 of 11 and 10 birds. The principal component analysis bi-plot labelled with the cluster categorization indicates that the groups with lower number of hens were located in the extreme of the plotted multivariate space and the larger group in the centre (Fig. 1). Distances between dots in the bi-plot are also an indicator of the within group similarities (see Supplementary Table S5 for PCA statistical details). Coherently, the characterization of the INE variables also informed that the two smaller groups have opposite responses in all variables studied and that the larger group was less homogeneous, consisting of animals with intermediate or partially shared responses with the extreme groups.
Figure 1.
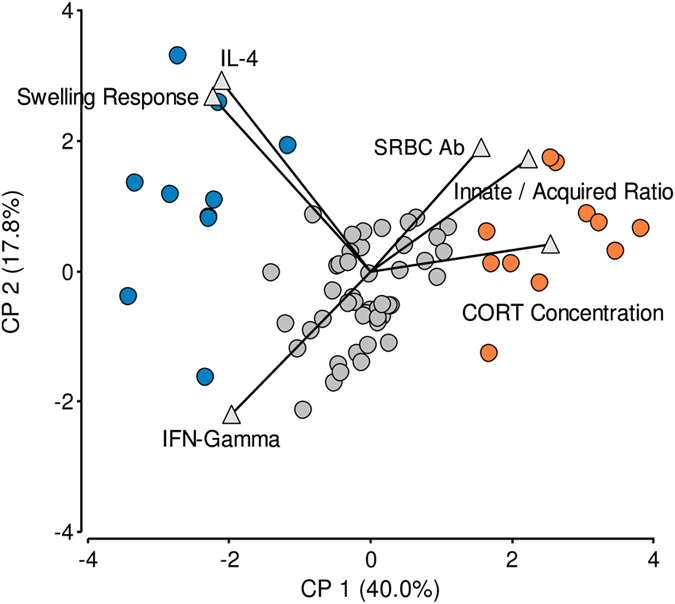
Exploration of data variability and clustering procedure. Principal component analysis bi-plot graphs. Each bird is represented by a dot and each explanatory and clustering variable used in the analysis is represented by triangle. Total distribution of the data categorized according to the birds’ assignation according to k-means clustering. Full blue dots and full orange dots represent extreme clusters with low and high basal CORT concentration respectively; full grey dots represent birds with intermediate configuration. The eigenvalues are shown in brackets next to each component. Number of birds in the study: 67.
Statistical evaluation of the INE variables after the cluster analysis showed that in the basal situation all measurements are also polarized, thus reinforcing the consistency of each cluster classified subgroup. Comparing the two extreme groups we found that they vary in their basal CORT concentration (F 2,51 = 91.82, p < 0.001; Fig. 2). Hens with lowest CORT concentration showed higher skin-swelling responses to PHA-P (F 2,51 = 46.39, p < 0.001; Fig. 3a), higher antibody response against SRBC (F 2,51 = 5.69, p < 0.005; Fig. 3b), and lower INN/ACQ ratio (F 2,51 = 19.28, p < 0.001; Fig. 3c) than their high CORT counterparts. To reveal if the clusters defined also exhibited a molecular substrate underlying the putative INE phenotypes we also evaluated the pro-inflammatory (IFN-γ) and anti-inflammatory (IL-4) cytokines. The analysis revealed that opposite groups differed in these mediator concentrations (Fig. 4). Animals with low basal CORT showed significantly higher levels of IFN-γ (F 2,51 = 7.07, p < 0.01; Fig. 4a) and IL-4 (F 2,51 = 38.61, p = 0.001; Fig. 4b) in comparison with their high basal CORT counterparts. It is worth mentioning that although animals with low CORT levels have significantly elevated both pro and anti-inflammatory cytokine production, IFN-γ values had a four-fold increment showing the predominance of this mediator determining a particular milieu.
Figure 2.
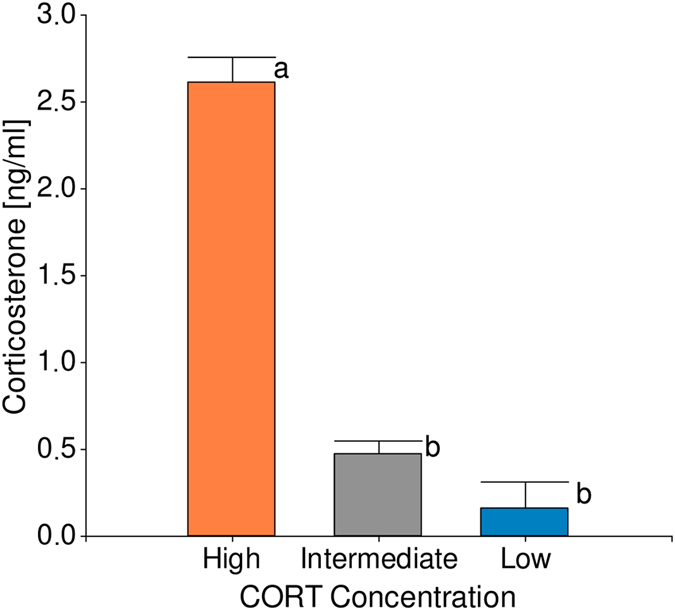
Divergent groups basal CORT concentration. Effect of clustering on basal CORT concentration. Low and High CORT birds where identified according to their hormonal concentration. Data are means ± SE. Different letters indicate significant (p < 0.05) differences between groups. Number of birds in the study = 67, number of birds per group Low = 10, High = 11, Intermediate = 46.
