Abstract
Pregnant women with polycystic ovary syndrome (PCOS) are often overweight or obese. To study the effects of maternal androgen excess in obese dams on metabolism, placental function and fetal growth, female C57Bl6J mice were fed a control (CD) or a high fat/high sucrose (HF/HS) diet for 4–10 weeks, and then mated. On gestational day (GD) 15.5–17.5, dams were injected with dihydrotestosterone (CD-DHT, HF/HS-DHT) or a vehicle (CD-Veh, HF/HS-Veh). HF/HS dams had higher fat content, both before mating and on GD18.5, with no difference in glucose homeostasis, whereas the insulin sensitivity was higher in DHT-exposed dams. Compared to the CD groups, the livers from HF/HS dams weighed more on GD18.5, the triglyceride content was higher, and there was a dysregulation of liver enzymes related to lipogenesis and higher mRNA expression of Fitm1. Fetuses from HF/HS-Veh dams had lower liver triglyceride content and mRNA expression of Srebf1c. Maternal DHT exposure, regardless of diet, decreased fetal liver Pparg mRNA expression and increased placental androgen receptor protein expression. Maternal diet-induced obesity, together with androgen excess, affects maternal and fetal liver function as demonstrated by increased triglyceride content and dysfunctional expression of enzymes and transcription factors involved in de novo lipogenesis and fat storage.
Introduction
Androgen excess in females is associated with insulin resistance, β-cell failure, ovarian dysfunction and infertility1. Moreover, around 28 to 64% of women with polycystic ovary syndrome (PCOS) are obese which increases the risk of poor pregnancy outcomes2, 3. Furthermore, when women with PCOS are pregnant, hyperandrogenemia is linked to adverse obstetric outcomes, including gestational diabetes mellitus (GDM), preeclampsia and low or high birth weight4, 5. It has been suggested that an impairment in the decidual trophoblast invasion6, and/or alterations of the microstructure of the placenta7, may be involved in the increased risk of pregnancy complications in women with PCOS. Exposure to an adverse intrauterine environment in pregnancies complicated by PCOS may program the fetus for long-term health consequences, including reproductive dysfunction as well as behavioral and metabolic abnormalities8–11.
It has been proposed that PCOS originates during fetal development and that this might be due, in part, to maternal androgen excess9–12. Maternal obesity, either established prior to pregnancy or due to an excessive weight gain during pregnancy, increases the risk of GDM and gestational hypertension and is associated with adverse obstetric outcomes including preterm birth and cesarean delivery13. Thus, maternal obesity is another factor causing an unfavorable intrauterine environment and it is associated with increased birth weight in the offspring and an augmented risk of being overweight at 4 years14, 15 and at 21 years of age16.
A large number of models of maternal obesity have been developed in various species. In some of these models maternal obesity is associated with altered placental function17, fetal overgrowth18, offspring hypertension, glucose intolerance19, stress20 and an altered methylation profile in oocytes and offspring liver, predisposing to the development of metabolic failures21.
Maternal (prenatal) androgen exposure is a commonly used animal model of PCOS that has been developed in rodents, sheep and non-human primates8–12, 22, 23. All these models reflect the lean PCOS phenotype and fail to reproduce the combination of maternal androgen excess and maternal obesity, and might not be relevant to overweight/obese pregnant women with PCOS. However, in the nonhuman primate PNA model, testosterone propionate have shown to increase the maternal body weight gain together with an altered maternal glucoregulation and higher body weight of the offspring at 8 weeks of postnatal life24. In fact, high levels of androgens during pregnancy have been related to metabolic25 and cardiovascular26 derangements. In animal models, we and others have also shown that prenatally androgenized (PNA) female rat offspring are born small for gestational age (SGA), but normal fetal weight has also been reported9–11, 22, 27, 28. However, in pregnant women with PCOS, especially those that are obese, both SGA and large for gestational age (LGA) occur29, 30. The PNA rat offspring are not obese but exhibit metabolic disturbances and dysfunction of adipose tissue, as reflected by altered adipocyte size, high circulating triglycerides (TG), non-alcoholic fatty liver and reproductive dysfunction22. Thus, there is a need for an animal model of both androgen excess and maternal obesity that is more reflective of human PCOS pregnancies complicated by obesity.
In the current study, we aimed to combine the PNA mouse model with a model of maternal obesity induced by a high fat/high sugar Western style diet that has been shown to result in fetal overgrowth18, 31 and to investigate maternal metabolism, placental function and fetal growth.
Results
Maternal Variables
The body weight development during pregnancy is shown in Fig. 1A. Dams in the HF/HS-Veh and HF/HS-DHT groups weighed more than CD and CD-DHT dams from GD0 until GD17.5 (p < 0.05), but did not differ in body weight on GD18.5 (Fig. 1A).
Figure 1.
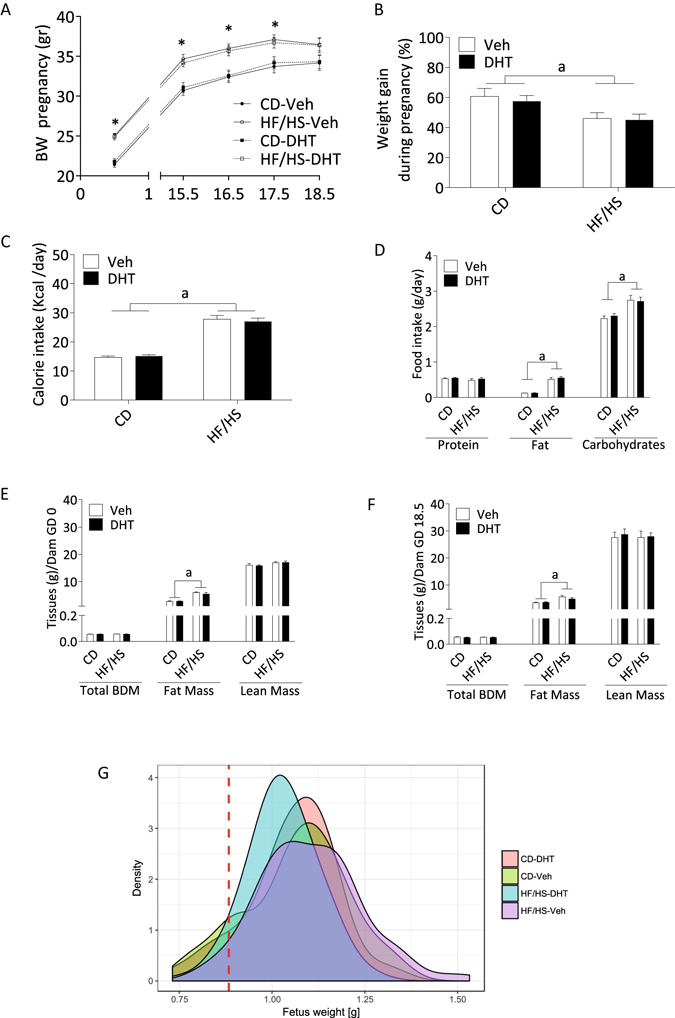
Maternal body weight development, calorie intake and body composition (A) Body weight gain during pregnancy; (B) Percentage of weight gain during pregnancy (CD-Veh, n = 12–13; HF/HS-Veh, n = 12–13; CD-DHT, n = 11–12; HF/HS-DHT, n = 14). (C) Caloric intake (Kcal/day) (CD-Veh, n = 12–13; HF/HS-Veh, n = 12–13; CD-DHT, n = 11–12; HF/HS-DHT, n = 14). (D) Food Intake (g/day) (E) DEXA the day before mating (GD0) and (F) DEXA GD18.5 (CD-Veh, n = 5; HF/HS-Veh, n = 5; CD-DHT, n = 4; HF/HS-DHT, n = 4). (G) Distribution of fetal weight across the groups. Values are means ± SEM. *P < 0.05 HF/HS vs CD-Veh and HF/HS-DHT vs CD-Veh or CD-DHT, a = Main effect of diet p < 0.05.
