Abstract
High density lipoprotein cholesterol (HDL-C) has been reported as a significant indicator of systemic inflammation. The association underlying HDL-C and persistent organ failure (POF), pancreatic necrosis (PNec) and mortality in acute pancreatitis (AP) has not been evaluated. From 2007 to 2016, consecutive AP patients with admission lipid profiles assessment were included in this study. The association of HDL-C value and other lipids with outcomes was explored with Cox proportional regression models, which were adjusted for confounding factors. 1131 consecutive AP patients were clinically eligible. Overall, 17.9% of the patients developed with POF, 27.1% experienced PNec, and 6.7% died during hospitalization. Lower HDL-C median (<1.06 mmol/L) was identified as an independent prognostic factor of the outcomes. Moreover, there was a positive trend for the association across increasing HDL-C quartiles and POF, PNec and mortality after multivariable analysis (p values were <0.001, <0.001 and 0.043, respectively). The AUC of HDL-C for the outcomes were comparable to that of Ranson score for diagnosing POF (0.778 vs. 0.678; P < 0.001), PNec (0.734 vs. 0.701; P = 0.143) and mortality (0.768 vs. 0.745; P = 0.516). Decreased HDL-C value is an independent risk factor for the incidence of POF, PNec and in-hospital mortality in AP.
Introduction
Acute pancreatitis (AP) is characterized by local and systemic inflammation, which is observed clinically from no systemic sign through the local and systemic inflammatory response, organ failure (OF), persistent organ failure (POF), pancreatic necrosis (PNec) and death1, 2. The underlying pathophysiology through which local pancreatic injury drives the systemic inflammatory response has not been fully elucidated, but cumulative data suggest that immune systems play pivotal roles3, 4. Impaired lipid metabolism plays a pivotal role in the pathogenesis of numerous diseases conditions, including cardiovascular conditions, infectious diseases diabetes and carcinoma5–9.
Compared with other lipoproteins, high density lipoprotein cholesterol (HDL-C) is carried within lipoprotein particles that are particularly heterogeneous, varying in size, composition, metabolism and function10. Decreased plasma concentrations of HDL-C is frequently observed in a serious of acute phase conditions associated with immune activation11. It is accepted that HDL may become dysfunctional in some disease conditions, and the concept that the anti-inflammatory status of HDL may be associated with the risk of cardiovascular and other disorders12–14. In addition to playing a central role in reverse cholesterol transport (RCT) in vascular protection, HDL and its mimetics have several other functions including antioxidant, antithrombotic and anti-apoptotic functions. The anti-inflammatory function of HDL is investigated intensively, affecting both local (such as expression of adhesion molecules on endothelium) and systemic immune systems (such as expression of adhesion molecules and cytokine secretion by monocytes)15.
HDL-C has been shown an association with disease severity and adverse outcomes in AP16–21. However, evidence supporting the relationship between HDL-C and incidence of POF, PNec and in-hospital mortality in a large population of AP, is currently lacking. In the present study, we aimed to evaluate whether there is an association between HDL-C and severe outcomes in patients with AP.
Materials and Methods
Study Population
A total of 3004 consecutive patients who admitted to the Pancreatic Disease Institute of Union Hospital (Wuhan, China) with a confirmed diagnosis of AP between January 2007 and January 2016 were included in this retrospective study. AP was defined as clinical findings based on the presence of two or more of the following three criteria: (1) abdominal pain consistent with AP; (2) serum amylase and/or lipase elevation ≥ three times the upper limit of normal; and/or (3) computed tomography (CT), magnetic resonance imaging (MRI) or abdominal ultrasonography findings characteristic of AP1. Of these, 1532 patients were admitted to hospital within 72 hours from symptom onset and underwent assessment of lipid profiles upon presentation. For this study, individuals were excluded if they met any of the following: age smaller than 18 years (n = 107), AP induced by trauma (n = 78), chronic pancreatitis (n = 223), a history of hyperlipidemia receiving statin treatment currently (n = 171), and unavailable laboratory measurements or medical records (n = 346). As some individuals met more than one exclusion criteria, the total number of eligible patients for the study was 1131 (Supplementary Figure 1). The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The local Ethics Committee of Union Hospital (Wuhan, China) approved this study. Informed consent was obtained from each patient or next of kin prior to the study.
Data Collection
Electronic medical records and paper charts were reviewed by one independent physician who was blinded to the study for information on demographics (age, sex, etiology), medical history, smoking habit, alcohol consumption, pre-existing co-morbidity such as diabetes mellitus, obesity, and pre-existing organ dysfunction (including chronic respiratory disease, chronic renal disease and cardiovascular disease). Blood samples were collected within 2 hours after hospitalization and analyzed using an automated clinical chemical analyzer within 6 hours of sampling in the same core clinical laboratory in Union Hospital (Wuhan, China). Whole blood samples were obtained for measurements of hemoglobin, and serum for creatinine, calcium, albumin, aspartate aminostransferase, triglyceride (TG), total cholesterol (T-CHO), LDL-C, and HDL-C levels. The values of TG, T-CHO, HDL-C and LDL-C were measured enzymatically. TG/HDL-C ratio was calculated as TG level (mmol/L) divided by HDL-C level (mmol/L). Non-HDL-C was defined as T-CHO (mmol/L) minus HDL-C level (mmol/L). Because the TG, T-CHO, TG/HDL-C ratio and non-HDL-C were not normally distributed in the AP patients, we constructed plots of them and HRs of the outcomes using the Lowess function. The results revealed a nonlinear relationship, suggesting the need for stratification of the patients into medians and quartiles according to their values for outcome analysis. Therefore, we stratified the patients into medians and quartiles according to their TG, T-CHO, HDL-C level, LDL-C level, TG/HDL-C ratio or non-HDL-C level. The primary predictor variable was the HDL-C in each quartile. We also selected TG, T-CHO, LDL-C, TG/HDL-C ratio and non-HDL-C as other predictors.
Outcomes
The outcomes were incidence of developing with POF, PNec and in-hospital mortality. OF was confirmed according to the 2012 Revised Atlanta criteria1 when the following cutoffs were exceeded: (1) cardiovascular failure if systolic blood pressure was <90 mmHg despite fluid replacement; (2) respiratory failure if the ratio of PaO2/FiO2 was <300 mmHg; and (3) renal failure if serum creatinine was ≥1.9 mg/dl. Presence of OF was assessed upon presentation and every 24 hours during hospitalization. POF was identified if OF lasting more than 48 hours. PNec was defined as appearance of pancreatic parenchymal and/or peri-pancreatic necrosis on contrast-enhanced computed tomography (CECT)1. In our department, patients will receive CT and/or MRI on admission to determine the presence of pancreatitis. As pancreatic and peri-pancreatic necrosis usually is not present upon presentation and may develop during the first few days, early imaging cannot reliably determine severity in the course of AP. A CECT 7–9 days after admission will be repeated to establish the presence and extent of PNec. The outcome information was centrally adjudicated, in accordance with above definitions, by trained clinicians and experienced radiologists who were blind to this study.
Statistical Analysis
Statistical analysis was performed using SPSS 20.0 (SPSS Inc, Chicago IL, USA). Continuous data are presented as mean and standard deviation (SD). Categorical data are reported as number (frequency). Student t test and Mann-Whitney U test were used to evaluate the differences of baseline characteristics between the study cohort and the control group. Multiple group comparisons were performed using the Chi-square test for categorical variables and the Kruskal-Wallis test for continuous data. Univariable, age and sex adjusted, or multivariable analyses for outcomes were performed using a logistic regression model. The multivariable covariates included age, sex, smoking habit, alcohol use, pre-existing comorbidities and admission laboratory data. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) are presented. P values were 2-sided, and a P value < 0.05 was considered to be statistically significant. For testing linear risk trends, we used the median and quartile rank as a continuous variable in the regression models. We checked the proportional HRs by examining graphs of estimated log (−log) survival. To further evaluate and compare the predictive performance of TG, T-CHO, HDL-C, LDL-C, TG/HDL-C ratio and non-HDL-C, we used receiver operating characteristic (ROC) curves, and the area under the ROC curve (AUC) was estimated. The predictive values were compared with conventional prognostic system of Ranson score.