Figure 3.
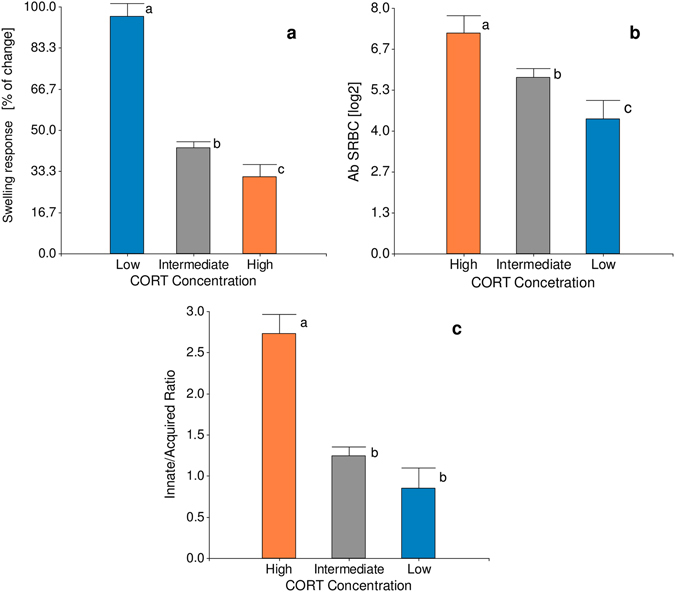
Immune effector analysis in divergent Low and High CORT groups. Effect of clustering on immune effectors is shown in (a) skin-swelling response to PHA-P, (b) antibody response against SRBC and (c) INN/ACQ. Data are means ± SE. Different letters indicate significant (p < 0.05) differences between groups. Number of birds per group Low = 10, High = 11, Intermediate = 46. INN/ACQ number was calculated using the following formula: INN/ACQ = (number of basophils + number of heterophils + number of monocytes)/(number of eosinophils + number of lymphocytes).
Figure 4.
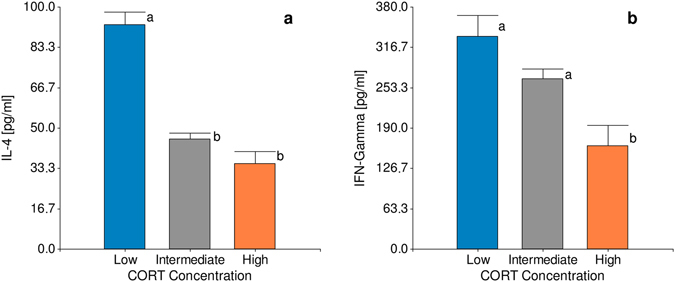
Analysis of molecular mediators in divergent Low and High CORT groups. Effect of clustering on the expression of (a) anti-inflammatory (IL-4) and (b) pro-inflammatory (IFN-γ) mediators. Data are adjusted means ± SE. Different letters indicate significant (p < 0.05) differences between groups. Number of birds per group: Low = 10, High = 11, Intermediate = 46.
Because of the physiological arrangements described, consisting of clusters of animals showing polarized INE responses comparable to the LEWIS/FISCHER paradigm10, 12, 14, 15 (Fig. 5) the two groups formerly defined are herein named ‘LEWIS-like’ or ‘FISCHER-like’ (animals with the lowest or the highest CORT concentration respectively and the other INE variables polarized accordingly).
Figure 5.
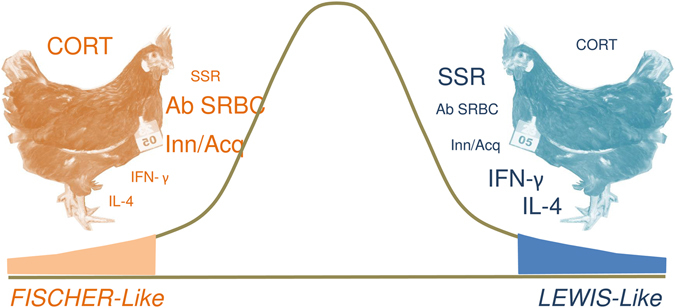
Schematic conceptual representation of INPs in Gallus gallus. The variables determining the existence of INPs are represented around each bird. The size of the variable indicates if the animals show high or low response in each of the parameters. SSR: skin-swelling response to PHA-P; Ab SRBC: antibody response against SRBC; Inn/Acq: innate/acquired leukocyte ratio; level of expression of pro-inflammatory (IFN-γ) and anti-inflammatory (IL-4) mediators. ‘FISCHER-like’ hens with high CORT concentration also manifest high Innate/Acquired and Ab SRBC, but low SSR, IFN-γ and IL-4 levels. ‘LEWIS-like’ counterparts have low CORT as well as low Innate/Acquired and AB SRBC responses, but high SSR, as well as INE mediators (IFN-γ and IL-4) response.
