ABSTRACT
Introduction
Physical endurance can be limited by muscle glycogen stores, in that glycogen depletion markedly reduces external work. During carbohydrate restriction, the liver synthesizes the ketone bodies, d-β-hydroxybutyrate, and acetoacetate from fatty acids. In animals and in the presence of glucose, d-β-hydroxybutyrate promotes insulin secretion and increases glycogen synthesis. Here we determined whether a dietary ketone ester, combined with plentiful glucose, can increase postexercise glycogen synthesis in human skeletal muscle.
Methods
After an interval-based glycogen depletion exercise protocol, 12 well-trained male athletes completed a randomized, three-arm, blinded crossover recovery study that consisted of consumption of either a taste-matched, zero-calorie control or a ketone monoester drink, followed by a 10-mM glucose clamp or saline infusion for 2 h. The three postexercise conditions were control drink then saline infusion, control drink then hyperglycemic clamp, or ketone ester drink then hyperglycemic clamp. Skeletal muscle glycogen content was determined in muscle biopsies of vastus lateralis taken before and after the 2-h clamps.
Results
The ketone ester drink increased blood d-β-hydroxybutyrate concentrations to a maximum of 5.3 versus 0.7 mM for the control drink (P < 0.0001). During the 2-h glucose clamps, insulin levels were twofold higher (31 vs 16 mU·L−1, P < 0.01) and glucose uptake 32% faster (1.66 vs 1.26 g·kg−1, P < 0.001). The ketone drink increased by 61 g, the total glucose infused for 2 h, from 197 to 258 g, and muscle glycogen was 50% higher (246 vs 164 mmol glycosyl units per kilogram dry weight, P < 0.05) than after the control drink.
Conclusion
In the presence of constant high glucose concentrations, a ketone ester drink increased endogenous insulin levels, glucose uptake, and muscle glycogen synthesis.
Key Words: d-β-HYDROXYBUTYRATE, GLUCOSE CLAMP, GLYCOGEN REPLETION, HYPERGLYCEMIA, INSULIN
In a series of experiments showing that endurance correlated with initial muscle glycogen stores, Jonas Bergström and Eric Hultman (5) demonstrated that muscle glycogen is the key determinant of endurance exercise capacity in man, and that glycogen exhaustion critically impairs the capacity for external muscular work (4,6). The work prompted decades of investigation into the optimal dose and formulation of both carbohydrate and amino acid supplements to enhance muscle glycogen recovery. No oral, postexercise regimen in humans is superior to an intake of 1.0–1.2 g·kg−1·h−1 carbohydrate for 4–6 h (8,34).
The ketone bodies, d-β-hydroxybutyrate (βHB) and acetoacetate, are naturally occurring four carbon substrates that are synthesized in the liver from circulating fatty acids under conditions of carbohydrate restriction. Ketone bodies supply fuel to the CNS during periods of starvation (9,31). The CNS has a constant, high-energy demand and accounts for 20% of the body's resting oxygen consumption (31). However, the brain is unable to oxidize fat, in which more than 99% of the body's energy stores are to be found (11). Ketone bodies are water-soluble, thermodynamically efficient substrates that connect the brain with its most abundant energy supply (24,31,35). In addition to providing an alternative to glucose for brain, skeletal muscle, and cardiac metabolism (10), ketone bodies act as a powerful signal to conserve precious stores of carbohydrate with a switch to the more abundant energy source, fat (30). Recently, a novel, safe, and orally bioavailable ketone monoester ((R)-3-hydroxybutyl (R)-3-hydroxybutyrate) drink has been developed (13), which can elevate βHB to 5–6 mM (equivalent to approximately a week of total fasting) within 30 min of consuming a single drink (37). We have demonstrated that ketone bodies can alter fuel preference during exercise, away from carbohydrate and toward fat oxidation (14). Here we tested whether elevated βHB influences fuel handling after exercise. Studies in animals have suggested that elevated ketone bodies, in the presence of abundant glucose, have the potential to increase glycogen synthesis (25,28). However, no study has determined whether ketones alter fuel handling after exercise in human muscle. The purpose of the present study was to test the hypothesis that exogenous ketone supplementation can increase glycogen repletion in human skeletal muscle.