Data availability statement
We declare that the data is available.
Results
Basic Characteristics and outcomes of the patients
Baseline characteristics of the AP patients are presented in Table 1. The mean age of the population was 46 years and 683 (60.4%) were males. The most common cause was biliary-induced AP (n = 565), followed by alcoholic-induced AP (n = 282) and hyperlipidemia-induced AP (n = 196), and 7.8% with other causes. The mean values of TG, T-CHO, HDL-C, LDL-C, TG/HDL-C and non-HDL-C upon admission for the entire population were 4.91 ± 8.49 mmol/L, 4.99 ± 3.01 mmol/L, 1.07 ± 0.40 mmol/L, 1.91 ± 0.89 mmol/L, 7.46 ± 18.54, and 3.92 ± 3.09 mmol/L, respectively (Table 1).
Table 1.
Baseline characteristics of the study patients with acute pancreatitis.
| All patients | |
|---|---|
| No. | 1131 |
| Age, years | 46.42 ± 14.87 |
| Male sex | 683 (60.4%) |
| Etiology | |
| Biliary | 565 (50.0%) |
| Alcoholic | 282 (24.9%) |
| Hyperlipidemia | 196 (17.3%) |
| Other cause | 88 (7.8%) |
| Lipid files | |
| TG, mmol/L | 4.91 ± 8.49 |
| TC, mmol/L | 4.99 ± 3.01 |
| HDL-C, mmol/L | 1.07 ± 0.40 |
| LDL-C, mmol/L | 1.91 ± 0.89 |
| TG/HDL-C | 7.46 ± 18.54 |
| Non-HDL-C, mmol/L | 3.92 ± 3.09 |
| Outcomes | |
| POF | 203 (17.9%) |
| Solitary POF | 119 |
| Respiratory | 119 |
| Renal | 0 |
| Cardiovascular | 0 |
| Multiple POF | 84 |
| Respiratory + renal | 65 |
| Respiratory + cardiovascular | 12 |
| Respiratory + cardiovascular + renal | 7 |
| PNec | 306 (27.1%) |
| <30% | 55 |
| 30–50% | 128 |
| >50% | 123 |
| In-hospital mortality | 76 (6.7%) |
Data are presented as mean (SD) and number (frequency).
Abbreviations: CI: confident interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; PNec, pancreatic necrosis; POF, persistent organ failure; T-CHO, total cholesterol; TG, triglycerides.
Overall, 203 (17.9%) of the patients were diagnosed with POF. 119 patients had solitary POF (all in respiratory system). Multiple POF was observed in 84 patients (65 of lung and kidney, 12 of lung and heart, and 7 of lung, kidney and heart). Accordingly, 306 (27.1%) patients developed PNec. During hospitalization, 76 patients with POF died with an overall mortality rate of 6.7%. No death was observed in non-POF ones. Accordingly, 21 patients died from persistent respiratory failure, 17 from persistent respiratory and renal failure, 13 from persistent respiratory and cardiac failure, and 25 from infected PNec.
When these patients were stratified according to medians of these lipid profiles, the incidence of POF was more prevalent in patients with higher levels of TG and TG/HDL-C, and lower levels of HDL-C and LDL-C. The incidence of PNec was statistically elevated in patients with higher levels of TG, TG/HDL-C and non-HDL-C, and lower levels of HDL-C and LDL-C. Mortality rate was significantly higher in patients with lower levels of T-CHO, HDL-C and LDL-C (Fig. 1). Meanwhile, when patients were stratified according to quartiles of various lipid parameters, the incidences of POF, PNec and in-hospital mortality remained significantly different among quartiles (Fig. 1).
Figure 1.
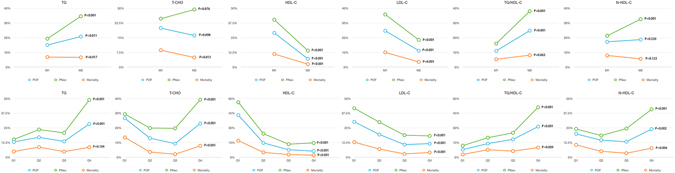
Comparison of the incidences of POF, PNec and in-hospital mortality in patients with AP according to medians and quartiles of different lipid profiles. Abbreviations: AP, acute pancreatitis; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; PNec, pancreatic necrosis; POF, persistent organ failure; T-CHO, total cholesterol; TG, triglycerides.
Comparison of clinical parameters and lipid profiles between patients with different outcomes
Table 2 shows that males and patients with drinking and smoking habits were more prone to develop PNec. POF ones and non-survivors showed elder ages. The values of total leukocytes, aspartate aminostransferase, serum glucose, creatinine, TG, TG/HDL-C, non-HDL-C detected upon presentation to hospital and Ranson score were significantly higher, while the admission levels of serum calcium, HDL-C and LDL-C were statistically lower in patients with severe outcomes (Table 2).
Table 2.
Comparison of baseline characteristics and outcomes between patients with acute pancreatitis.