Post-chronic social stress disruption: sequential changing of the group members’ appearance
Similar procedures and analyses of data described for the basal situation were performed to evaluate individual and population immune-neuroendocrine responses post-chronic social stress disruption.
K-means analysis shows again 3 differentially-sized groups. Nevertheless, post-chronic social stress the size of the groups appeared more balanced than prior stress. The largest group in this situation consists of 30 birds and the other 2 of 18 and 17 birds. Figure 6 shows the representation of individual responses obtained in the multivariate space. The PCA bi-plot labelled with the cluster categorization again localises the two minor groups in the extremes of the plotted multivariate space and the largest one in the centre. INE arrangements found (see Supplementary Figs S2, S3 and S4 and Supplementary Table S6) and numerical analyses (Fig. 7) indicate that post-chronic social stress, 18 birds express an extreme INE association equivalent to a FISCHER-like configuration and 17 birds express the opposite configuration of the INE variables analysed, comparable to a LEWIS-like INP. The remaining 30 birds expressed an intermediate INE configuration (Figs 6 and 7).
Figure 6.
Exploration of data variability and clustering procedure post- chronic social stress. Principal component analysis bi-plot graph. Each bird is represented by a dot and each explanatory and clustering variable used in the analysis is represented by triangle. Total distribution of the data categorized according to the birds’ INP after stress disruption. Full blue dots and full orange dots represent extreme LEWIS- and FISCHER-like birds respectively; full grey dots represent birds with intermediate configuration. The eigenvalues are shown in brackets next to each component. Number of birds in the study: 65. Number of birds per group: LEWIS-Like = 17, FISCHER-Like = 18, Intermediate = 30.
Figure 7.
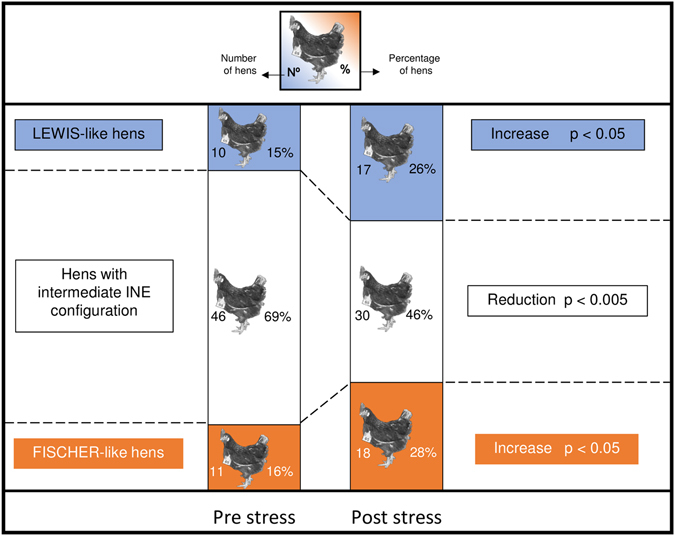
Proportion analysis of stress induced effects. The studied population is schematized previous and post chronic social stress impairment, as cumulative bars. In each bar the different INE configurations are represented in sections with blue (LEWIS-like), white (intermediate) or orange (FISCHER-like) stuffing. Information regarding absolute number and percentage of birds is also given. The final statistical and biological effect of the stress impairment is shown together with p-values. In every case, a p-value < 0.05 was considered to represent significant differences. Total number of animals previous stress = 67, total number of animals post stress = 65.
The subgroups with the extreme INE configurations were mostly composed with the same individuals already categorized prior to stress. The 86% (18 out of 21) of the birds remained showing the same profile post-chronic social stress disruption. However, other situations were also observed within the population. A wide percentage of birds showing intermediate profile (40%) did switch its profile towards one or the other of the two extreme INP. Proportion analyses showed (Fig. 7) that the number of birds expressing FISCHER-like and LEWIS-like configuration were respectively increased from 11 to 18 birds (p < 0.05) and from 10 to 17 birds (p < 0.05) post-chronic social stress. The described increments are consistent with the reduction found in the number of birds showing an intermediate configuration post-chronic social stress (reduced from 46 to 30 individuals, p < 0.005). Moreover, the percentage of birds maintaining an extreme INE configuration post-chronic social stress (86%) was significantly higher than the percentage of birds (60%) conserving their intermediate configuration (p < 0.01). The latest implies that the 40% of those birds initially expressing an intermediate configuration polarized their INE interplay towards an extreme INP post-chronic social stress.