METHODS
Subjects
Twelve male, well-trained athletes (mean ± SD: age = 33.0 ± 6.5 yr, body weight = 75.8 ± 5.0 kg, height = 1.70 ± 0.10 m, V˙O2max = 57.0 ± 4.8 mL·kg−1·min−1, peak power output [PPO] = 316 ± 34 W) who trained for 6–8 h·wk−1 volunteered to take part in the study. Experimental procedures and potential side effects were explained, and all participants gave written informed consent. Participants were instructed to refrain from alcohol and caffeine intake for 48 h before study visits. None of the participants had a history of neuromuscular or metabolic illness, and none were taking regular medication or dietary supplements. The study was conducted at the University of Oxford, was approved by the Ministry of Defence Research Ethics Committee (MODREC), and was conducted in accordance with the Declaration of Helsinki.
Measurement of maximal oxygen uptake
V˙O2max and PPO were determined in an incremental exercise protocol to volitional fatigue on an electrically braked upright cycle ergometer (Ergoselect 100; Ergoline, Baden-Württemberg, Germany). The test commenced with a 3-min warm-up at 50 W, followed by 25-W increments in workload every 3 min. Breath-by-breath measurements were performed using indirect calorimetry (Metalyser 3BR2Cortex Biophysik, Leipzig, Germany). A maximal test was defined by a respiratory exchange ratio exceeding 1.1 and/or a plateau in V˙O2 despite increasing workload. All participants achieved a maximal test and reached, or exceeded, 90% of their age-predicted maximal heart rate.
Glycogen depletion protocol
Participants attended the laboratory after a 12-h overnight fast. Glycogen depletion used the method described by van Loon et al. (39). After a 10-min warm-up at 50% V˙O2max, participants commenced exercise at intermittent intensity for 2-min intervals, alternating 90% PPO efforts with 50% PPO recovery. When fatigued at this intensity, the upper interval workload was decreased progressively in 10% PPO increments. Exhaustion, and protocol completion, was defined by the inability to complete 2 min at 60% PPO. Each participant's heart rate was monitored throughout (Polar H7 heart rate monitor; Polar, Kempele, Finland), and water was consumed ad libitum.
Ketone and control drinks
After completion of glycogen depletion, participants consumed either a ketone drink or an acaloric taste- and appearance-matched control drink of equal volume. The ketone drink, which had no side effects, contained 0.573 mL·kg−1 of the ketone ester, (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (12,13). The natural bitter taste was partially masked with citrus flavoring (Symrise, Holzminden, Germany) and proprietary sweetener (aspartame; NutraSweet™, Chicago, IL). The control drink contained the same citrus and sweetener components as the ketone drink. The bitter taste was matched with the addition of a weight-dosed commercial bitter agent (Symrise). The drinks were given immediately after the postglycogen depletion muscle biopsy, and the clamps started 30 min after ingestion.
Hyperglycemic and saline clamps
The hyperglycemic clamp was conducted according to the method described by DeFronzo et al. (15). A clamp method of providing carbohydrate after exhaustive exercise was chosen for three reasons. First, the technique provided a way to standardize glucose available to skeletal muscle across different visits for the same participant and between different individuals. Second, by delivering glucose intravenously, it is possible to avoid the variations in time and magnitude of glucose delivery caused by oral ingestion and enteral absorption. Third, it is possible to ensure that glucose delivery to the muscle is at least as high as would be provided by the recommended optimum postexercise carbohydrate feeding of 1.0–1.2 g·kg−1·h−1 for 4–6 h (34). The selection of a supraphysiological target whole blood glucose of 10 mM was made so that, in the event that an increase in glucose uptake was evident after a ketone supplement, it would be likely to represent a biologically significant intervention, which would increase glucose uptake, and potentially glycogen storage, beyond that achieved by high-dose oral carbohydrate feeding alone.
Participants had two intravenous cannulae sited (VenflonTM; Becton Dickinson, Plymouth, UK): one in the antecubital fossa (22 gauge), for infusion of 20% glucose (20% dextrose; Baxter Corporation, Staines, UK), and one in the dorsum of the contralateral hand (22 gauge) for sampling whole blood glucose at 5-min intervals. The limb containing the sampling cannula was heated to 40°C–44°C using two therapeutic heat pads (HK35 Beurer, Ulm, Germany) wrapped around the hand and forearm to cause maximal vasodilatation and generate a “pseudoarterialized” blood sample. A priming dose of 240 mg·kg−1 glucose was given during the first 15 min of the clamp, after which the glucose infusion rate was adjusted to maintain a whole blood glucose of 10 mM (180 mg·dL−1), measured in the pseudoarterialized blood sample using a benchtop analyzer (HemoCue 201+; Radiometer, Copenhagen, Denmark). At the end of the 2-h clamp, a second muscle biopsy was taken. The participant was then given a moderate glycemic index meal, and the dextrose infusion rate was gradually lowered to zero. Participants were observed until euglycemia was maintained for a period of 30 min after cessation of glucose infusion. For the saline clamp, all outward appearances and associated measurements were identical, but a 0.9% NaCl solution was infused instead of 20% glucose.