| Non-POF | POF | P | Non-PNec | PNec | P | Survior | Non-survior | P | |
|---|---|---|---|---|---|---|---|---|---|
| 928 | 203 | 825 | 306 | 1055 | 76 | ||||
| Male sex | 563 (60.7%) | 120 (59.1%) | 0.682 | 473 (57.3%) | 210 (68.6%) | <0.001 | 643 (60.9%) | 40 (52.6%) | 0.152 |
| Age, years | 44.02 ± 14.16 | 51.51 ± 16.06 | <0.001 | 44.91 ± 14.83 | 46.59 ± 14.65 | 0.212 | 44.57 ± 14.30 | 56.39 ± 17.08 | <0.001 |
| Etiology | <0.001 | <0.001 | 0.077 | ||||||
| Biliary | 461 (49.7%) | 104 (51.2%) | 442 (53.6%) | 123 (40.2%) | 519 (49.2%) | 46 (60.5%) | |||
| Alcoholic | 216 (23.3%) | 66 (32.5%) | 147 (17.8%) | 135 (44.1%) | 262 (24.8%) | 20 (26.3%) | |||
| Hyperlipidemia | 167 (18.0%) | 29 (14.3%) | 158 (19.2%) | 38 (12.4%) | 188 (17.8%) | 8 (10.5%) | |||
| Other cause | 84 (9.1%) | 4 (2.0%) | 78 (9.5%) | 10 (3.3%) | 86 (8.2%) | 2 (2.6%) | |||
| Daily drinker | 416 (44.8%) | 96 (47.3%) | 0.523 | 333 (40.4%) | 179 (58.5%) | <0.001 | 482 (45.7%) | 30 (39.5%) | 0.239 |
| Current smoker | 468 (50.4%) | 100 (49.3%) | 0.763 | 376 (45.6%) | 192 (62.7%) | <0.001 | 540 (51.2%) | 28 (36.8%) | 0.016 |
| Diabetes mellitus | 85 (9.2%) | 77 (37.9%) | <0.001 | 85 (10.3%) | 77 (25.2%) | <0.001 | 126 (11.9%) | 36 (47.4%) | <0.001 |
| Obesity | 146 (15.8%) | 46 (22.7%) | <0.001 | 122 (14.8%) | 70 (22.9%) | 0.001 | 180 (17.1%) | 12 (15.8%) | 0.773 |
| Pre-existing organ dysfunctions | 218 (23.5%) | 91 (44.8%) | 0.118 | 215 (26.1%) | 94 (30.7%) | 0.102 | 265 (25.1%) | 44 (57.9%) | <0.001 |
| Chronic respiratory disease | 195 (21.0%) | 78 (38.4%) | 0.247 | 199 (24.1%) | 74 (24.2%) | 0.978 | 229 (21.7%) | 44 (57.9%) | <0.001 |
| Chronic renal disease | 9 (1.0%) | 16 (7.9%) | <0.001 | 3 (0.4%) | 22 (2.7%) | <0.001 | 2 (0.2%) | 23 (30.3%) | <0.001 |
| Cardiovascular disease | 34 (3.7%) | 36 (17.7%) | <0.001 | 28 (3.4%) | 42 (13.7%) | <0.001 | 32 (3.0%) | 38 (50.0%) | <0.001 |
| Laboratory data | |||||||||
| Total leukocyte count, ×109/L | 11.28 ± 4.23 | 14.49 ± 6.42 | <0.001 | 11.10 ± 4.21 | 13.90 ± 5.80 | <0.001 | 11.71 ± 4.80 | 13.93 ± 5.14 | <0.001 |
| Aspartate aminostransferase, U/L | 73.38 ± 118.23 | 92.59 ± 102.17 | <0.001 | 73.65 ± 119.48 | 85.40 ± 104.55 | <0.001 | 73.98 ± 115.80 | 116.34 ± 107.41 | <0.001 |
| Serum glucose, mmol/L | 7.23 ± 2.65 | 11.53 ± 6.67 | <0.001 | 7.01 ± 2.36 | 10.68 ± 6.01 | <0.001 | 7.59 ± 3.07 | 13.72 ± 8.98 | <0.001 |
| Serum calcium, mmol/L | 2.14 ± 0.24 | 1.71 ± 0.37 | <0.001 | 2.16 ± 0.22 | 1.81 ± 0.38 | <0.001 | 2.10 ± 0.27 | 1.60 ± 0.40 | <0.001 |
| Creatinine, mmol/L | 64.94 ± 21.07 | 143.35 ± 125.64 | <0.001 | 64.00 ± 19.55 | 119.51 ± 108.96 | <0.001 | 70.04 ± 36.36 | 203.64 ± 161.87 | <0.001 |
| Ranson score | 3.32 ± 1.82 | 5.00 ± 1.83 | <0.001 | 3.13 ± 1.71 | 4.95 ± 1.87 | <0.001 | 3.50 ± 1.90 | 5.34 ± 1.49 | <0.001 |
| TG, mmol/L | 4.14 ± 6.85 | 8.44 ± 13.14 | <0.001 | 3.52 ± 6.25 | 8.65 ± 11.93 | <0.001 | 4.77 ± 8.14 | 6.86 ± 12.31 | 0.038 |
| T-CHO, mmol/L | 4.81 ± 2.44 | 5.82 ± 4.76 | <0.001 | 4.62 ± 2.31 | 5.99 ± 4.22 | <0.001 | 4.96 ± 2.82 | 5.36 ± 4.99 | 0.264 |
| HDL-C, mmol/L | 1.14 ± 0.37 | 0.75 ± 0.39 | <0.001 | 1.16 ± 0.38 | 0.83 ± 0.40 | <0.001 | 1.04 ± 0.43 | 0.70 ± 0.39 | <0.001 |
| LDL-C, mmol/L | 1.99 ± 0.87 | 1.54 ± 0.90 | <0.001 | 2.03 ± 0.84 | 1.59 ± 0.94 | <0.001 | 1.94 ± 0.87 | 1.48 ± 1.03 | 0.068 |
| TG/HDL-C | 6.42 ± 14.50 | 18.64 ± 35.00 | <0.001 | 5.59 ± 13.77 | 16.66 ± 30.30 | <0.001 | 7.70 ± 17.64 | 20.47 ± 40.50 | <0.001 |
| Non-HDL-C, mmol/L | 3.75 ± 2.49 | 5.09 ± 4.83 | <0.001 | 3.55 ± 2.34 | 5.17 ± 4.29 | <0.001 | 3.93 ± 2.86 | 4.74 ± 5.14 | 0.027 |
Data are presented as mean (SD) and number (frequency).
Abbreviations: CI: confident interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; PNec, pancreatic necrosis; POF, persistent organ failure; T-CHO, total cholesterol; TG, triglycerides.
Association between different lipid profiles and the three outcomes
-
HDL-C and LDL-C are independently associated with outcomes
We next evaluated the association of various lipid parameters at presentation with the three AP outcomes. Table 3 shows HRs and 95% CIs for POF, PNec and in-hospital mortality using lipid profiles as continuous variables. In univariate analysis, lipid files such as TG, HDL-C, LDL-C, TG/HDL-C and non-HDL-C were significantly associated with the three outcomes.
After multivariate adjustments for confounding factors, HDL-C and LDL-C remained independent prognostic factors for POF (HR 0.09, 95% CIs 0.05 to 0.17; P < 0.001 and HR 0.75, 95% CIs 0.57 to 0.98; P = 0.037) and PNec (HR 0.22, 95% CIs 0.13 to 0.36; P < 0.001 and HR 0.73, 95% CIs 0.59 to 0.92; P = 0.006). Meanwhile, HDL-C was statistically associated with in-hospital mortality (HR 0.13, 95%CIs 0.05 to 0.32; P < 0.001).
-
Higher HDL-C median is independently correlated with outcomes
Table 4 shows HRs and 95% CIs for outcomes by medians of lipid values with median 1 (lower median) as reference. In unadjusted and age- and sex- adjusted analyses, higher medians of TG, HDL-C, LDL-C and TG/HDL-C were significantly associated with POF, and higher medians of lipid parameters (except for T-CHO) were statistically correlated with PNec. Higher medians of TG, T-CHO, HDL-C, LDL-C and TG/HDL-C were significantly associated with mortality in unadjusted analysis. After adjustments for sex and age, these lipid profiles (except for T-CHO) remained consistent as statistically predictive factors. In multivariate-adjusted analysis, results suggested higher median of HDL-C was independently related to POF (HR 0.28, 95% CIs 0.17 to 0.45; P < 0.001); PNec (HR 0.42, 95% CIs 0.30 to 0.61; P < 0.001) and in-hospital mortality (HR 0.51, 95% CIs 0.24 to 1.05; P = 0.036). Moreover, higher median of TG/HDL-C remained significantly associated with POF (HR 1.87, 95% CIs 1.05 to 3.31; P = 0.033), and higher median of T-CHO was statistically related to mortality (HR 0.45, 95% CIs 0.21 to 0.96; P = 0.038).
Higher HDL-C quartiles are independently correlated with outcomes
Table 3.