Discussion
Gallus gallus (the domestic chicken) is an excellent avian model involved in most avian research immunology and physiology due to its economic importance6, 27, 39, 52–54. In the present study, we explored and characterized the INPs’ distribution in a chicken laying strain. We also characterized INE interactions post-chronic social stress in order to reveal the effects of the challenging situation in the described interplay. In particular, we analysed whether a chronic social stress disturbance can modulate the INE interplay of each individual consequently affecting the INPs’ proportions and distributions at a population level. As previously mentioned, the reported data may have diverse interpretations regarding plasticity, polarization, and other concepts of eco-immunological and physiological importance.
Our results shows that the INP physiological arrangement previously described for Coturnix coturnix 14 are also observed in Gallus gallus providing further consistency to the phenomenon in focus. INPs have a physiological substrate underlying the arrangement of each variable measured. LEWIS-like birds show in basal situation higher PHA response, a marker of the individual general proinflammatory potential42, linked to a lower humoral immune response to the T-dependent antigen SRBC39. According to previous reports, adrenocorticotropic hormone and CORT are immunosuppressive and hypothalamus-pituitary-adrenal axis activity generally reduces lymphocyte and increases heterophil numbers 6, 55–57. This phenomenon could also explain the different distribution of leukocyte subpopulation found in the two extreme CORT groups. Findings imply that animals differing in their basal CORT levels also show dissimilar and opposite INE associated responses.
The observed generalization of the INPs in Coturnix coturnix to a Gallus gallus laying strain also leads to interrogations related to the potential relationship between domestication and the expression of INPs. Up to this point, these phenotypes have been described in homo sapiens sapiens 13 or in other species (birds or mammals)14, 15 that during the last decades have been living in environments highly influenced by humans. Moreover, their development has taken place under direct human manipulation through many generations of selective pressure with either scientific or commercial/productive purposes6, 58–60. In this context, the expression of different phenotypical arrays within an avian population could represent a strategy to keep the higher diversity of INE responses in human-related environments.
Relations between INE components and behaviour have already been studied showing a strong interaction between them10, 23, 61–63. Behavioural changes, such as induced flight-or-fight responses or social defeat, resulted in immune modulations and simultaneous changes in endocrine profiles10. Ritschoff et al.61 demonstrated that a territorial intrusion induced upregulation in NF-kappa-β (the key transcription factor that triggers pro-inflammatory responses) in different species (Mus musculus, Gasterosteus aculeatus and Apis mellifera). Thus, the different INPs found could partially or in full be a reflection of the environment within pens during development (social interactions established between hens); however, further studies are needed to confirm that hypothesis.
It is worth mentioning that both basal and post-chronic social stress exposure all animals were deemed healthy (free from helminthic parasite indicators in the faeces, blood smears with no indications of infections, and plumage and footpath condition suggesting a good welfare status). From the eco-immunological perspective, INPs diversity could be interpreted considering the ‘dilution effect’, which relates diversity with the success of parasites in a particular community associating diverse host communities with lower levels of parasitism64–66. The expression of different INPs in peer birds within the same population could be considered an application of the community original hypothesis mentioned above. For a given population of animals, to hold a diversity of responses, amplifies the potential collection of challenging organisms they can deal with. Thereby, the observed INE diversity would function as the bases of the ‘dilution effect’.
Regarding post-stress stability of the INPs proportions at the population level, the larger number of birds that changed their phenotype post-chronic stress belonged to the intermediate INP configuration. Complementary analyses informed that this flux was not polarized towards one profile selectively; on the contrary, it was balanced between both extreme INPs arrangements described. Plasticity is a central concept in biological sciences and has been defined as the ability of a certain genotype to produce distinct phenotypes when exposed to different environments throughout its ontogeny67–71. Herein the social stressor may have determined a different environment for birds compared to the basal situation in which they were reared (undisturbed condition). Taking into consideration that the same individuals were sampled, the genotype could be considered as a component that remained stable67, 71. Plasticity would have allowed the fluxes from intermediate to extreme configurations and vice versa that were observed in the INPs distribution post chronic stress. In this context, the major flux observed from intermediate to extreme configurations could be interpreted as a response to the social chronic stress disruption. Nevertheless, it is worth emphasizing that the polarization is not complete. In particular, about 60% of the initial subgroup still remains with an intermediate INE configuration. This new distribution leads to a different and new configuration for future challenges demanding plastic responses from the INE interplay and, at the same time, still shows a potential variability of responses. However further research is needed to evaluate the last hypothesis.