Muscle biopsies
Muscle biopsies were taken under local anesthetic (2% lidocaine hydrochloride) injected subcutaneously and infiltrated up to 3 cm deep into underlying muscle. Biopsies were taken from lateral incisions (approximately 8 mm in length) 2–3 cm apart, over the distal third of the vastus lateralis. Sampling was performed using a Bard MonoptyTM Core Biopsy Instrument, 12-F gauge, 10 cm long (Bard Biopsy Systems, Tempe, AZ). Four passes were made with the biopsy instrument yielding approximately 100 mg of tissue per biopsy. Samples were immediately frozen in liquid nitrogen and then stored at −80°C for later analysis.
Blood analyses
Peripheral blood samples were aspirated from the 22-gauge venous catheter inserted in the dorsum of the hand. Samples were collected in ethylenediaminetetraacetic acid tubes and stored at 4°C until centrifugation (1500g for 10 min at 4°C). Multiple aliquots of plasma were stored at −80°C until analysis. Samples were analyzed for glucose, lactate, nonesterified fatty acids (NEFA), and triglyceride, by automated benchtop analyzer (ABX Pentra, Montpellier, France). βHB and acetoacetate were analyzed using commercially available colorimetric assays (Sigma-Aldrich, St. Louis, MO). Insulin was measured using a commercial ELISA kit (Mercodia, Uppsala, Sweden).
Glycogen analysis
Glycogen in muscle samples was determined according to the method described by van Loon et al. (39). Freeze-dried skeletal muscle (3–6 mg) was powdered and hydrolyzed in 1 M hydrochloric acid at 99°C for 4 h. After passive cooling to room temperature, samples were neutralized using 250 μL of 0.12 mol·L−1 Tris/2.1 mol·L−1 potassium hydroxide saturated with potassium chloride. After centrifugation, 150 μL of supernatant was analyzed (in duplicate) for glucose using an automated benchtop analyzer (ABX Pentra). Glycogen content was expressed as millimoles of glycosyl units per kilogram dry weight of muscle.
Study design
The study was of a randomized, blinded, crossover design with three arms (Fig. 1). Participants attended a baseline visit for familiarization with experimental conditions and to complete an incremental maximum exercise test, measuring maximum oxygen uptake (V˙O2max) and PPO, to permit the prescription of the glycogen depletion protocol. Each study visit followed an overnight fast and commenced with a validated, exercise-interval protocol to deplete muscle glycogen (38,39). Participants ingested a taste- and appearance-matched ketone or control drink before either a 2-h hyperglycemic clamp with intravenous 20% glucose infusion to clamp whole blood glucose at 10 mM (180 mg·dL−1) or a sham clamp using a 0.9% saline infusion. The three recovery conditions used were (i) control drink followed by saline clamp (control saline), (ii) control drink followed by hyperglycemic clamp (control glucose), and (iii) ketone ester drink ((R)-3-hydroxybutyl (R)-3-hydroxybutyrate at 0.573 mL·kg−1 [615 mg·kg−1]) followed by hyperglycemic clamp (ketone glucose). Muscle samples were obtained from the vastus lateralis immediately after the glycogen depletion exercise and after the 2-h recovery period. Venous blood was sampled at 5-min intervals for whole blood glucose and at regular intervals to measure substrate and insulin concentrations.
FIGURE 1.

Schematic of the study protocol.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 7; GraphPad Software Inc., La Jolla, CA). Energy substrate and insulin data were analyzed using a two-way ANOVA, with time and recovery condition as factors. Comparisons included both substrate concentrations at predetermined time intervals and area under the curve (AUC) measurements. Where significance of the recovery intervention or interaction of recovery intervention and time was detected, Tukey post hoc corrections were made for multiple comparisons to identify specific significant differences. Results for substrates are presented as two-way ANOVA (AUC), followed by Tukey post hoc comparisons of experimental conditions describing the mean difference and P value. Glycogen results were analyzed using Student's paired t-test. All results are presented as mean ± SEM. A P value ≤0.05 was taken to indicate statistical significance.