There was a main effect of diet on weight gain during pregnancy; dams fed HF/HS had a lower percentage of weight gain than those fed control chow [F(1,44) = 10.4. p < 0.05] (Fig. 1B). Calorie intake (Fig. 1C) and also food intake per day, i.e. the number of grams of the sucrose solution and pellets, was higher in dams fed HF/HS [F(1,48) = 150.4. p < 0.001] (Fig. 1D).
There was a significant main effect of diet on body composition measured by DEXA prior to mating and on GD 18.5 with higher fat mass in dams fed HF/HS both on GD0 [F(1,14) = 129.1, p < 0.001] (Fig. 1E) and on GD18.5 [F (1,14) = 19.44, p < 0.001] (Fig. 1F), with no effect of the DHT injection.
The litter size and placental weight were not affected by maternal diet or DHT injection (Table 1). Although not significant, there was a trend for lower fetal weight in DHT injected dams than in vehicle-injected dams [F (1,48) 3.16, p = 0.082] (Table 1). Posthoc analyses revealed that it was fetuses from HF/HS-DHT dams that weighed less than fetuses from HF/HS-Veh dams (p = 0.035) (Table 1). The body composition of fetuses, assessed by BCA, was not affected by maternal diet or DHT injection (Table 2). The distribution of fetal weight is displayed in Fig. 1G.
Table 1.
Litter size, fetal and placental weight.
| CD-Veh (n = 13) | CD-DHT (n = 13) | HF/HS-Veh (n = 12) | HF/HS-DHT (n = 14) | Main Effect | |
|---|---|---|---|---|---|
| Litter size | 7.62 ± 0.72 | 7.75 ± 0.69 | 7.69 ± 0.64 | 8.21 ± 0.59 | ns |
| Fetal Weight at GD 18.5 | 1.07 ± 0.04 | 1.06 ± 0.03 | 1.11 ± 0.02 | 1.03 ± 0.02 | ns |
| Placenta Weight at GD 18.5 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 | ns |
| Fetus/Placenta Weight | 10.99 ± 0.47 | 11.02 ± 0.30 | 11.75 ± 0.46 | 10.86 ± 0.29 | ns |
GD: Gestational day; CD, control diet; HF/HS, high fat diet; Veh, vehicle; DHT, dihydrotestosterone. ns: no significance. Data are Mean values ± SEM and were analyzed by 2-way ANOVA to investigate interactions between maternal diet and DHT injection or main effect of either maternal diet or DHT. There was no interaction between maternal diet and the DHT injection in any variables studied.
Table 2.
Fetal body composition was assessed by Body Composition Analyzer (BCA), Bruker minispec LF110.
| CD-Veh n = 4 | CD-DHT n = 5 | HF/HS-Veh n = 4 | HF/HS-DHT n = 4 | Main Effect | |
|---|---|---|---|---|---|
| Body Mass (g) | 1.07 ± 0.05 | 0.99 ± 0.12 | 1.12 ± 0.02 | 1.04 ± 0.03 | ns |
| Lean Mass (g) | 0.52 ± 0.11 | 0.66 ± 0.05 | 0.54 ± 0.09 | 0.53 ± 0.03 | ns |
| Fat (g) | 0.38 ± 0.06 | 0.32 ± 0.04 | 0.39 ± 0.05 | 0.34 ± 0.04 | ns |
CD, control diet; HF/HS, high fat diet; Veh, vehicle; DHT, dihydrotestosterone. ns: no significance. Data are Mean values ± SEM and were analyzed by 2-way ANOVA to investigate interactions between maternal diet and DHT injection or main effects of either maternal diet or DHT.
Maternal circulating sex steroids and glucose metabolism
Maternal sex steroid levels measured by LC-MS/MS are presented in Table 3. There was a main effect of maternal diet on circulating progesterone levels [F(1,30) 5.77, p = 0.023] with higher concentrations in dams fed HF/HS and injected with DHT than in dams on control chow and injected with DHT (p < 0.05). There was a main effect of the DHT injection with higher circulating DHT concentrations in dams fed control chow and injected with DHT than in the vehicle controls [F(1,30) 9.97, p = 0.004], with no difference in dams fed HF/HS. There were no interaction effects or main effects of diet or DHT injection on circulating estradiol and testosterone.
Table 3.
Sex steroids in the serum of dams on GD 18.5.
| CD-Veh n = 7–8 | CD-DHT n = 8 | HF/HS n = 8 | HF/HS-DHT n=10 | Main Effect | Posthoc test (p < 0.05) | |
|---|---|---|---|---|---|---|
| Estradiol (pg/ml) | 19.21 ± 1.87 | 16.68 ± 1.48 | 23.77 ± 4.47 | 17.26 ± 2.19 | ns | ns |
| Testosterone (pg/ml) | 117.58 ± 15.77 | 155.48 ± 32.39 | 108.84 ± 10.77 | 116.60 ± 11.13 | ns | ns |
| Progesterone (ng/ml) | 16.20 ± 5.89 | 11.99 ± 4.42 | 26.40 ± 11.70 | 36.57 ± 8.21 | a: p = 0.023 | HF/HS-DHT vs CD-DHT |
| DHT (pg/ml) | 45.80 ± 14.58 | 1553.51 ± 783.59 | 52.40 ± 11.44 | 961.44 ± 173.67 | b: p = 0.004 | CD-DHT vs CD-Veh |
CD, control; HF/HS, high fat/high sucrose diet; DHT, dihydrotestosterone. Data are Mean values ± SEM. a = Main effect of diet [F (1,30) 5.77, p = 0.023]. b = Main effect of injection [F (1.30) 9.97, p = 0.004] There was no interaction between diet and DHT injection for any of the variables.
The glucose levels from the oral glucose tolerance test (OGTT), performed on GD18.5, are presented in Fig. 2A. There was a main effect of the DHT injection with lower Homeostasis Model Assessment Insulin Resistance (HOMA-IR) in DHT injected pregnant dams than in vehicle-injected dams [F(1,11) 5.40. p = 0.040] (Fig. 2B). There were no interaction or main effects of diet or DHT injection on the Area Under the Curve (AUC) of glucose T0-90′ (Fig. 2C). However, there was a main effect of the DHT injection with lower AUC of insulin T0-15′ [F(1,10) 8.06. p = 0.018] (Fig. 2D), specifically when dams fed control chow and injected with DHT were compared to vehicle controls (p = 0.043) (Fig. 2D).
Figure 2.
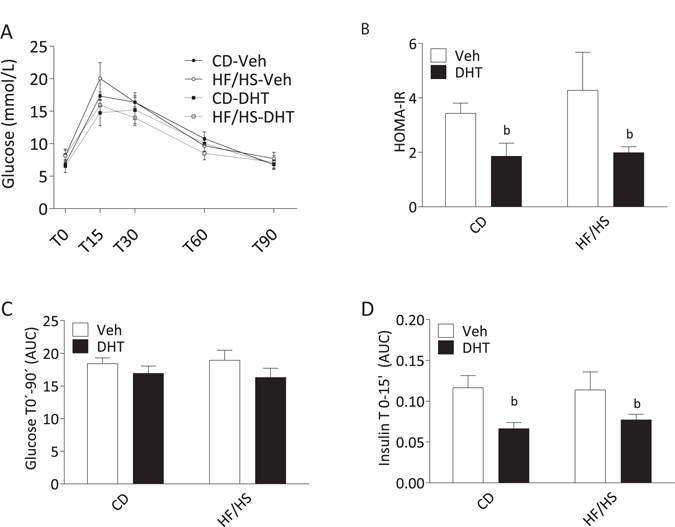
Glucose metabolism on GD 18.5. (A) Blood glucose levels during oral glucose tolerance test (OGTT) (n = 4–5 per group). (B) The Homeostasis Model Assessment (HOMA-IR). (C) Glucose area under the curve (AUC) T0-90 minutes and (D) Insulin AUC T0-15 minutes (n = 3–5 per group). Values are means ± SEM; b = Main effect of injection p < 0.05.