Factors associated with outcomes using univariate and multivariate Cox proportional analyses.
| POF | P-value | PNec | P-value | Mortality | P-value | |
|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | ||||
| Univariate | ||||||
| Female sex (1 = yes, 0 = no) | 1.07 (0.78, 1.45) | 0.682 | 0.61 (0.47, 0.81) | <0.001 | 1.40 (0.88, 2.24) | 0.153 |
| Age (years) | 1.04 (1.02, 1.05) | <0.001 | 1.01 (1.00, 1.02) | 0.090 | 1.06 (1.04, 1.07) | <0.001 |
| Biliary etiology (1 = yes, 0 = no) | 1.06 (0.79, 1.44) | 0.688 | 0.58 (0.45, 0.76) | <0.001 | 1.58 (0.98, 2.25) | 0.058 |
| Daily drinker (1 = yes, 0 = no) | 1.10 (0.81, 1.50) | 0523 | 2.08 (1.60, 2.72) | <0.001 | 0.78 (0.48, 1.25) | 0.294 |
| Current smoker (1 = yes, 0 = no) | 0.95 (0.70, 1.29) | 0.763 | 2.01 (1.54, 2.63) | <0.001 | 0.56 (0.34, 0.90) | 0.017 |
| Diabetes mellitus (1 = yes, 0 = no) | 6.06 (4.23, 8.69) | <0.001 | 2.93 (2.08, 4.12) | <0.001 | 6.64 (4.08, 10.80) | <0.001 |
| Obesity (1 = yes, 0 = no) | 1.57 (1.08, 2.28) | 0.018 | 1.71 (1.23, 2.37) | 0.001 | 0.91 (0.48, 1.72) | 0.773 |
| Pre-existing organ dysfunctions (1 = yes, 0 = no) | 2.65 (1.93, 3.63) | <0.001 | 1.26 (0.94, 1.68) | 0.119 | 4.10 (2.55, 6.60) | <0.001 |
| Total leukocyte count (×109/L) | 1.10 (1.07, 1.14) | <0.001 | 1.13 (1.10, 1.17) | <0.001 | 1.08 (1.03, 1.12) | <0.001 |
| Aspartate aminostransferase (U/L) | 1.00 (1.00, 1.00) | 0.034 | 1.00 (1.00, 1.00) | 0.131 | 1.00 (1.00, 1.00) | 0.003 |
| Serum glucose (mmol/L) | 1.26 (1.21, 1.32) | <0.001 | 1.32 (1.26, 1.39) | <0.001 | 1.24 (1.18, 1.30) | <0.001 |
| Serum calcium (mmol/L) | 0.01 (0.00, 0.02) | <0.001 | 0.01 (0.01, 0.02) | <0.001 | 0.02 (0.01, 0.05) | <0.001 |
| Creatinine (mmol/L) | 1.03 (1.03, 1.04) | <0.001 | 1.03 (1.02, 1.03) | <0.001 | 1.03 (1.02, 1.03) | <0.001 |
| Ranson score | 1.60 (1.47, 1.76) | <0.001 | 1.77 (1.62, 1.94) | <0.001 | 1.59 (1.41, 1.79) | <0.001 |
| TG (mmol/L) | 1.05 (1.03, 1.06) | <0.001 | 1.07 (1.05, 1.09) | <0.001 | 1.02 (1.00, 1.04) | 0.042 |
| T-CHO (mmol/L) | 1.09 (1.05, 1.14) | <0.001 | 1.15 (1.10, 1.20) | <0.001 | 1.04 (0.97, 1.11) | 0.266 |
| HDL-C (mmol/L) | 0.16 (0.10, 0.23) | <0.001 | 0.25 (0.18, 0.34) | <0.001 | 0.14 (0.08, 0.26) | <0.001 |
| LDL-C (mmol/L) | 0.51 (0.42, 0.63) | <0.001 | 0.53 (0.45, 0.63) | <0.001 | 0.50 (0.36, 0.69) | <0.001 |
| TG/HDL-C | 1.02 (1.02, 1.03) | <0.001 | 1.03 (1.02, 1.04) | <0.001 | 1.02 (1.01, 1.02) | <0.001 |
| Non-HDL-C (mmol/L) | 1.12 (1.07, 1.17) | <0.001 | 1.17 (1.12, 1.23) | <0.001 | 1.07 (1.01, 1.13) | 0.030 |
| Multivariate | ||||||
| Female sex (1 = yes, 0 = no) | 1.15 (0.54, 2.42) | 0.721 | 1.50 (0.79, 2.87) | 0.218 | 1.11 (0.42, 2.30) | 0.836 |
| Age (years) | 1.03 (1.01, 1.06) | 0.003 | 1.01 (0.99, 1.02) | 0.506 | 1.05 (1.02, 1.09) | 0.003 |
| Biliary etiology (1 = yes, 0 = no) | 1.37 (0.82, 2.29) | 0.227 | 0.95 (0.63, 1.44) | 0.815 | 1.18 (0.53, 2.61) | 0.686 |
| Daily drinker (1 = yes, 0 = no) | 2.139 (1.16, 4.95) | 0.018 | 1.51 (0.86, 2.67) | 0.151 | 3.61 (1.18, 11.07) | 0.023 |
| Current smoker (1 = yes, 0 = no) | 0.51 (0.25, 1.04) | 0.064 | 1.79 (0.96, 3.34) | 0.066 | 0.22 (0.08, 0.60) | 0.003 |
| Diabetes mellitus (1 = yes, 0 = no) | 3.74 (2.29, 6.11) | <0.001 | 2.54 (1.62, 3.09) | <0.001 | 2.19 (1.14, 4.22) | 0.018 |
| Obesity (1 = yes, 0 = no) | 0.98 (0.56, 1.72) | 0.940 | 0.94 (1.23, 2.37) | 0.001 | 0.67 (0.27, 1.67) | 0.386 |
| Pre-existing organ dysfunctions (1 = yes, 0 = no) | 1.34 (0.70, 2.57) | 0.378 | 1.07 (0.60, 1.91) | 0.817 | 1.25 (0.46, 3.39) | 0.663 |
| Ranson score* | 1.23 (1.09, 1.38) | <0.001 | 1.40 (1.27, 1.55) | <0.001 | 1.35 (1.13, 1.61) | <0.001 |
| TG (mmol/L) | 1.02 (0.97, 1.08) | 0.365 | 1.02 (0.98, 1.08) | 0.333 | 0.90 (0.83, 0.99) | 0.032 |
| T-CHO (mmol/L) | 1.07 (0.96, 1.17) | 0.183 | 1.06 (0.96, 1.17) | 0.249 | 1.11 (0.99, 1.25) | 0.075 |
| HDL-C (mmol/L) | 0.09 (0.05, 0.17) | <0.001 | 0.22 (0.13, 0.36) | <0.001 | 0.13 (0.05, 0.32) | <0.001 |
| LDL-C (mmol/L) | 0.75 (0.57, 0.98) | 0.037 | 0.73 (0.59, 0.92) | 0.006 | 0.70 (0.46, 1.06) | 0.091 |
| TG/HDL-C | 1.00 (0.99, 1.02) | 0.756 | 1.00 (0.98, 1.02) | 0.883 | 1.04 (1.01, 1.07) | 0.006 |
| Non-HDL-C (mmol/L)** | — | — | — | |||
*As data regarding total leukocyte count, aspartate aminostransferase, glucose, calcium and creatinine are parts of Ranson scoring system, we decide to only include Ranson score in multivariate analysis.
**Since non-HDL-C was defined as T-CHO minus HDL-C level, we decide to exclude it in multivariate analysis.
Abbreviations: CI: confident interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; PNec, pancreatic necrosis; POF, persistent organ failure; T-CHO, total cholesterol; TG, triglycerides.
Table 4.