Studies in ecology and evolution lead Cohen et al. to propose in 2012 ‘physiological regulated networks’ (PRN) as the body of molecules and regulatory relationships that maintain and adjust homeostasis and facilitate performance at the whole-organism level. In these networks, single molecules defined as ‘integrators’ have the crucial role of synthesizing internal or external information thereby determining multiple aspects of PRN functioning. The findings of our study could also be discussed within the theoretical context of PRN70, 72. One of the key functions of PRN is to facilitate transitions among phenotype states, which is consistent with the observed INE adjustments from the basal to the post-chronic social stress state. The different variables selected to determine INPs would represent part of the scaffold in which external challenges are physiologically integrated and solved. At the same time, not every bird responded to challenging situations with a phenotypic transition (some of them retained their previous INP). This was particularly evident in individuals with extreme phenotypes in basal conditions that in most of the cases remained with extreme configurations post-chronic social stress. Different scenarios should be considered at the individual level regarding PRN background70–72. Firstly, each phenotype could represent a potential ‘state’ of the PRN70. These phenotypes coexist as ‘different states’ to give appropriate responses to the potential challenges presented. Secondly, focusing on the birds that changed their INP configuration post-chronic social stress exposure, the different INPs could be considered as distinct PRN states equally valid for an individual to adjust the organism physiology to environmental demands based on the possibilities that individual plasticity offers. On the other hand, we believe that the fact that some individuals retained extreme INP configurations does not invalidate the concept of plasticity but reinforces the idea that biological phenomena rarely happen in an ‘all or nothing’ manner.
Lastly, focusing the attention in the phenomenon at a population level, again two different scenarios could be described. In basal conditions 16% of the population expressed a Th1 biased response, 16% an opposite Th2 biased response and 58% an intermediate response. Post-chronic social stress the extreme percentages increased reaching 29% each at expenses of a loss in the intermediate subgroup of animals (final 32%). A remarkable positive aspect of both circumstances is the conserved variability intra-population (with the associated advantages previously mentioned). But, and very important in this case, the change in the frequencies post-chronic social stress led to a more balanced proportion of the three INPs. In the case of an eventual situation implying a significant reduction in the overall population, (e.g.: a bottle-neck or founding event)73–77 the chances of representation of each INE configuration could be higher when the substrate is a more balanced population. Again, the fact that biological phenomena do not occur at ‘all or nothing’ gains coherence and empirical consistency.
INPs observed in mammals and quail seems a more general phenomenon that also appears in an avian species with a much greater commercial relevance and, more importantly, that has been intensely manipulated through a long history of selective breeding. Findings suggest that at a population level, hens are prepared to deal with a wide spectrum of INE challenges. After chronic stress exposure, an increase in the number of birds showing extremes INP frequencies was found at the expense of a reduction in the number of hens showing intermediate responses. Observations suggest a stress disruptive effect on INPs distribution. The increased frequency in polarized INPs induced by chronic stress leads to a more balanced INPs distribution, which would be the new substrate for an eventual challenge demanding an INE response.
Ethics approval
Animal care was provided in adherence with Institutional animal Care and Use Committee guidelines. The experiment was approved by the ethical committee at Neiker-Tecnalia in compliance with the Spanish legislation regarding the use of animals for experimental and other scientific purposes (Real Decreto 1201/2005).
All the data on which the conclusions of the paper are based are presented in the paper. Nevertheless, datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The authors wish to thank the excellent contribution of Iurancha González, the maintenance personnel at Neiker-Tecnalia and Avícola Gorrotxategui S.L. We also thank Raquel Marquinez for her assistance during sample analyses, the veterinary assistance of Ignacia Beltrán de Heredia, Dario C. Arbelo for his technical advice during leukocyte determinations and Ana Georgina Flesia for her advice during data statistical analyses. F.N.N, S.G.C and R.H.M are career members of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) Argentina. I.E. is an Ikerbasque Research Professor. The authors also wish to thank two anonymous reviewers and the Editor for their comments that helped improving the quality of the manuscript. This project was funded by the Ministry of Economy and Competitiveness of Spain (Agro resources and technologies (AGL) 2010-18276, MODELAY). This work done while R.H.M. was on a sabbatical visit to Neiker-Tecnalia, Arkaute Agrifood Campus, Department of Animal Production, P.O. Box 46, 01080 Vitoria-Gasteiz, under an IKERBASQUE (Basque Foundation for Science, Alameda Urquijo 36-5 Plaza Bizkaia, 48011 Bilbao, Spain) visiting professor support program.
Author Contributions
F.N.N., R.H.M. and I.E. conceived and planned the experiments. F.N.N. and R.H.M. performed the experiments. All authors wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08733-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
F. Nicolas Nazar, Email: franco.nicolas.nazar@unc.edu.ar.
Raul H. Marin, Email: raul.marin@unc.edu.ar
References
- 1.Besedovsky H, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr. Rev. 2013;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 2.Ottaviani E, Malagoli D, Franceschi C. Common evolutionary origin of the immune and neuroendocrine systems: from morphological and functional evidence to in silico approaches. Trends Immunol. 2007;28:497–502. doi: 10.1016/j.it.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 4.Flajnik MF, Du Pasquier L. Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 2004;25:640–4. doi: 10.1016/j.it.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman J. Evolution and immunity. Immunology. 2010;130:459–62. doi: 10.1111/j.1365-2567.2010.03294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scanes, C. G. Sturkie’s avian physiology. (Elsevier, 2014).