RESULTS
Physiological response to the glycogen depletion exercise
The time to exhaustion for all glycogen depletion protocols was 115 ± 2 min. The mean heart rate was 165 ± 0 bpm. The mean duration and heart rates were the same for the three recovery conditions.
Blood substrate concentrations
With control saline, blood βHB concentrations rose steadily from 0.8 ± 0.1 mM at the start of the clamp to 1.6 ± 0.2 mM by the end (Fig. 2A). With control glucose, βHB started at 0.7 ± 0.1 mM and fell to 0.15 ± 0.0 mM by the end of the clamp. After the ketone ester drink, βHB rose to peak at 5.3 ± 0.5 mM and decreased to 3.3 ± 0.2 mM by the end of the clamp. The βHB AUC from 0 to 120 min (AUC0–120) for control saline, control glucose, and ketone glucose were 190 ± 14, 97 ± 14, and 650 ± 38 mmol·min−1, respectively (P < 0.0001 for comparison between each condition) (Fig. 2B).
FIGURE 2.

A, βHB, glucose, and NEFA concentrations during each study visit. B, AUC measures of βHB, glucose, and NEFA during the 2-h recovery clamp. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Error bars are ±SEM.
With control saline, the glucose at the start of the clamp was 4.5 ± 0.2 mM and remained ≤4.6 ± 0.2 mM throughout (Fig. 2A). After both control glucose and ketone glucose, the starting glucose, at ≤4.6 ± 0.2 mM, rose rapidly to a plateau between 9 and 12 mM throughout the 10-mM glucose clamp. There was no significant difference in glucose availability between control glucose and ketone glucose. The glucose AUC0–120 (Fig. 2B) was the same for control glucose and ketone glucose, at 2137 ± 43 and 1927 ± 151 mmol·min−1, respectively.
For all conditions, NEFA was 0.42 ± 0.0 mM after an overnight fast (Fig. 2A). During the glycogen depleting exercise, NEFA rose to 0.75 ± 0.1 mM, with no difference between groups before consuming the drinks. At the start and throughout the saline infusion, NEFA was elevated at ≥1.2 ± 0.2 mM. For control glucose, NEFA was 1.3 ± 0.2 mM at the start of the clamp but fell rapidly to 0.6 ± 0.1 mM after 25 min. After the ketone ester drink, NEFA was significantly lower than both control saline and control glucose at the start of the clamp, at 0.7 ± 0.1 mM (P < 0.0001). NEFA levels fell further, to 0.1 ± 0.0 mM, by 1 h of the glucose clamp and remained at this level for the following hour. The NEFA AUC0–120 (Fig. 2B) for control saline, control glucose, and ketone glucose were 272 ± 33, 165 ± 21, and 110 ± 13 mmol·min−1, respectively (P < 0.05 for comparisons between all conditions).
Glucose uptake and endogenous insulin concentration
During the 2-h hyperglycemic clamp, glucose uptake was 32% higher after the ketone ester drink compared with the control drink (Fig. 3, lower panel). Total glucose uptake was 1.26 ± 0.04 g·kg−1 for control glucose and 1.66 ± 0.06 g·kg−1 for ketone glucose, the difference being 0.4 ± 0.0 g·kg−1 (P < 0.0001). On the basis of the mean body weight of the participants (75.8 kg), and 2 h of clamping, this represents a 32% increase, from 197 to 258 g, an additional 61 g or 340 mmol of glucose. The greater glucose uptake was associated with a twofold higher insulin concentration by the end of the clamp (Fig. 3, upper panel), the insulin concentrations being 16 ± 3 mU·L−1 for control glucose and 31 ± 6 mU·L−1 for ketone glucose, with the mean difference being 15 ± 3 mU·L−1 (P < 0.001).
FIGURE 3.