Placental steroidogenesis
There was a main effect of the DHT injection with higher androgen receptor expression in DHT injected dams than in vehicle-injected dams [F(1,25) 5.90, p = 0.023] (Fig. 3A). There were no interaction or main effects of diet or DHT injection on the expression of estrogen receptors α and β, progesterone receptor A and B or glucocorticoid (Fig. 3B–F), nor in the expression of steroidogenic enzymes CYP11A1 and 17-βHSD2 (Supplementary Fig. 2A,B).
Figure 3.
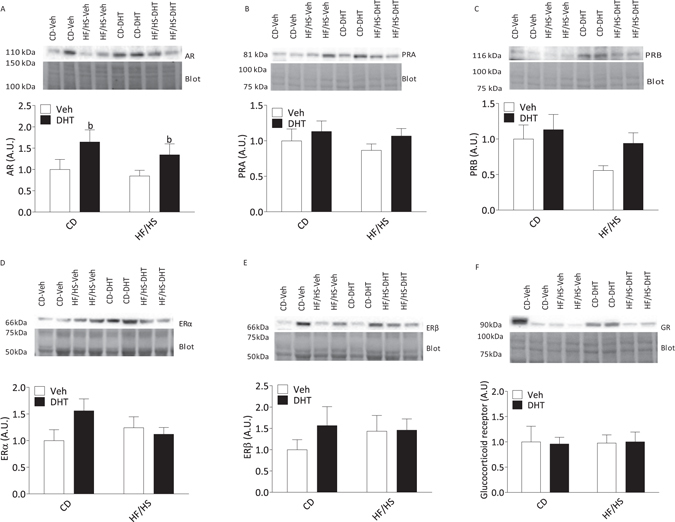
Protein expression of sex steroid receptors in placental homogenates on GD 18.5 (A) Androgen receptor (AR), (B) Progesterone receptor A (PRA). (C) Progesterone receptor B (PRB), (D) Estrogen receptor (ER) α, ERα. (E), Estrogen receptor β, ERβ. (F) Glucocorticoid Receptor (GR). (CD-Veh, n = 8; HF/HS-Veh, n = 8; CD-DHT, n = 8; HF/HS-DHT, n = 10). Protein expression was quantified by Western blot and normalized to total protein loaded in each line in the stain free blot. All blot images were cropped for publication, and the full images are is shown in Supplementary Fig. 5. Values are means ± SEM. a = Main effect of diet p < 0.05.
Circulating adiponectin and local adiponectin receptor in placenta
Circulating HMW adiponectin was not affected by diet or DHT injection (Supplementary Fig. 3A). There were no interaction or main effects of diet or DHT injection on the placenta protein expression of adiponectin receptor 2 [F(1,29) 3.781, p = 0.059] (Supplementary Fig. 3B).
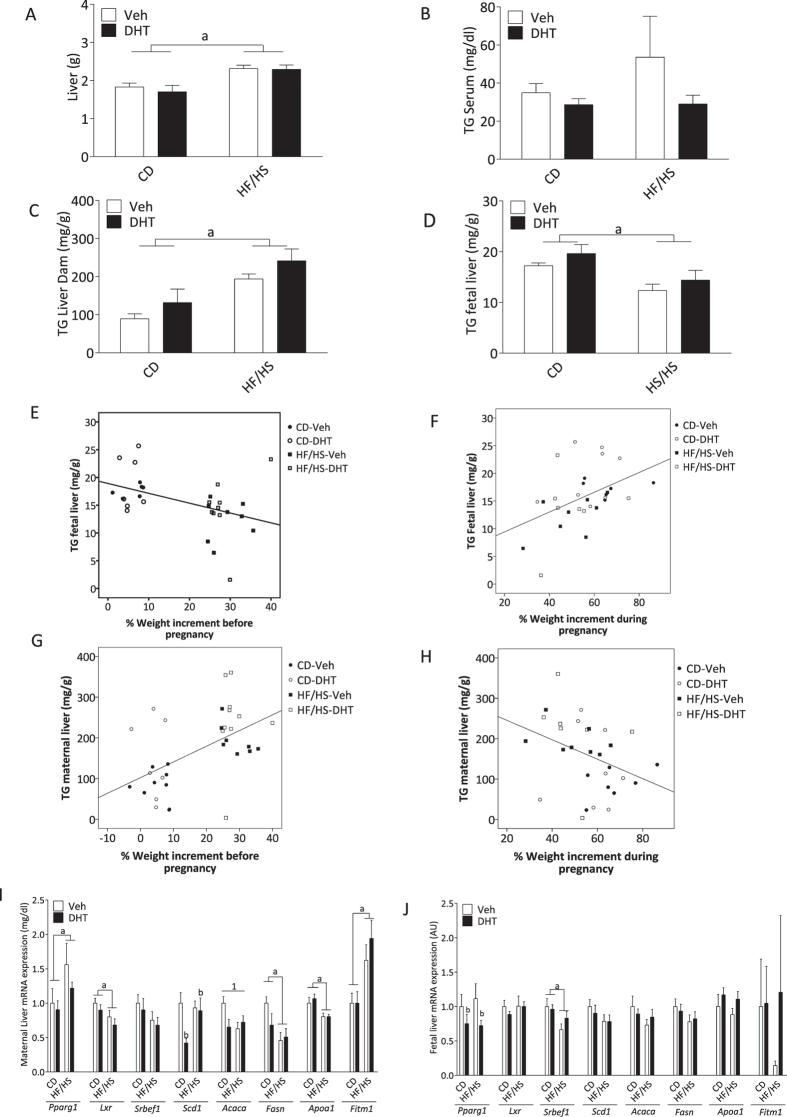
Liver weight and triglyceride content
There was a main effect of diet with higher liver weight in dams fed HF/HS than in dams fed CD [F(1,30) 52.40, p < 0.001] (Fig. 4A). Circulating TG levels were not affected by diet or injection. Although no interaction was found, there was a main effect of diet on maternal liver TG content with higher content in dams fed HF/HS [F(1,30) 16.50. p < 0.001], the posthoc test revealed that maternal liver TG content was higher in HF/HS-Veh and HF/HS-DHT than in the CD and CD-DHT groups, respectively (p < 0.05). Livers from DHT injected animals tended to have a higher content of DHT than their vehicle injected counterparts, although this effect was not statistically significant [F(1,30) 2.902. p = 0.09], (Fig. 4C). There was a main effect of diet on fetal liver TG content with lower content in fetuses from dams fed HF/HS than in fetuses from dams fed control chow [F(1,28) 6.92, p = 0.014] (Fig. 4D). Fetal TG content was inversely correlated with maternal weight gain before pregnancy (−0.452, p = 0.009, Fig. 4E), but positively correlated to pregnancy weight gain (0.446, p = 0.015, Fig. 4F). Contrary, the TG concentration in maternal liver correlated positively with pre-pregnancy weight gain (0.517, p = 0.002; Fig. 4G) and negatively with maternal weight gain in pregnancy (−0.365, p = 0.048, p = 0.015, Fig. 4H).