- 7.Jafarian-Tehrani M, Sternberg EM. Neuroendocrine and other factors in the regulation of inflammation. Animal models. Ann. N. Y. Acad. Sci. 2000;917:819–24. doi: 10.1111/j.1749-6632.2000.tb05447.x. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg, E. M. Neuroendocrine regulation of autoimmune/inflammatory disease. J. Endocrinol. 429–435 (2001). [DOI] [PubMed]
- 9.Murphy, K. Inmunobiología de Janeway. (Interamericana de México, 2009).
- 10.Ashley NT, Demas GE. Neuroendocrine-immune circuits, phenotypes, and interactions. Horm. Behav. 2017;87:25–34. doi: 10.1016/j.yhbeh.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demas GE, Carlton ED. Ecoimmunology for psychoneuroimmunologists: Considering context in neuroendocrine–immune–behavior interactions. Brain. Behav. Immun. 2015;44:9–16. doi: 10.1016/j.bbi.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sternberg EM, et al. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc. Natl. Acad. Sci. USA. 1989;86:4771–5. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elenkov IJ, et al. Low- versus high-baseline epinephrine output shapes opposite innate cytokine profiles: presence of Lewis- and Fischer-like neurohormonal immune phenotypes in humans? J. Immunol. 2008;181:1737–1745. doi: 10.4049/jimmunol.181.3.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazar FN, Barrios BE, Kaiser P, Marin RH, Correa SG. Immune neuroendocrine phenotypes in Coturnix coturnix: do avian species show LEWIS/FISCHER-like profiles? PLoS One. 2015;10 doi: 10.1371/journal.pone.0120712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sternberg EM, Hill J. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc. Natl. Acad. Sci. USA. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei R, Listwak SJ, Sternberg EM. Lewis hypothalamic cells constitutively and upon stimulation express higher levels of mRNA for pro-inflammatory cytokines and related molecules: comparison with inflammatory resistant Fischer rat hypothalamic cells. J. Neuroimmunol. 2003;135:10–28. doi: 10.1016/S0165-5728(02)00429-0. [DOI] [PubMed] [Google Scholar]
- 17.Zelazowski P, et al. In vitro regulation of pituitary ACTH secretion in inflammatory disease susceptible Lewis (LEW/N) and inflammatory disease resistant Fischer (F344/N) rats. Neuroendocrinology. 1992;56:474–82. doi: 10.1159/000126264. [DOI] [PubMed] [Google Scholar]
- 18.Mashaly MM, et al. Effect of Heat Stress on Production Parameters and Immune Responses of Commercial Laying Hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- 19.Appleby, M. C., Hughes, B. O. & Elson, H. A. Poultry production systems: behaviour, management and welfare (C.A.B. International, 1992).
- 20.van der Most PJ, de Jong B, Parmentier HK, Verhulst S. Trade-off between growth and immune function: a meta-analysis of selection experiments. Funct. Ecol. 2011;25:74–80. doi: 10.1111/j.1365-2435.2010.01800.x. [DOI] [Google Scholar]
- 21.Lara L, Rostagno M. Impact of Heat Stress on Poultry Production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calandreau L, et al. Effect of one week of stress on emotional reactivity and learning and memory performances in Japanese quail. Behav. Brain Res. 2011;217:104–10. doi: 10.1016/j.bbr.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Koolhaas JM. Coping style and immunity in animals: making sense of individual variation. Brain. Behav. Immun. 2008;22:662–7. doi: 10.1016/j.bbi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Renaudeau D, et al. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 2012;6:707–728. doi: 10.1017/S1751731111002448. [DOI] [PubMed] [Google Scholar]
- 25.Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochem. Int. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Butts CL, Sternberg EM. Neuroendocrine factors alter host defense by modulating immune function. Cell. Immunol. 2008;252:7–15. doi: 10.1016/j.cellimm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuenzel WJ, Jurkevich A. Molecular neuroendocrine events during stress in poultry. Poult. Sci. 2010;89:832–840. doi: 10.3382/ps.2009-00376. [DOI] [PubMed] [Google Scholar]
- 28.Gross WB, Siegel PB. Selective Breeding of Chickens for Corticosterone Response to Social Stress. Poult. Sci. 1985;64:2230–2233. doi: 10.3382/ps.0642230. [DOI] [PubMed] [Google Scholar]
- 29.Ericsson M, Fallahsharoudi A, Bergquist J, Kushnir MM, Jensen P. Domestication effects on behavioural and hormonal responses to acute stress in chickens. Physiol. Behav. 2014;133:161–9. doi: 10.1016/j.physbeh.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 30.Fallahsharoudi A, et al. Domestication Effects on Stress Induced Steroid Secretion and Adrenal Gene Expression in Chickens. Sci. Rep. 2015;5 doi: 10.1038/srep15345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirén A, Wright D, Jensen P. Domestication-related variation in social preferences in chickens is affected by genotype on a growth QTL. Genes. Brain. Behav. 2013;12:330–7. doi: 10.1111/gbb.12017. [DOI] [PubMed] [Google Scholar]