Risk of POF, PNec and in-hospital mortality by medians of lipid profiles.
| POF | P-value | PNec | P-value | In-hospital mortality | P-value | |
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | ||||||
| TG (M2 vs. M1) | 1.49 (1.09, 2.02) | 0.012 | 2.21 (1.68, 2.90) | <0.001 | 0.98 (0.61, 1.55) | <0.001 |
| T-CHO (M2 vs. M1) | 0.77 (0.57, 1.05) | 0.099 | 1.27 (0.97, 1.65) | 0.077 | 0.55 (0.34, 0.89) | 0.014 |
| HDL-C (M2 vs. M1) | 0.20 (0.14, 0.29) | <0.001 | 0.24 (0.18, 0.32) | <0.001 | 0.20 (0.11, 0.37) | <0.001 |
| LDL-C (M2 vs. M1) | 0.39 (0.28, 0.53) | <0.001 | 0.41 (0.31, 0.54) | <0.001 | 0.33 (0.20, 0.56) | <0.001 |
| TG/HDL-C (M2 vs. M1) | 2.56 (1.84, 3.55) | <0.001 | 3.22 (2.43, 4.28) | <0.001 | 1.57 (0.97, 2.52) | 0.035 |
| Non-HDL-C (M2 vs. M1) | 1.08 (0.79, 1.47) | 0.634 | 1.78 (1.36, 2.34) | <0.001 | 0.72 (0.45, 1.16) | 0.182 |
| Age- and sex-adjusted HR (95% CI) | ||||||
| TG (M2 vs. M1) | 3.51 (2.37, 5.20) | <0.001 | 2.96 (2.13, 4.09) | <0.001 | 2.96 (1.66, 5.29) | <0.001 |
| T-CHO (M2 vs. M1) | 0.93 (0.68, 1.28) | 0.665 | 1.32 (1.00, 1.73) | 0.057 | 0.74 (0.45, 1.22) | 0.240 |
| HDL-C (M2 vs. M1) | 0.17 (0.12, 0.25) | <0.001 | 0.29 (0.21, 0.38) | <0.001 | 0.16 (0.09, 0.30) | <0.001 |
| LDL-C (M2 vs. M1) | 0.36 (0.26, 0.50) | <0.001 | 0.42 (0.32, 0.55) | <0.001 | 0.29 (0.17, 0.49) | <0.001 |
| TG/HDL-C (M2 vs. M1) | 6.61 (4.32, 10.11) | <0.001 | 4.20 (3.04, 5.79) | <0.001 | 4.35 (2.45, 7.74) | <0.001 |
| Non-HDL-C (M2 vs. M1) | 1.37 (0.99, 1.89) | 0.062 | 1.85 (1.40, 2.44) | <0.001 | 1.05 (0.64, 1.74) | 0.850 |
| Multivariate-adjusted HR (95% CI)* | ||||||
| TG (M2 vs. M1) | 0.85 (0.47, 1.54) | 0.599 | 0.69 (0.44, 1.10) | 0.122 | 0.58 (0.23, 1.49) | 0.258 |
| T-CHO (M2 vs. M1) | 0.68 (0.42, 1.09) | 0.106 | 0.98 (0.68, 1.43) | 0.929 | 0.45 (0.21, 0.96) | 0.038 |
| HDL-C (M2 vs. M1) | 0.28 (0.17, 0.45) | <0.001 | 0.42 (0.30, 0.61) | <0.001 | 0.51 (0.24, 1.05) | 0.066 |
| LDL-C (M2 vs. M1) | 0.83 (0.54, 1.29) | 0.407 | 0.86 (0.61, 1.22) | 0.391 | 0.79 (0.39, 1.61) | 0.520 |
| TG/HDL-C (M2 vs. M1) | 1.87 (1.05, 3.31) | 0.033 | 1.06 (0.67, 1.67) | 0.806 | 0.98 (0.41, 2.32) | 0.961 |
| Non-HDL-C (M2 vs. M1) | 1.09 (0.67, 1.77) | 0.723 | 1.36 (0.92, 1.99) | 0.122 | 0.54 (0.25, 1.15) | 0.109 |
*Multivariate analysis: adjustment for sex, age, biliary etiology, smoking habit, alcohol intake, diabetes mellitus, obesity, history of pre-existing organ dysfunctions including history of chronic pulmonary disease, chronic renal disease and cardiovascular disease, admission laboratory data including total leukocyte count, serum glucose, and calcium.
M1: below the median value, M2: above the median value. HDL-C medians: median 1, <1.06 mmol/L; median 2, ≥1.06 mmol/L; LDL-C medians: median 1, <1.85 mmol/L; median 2, ≥1.85 mmol/L; Non-HDL-C medians: median 1, <3.02 mmol/L; median 2, ≥3.02 mmol/L; T-CHO medians: median 1, <4.14 mmol/L; median 2, ≥4.14 mmol/L; TG medians: median 1, <1.52 mmol/L; median 2, ≥1.52 mmol/L; TG/HDL-C medians: median 1, <1.52; median 2, ≥1.52.
Abbreviations: CI: confident interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; PNec, pancreatic necrosis; POF, persistent organ failure; T-CHO, total cholesterol; TG, triglycerides.
Table 5 shows multivariable-adjusted HRs and 95% CIs for outcomes by quartile of lipid profiles with quartile 1 (lowest quartile) as reference. In contrast to the results for TG, LDL-C, and TG/HDL-C ratio quartiles, there was a negative trend for the association across increasing HDL-C quartiles and incidence of POF, PNec and in-hospital death, p values for trends across quartiles were <0.001, <0.001 and 0.043, respectively. The adjusted HRs for highest HDL-C versus the lowest quartile were 0.20 (95% CIs 0.10 to 0.39) for POF; 0.43 (95% CIs 0.26 to 0.71) for PNec and 0.42 (95% CIs 0.15 to 1.14) for in-hospital mortality, respectively. These data were consistent with, and supported the data, that we obtained for HDL-C and each of the three outcomes.
Table 5.
HRs for POF, PNec and in-hospital mortality by quartiles of lipid profiles using multivariable analysis.
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | P-value for trend | |
|---|---|---|---|---|---|
| POF | |||||
| TG | 1.00 (reference) | 1.61 (0.86, 3.00) | 0.95 (0.46, 1.97) | 1.99 (0.80, 4.99) | 0.352 |
| T-CHO | 1.00 (reference) | 0.83 (0.45, 1.51) | 0.54 (0.29, 1.01) | 0.74 (0.38, 1.47) | 0.169 |
| HDL-C | 1.00 (reference) | 0.53 (0.31, 0.92) | 0.23 (0.12, 0.43) | 0.20 (0.10, 0.39) | <0.001 |
| LDL-C | 1.00 (reference)1 | 0.77 (0.45, 1.34) | 0.83 (0.45, 1.53) | 0.66 (0.36, 1.20) | 0.207 |
| TG/HDL-C | 1.00 (reference) | 1.67 (0.84, 3.33) | 2.02 (0.99, 4.13) | 3.68 (1.53, 8.84) | 0.005 |
| Non-HDL-C | 1.00 (reference) | 0.72 (0.39, 1.33) | 0.96 (0.52, 1.76) | 0.87 (0.43, 1.77) | 0858 |
| PNec | |||||
| TG | 1.00 (reference) | 1.83 (1.10, 3.04) | 0.75 (0.42, 1.36) | 1.95 (0.97, 3.88) | 0.314 |
| T-CHO | 1.00 (reference) | 1.07 (0.64, 1.77) | 0.98 (0.60, 1.61) | 1.07 (0.61, 1.89) | 0.911 |
| HDL-C | 1.00 (reference) | 0.64 (0.41, 0.99) | 0.27 (0.17, 0.45) | 0.43 (0.26, 0.71) | <0.001 |
| LDL-C | 1.00 (reference) | 0.88 (0.56, 1.37) | 0.96 (0.59, 1.56) | 0.69 (0.42, 1.12) | 0.183 |
| TG/HDL-C | 1.00 (reference) | 1.53 (0.89, 2.61) | 0.98 (0.55, 1.75) | 2.06 (1.06, 4.02) | 0.111 |
| Non-HDL-C | 1.00 (reference) | 0.76 (0.45, 1.27) | 1.28 (0.78, 2.09) | 1.04 (0.59, 1.83) | 0.474 |
| In-hospital mortality | |||||
| TG | 1.00 (reference) | 2.13 (0.90, 5.05) | 1.04 (0.33, 3.23) | 0.75 (0.17, 3.36) | 0.861 |
| T-CHO | 1.00 (reference) | 0.39 (0.15, 0.97) | 0.23 (0.07, 0.70) | 0.42 (0.15, 1.14) | 0.017 |
| HDL-C | 1.00 (reference) | 0.79 (0.34, 1.83) | 0.50 (0.19, 1.32) | 0.42 (0.15, 1.18) | 0.043 |
| LDL-C | 1.00 (reference) | 0.91 (0.42, 1.96) | 0.82 (0.30, 2.23) | 0.70 (0.27, 1.82) | 0.457 |
| TG/HDL-C | 1.00 (reference) | 1.78 (0.67, 4.73) | 1.67 (0.57, 4.89) | 0.71 (0.17, 2.90) | 0.942 |
| Non-HDL-C | 1.00 (reference) | 0.54 (0.23, 1.26) | 0.50 (0.20, 1.26) | 0.30 (0.10, 0.91) | 0.027 |
*Multivariate analysis: adjustment for sex, age, biliary etiology, smoking habit, alcohol intake, diabetes mellitus, obesity, history of pre-existing organ dysfunctions including history of chronic pulmonary disease, chronic renal disease and cardiovascular disease, admission laboratory data including total leukocyte count, serum glucose, and calcium.