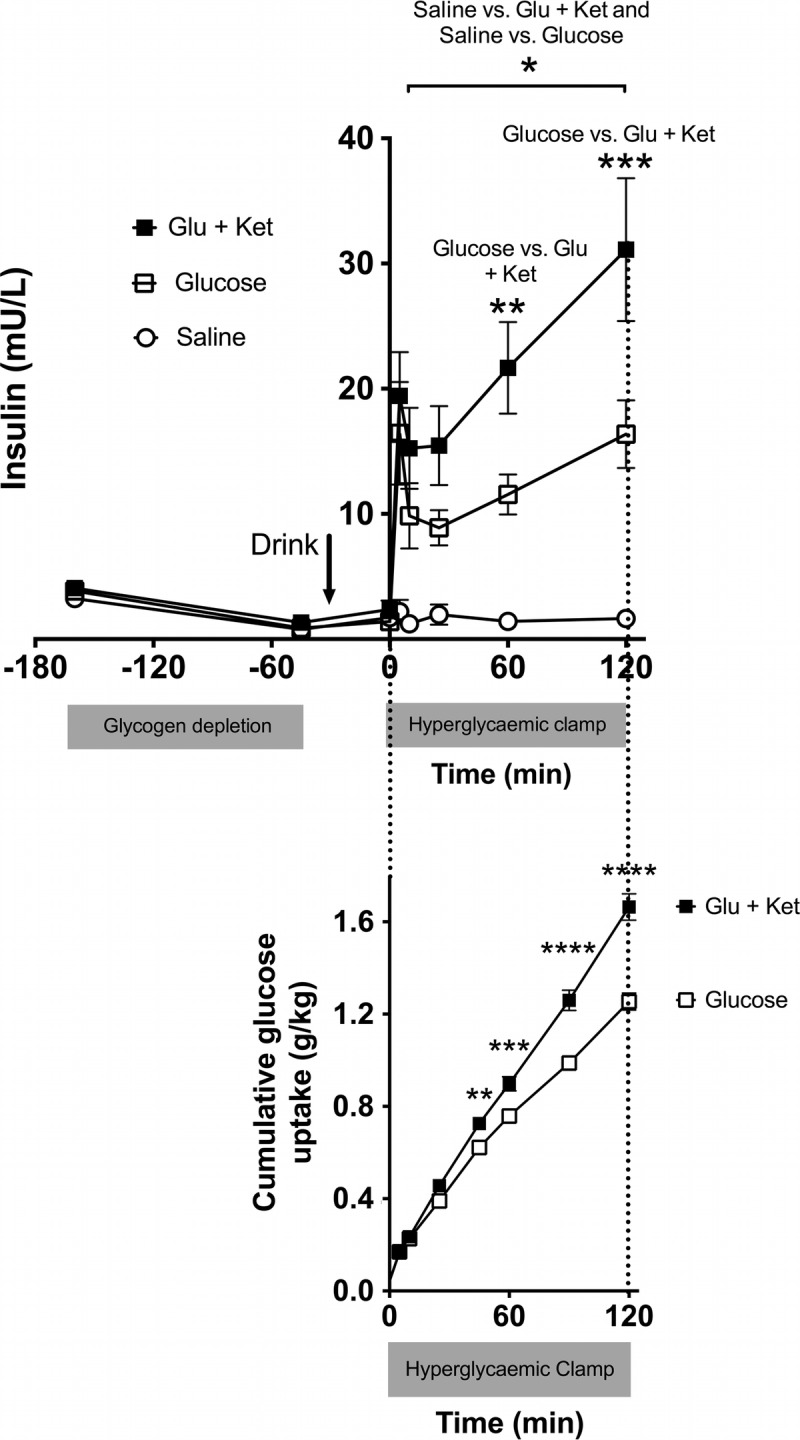
Insulin concentration throughout the study visits and cumulative glucose uptake during the 2-h recovery clamp. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Error bars are ±SEM.
Muscle glycogen
Muscle glycogen increased during the 2 h infusion after exercise under all conditions, but the levels after the ketone ester drink were 50% higher than those following the other two conditions (Table 1 and Fig. 4). During the saline infusion, glycogen synthesis was 70% lower than after the ketone drink plus 10-mM glucose clamp.
TABLE 1.
Muscle glycogen concentrations before and after glucose/saline infusions.
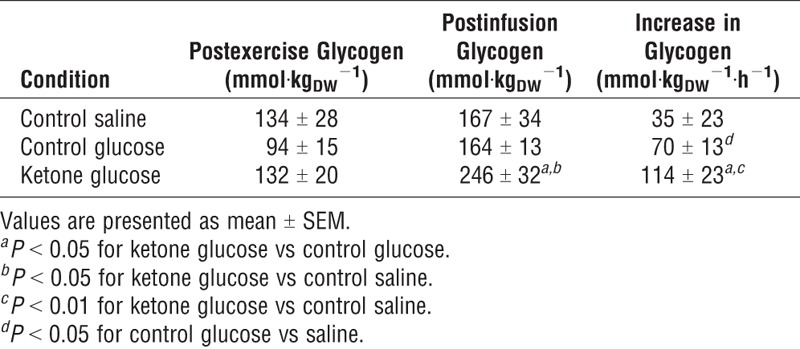
FIGURE 4.