- 32.Eklund B, Jensen P. Domestication effects on behavioural synchronization and individual distances in chickens (Gallus gallus) Behav. Processes. 2011;86:250–256. doi: 10.1016/j.beproc.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Soleimani AF, Zulkifli I, Omar AR, Raha AR. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011;90:1435–40. doi: 10.3382/ps.2011-01381. [DOI] [PubMed] [Google Scholar]
- 34.Marin RH, Liste MG, Campderrich I, Estevez I. The impact of phenotypic appearance on body weight and egg production in laying hens: a group-size- and experience-dependent phenomenon. Poult. Sci. 2014;93:1623–35. doi: 10.3382/ps.2013-03705. [DOI] [PubMed] [Google Scholar]
- 35.Cornetto T, Estevez I. Influence of vertical panels on use of space by domestic fowl. Appl. Anim. Behav. Sci. 2001;71:141–153. doi: 10.1016/S0168-1591(00)00171-4. [DOI] [PubMed] [Google Scholar]
- 36.Dennis RL, Newberry RC, Cheng H-W, Estevez I. Appearance matters: artificial marking alters aggression and stress. Poult. Sci. 2008;87:1939–1946. doi: 10.3382/ps.2007-00311. [DOI] [PubMed] [Google Scholar]
- 37.Nazar, F. N. Determinación de fenotipos inmunoneuroendócrinos y su modulación por situaciones de estrés crónico en 2 modelos de aves que difieren en el grado de domesticación: Coturnix coturnix y Gallus gallus. Univ. Nac. CórdobaCórdoba, 140 (2015).
- 38.Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol. Behav. 2001;73:261–271. doi: 10.1016/S0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 39.Davison, F. Avian Immunology. Avian Immunology (Elsevier, 2014), doi:10.1016/B978-0-12-396965-1.00001-7.
- 40.Kogut MH, Rothwell L, Kaiser P. IFN-gamma priming of chicken heterophils upregulates the expression of proinflammatory and Th1 cytokine mRNA following receptor-mediated phagocytosis of Salmonella enterica serovar enteritidis. J. Interferon Cytokine Res. 2005;25:73–81. doi: 10.1089/jir.2005.25.73. [DOI] [PubMed] [Google Scholar]
- 41.Hong YH, Lillehoj HS, Lee SH, Dalloul RA, Lillehoj EP. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol. 2006;114:209–23. doi: 10.1016/j.vetimm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Vinkler, M. & Bainova, H. Functional analysis of the skin-swelling response to phytohaemagglutinin. 1081–1086, doi:10.1111/j.1365-2435.2010.01711.x (2010).
- 43.Nazar FN, Marin RH. Chronic stress and environmental enrichment as opposite factors affecting the immune response in Japanese quail (Coturnix coturnix japonica) Stress. 2011;14:166–73. doi: 10.3109/10253890.2010.523093. [DOI] [PubMed] [Google Scholar]
- 44.Roberts ML, Buchanan KL, Evans MR, Marin RH, Satterlee DG. The effects of testosterone on immune function in quail selected for divergent plasma corticosterone response. J. Exp. Biol. 2009;212:3125–31. doi: 10.1242/jeb.030726. [DOI] [PubMed] [Google Scholar]
- 45.Stadecker, M. & Lukic, M. The cutaneous basophil response to phytohemagglutinin in chickens. J. Immunol. 1564–1568 (1977). [PubMed]
- 46.Fair JM, Hansen ES, Ricklefs RE. Growth, developmental stability and immune response in juvenile Japanese quails (Coturnix coturnix japonica) Proc. Biol. Sci. 1999;266:1735–1742. doi: 10.1098/rspb.1999.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazar FN, Marin RH, Liste G, Campderrich I, Estevez I. Manipulation of the phenotypic appearance of individuals in groups of laying hens: effects on stress and immune-related variables. Stress. 2015;18:710–7. doi: 10.3109/10253890.2015.1078306. [DOI] [PubMed] [Google Scholar]
- 48.Sever, J. Application of a microtechnique to viral serological investigations. J. Immunol. (1962). [PubMed]
- 49.Adriaansen-Tennekes R, Decuypere E, Parmentier HK, Savelkoul HFJ. Chicken lines selected for their primary antibody response to sheep red blood cells show differential hypothalamic-pituitary-adrenal axis responsiveness to mild stressors. Poult. Sci. 2009;88:1879–82. doi: 10.3382/ps.2009-00150. [DOI] [PubMed] [Google Scholar]
- 50.Davies S, Rodriguez NS, Sweazea KL, Deviche P. The effect of acute stress and long-term corticosteroid administration on plasma metabolites in an urban and desert songbird. Physiol. Biochem. Zool. 2013;86:47–60. doi: 10.1086/667990. [DOI] [PubMed] [Google Scholar]
- 51.Di Rienzo, J. A. et al. InfoStat, versión 2013. Grupo InfoStat, FCA, Univ Nac Cba, Argentina. (2013).