HDL-C quartiles: quartile 1, <0.81 mmol/L; quartile 2, 0.81–1.06 mmol/L; quartile 3, 1.07–1.34 mmol/L; quartile 4, ≥1.35 mmol/L; LDL-C quartiles: quartile 1, <1.26 mmol/L; quartile 2, 1.26–1.84 mmol/L; quartile 3, 1.85–2.36 mmol/L; quartile 4, ≥2.37 mmol/L; Non-HDL-C quartiles: quartile 1, <2.29 mmol/L; quartile 2, 2.29–3.01 mmol/L; quartile 3, 3.02–4.40 mmol/L; quartile 4, ≥4.41 mmol/L; TG quartiles: quartile 1, <0.87 mmol/L; quartile 2, 0.87–1.51 mmol/L; quartile 3, 1.52–4.29 mmol/L; quartile 4, ≥4.30 mmol/L; T-CHO quartiles: quartile 1, <3.36 mmol/L; quartile 2, 3.36–4.12 mmol/L; quartile 3, 4.13–5.45 mmol/L; quartile 4, ≥5.46 mmol/L; TG/HDL-C quartiles: quartile 1, <0.76; quartile 2, 0.76–1.52; quartile 3, 1.52–6.65; quartile 4, ≥6.66.
Abbreviations: CI: confident interval; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; PNec, pancreatic necrosis; POF, persistent organ failure; T-CHO, total cholesterol; TG, triglycerides.
Moreover, TG/HDL-C quartile was positively related to POF (adjusted HR for highest quartile was 3.68 [95% CI 1.53 to 8.84]; p value for the trend across quartiles was 0.005). Besides, T-CHO and non-HDL-C concentration quartiles were inversely associated with in-hospital mortality (adjusted HR for highest quartile was 0.42 [95% CI 0.15 to 1.14) and 0.30 [95% CI 0.10 to 0.91], p value for the trend across quartiles was 0.017 and 0.027], with similar but non-significantly different trends for POF and PNec.
We also investigated associations between HDL-C and outcomes in subgroup analyses. The results are shown in Supplementary Tables S1–S3.
ROC analysis of HDL-C and other lipid profiles as predictors of the three outcomes
We next analyzed the predictive value of these lipid parameters detected upon presentation. Compared to TG, T-CHO, LDL-C, TG/HDL-C and non-HDL-C, HDL-C showed a superior prognostic performance for predicting severe outcomes (Supplementary Table S4). The predictive values of HDL-C for PNec and in-hospital mortality were higher (but not statistically different) than those of Ranson score (AUC 0.734 [95% CIs 0.700 to 0.769] vs. AUC 0.701 [95% CIs 0.668 to 0.734]; P = 0.143 and AUC 0.768 [95% CIs 0.710 to 0.827] vs. AUC 0.745 [95% CIs 0.694 to 0.795]; P = 0.516). Moreover, HDL-C performed significantly better than Ranson score in diagnosing POF (AUC 0.778 [95% CIs 0.740 to 0.85] vs. AUC 0.678 [95% CIs 0.638 to 0.719]; P < 0.001) (Fig. 2). The cut-off value of HDL-C for the prediction of the three outcomes were 0.94 mmol/L (POF), 1.03 mmol/L (PNec) and 0.82 mmol/L (mortality), respectively.
Figure 2.
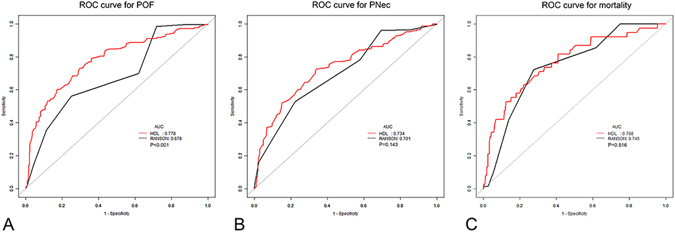
ROC curve of HDL-C levels and Ranson score as pedictors of POF, PNec and in-hospital mortality. Abbreviations: AUC: area under the curve; HDL-C, high-density lipoprotein cholesterol; PNec, pancreatic necrosis; POF, persistent organ failure; ROC, receiver operating characteristic.
Discussion
In the present research, we examined the involvement of admission lipid parameters (TG, T-CHO, HDL-C, LDL-C, TG/HDL-C and non-HDL-C) with incidence of POF, PNec and in-hospital mortality in patients with AP. Our results showed for the first time that, in a single-ethnicity Asian Chinese population of patients with AP, even after adjustments for relevant potential confounding factors, a decreased HDL-C level was an independent prognostic factor of adverse outcomes, not only for POF, PNec, but also for death. We also found a decreased LDL-C level was correlated with POF and PNec, but the significance and predictive value were inferior to HDL-C.
AP is an immune disorder featured by the activations of both innate and adaptive immune systems, with release of pro-and anti-inflammatory cytokines. Exaggerated and uncontrollable host response leads to systemic inflammation, and eventually progresses into organ dysfunction and even POF22. There exist several pathogeneses contributing to the development of regional pancreatic infarctions that cause PNec, including infiltration of inflammatory cytokines, elevated vascular permeability, hypovolemia with shunting of blood from vital organs, vascular spasm, and free fatty acid (FFA)-induced acinar cell disruption23, 24.
HDL plays a central role in FFA clearance and reverse cholesterol transport. Besides, HDL and its mimetics also show anti-oxidant, anti-thrombotic and anti-apoptotic functions15. Several researchers figured out that a low amount of HDL-C, which has anti-inflammatory properties, can in turn lead to a more severe systemic inflammatory response25. Other laboratories found that HDL can repress inflammatory gene expression in cytokine activated endothelial cells and other cell types26, 27.
Apart from the basic function of lipid transference, human LDL is also shown to be involved in organismal protein transfer and delivering pro-inflammatory and pro-thrombotic protein mediators from synthetic place to the site of inflammatory organ systems28. In response to inflammatory conditions, native LDL is chemically modified, forming LDL-containing circulating immune complexes (LDL-CIC), which leads to local accumulation and activation of macrophages, releasing pro-inflammatory cytokines and mediators29. Modified LDL, especially oxidized LDL, is a key molecule in the early progression of endothelial dysfunction. Studies have demonstrated that ox-LDL plays a central role in the induction of both pro-inflammatory mediators and anti-inflammatory cytokines such as tumor necrosis factor-a (TNF-a), interleukin-6 (IL-6) and interleukin-1-1(IL-1) in human peripheral blood mononuclear cells30, 31.