A, Skeletal muscle glycogen pre- and postrecovery period. B, The increase in glycogen during 2 h infusion. *P < 0.05; **P < 0.01. Error bars are ±SEM.
DISCUSSION
Here, under conditions of matched glucose availability, a ketone monoester drink allowed significantly greater endogenous insulin release, glucose uptake, and muscle glycogen synthesis compared with hyperglycemic glucose alone. Richter et al. (32) demonstrated that moderate-intensity exercise causes both an increase in insulin sensitivity (defined as a left-shift of the glucose uptake vs insulin concentration relationship) and insulin responsiveness (a state in which supraphysiological doses of insulin can effect a further increase in glycogen synthesis; defined as upward displacement of the glucose uptake vs insulin concentration relationship) in rodents. Increases in both insulin sensitivity and insulin responsiveness enhance glycogen synthesis. Exercise alone can enhance insulin sensitivity, independent of glycogen depletion, but the increased insulin responsiveness is specifically mediated by glycogen depletion itself (41). AMP-activated protein kinase activity has been implicated in the increased insulin sensitivity postexercise (18), and ketone bodies may increase AMPK activity in rodents (40). AMPK activity is inversely correlated with glycogen concentration and glycogen is thought to inhibit AMPK by binding to the carbohydrate-binding domain on its β-subunit (29). Taken together, these findings have led to speculation that increasing AMPK release from glycogen during exercise increases insulin sensitivity (23).
The close similarity between the additional amount of glucose taken up after the ketone ester and the additional amount of glycogen estimated to be stored in muscle supports the argument that the infused glucose was stored as glycogen. The ketone ester increased whole-body glucose uptake from 1.26 to 1.66 g·kg−1, equivalent to 340 mmol of glucose. The ketone drink increased glycogen by 44 mmol (from 70 to 114 mmol) glycosyl units per kilogram (dry weight of muscle). In a whole-body MRI study of 66 males 18–29 yr of age, of a similar height and weight to our participants, Janssen et al. (22) estimated the lower limb skeletal muscle mass to be 18.5 kg. Applying a wet–dry weight correction, based on a water content of 75% (36), 18.5 kg lower limb muscle mass would be equivalent to a dry weight of 4.4 kg in our participants. Assuming that trained individuals could recruit 90% of this muscle, then the increase seen in the glycogen content of our skeletal muscle biopsies after exogenous ketones would require an additional 352-mmol glucose. Thus, the fate of the additional infused glucose was probably incorporated into skeletal muscle glycogen, which is consistent with the findings of Maehlum et al. (27), who concluded that skeletal muscle glycogen synthesis takes precedence over hepatic glycogen synthesis postexercise.
It seems likely that elevated circulating βHB augmented insulin release from pancreatic beta cells, to double circulating insulin concentrations in response to 10 mM blood glucose, thereby explaining the 32% higher whole-body glucose uptake after a ketone ester versus control drink. The first report of ketones causing endogenous insulin release was in 1964 in dogs (26). Early studies in humans failed to demonstrate any increase in insulin secretion in response to ketone bodies (1,2,17). Subsequently, using catheters placed in the hepatic portal vein (for the purposes of hepatic imaging in two colorectal cancer patients), Balasse et al. (3) identified an increase in hepatic portal vein insulin in response to acetoacetate infusion. However, the same group failed to reproduce the insulinotropic effect in the peripheral blood of obese subjects, admitted to hospital for therapeutic fasting, but their mean glucose concentration was low at 3.9 mM (~71 mg·dL−1). In all cases in which no increase in peripheral blood insulin was demonstrated, the blood glucose concentrations were 5 mM (90 mg·dL−1) or lower (1–3,16,17). It therefore appears likely that the augmentation of insulin release from beta cells in response to βHB requires a simultaneous presentation of high-normal, or frankly elevated, blood glucose. Here, increased insulin after ketone ester versus control glucose was only observed after the start of glucose infusion and the rapid elevation of plasma glucose above the starting concentration of 4.6 mM.
βHB promotes insulin secretion from isolated rat pancreatic islets in the presence of 5 mM glucose but is ineffective in the absence of glucose (7). Here, βHB did not increase insulin levels before the glucose infusion, when glucose concentrations were 4.6 mM (Fig. 3), but it did lower circulating free fatty acid concentrations (Fig. 2). Consequently, we do not know if βHB is used by the pancreatic beta cells for energy and thereby increases insulin release in proportion to the glucose taken up by tissues or if the βHB decreases free fatty acid concentrations, thereby stimulating glucose uptake into tissues (the “Randle effect”) or both.