Figure 4.
Liver weight, triglyceride content and gene expression of lipid metabolism markers in the maternal and fetal liver. (A) Weight of dams liver (CD-Veh, n = 8; HF/HS, n = 8; CD-DHT, n = 8; HF/HS-DHT, n = 10). (B) Circulating Triglycerides (TG) in serum and (C) TG content in dams liver, (CD-Veh, n = 8; HF/HS-Veh, n = 8; CD-DHT, n = 8; HF/HS-DHT, n = 9). (D) TG content in fetal liver (CD-Veh, n = 7; HF/HS, n = 8; CD-DHT, n = 8; HF/HS-DHT, n = 9). (E) Correlation analyses between TG content in fetal liver and maternal weight gain before pregnancy, (F) Correlation analyses between TG content in fetal liver and percentage of maternal weight gain during pregnancy, (G) Correlation analyses between TG content in maternal liver and percentage of maternal weight gain before pregnancy, (H) Correlation analyses between TG content in maternal liver and percentage of maternal weight gain during pregnancy, (I) Transcription factors, lipogenic enzymes, cholesterol and fat storage mediators in Maternal liver and (J) fetal liver (CD, n = 8; HF/HS, n = 8; CD-DHT, n = 7–8; HF/HS-DHT, n = 10). Values are means ± SEM. a = Main effect of diet, b = main effect of injection, 1 = Interaction between diet and injection.
Expression of genes related to triglyceride metabolism in the liver from dams and fetuses
In the maternal livers, there was a main effect of diet on mRNA expression of transcription factors; dams fed HF/HS had higher Pparg and lower Lxr than dams fed control chow [Pparg: F(1,30) 5.14, p = 0.031; Lxr: F(1,30) 6.12, p = 0.019] (Fig. 4I). There were both interaction effects and main effects of diet and DHT injection in the expression of enzymes related to the fatty acid synthesis. For the mRNA expression of Scd1, there was a main effect of the DHT injection [F(1,30) 4.69, p = 0.038] (Fig. 4I) with lower expression in DHT-injected than in vehicle-injected dams fed control chow (p < 0.01) and higher in the HF/HS-DHT than in the CD-DHT group (p = 0.023). For the mRNA expression of Acaca, there was an interaction between diet and DHT injection [F(1,30) 5.16, p = 0.030] (Fig. 4I). The posthoc analysis showed that Acaca had a lower expression in DHT-injected dams than in vehicle-injected dams fed control chow (p < 0.05), and lower expression in dams fed HF/HS than CD (p < 0.05). For enzymes related to fatty acid synthesis, there was a main effect of diet with lower mRNA expression of Fasn in dams fed HF/HS [F(1,30) 7.989. p < 0.01] (Fig. 4I) and of Apoa1, an important component of high density lipoprotein (HDL) [F(1,30) 14.66, p < 0.01] (Fig. 4I). Finally, there was a main effect of diet with higher mRNA expression of Fitm1 in both HF/HS groups [F(1,30) 14.66, p < 0.01] (Fig. 4I).
There was a main effect of the DHT injection with lower mRNA expression of Pparg in fetal livers from DHT injected dams, than in fetal livers from vehicle-injected dams [F(1.29) 4.42, p < 0.05] (Fig. 4J), and a main effect of diet on Srebfc with lower mRNA expression in fetal livers from dams fed HF/HS [F(1.29) 6.44, p < 0.05] (Fig. 4J).
Kidney
There was a main effect of diet with higher kidney weight in dams fed HF/HS than in controls [F(1,30) 4,250, p = 0.048] (Fig. 5A). The posthoc analysis demonstrated that kidneys in the HF/HS-DHT dams were heavier than the kidneys in the HF/HS dams injected with vehicle. Morphological evaluation of the renal tissues stained with PAS showed normally looking glomeruli with no difference between the four groups (data not shown).
Figure 5.
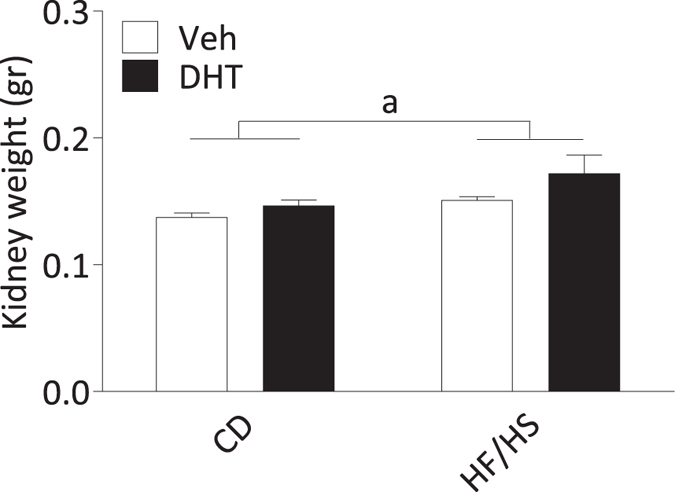
Kidney weight. The weight of the kidneys. a = Main effect of diet; p < 0.05 vs CD. Values are means ± SEM.
Discussion
Women with PCOS are often obese which increases the risk to deliver a LGA or SGA newborn. Many animal models of PCOS represent the lean phenotype of the syndrome with normal or growth restricted fetuses22, 32. The novelty of the present study is that we combine the well characterized PNA mouse model9–11, 28 with a maternal obesity model18. We demonstrate that diet-induced obesity results in enlarged maternal livers accompanied with a marked increase in maternal liver TG content, which was most pronounced in the DHT-treated animals. Moreover, HF/HS was associated with a decreased fetal liver TG content indicating a compensatory mechanism in the intrauterine life. As indicated by the HOMA-IR results, the HF/HS-diet induced obesity was not associated with changes in insulin sensitivity, whereas maternal androgen excess increased insulin sensitivity.
Studies in pregnant women with PCOS show that they gain more weight during pregnancy than controls, independent of body mass index (BMI)30, 33, and is more likely to give birth to either SGA5 or LGA29 babies. We here demonstrate that the total percentage of body weight gain in dams fed HF/HS was lower than in dams on control chow with no effect of the DHT injection during late pregnancy. The lower increase in body weight during late pregnancy is in line with another study using a similar HF/HS-induced obesity model with the same litter size34. Despite this, DEXA measurements show that dams fed HF/HS have higher fat mass than controls, both on GD0 and GD18.5, which is in line with a previous report with a similar HF/HS protocol18. In contrast to the results of Rosario et al.18, fetal weight was not higher in in fetuses from dams fed HF/HS. One plausible explanation could be the lower weight gain at the end of the pregnancy in dams fed HF/HS compared to dams on control chow.
As expected, the serum levels of DHT were higher in mice injected with DHT demonstrating that the dams were hyperandrogenized at the end of pregnancy. No previous studies using the maternal androgen excess model have measured DHT in maternal serum in mice9–11, 28. Since maternal androgens have the potential to affect the fetus and/or placental function directly32, it is important to investigate the circulating levels of this hormone. Thus, this is the first study to quantify maternal sex steroid concentrations in mice exposed to maternal androgen excess, with or without obesity. Circulating estradiol and testosterone was not affected by diet or the DHT injection, indicating that any changes observed in the DHT-exposed mice most likely are related to androgen receptor activation by DHT. However, an estrogen effect cannot completely be ruled out as DHT can be metabolized to 5α-androstane-3β,17β-diol (3β-diol) that could activate the estrogen receptor beta35.