The possible mechanisms causing decreased values of serum lipid cholesterol (including HDL-C and LDL-C) in AP are as follows: the reduction of lipoprotein synthesis in the liver, a lower rate of general catabolic metabolism, and the activation of immune system during the acute phase response. In contrast, the production of triglycerides in the liver increases during the acute inflammatory response32.
Decreased values of serum HDL-C in AP represents impaired anti-inflammatory function, which may lead to increase in FFA, creating acidic microenvironment and damaging pancreatic acinar cells. Decreased serum LDL-C is representative of endothelial dysfunction, with elevated vascular permeability, hypovolemia of vital organ systems, and release of both pro- and anti-inflammatory cytokines from immune cells. All of these mechanisms will cause uncontrollable self-immune response and result in the presence of POF, PNec and mortality in AP.
With respect to the relationship between lipoprotein and immune disorders, several studies, including ours, have investigated the association among lipid profiles and outcome in AP. Decreased serum level of HDL-C and decreased activity of paraoxonase 1 (which is responsible for the antioxidant ability of HDL-C) were observed by Unal and colleagues16 in an experimental AP model. HDL-C is shown to suppress immune response mediated by toll-like receptor 4 (TLR-4) in macrophages, which is another pathway mediating the anti-inflammatory effects of HDL besides paraoxonase 117. Bugdaci et al.18 suggested that low levels of HDL in patients with AP during early phase are associated with disease severity. In another study, Khan et al.19 reported decreased concentrations of serum T-CHO, LDL-C and HDL-C during acute phase in patients with alcohol induced AP compared to normal ones. But the sample size was small and the study did not include other etiologies of AP. Later, they analyzed blood samples obtained within a few days after admission and follow-up samples of patients with AP, and found that serum T-CHO, HDL-C and LDL-C levels measured within 2 days of hospitalization were significantly lower in patients with severe pancreatitis20. Peng et al. reported serum levels of HDL and Apo A-I at admission can differentiate POF from transient OF in AP, but they failed to include hyper-triglyceridemic pancreatitis21.
In spite of AP, low plasma levels of HDL-C is shown as a poor prognostic factor with increased mortality and adverse outcomes in patients with endotoxemia and sepsis33. Stachon et al.34, 35 reported lower serum lipid levels during admission to the intensive care unit in non-survivors compared to survivors. Other researchers reported there was a positive trend for the association across increasing HDL/Apo A-I ratio quartiles and mortality from cardiovascular disease, carcinoma and all cause36.
In our previous researches, we found that admission parameters such as peripheral blood CD4+ T lymphocytes22, neutrophil to lymphocyte ratio37, mean platelet volume38, serum calcium39 and lactate dehydrogenase40 were independent risk factors for POF. The present finding that lower concentrations of admission HDL-C and LDL-C are associated with increased risk of adverse outcomes complements the evidence that decreased HDL-C correlates with acute phase responses. In other words, AP patients presented with low HDL-C and LDL-C levels are at high risk of developing a poor outcome. Moreover, the predictive value of HDL-C is superior to that of LDL-C. Our data suggest that HDL-C levels may have critical influences for other clinical conditions beyond cardiovascular diseases.
Several limitations are evident in our study that need to be considered. First, the observational nature of this study precludes the conclusions of a causal relationship, and as all patients were Asian Chinese, a confounding by ethnic variety cannot be excluded from a theoretical point of view. Second, as a retrospective study, we were not able to measure the concentrations of lipoproteins such as Apo A-I, Apo A-II and Apo B, which may be more specific to HDL-C and LDL-C particles. Third, lipid parameters were measured only once and the time elapsed between assessment and the attack of AP was long in some patients. Moreover, we excluded a large number of patients who do not undergo assessments of lipid profiles upon presentation. This selection bias may influence the results. Besides, we did not include patients with AP induced by trauma as well as patients with chronic pancreatitis. Thus, the conclusion may not be generalized to these kinds of patients. Furthermore, since the baseline values of lipid parameters in AP patients with statin therapy are not within normal range, and statin therapy may influence the outcome in established AP41–43, the conclusion may not be generalized to these patients. Due to retrospective nature of our study, we did not record other laboratory parameters beside lipid profiles in these patients. The relationship between lipid profiles and outcomes in AP patients with statin therapy should be explored separately.
There are also several strengths of the present study. First, to the best of our knowledge, this is the first detailed study of the impact of HDL-C on incidence of POF, PNec and in-hospital mortality from a single medical center in an Asian Chinese population of AP. Second, we only include patients who presented within 72 hours from AP symptom onset and without any medical treatment. It is possible that the use of medication such as statin treatment may rapidly influence the levels of peripheral lipid profiles. Furthermore, multivariate adjustments for severity and survival predictors, especially calcium and glucose, were utilized in the outcome analysis.
In conclusion, our results suggest that HDL-C level can independently predict POF, PNec and mortality in patients with AP in an Asian-Chinese population.
Electronic supplementary material
Author Contributions
Yushun Zhang, Bo Wang, Ping Fan and Heshui Wu designed the study. Feng Guo, Shoukang Li and Feiyang Wang collected and organized the data. Zibo Meng, Jingyuan Zhao and Zhiqiang Liu analyzed the data and illustrated the results. Yushun Zhang, and Feng Guo wrote the paper. Bo Wang, Ping Fan, Chunyou Wang and Heshui Wu revised the paper. All the authors discussed and agreed on the results. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Yushun Zhang and Feng Guo contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06618-w
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bo Wang, Email: 67289331@qq.com.
Ping Fan, Email: 85435895@qq.com.
Heshui Wu, Email: heshuiwu@hust.edu.cn.