The doubling of endogenous insulin release in response to elevated βHB concentrations has implications beyond enhanced glucose uptake and glycogen synthesis. Insulin has an anabolic action on skeletal muscle by inhibiting muscle catabolism that normally follows exercise (19,20). Therefore, this large, sustained increase in insulin release with abundant carbohydrate supply immediately after exercise potentially preserves skeletal muscle.
Speculation regarding the adaptive advantage of an insulinotropic effect of ketone bodies has centered on negative feedback mechanisms (33). Insulin inhibits peripheral lipolysis, thereby limiting the supply of circulating NEFA to the liver and preventing uncontrolled ketogenesis. It is plausible that an insulinotropic action of ketones is an adaptation specifically to confer advantage at times of transition from the starved to the fed state. As glucose concentrations increase after a meal ending a prolonged fast, the enhanced insulin release in the presence of significant endogenous ketosis would serve both to protect the carbohydrate stores of the liver, by limiting hepatic glucose output, and to ensure maximal assimilation of the circulating glucose into body tissues in the form of glycogen.
Limitations
In this laboratory-based study, we used rigidly controlled, intravenous high-dose glucose delivery, aiming for constant 10 mM (180 mg·dL−1) whole blood glucose (the upper limit of glucose concentrations seen postprandially). It is therefore necessary to investigate whether the findings of this study can be reproduced in athletes consuming a ketone ester drink in addition to recommended postexercise dietary carbohydrate regimens. Not run was a fourth (control) protocol, a ketone drink plus saline infusion, which would presumably have resulted in low glycogen recovery, similar to that observed with the zero-calorie drink plus saline infusion, owing to the lack of exogenous glucose.
Given that low muscle glycogen stores impair both moderate- and high-intensity exercise (21), and that the exhaustion of glycogen reserves during exercise causes a marked reduction in external work (4,6), any intervention that enhances glycogen synthesis is of potential benefit to human exercise performance. Here, we have shown that ketone and glucose together augment and accelerate glucose uptake, probably by elevating insulin, thereby hastening glycogen recovery.
Acknowledgments
The UK Defence Science and Technology Laboratories is gratefully acknowledged for funding this study. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation and do not constitute endorsement by the American College of Sports Medicine.
The intellectual property and patents covering the uses of ketone bodies and esters are owned by BTG Ltd,, the University of Oxford, the National Institutes of Health, and TΔS® Ltd. Should royalties ever accrue from these patents, K. C., P. J. C., and D. A. H., as named inventors, may receive a share of royalties as determined by the terms of their respective institutions. K. C. is a director of TΔS®, a University of Oxford spin out company that aims to develop and commercialize products based on the ketone ester.
REFERENCES
- 1.Balasse E, Ooms HA. Changes in the concentrations of glucose, free fatty acids, insulin and ketone bodies in the blood during sodium beta-hydroxybutyrate infusions in man. Diabetologia. 1968;4(3):133–5. [DOI] [PubMed] [Google Scholar]
- 2.Balasse EO, Neef MA. Inhibition of ketogenesis by ketone bodies in fasting humans. Metabolism. 1975;24(9):999–1007. [DOI] [PubMed] [Google Scholar]
- 3.Balasse EO, Ooms HA, Lambilliotte JP. Evidence for a stimulatory effect of ketone bodies on insulin secretion in man. Horm Metab Res. 1970;2(6):371–2. [DOI] [PubMed] [Google Scholar]
- 4.Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71(2): 140–50. [DOI] [PubMed] [Google Scholar]
- 5.Bergström J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1966;210(5033):309–10. [DOI] [PubMed] [Google Scholar]
- 6.Bergström J, Hultman E. A study of the glycogen metabolism during exercise in man. Scand J Clin Lab Invest. 1967;19(3):218–28. [DOI] [PubMed] [Google Scholar]
- 7.Biden TJ, Taylor KW. Effects of ketone bodies on insulin release and islet-cell metabolism in the rat. Biochem J. 1983;212(2):371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke LM, Kiens B, Ivy JL. Carbohydrates and fat for training and recovery. J Sports Sci. 2004;22(1):15–30. [DOI] [PubMed] [Google Scholar]
- 9.Cahill G, Jr, Felig P, Owen O, Wahren J. Metabolic adaptation to prolonged starvation in man. Nord Med. 1970;83(3):89. [PubMed] [Google Scholar]
- 10.Cahill GF. Starvation in man. N Engl J Med. 1970;282(12):668–75. [DOI] [PubMed] [Google Scholar]