Surprisingly, dams fed either HF/HS or CD and injected with DHT displayed a decrease in HOMA-IR. This indicates improved insulin sensitivity, rather than decreased, as hypothesized. Most likely, this reflects an acute effect of the DHT exposure. Our results are supported by a previous in vitro study of glucose-induced insulin secretion in cultured beta cells from normal rats36. Exposure for 72 hours to either DHT or testosterone reduced the insulin secretion, and the effect was reversed by flutamide, demonstrating the capacity of androgens to modulate insulin secretion via androgen receptors36.
As expected, the liver weight was increased in dams fed HF/HS. Although no effects of diet or DHT injection were found on circulating TG, the liver TG content was higher in dams fed HF/HS and in dams exposed to DHT. This finding is supported by a clinical study in hyperandrogenic women with PCOS, who exhibit higher liver fat content than women with PCOS without hyperandrogenism, independent of BMI and insulin resistance37. Moreover, the prevalence of nonalcoholic fatty liver disease in women with PCOS has been shown to be 25%38 often together with metabolic syndrome. All non-invasive indices of hepatic steatosis and hepatic fibrosis are higher than normal in women with PCOS, and even higher when the metabolic syndrome is present39. In addition, kidney weights were also significantly increased in the dams fed HF/HS and injected with DHT (Fig. 5A). Hyperandrogenic women with PCOS have previously been shown to have an increased risk for pre-term birth and preeclampsia40. However, in this study, we found no histological evidence of preeclampsia or other kidney injuries, which in this case may be due to a too short treatment time.
In order to investigate molecular signatures that may underlie the liver TG accumulation, and if the increased TG was an effect of diet or androgen injection, we analyzed the expression of the enzymes related to the de novo lipogenesis pathway: Acaca, Fasn and Scd1, and their regulators: Lxr and Srebf1c, in the liver of dams and fetuses (Fig. 6). Further, the lipid accumulation in the maternal liver does not seem to be related to an active de novo lipogenesis, as was demonstrated by the dysregulation of the gene expression of the enzymes that constitute the pathway. In fact, the gene expression of Acetyl-CoA Carboxylase alpha, Acaca was lower in DHT-injected dams fed either control chow or HF/HS. Moreover, Stearoyl-Coenzyme-A desaturase 1, Scd1, was also decreased in dams exposed to DHT. The regulation of the gene expression of Acaca and Scd1 by androgens is supported by Kelly et al.41 who demonstrated that testosterone reversed the hepatic TG accumulation in androgen-deficient male mice41.
Figure 6.
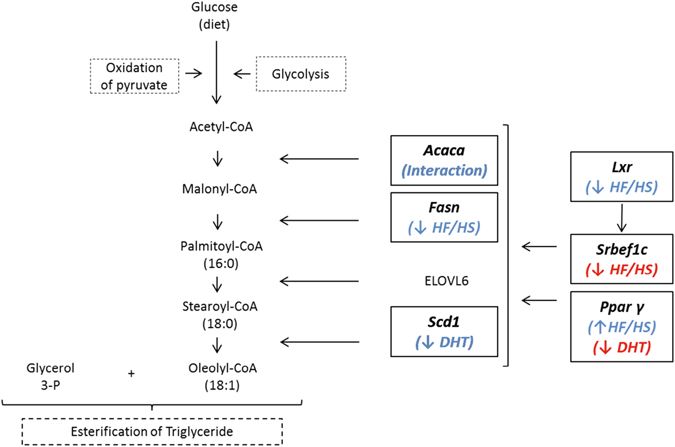
Simplified scheme of the enzymes and transcription factors involved in de novo lipogenesis pathway. Briefly, the enzymes Acetyl-CoA Carboxylase alpha (Acaca), Fatty acid synthase (Fasn), Elongation of very long chain fatty acids protein 6, (Elovl6), and Stearoyl-CoenzimeA desaturase, (Scd1) participate directly in the synthesis and elongation of fatty acids starting from Acetyl-CoA. Liver X receptor regulates the transcription of Sterol regulatory element-binding factor 1, (Srebf1), which in turn, stimulates the transcription of the enzymes mentioned above to increase the lipid biosynthesis in the liver. Peroxisome proliferator-activated receptor gamma (Pparg), a master regulator of transcription, also affects the expression level of the proteins in the de novo lipogenesis process. In the figure the molecules studied in this research has been highlighted by bold letters in a square. The blue letters are the changes occurring in the maternal liver. In red are the changes found in fetal livers.
The transcription factor Srebf1c is recognized to be the main regulator of the transcription of the enzymes Acaca, Fasn and Scd1, and is at the same time regulated by Lxr 42. Srebf1c has been reported to have increased expression in the liver from animals exposed to a diet with a high carbohydrate content43. In this study, the expression level of Srebf1c was unaltered, probably related to the lower transcription level of Lxr in dams fed HF/HS. This might be a protective mechanism that reduces the de novo lipogenesis pathway in the liver as a response to the lipid accumulation in the tissue.
Obese dams had increased expression of Pparg in the liver, which is known to be related to HF/HS-induced steatosis44, 45. Pparg overexpression has been reported to result in increased expression of fat storage-inducing transmembrane protein Fitm1 46, an important precursor of lipid droplet formation47. Therefore, it is possible that the increased expression of Fitm1 that we observed in the livers of dams fed with HF/HS constitutes a link between increased expression of Pparg and accumulation of TG in the liver of obese dams. Apoa-1 was decreased in both HF/HS groups. ApoA1 is synthesized in liver and intestine and is an important component of HDL, a marker of cardiovascular risk48, suggesting a decreased capacity to remove cholesterol from macrophages in the intestine wall and its posterior excretion in the bile49.
Contrary to the increase in TG liver content in dams, the fetal liver TG content was decreased in dams fed with HF/HS with no significant changes in mRNA expression of genes related to de novo lipogenesis. The decrease in lipid accumulation contrasts what has previously been reported with higher lipid droplet accumulation in fetal livers from dams fed HF/HS50. Moreover, we found a negative correlation between fetal TG content and maternal pre-pregnancy weight gain (Fig. 4E) and a positive correlation during pregnancy (Fig. 4F) indicating that the TG accumulation in the fetus is determined by the weight increment of the dam before and during the pregnancy. Similar to these results, Abbott et al. showed a lower concentration of circulating free fatty acids (FFA) in female rhesus monkey fetuses that were prenatally androgenized (and whose mother also demonstrated an increase in their body weight) compared to the fetuses from dams injected with vehicle24, whereas the FFA was increased in adult female offspring51. Collectively, these observations raise the possibility that the lower accumulation of TG in the fetal liver in obese dams could be a result of modifications of placental transfer of FFA50, which is believed to be an important source for TG synthesis in the fetal liver52.
Another possibility is that β-oxidation is activated in the fetal liver in obese dams, which was not the focus of this study. The expression of the transcription factor Pparg was decreased in fetal livers exposed to DHT, which most likely is related to a direct action of the androgens on the fetal liver. An in vitro study demonstrated that testosterone and DHT inhibit adipocyte differentiation by decreasing the gene expression of PPARγ, C/EBPα and C/EBPβ, through the impairment of Bone Morphogenic Protein 4 (BMP4) signaling53. Moreover, we have demonstrated that Pparg mRNA expression is decreased in adipose tissue of women with PCOS compared to controls54. Furthermore, the fetal expression of Srebf1c was decreased in fetal livers from dams fed HF/HS which could be related to the lower TG content55, and with the absence of changes in enzymes involved in de novo lipogenesis in the liver56.
In order to investigate if HF/HS or DHT injection affect the steroidogenic environment, we assessed steroid- and glucocorticoid receptors and also enzymes related to the local steroidogenesis in the placenta. The placental protein expression of AR in DHT exposed dams was higher independent of diet, similar to what we have previously reported in the placenta from testosterone-exposed pregnant rats22. Of note, at adult age, female offspring displayed higher levels of red oil staining in livers and high circulating testosterone and TG in the absence of any disruption in the glucose metabolism22.