- 52.Shini S, Huff GR, Shini A, Kaiser P. Understanding stress-induced immunosuppression: exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes. Poult. Sci. 2010;89:841–51. doi: 10.3382/ps.2009-00483. [DOI] [PubMed] [Google Scholar]
- 53.Kaiser P. Advances in avian immunology–prospects for disease control: a review. Avian Pathol. 2010;39:309–24. doi: 10.1080/03079457.2010.508777. [DOI] [PubMed] [Google Scholar]
- 54.Puvadolpirod S, Thaxton JP. Model of physiological stress in chickens 1. Response parameters. Poult. Sci. 2000;79:363–9. doi: 10.1093/ps/79.3.363. [DOI] [PubMed] [Google Scholar]
- 55.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain. Behav. Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 56.Dhabhar, F. S., Miller, A. H., McEwen, B. S. & Spencer, R. Stress-Induced Changes in Blood leukocyte Distribution. J. Immunol. 1638–1644 (1996). [PubMed]
- 57.Dhabhar FS. Enhancing versus Suppressive Effects of Stress on Immune Function: Implications for Immunoprotection versus Immunopathology. Allergy, Asthma Clin. Immunol. 2008;4:2–11. doi: 10.1186/1710-1492-4-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rauw WM. Immune response from a resource allocation perspective. Front. Genet. 2012;3 doi: 10.3389/fgene.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huss D, Poynter G, Lansford R. Japanese quail (Coturnix japonica) as a laboratory animal model. Lab Anim. (NY). 2008;37:513–9. doi: 10.1038/laban1108-513. [DOI] [PubMed] [Google Scholar]
- 60.Cheema MA, Qureshi MA, Havenstein GB. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1519–29. doi: 10.1093/ps/82.10.1519. [DOI] [PubMed] [Google Scholar]
- 61.Rittschof CC, et al. Neuromolecular responses to social challenge: Common mechanisms across mouse, stickleback fish, and honey bee. Proc. Natl. Acad. Sci. 2014;111:17929–17934. doi: 10.1073/pnas.1420369111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hau M, Goymann W. Endocrine mechanisms, behavioral phenotypes and plasticity: known relationships and open questions. Front. Zool. 2015;12 doi: 10.1186/1742-9994-12-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng HW, Singleton P, Muir WM. Social stress in laying hens: differential dopamine and corticosterone responses after intermingling different genetic strains of chickens. Poult. Sci. 2002;81:1265–1272. doi: 10.1093/ps/81.9.1265. [DOI] [PubMed] [Google Scholar]
- 64.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA. 2003;100:567–71. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt KA, Ostfeld RS. Biodiversity and The Dilution Effect in Disease Ecology. Ecology. 2001;82:609–619. doi: 10.1890/0012-9658(2001)082[0609:BATDEI]2.0.CO;2. [DOI] [Google Scholar]
- 66.Schmid-Hempel, P. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. (Oxford University Press New York, 2011).
- 67.DeWitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998;13:77–81. doi: 10.1016/S0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- 68.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 2005;20:685–692. doi: 10.1016/j.tree.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Fusco G, Minelli A. Phenotypic plasticity in development and evolution: facts and concepts. Philos. Trans. R. Soc. B Biol. Sci. 2010;365:547–556. doi: 10.1098/rstb.2009.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen AA, Martin LB, Wingfield JC, McWilliams SR, Dunne JA. Physiological regulatory networks: Ecological roles and evolutionary constraints. Trends Ecol. Evol. 2012;27:428–435. doi: 10.1016/j.tree.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 71.Ghalambor, C. K., Martin, L. B. & Woods, H. A. Plasticity, Complexity, and the Individual. Integr. Org. Biol. 1–22, doi:10.1002/9781118398814.ch1 (2014).
- 72.Martin LB, et al. Integrator Networks: Illuminating the Black Box Linking Genotype and Phenotype. Integr. Comp. Biol. 2011;51:514–527. doi: 10.1093/icb/icr049. [DOI] [PubMed] [Google Scholar]
- 73.Futuyma, D. J. Evolutionary Biology. (Sinauer Associates, 1998).
- 74.Hartl, D. L. & Clark, A. G. Principles of Population Genetics. (Sinauer Associates, 2007).
- 75.Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol. Ecol. 2008;17:431–49. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- 76.Carson HL, Templeton AR. Genetic Revolutions in Relation to Speciation Phenomena: The Founding of New Populations. Annu. Rev. Ecol. Syst. 1984;15:97–131. doi: 10.1146/annurev.es.15.110184.000525. [DOI] [Google Scholar]
- 77.Maruyama T, Fuerst PA. Population bottlenecks and nonequilibrium models in population genetics. II. Number of alleles in a small population that was formed by a recent bottleneck. Genetics. 1985;111:675–89. doi: 10.1093/genetics/111.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