- 11.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1–22. [DOI] [PubMed] [Google Scholar]
- 12.Clarke K, Tchabanenko K, Pawlosky R, et al. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol. 2012;63(2):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke K, Tchabanenko K, Pawlosky R, et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63(3):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256–68. [DOI] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. [DOI] [PubMed] [Google Scholar]
- 16.Devecerski M, Pierce CE, Frawley TF. Effect of ketone acids on glucose and fat metabolism in adipose tissue of the rat. Metabolism. 1968;17(10):877–84. [DOI] [PubMed] [Google Scholar]
- 17.Fajans SS, Floyd JC, Knopf RF, Conn JW. A comparison of leucine and acetoacetate-induced hypoglycemia in man. J Clin Invest. 1964;43:2003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282(1):E18–23. [DOI] [PubMed] [Google Scholar]
- 19.Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J Clin Invest. 1995;96(4):1722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukagawa NK, Minaker KL, Rowe JW, et al. Insulin-mediated reduction of whole body protein breakdown. Dose–response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985;76(6):2306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hargreaves M, Richter EA. Regulation of skeletal muscle glycogenolysis during exercise. Can J Sport Sci. 1988;13(4):197–203. [PubMed] [Google Scholar]
- 22.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985). 2000;89(1):81–8. [DOI] [PubMed] [Google Scholar]
- 23.Jensen TE, Richter EA. Regulation of glucose and glycogen metabolism during and after exercise. J Physiol. 2012;590(5): 1069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashiwaya Y, King MT, Veech RL. Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart. Am J Cardiol. 1997;80(3A):50A–64. [DOI] [PubMed] [Google Scholar]
- 25.Laughlin MR, Taylor J, Chesnick AS, Balaban RS. Nonglucose substrates increase glycogen synthesis in vivo in dog heart. Am J Physiol. 1994;267(1 Pt 2):H219–23. [DOI] [PubMed] [Google Scholar]
- 26.Madison LL, Mebane D, Unger RH, Lochner A. The hypoglycemic action of ketones. II. Evidence for a stimulatory feedback of ketones on the pancreatic beta cells. J Clin Invest. 1964;43:408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maehlum S, Felig P, Wahren J. Splanchnic glucose and muscle glycogen metabolism after glucose feeding during postexercise recovery. Am J Physiol. 1978;235(3):E255–60. [DOI] [PubMed] [Google Scholar]
- 28.Maizels EZ, Ruderman NB, Goodman MN, Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J. 1977;162(3): 557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain metabolism during fasting. J Clin Invest. 1967; 46(10):1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69(4):785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980;60(1):143–87. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez NR, Di Marco NM, Langley S, American Dietetic Association; Dietitians of Canada. American College of Sports Medicine Position Stand: nutrition and athletic performance. Med Sci Sports Exerc. 2009;41(3):709–31. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Kashiwaya Y, Keon CA, et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9(8):651–8. [DOI] [PubMed] [Google Scholar]
- 36.Sawka MN. Physiological consequences of hypohydration: exercise performance and thermoregulation. Med Sci Sports Exerc. 1992;24(6):657–70. [PubMed] [Google Scholar]
- 37.Shivva V, Cox PJ, Clarke K, Veech RL, Tucker IG, Duffull SB. The population pharmacokinetics of d-β-hydroxybutyrate following administration of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. AAPS J. 2016;18(3):678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor C, Bartlett JD, van de Graaf CS, et al. Protein ingestion does not impair exercise-induced AMPK signalling when in a glycogen-depleted state: implications for train-low compete-high. Eur J Appl Physiol. 2013;113(6):1457–68. [DOI] [PubMed] [Google Scholar]
- 39.van Loon LJ, Saris WH, Kruijshoop M, Wagenmakers AJ. Maximizing postexercise muscle glycogen synthesis: carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am J Clin Nutr. 2000;72(1):106–11. [DOI] [PubMed] [Google Scholar]
- 40.Veech RL. Ketone esters increase brown fat in mice and overcome insulin resistance in other tissues in the rat. Ann N Y Acad Sci. 2013;1302:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zorzano A, Balon TW, Goodman MN, Ruderman NB. Glycogen depletion and increased insulin sensitivity and responsiveness in muscle after exercise. Am J Physiol. 1986;251(6 Pt 1):E664–9. [DOI] [PubMed] [Google Scholar]