Because adiponectin has been proposed to be a major regulator of fetal growth and linked with maternal metabolism and placenta function57–59, we decided to measure circulating adiponectin in dams and adiponectin receptor 1 and 2 in the placenta. Neither circulating levels of HMW adiponectin nor protein expression of adiponectin receptor 2 in the placenta were affected by diet or the DHT injection suggesting that mothers fed HF/HS were not extremely affected in their metabolism as previously reported58 and may explain the lack of difference in fetal weight between the groups.
The strength of this study is the combination of two established mouse models which may reflect a pregnant woman with PCOS that frequently are overweight or obese. The dramatical increase in liver TG content in obese dams exposed to androgens is in line with the increased prevalence of nonalcoholic fatty liver disease in hyperandrogenic women with PCOS, allowing us to investigate the molecular signature and development of hepatic steatosis. The weakness is that the dose of DHT was adjusted to body weight, which could affect the variability of the results but also that we could not reproduce the LGA phenotype.
In conclusion, in our combined model, maternal obesity and androgen excess do not alter fetal and placenta weight, but negatively affect maternal and fetal liver function as demonstrated by increased TG content and dysfunctional expression of enzymes and transcription factors involved in the de novo lipogenesis.
Material and Methods
Animals
12 weeks old virgin C57/Bl6J mice were purchased from Janvier Labs (Le Genest-Saint-Isle, France). Animals were housed ten per cage under a 12 h light/dark cycle, at a temperature of 21–22 °C and 55–65% humidity. After 1 week of acclimatization, they were fed a control (CD) diet (98121701) (Research Diet, NJ, USA) containing fat: 10 kcal%, carbohydrate: 73 kcal%, and protein: 17 kcal%, or a hypercaloric (HF/HS) diet (D12079B) (Research Diet, NJ, USA) containing fat: 41 kcal%, carbohydrate: 43 kcal%, and protein; 17 kcal% together with (ii) and a 20% sucrose solution (S9378) (Sigma-Aldrich) supplemented with Vitamin mix of 10 gm/4000 kcal (V10001) (Research Diet, NJ, USA) and Mineral mix of 35 gm/4000 kcal (S10001) (Research Diet, NJ, USA). Nutrition and tap water was provided ad libitum. All animal procedures were approved by the Animal Ethics Committee of the University of Gothenburg (Dnr: 116–2014), in accordance with the legal requirements of the European Community (Decree 86/609/EEC).
Experimental design and methods
Females were fed with CD (n = 25) or HF/HS (n = 27) during 4 to 10 weeks until mice in the HF/HS group had increased their weight by at least 25% compared to the beginning of the study (Supplementary Fig. 1). At this time, females in estrus phase, as determined by vaginal smears, were mated overnight with a male on the control diet. The presence of a post-copulation plug was confirmed the following morning which was defined as gestational day (GD) 0.5. Plug positive animals were placed in a separate cage and continued on the same diet. On GD 15.5 the HF/HS and CD groups were subdivided and assigned to receive a 100 µl subcutaneous injection in the interscapular area of 250 µg of 5α-Androstan-17β-ol-3-one (DHT) (cat A8380, Sigma-Aldrich, St. Louis, MO, USA), dissolved in a mixture of 5 µl Benzil Benzoate (B6630, Sigma) and 95 µl Sesame oil (S3547, Sigma) or Vehicle alone for two days (until GD 17.5). On day 18.5 animals were fasted for 4 hours and then anesthetized with 4% isoflurane inhalation. Axillary blood was collected for sex steroid analyses, and the animals were sacrificed by heart puncture. The uterus was dissected, and fetuses and placentas were collected and weighed. The fetuses were sacrificed by anesthesia inhalation prior to dissection.
Experiment 1
Tissue collection
All placentas from one dam were pooled and washed in ice cold Tris-Saline buffer (10 mM Tris-HEPES, 154 mM NaCl) and homogenized on ice in buffer D (10 mM Tris-HEPES, 250 mM sucrose, 1 mM EDTA, and protease and phosphatase cocktail inhibitors (cat P8340, P5276 and P0044, Sigma) using a polytron (T 18 digital ULTRA-TURRAX®). Aliquots were snap frozen and stored at −80 °C. Fetuses were dissected from the dorsal region, and the kidneys and livers, respectively, from all pups in one litter, were pooled and snap frozen in liquid nitrogen and stored at −80 °C until further analyses. Maternal kidneys and liver were dissected, weighed, and snap frozen individually in liquid nitrogen and stored at −80 °C.
Experiment 2
In a subset of mice under the same design (n = 5/group) lean and fat mass was examined using Dual Energy X-ray Absorption technique (DEXA) Lunar PIXImus2tm, (GE Medical System Lunar, Madison, USA) before the start of mating and on GD 18.5.
After 6 hours of fasting, immediately after the DEXA measurement, glucose tolerance was measured using the oral glucose tolerance test (OGTT) in conscious dams. Mice were gavaged with glucose (20% in saline), and blood glucose was measured from the tail vein at 0, 15, 30, 60, and 90 min after the glucose load. Blood glucose was measured immediately using a glucose meter (One Touch Ultra-2). At times 0 and 15 min blood was collected for insulin analysis.
In this subset of animals, the fetuses were dissected and saved in 15 ml polypropylene tubes until the next day for body composition analysis (lean and fat mass) by the Body Composition Analyzer (BCA), Bruker minispec LF110 (Bruker Biospin, Germany).
Analytical methods
Maternal serum concentrations of progesterone, testosterone, 17β-estradiol and dihydrotestosterone were measured using a previously described validated assay at Endoceutics Inc. (Quebec City, Canada)60. Briefly, 75 µl of serum were treated by liquid-liquid extraction. Samples were analyzed by UPLC-ESI-MS/MS using a Shimadzu Nexera ultra high-performance liquid chromatography (UPLC) coupled with an AB Sciex Qtrap 6500™ mass spectrometer in MRM mode60. Chromatograms were processed and verified blindly using a validated Analyst® 1.6.2 software. Upon transfer to the validated Watson LIMS 7.4.1 data management system, data was further processed and unblinded without possible alteration.
Five µl of plasma were used for insulin measurement by the ELISA method using the Ultra-Sensitive Mouse Insulin ELISA Kit, cat# 90080 (Crystalchem.Inc, IL, USA) according to the manufacturer’s instructions. The limit of detection was 0.05 ng/ml. The intra-assay coefficient of the variations was ≤10%. The HOMA-IR index was calculated following the formula (gluc/mmol/L) x Ins(mIU/L))/22.5 assessed in mice by Bowe et al.61.
In the OGTT, the area under the curve (AUC) for glucose and insulin was calculated according to the trapezoid method:
With g(t) the glucose concentration at time t and i the intervals of time at which measurements of glucose concentration were taken (0.0, 0.25, 0.5, 1.0 and 1.5 hours). Serum High Molecular Weight (HMW) adiponectin levels were determined using the Adiponectin (Mouse) Total, HMW ELISA kit, cat # 47-ADPMS-E01 from ALPCO (NH, USA) according to the manufacturer instructions. The intra-assay variation was <10%.
Triglyceride liver content
Liver TGs were extracted from the dam; 100 mg of the left lateral lobe of the liver was homogenized in the Tissue Lyzer (Qiagen) for two minutes in a mixture of 5% NP-40 Alternative from Calbiochem (cat. 492016) in distilled water. Then the samples were heated at 90 °C for 5 minutes in a water bath, until the samples became cloudy, cooled down at room temperature and heated for five minutes, cooled again and centrifuged at 13,000 g × 2 minutes. The supernatant was removed and analyzed using the TG kit TR210 Randox (London, UK) according to instructions from the manufacturer.
Western Blot
Briefly, placental homogenates were centrifuged at 13,000 g for 10 min at 4 °C, the supernatant was collected, and the protein concentration was measured using a spectrometer (Direct detect, Millipore, USA). Thirty µg of total protein was loaded in Criterion™ TGX (Tris-Glycine eXtended) Stain-Free™ precast gels (Biorad, City, Country). The gel was activated with the ChemiDoc MP imager (Biorad) and transferred to polyvinylidene difluoride membranes (PVDF) in the turbo system Trans-Blot turbo transfer (Biorad). Thereafter, membranes were blocked and incubated with primary antibodies for progesterone receptor (PR), androgen receptor (AR), estrogen receptor (ER) α and β, glucocorticoid receptor (GR), CYP11A1, 17 βHSD2 and adiponectin receptor 2 (ADIPOR2) (Supplementary Table 1). The protein expression in each sample was normalized to total amount of loaded protein in each sample using Image Lab 5.0 (Biorad).
Periodic Acid-Schiff (PAS) staining of glomeruli
Frozen renal tissue obtained from 3 mice from each treatment group was used for immunostaining using periodic acid Schiff staining (PAS) to visualize the morphology of the glomeruli.
RNA isolation and mRNA expression
Maternal and random pieces of the pool of fetal liver samples were homogenized in the Tissue Lyzer and using the ReliaPrep™ RNA Tissue Miniprep kit from Promega, cat #Z6112 (Madison, USA). RNA quantification was assessed by spectrophotometric measurements (Nanodrop 1000, NanoDrop Technologies). cDNA synthesis was carried out using the High Capacity RNA-to-cDNA kit, cat # 4387406 (Applied Biosystems, USA). Oligonucleotides for target genes and reference genes were purchased from Eurofins (Ebersberg, Germany) (Supplementary Table 2). Quantitative real-time PCR was performed using in Applied Biosystems® ViiA 7 RUO System (Life Technologies), and the data were analyzed by QuantStudioTM Real Time PCR software version 1.2 (Life Technologies ®). We tested Gapdh, Rpl0 and Rpl19 as endogenous control, but according to NormFinder, the best one was Rpl19 that was subsequently used as endogenous control for all our maternal and fetal samples.
Statistical analysis
Data are presented as mean values ± SEM and were analyzed with SPSS version 21.0 (SPSS, Chicago, IL, USA). Body weight gain during pregnancy was analyzed using repeated measures ANOVA followed by Bonferroni posthoc test for group comparisons. In order to investigate if there is an interaction (simple effects) between maternal diet and maternal DHT injection or if there is a main effect of either maternal diet or maternal DHT injection, two-way ANOVA was performed followed by Bonferroni post hoc test. The results reported in brackets, [], show the degree of freedom for the groups tested, the degree of freedom of the total samples that were tested, the F value and p-value. Furthermore, if there was a significant main effect, Bonferroni posthoc test was performed. P-values < 0.05 were considered statistically significant. In graphs, all the data are presented as mean values ± SEM. Figures show the interaction between the diet and DHT injection effects are marked as (1) or the main effects of diet (a) or DHT treatment (b).
Electronic supplementary material
Acknowledgements
We thank Carolina Johansson, research administrator at the Department of Physiology and Pharmacology, Karolinska Institutet, for language editing of the final version of the manuscript, and Isabel Barragán for her help in the interpretation of molecular analyses in the liver. We also thank the Centre for Physiology and Bio-Imaging (CPI) core facility and Experimental Biomedicine at the Sahlgrenska Academy, the University of Gothenburg for technical assistance and animal housing. The work was supported by the Swedish Medical Research Council (Project No. 2014–2775); Jane and Dan Ohlsson Foundation; Wilhelm and Martina Lundgren’s Science Fund; Hjalmar Svensson Foundation; Adlerbert Research Foundation; Novo Nordisk Foundation (NNF16OC0020744); Strategic Research Programme (SRP) in Diabetes at Karolinska Institutet; Swedish federal government under the LUA/ALF agreement ALFGBG-429501, and Regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet (all ESV) and FONDECYT 11130250 (MM). RF thanks the National Commission for Scientific and Technological Research (CONICYT, Chile) for financial support of Ph.D. study training at Karolinska Institutet. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author Contributions
E.S.-V., R.F. and M.M. designed the study. R.F., M.H., L.H. performed the animal experiment. R.F., M.M., M.H., M.J., F.L., T.J. performed the laboratory work. K.E. and J.N. made the kidneys analysis. F.L. contributed with the hormone analysis in plasma. R.F. and E.S.V. wrote the manuscript. All the authors revised critically and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08559-w
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Navarro G, Allard C, Xu WW, Mauvais-Jarvis F. The Role of Androgens in Metabolism, Obesity, and Diabetes in Males and Females. Obesity. 2015;23:713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9 doi: 10.1186/1477-7827-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezeh U, Yildiz BO, Azziz R. Referral Bias in Defining the Phenotype and Prevalence of Obesity in Polycystic Ovary Syndrome. Journal of Clinical Endocrinology & Metabolism. 2013;98:E1088–E1096. doi: 10.1210/jc.2013-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falbo A, et al. Changes in androgens and insulin sensitivity indexes throughout pregnancy in women with polycystic ovary syndrome (PCOS): relationships with adverse outcomes. J Ovarian Res. 2010;3 doi: 10.1186/1757-2215-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palomba S, et al. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21:575–592. doi: 10.1093/humupd/dmv029. [DOI] [PubMed] [Google Scholar]
- 6.Palomba S, et al. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. J Clin Endocrinol Metab. 2012;97:2441–2449. doi: 10.1210/jc.2012-1100. [DOI] [PubMed] [Google Scholar]
- 7.Palomba S, et al. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod. 2013;28:2838–2847. doi: 10.1093/humrep/det250. [DOI] [PubMed] [Google Scholar]
- 8.Hu M, et al. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci USA. 2015;112:14348–14353. doi: 10.1073/pnas.1507514112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roland AV, Nunemaker CS, Keller SR, Moenter SM. Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol. 2010;207:213–223. doi: 10.1677/JOE-10-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witham EA, Meadows JD, Shojaei S, Kauffman AS, Mellon PL. Prenatal exposure to low levels of androgen accelerates female puberty onset and reproductive senescence in mice. Endocrinology. 2012;153:4522–4532. doi: 10.1210/en.2012-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldwell AS, et al. Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology. 2015;156:1441–1452. doi: 10.1210/en.2014-1887. [DOI] [PubMed] [Google Scholar]
- 12.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71:776–784. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun SYR, Chao S. Prepregnancy Obesity and Associated Risks of Adverse Pregnancy Outcomes. Obstetrics and Gynecology. 2015;125:89S–89S. doi: 10.1097/01.AOG.0000463527.58524.d6. [DOI] [Google Scholar]
- 14.Gaillard R, et al. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013;21:1046–1055. doi: 10.1002/oby.20088. [DOI] [PubMed] [Google Scholar]
- 15.Valsamakis G, Kyriazi EL, Mouslech Z, Siristatidis C, Mastorakos G. Effect of maternal obesity on pregnancy outcomes and long-term metabolic consequences. Hormones (Athens) 2015;14:345–357. doi: 10.14310/horm.2002.1590. [DOI] [PubMed] [Google Scholar]
- 16.Mamun AA, et al. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119:1720–1727. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 17.Sferruzzi-Perri AN, et al. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. Faseb Journal. 2013;27:3928–3937. doi: 10.1096/fj.13-234823. [DOI] [PubMed] [Google Scholar]
- 18.Rosario FJ, Kanai Y, Powell TL, Jansson T. Increased placental nutrient transport in a novel mouse model of maternal. obesity with fetal overgrowth. Obesity (Silver Spring) 2015;23:1663–1670. doi: 10.1002/oby.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuelsson AM, Matthews PA, Jansen E, Taylor PD, Poston L. Sucrose feeding in mouse pregnancy leads to hypertension, and sex-linked obesity and insulin resistance in female offspring. Front Physiol. 2013;4 doi: 10.3389/fphys.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuduki T, Yamamoto K, E S, Hatakeyama Y, Sakamoto Y. High Dietary Fat Intake during Lactation Promotes the Development of Social Stress-Induced Obesity in the Offspring of Mice. Nutrients. 2015;7:5916–5932. doi: 10.3390/nu7075257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge ZJ, et al. DNA Methylation in Oocytes and Liver of Female Mice and Their Offspring: Effects of High-Fat-Diet-Induced Obesity. Environmental Health Perspectives. 2014;122:159–164. doi: 10.1289/ehp.1307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun M, et al. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. Am J Physiol Endocrinol Metab. 2012;303:E1373–1385. doi: 10.1152/ajpendo.00421.2012. [DOI] [PubMed] [Google Scholar]
- 23.Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology. 2010;151:595–605. doi: 10.1210/en.2009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott DH, et al. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010;299:E741–751. doi: 10.1152/ajpendo.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sir-Petermann T, et al. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 26.Hu S, Leonard A, Seifalian A, Hardiman P. Vascular dysfunction during pregnancy in women with polycystic ovary syndrome. Hum Reprod. 2007;22:1532–1539. doi: 10.1093/humrep/dem028. [DOI] [PubMed] [Google Scholar]
- 27.Roland AV, Moenter SM. Prenatal androgenization of female mice programs an increase in firing activity of gonadotropin-releasing hormone (GnRH) neurons that is reversed by metformin treatment in adulthood. Endocrinology. 2011;152:618–628. doi: 10.1210/en.2010-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Homa LD, Burger LL, Cuttitta AJ, Michele DE, Moenter SM. Voluntary Exercise Improves Estrous Cyclicity in Prenatally Androgenized Female Mice Despite Programming Decreased Voluntary Exercise: Implications for Polycystic Ovary Syndrome (PCOS) Endocrinology. 2015;156:4618–4628. doi: 10.1210/en.2015-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos N, et al. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ. 2011;343 doi: 10.1136/bmj.d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sir-Petermann T, et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–2126. doi: 10.1093/humrep/dei009. [DOI] [PubMed] [Google Scholar]
- 31.Rosario FJ, et al. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J Physiol. 2012;590:1495–1509. doi: 10.1113/jphysiol.2011.226399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sathishkumar K, et al. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol. 2011;9 doi: 10.1186/1477-7827-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palomba S, et al. Pregnancy in women with polycystic ovary syndrome: the effect of different phenotypes and features on obstetric and neonatal outcomes. Fertil Steril. 2010;94:1805–1811. doi: 10.1016/j.fertnstert.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 34.Panchenko PE, et al. Expression of epigenetic machinery genes is sensitive to maternal obesity and weight loss in relation to fetal growth in mice. Clin Epigenetics. 2016;8 doi: 10.1186/s13148-016-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: Activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5α-androstane 3β, 17β diol. Hormones and behavior. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, et al. Increased androgen levels in rats impair glucose-stimulated insulin secretion through disruption of pancreatic beta cell mitochondrial function. The Journal of Steroid Biochemistry and Molecular Biology. 2015;154:254–266. doi: 10.1016/j.jsbmb.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Jones H, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 38.Romanowski MD, et al. Prevalence of non-alcoholic fatty liver disease in women with polycystic ovary syndrome and its correlation with metabolic syndrome. Arq Gastroenterol. 2015;52:117–123. doi: 10.1590/S0004-28032015000200008. [DOI] [PubMed] [Google Scholar]
- 39.Polyzos SA, et al. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: assessment of non-invasive indices predicting hepatic steatosis and fibrosis. Hormones (Athens) 2014;13:519–531. doi: 10.14310/horm.2002.1493. [DOI] [PubMed] [Google Scholar]
- 40.Naver KV, et al. Increased risk of preterm delivery and pre-eclampsia in women with polycystic ovary syndrome and hyperandrogenaemia. BJOG: An International Journal of Obstetrics & Gynaecology. 2014;121:575–581. doi: 10.1111/1471-0528.12558. [DOI] [PubMed] [Google Scholar]
- 41.Kelly DM, et al. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sciences. 2014;109:95–103. doi: 10.1016/j.lfs.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12(Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 43.Korach-Andre M, et al. Liver X receptors regulate de novo lipogenesis in a tissue-specific manner in C57BL/6 female mice. Am J Physiol Endocrinol Metab. 2011;301:E210–222. doi: 10.1152/ajpendo.00541.2010. [DOI] [PubMed] [Google Scholar]
- 44.Inoue M, et al. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 45.Tailleux A, Wouters K, Staels B. Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta. 2012;1821:809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Hasenfuss SC, et al. Regulation of Steatohepatitis and PPARγ Signaling by Distinct AP-1 Dimers. Cell Metabolism. 2014;19:84–95. doi: 10.1016/j.cmet.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadereit B, et al. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci USA. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhee, E. J., Byrne, C. D. & Sung, K. C. The HDL cholesterol/apolipoprotein A-I ratio: an indicator of cardiovascular disease. Curr Opin Endocrinol Diabetes Obes (2017). [DOI] [PubMed]
- 49.Heinecke JW. The not-so-simple HDL story: A new era for quantifying HDL and cardiovascular risk? Nat Med. 2012;18:1346–1347. doi: 10.1038/nm.2930. [DOI] [PubMed] [Google Scholar]
- 50.Diaz P, Harris J, Rosario FJ, Powell TL, Jansson T. Increased placental fatty acid transporter 6 and binding protein 3 expression and fetal liver lipid accumulation in a mouse model of obesity in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1569–1577. doi: 10.1152/ajpregu.00385.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keller E, et al. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;155:2696–2703. doi: 10.1210/en.2014-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012 doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chazenbalk G, et al. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids. 2013;78:920–926. doi: 10.1016/j.steroids.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokosar M, et al. Epigenetic and Transcriptional Alterations in Human Adipose Tissue of Polycystic Ovary Syndrome. Sci Rep. 2016;6 doi: 10.1038/srep22883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Viscarra J, Kim S-J, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang G, et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 57.Aye IL, Powell TL, Jansson T. Review: Adiponectin–the missing link between maternal adiposity, placental transport and fetal growth? Placenta. 2013;34(Suppl):S40–45. doi: 10.1016/j.placenta.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aye I, Rosario FJ, Powell TL, Jansson T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:12858–12863. doi: 10.1073/pnas.1515484112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiao L, et al. Knockout maternal adiponectin increases fetal growth in mice: potential role for trophoblast IGFBP-1. Diabetologia. 2016;59:2417–2425. doi: 10.1007/s00125-016-4061-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ke Y, Bertin J, Gonthier R, Simard J-N, Labrie F. A sensitive, simple and robust LC–MS/MS method for the simultaneous quantification of seven androgen- and estrogen-related steroids in postmenopausal serum. The Journal of Steroid Biochemistry and Molecular Biology. 2014;144(Part B):523–534. doi: 10.1016/j.jsbmb.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 61.Bowe JE, et al. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol. 2014;222:G13–25. doi: 10.1530/JOE-14-0182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.