References
- 1.Banks PA, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 2.Malledant Y, Malbrain ML, Reuter DA. What’s new in the management of severe acute pancreatitis? Intensive Care Medicine. 2015;41:1957–1960. doi: 10.1007/s00134-015-3903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oiva J, et al. Acute pancreatitis with organ dysfunction associates with abnormal blood lymphocyte signaling: controlled laboratory study. Critical Care. 2010;14:R207. doi: 10.1186/cc9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermejo-Martín JF, et al. Circulating neutrophil counts and mortality in septic shock. Critical Care. 2014;18:407. doi: 10.1186/cc13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rye KA, Barter PJ. Cardioprotective functions of HDLs. Journal of Lipid Research. 2015;55:168–179. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sviridov D, Mukhamedova N, Remaley AT, Chin-Dusting J, Nestel P. Antiatherogenic functionality of high density lipoprotein: how much versus how good. Journal of Atherosclerosis and Thrombosis. 2008;15:52–62. doi: 10.5551/jat.E571. [DOI] [PubMed] [Google Scholar]
- 7.McMahon KM, et al. Synthetic high-density lipoprotein-like nanoparticles as cancer therapy. Cancer Treatment Research. 2015;166:129–150. doi: 10.1007/978-3-319-16555-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, et al. Biomimetic, synthetic HDL nanostructures for lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2511–2516. doi: 10.1073/pnas.1213657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su F, et al. HDL mimetics inhibit tumor development in both induced and spontaneous mouse models of colon cancer. Molecular Cancer Therapy. 2012;11:1311–1319. doi: 10.1158/1535-7163.MCT-11-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asztalos BF, Tani M, Schaefer EJ. Metabolic and functional relevance of HDL subspecies. Current Opinion in Lipidology. 2011;22:176–185. doi: 10.1097/MOL.0b013e3283468061. [DOI] [PubMed] [Google Scholar]
- 11.Catapano AL, Pirillo A, Bonacina F, Norata GD. HDL in innate and adaptive immunity. Cardiovascular Research. 2014;103:372–383. doi: 10.1093/cvr/cvu150. [DOI] [PubMed] [Google Scholar]
- 12.Luscher TF, Landmesser U, von Eckardstein A, Fogelman AM. High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circulation Research. 2014;114:171–182. doi: 10.1161/CIRCRESAHA.114.300935. [DOI] [PubMed] [Google Scholar]
- 13.Rye KA, Barter PJ. Regulation of high-density lipoprotein metabolism. Circulation Research. 2014;114:143–156. doi: 10.1161/CIRCRESAHA.114.300632. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls SJ, Puri R. Is it time for HDL to change its tune? Circulation. 2013;128:1175–1176. doi: 10.1161/CIRCULATIONAHA.113.005090. [DOI] [PubMed] [Google Scholar]
- 15.Sviridoc D, Temaley AT. High-density lipoprotein mimetics: promises and challenges. Biochemical Journal. 2015;472:249–259. doi: 10.1042/BJ20150832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unal E, et al. Serum paraoxonase (a high density lipoprotein-associated lipophilic antioxidant) activity and lipid profile in experimental acute pancreatitis. Pancreas. 2005;31:84–87. doi: 10.1097/01.mpa.0000168227.74203.e4. [DOI] [PubMed] [Google Scholar]
- 17.Yvan-Charvet L, et al. Cholesterol efflux potential and anti-inflammatory properties of high-density lipoprotein after with niacin or anacetrapib. Arteriosclerosis Thrombosis and Vascular Biology. 2010;307:1430. doi: 10.1161/ATVBAHA.110.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bugdaci MS, Sokmen M, Zuhur SS, Altuntas Y. Lipid profile changes and importance of low serum alpha-lipoprotein fraction (highdensity lipoprotein) in cases with acute pancreatitis. Pancreas. 2011;40:241–1244. doi: 10.1097/MPA.0b013e3182211bbf. [DOI] [PubMed] [Google Scholar]
- 19.Khan J, et al. Serum lipid and fatty acid profiles are highly changed in patients with alcohol induced acute pancreatitis. Pancreatology. 2012;128:44–48. doi: 10.1016/j.pan.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Khan J, Nordback I, Sand J. Serum lipid levels are associated with the severity of acute pancreatitis. Digestion. 2013;87:223–228. doi: 10.1159/000348438. [DOI] [PubMed] [Google Scholar]
- 21.Peng YS, et al. Serum levels of apolipoprotein A-I and high-density lipoprotein can predict organ failure in acute pancreatitis. Critical Care. 2015;19:88. doi: 10.1186/s13054-015-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang ZY, et al. The reduction of peripheral blood CD4+ T cell indicates persistent organ failure in acute pancreatitis. PLoS One. 2015;10:e0125529. doi: 10.1371/journal.pone.0125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. American Journal of Gastroenterology. 2009;104:164–170. doi: 10.1038/ajg.2008.66. [DOI] [PubMed] [Google Scholar]
- 24.Asztalos BL, et al. Role of LCAT in HDL remodelling: investigation of LCAT deficiency states. Journal of Lipid Research. 2007;48:592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Murphy AJ, Woollard KJ. High-density lipoprotein: a potent inhibitor of inflammation. Clinical and Experimental Pharmacology and Physiology. 2010;37:710–718. doi: 10.1111/j.1440-1681.2009.05338.x. [DOI] [PubMed] [Google Scholar]
- 26.Higashi Y, et al. Endothelial function in subjects with isolated low HDL cholesterol: role of nitric oxide and circulating progenitor cells. American Journal of Physiology Endocrinology and Metabolism. 2010;298:E202–E209. doi: 10.1152/ajpendo.00394.2009. [DOI] [PubMed] [Google Scholar]
- 27.Tabet F, et al. HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nature Communications. 2014;5:3292. doi: 10.1038/ncomms4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orekhov AN, Bobryshev YV, Sobenin IA, Melnichenko AA, Chistiakov DA. Modified low density lipoprotein and lipoprotein-containing circulating immune complexes as diagnostic and prognostic biomarkers of atherosclerosis and type 1 diabetes macrovascular disease. International Journal of Molecular Science. 2014;15:12807–12841. doi: 10.3390/ijms150712807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobenin IA, et al. Low density lipoprotein-containing circulating immune complexes: role in atherosclerosis and diagnostic value. BioMed Research International. 2014;2014:205697. doi: 10.1155/2014/205697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groeneweg M, et al. Lipopolysaccharide induced gene expression in murine macrophages is enhanced by prior exposure to oxLDL. Journal of Lipid Research. 2006;47:2259–2267. doi: 10.1194/jlr.M600181-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Bonaterra GA, et al. Increased gene expression of scavenger receptors and proinflammatory markers in peripheral blood mononuclear cells of hyperlipidemic males. Journal of Molecular Medicine. 2007;85:181–190. doi: 10.1007/s00109-006-0117-6. [DOI] [PubMed] [Google Scholar]
- 32.Carpentier YA, Scruel O. Changes in the concentration and composition of plasma lipoproteins during the acute phase response. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5:153–158. doi: 10.1097/00075197-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Chien JY, Jerng JS, Yu CJ, Yang PC. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Critical Care Medicine. 2005;33:1688–1693. doi: 10.1097/01.CCM.0000171183.79525.6B. [DOI] [PubMed] [Google Scholar]
- 34.Stachon A, et al. Estimation of the mortality risk of surgical intensive care patients based on routine laboratory parameters. European Surgical Research. 2008;40:263–272. doi: 10.1159/000113106. [DOI] [PubMed] [Google Scholar]
- 35.Stachon A, et al. A laboratory-based risk score for medical intensive care patients. Clinical Chemistry and Laboratory Medicine. 2008;46:855–862. doi: 10.1515/CCLM.2008.136. [DOI] [PubMed] [Google Scholar]
- 36.Sung KC, Ryu S, Wild SH, Byrne CD. An increased high-density lipoprotein cholesterol/apolipoprotein A-I ratio is associated with increased cardiovascular and all-cause mortality. Heart. 2015;101:553–558. doi: 10.1136/heartjnl-2014-306784. [DOI] [PubMed] [Google Scholar]
- 37.Zhang YS, et al. Neutrophil to lymphocyte ratio predicts persistent organ failure and in-hospital mortality in an Asian Chinese population of acute pancreatitis. Medicine. 2016;95:37. doi: 10.1097/MD.0000000000004746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang P, Zhang YS, Wu HS. Mean platelet volume as an indicator of persistent organ failure in acute pancreatitis. International Journal of Clinical and Experimental Pathology. 2016;9:12883–12889. [Google Scholar]
- 39.Peng, T. et al. Serum calcium as an indicator of persistent organ failure in acute pancreatitis. American Journal of Emergency Medicine Feb 4. pii: S0735-6757(17)30095-5. doi:10.1016/j.ajem.2017.02.006 (2017). [DOI] [PubMed]
- 40.Cui J, et al. Serum lactate dehydrogenase is predictive of persistent organ failure in acute pancreatitis. Journal of Critical Care. 2017;41:161–165. doi: 10.1016/j.jcrc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Almeida JL, et al. Statin pretreatment in experimental acute pancreatitis. JOP. 2008;9:431–439. [PubMed] [Google Scholar]
- 42.Preiss D, et al. Lipid-modifying therapies and risk of pancreatitis. JAMA. 2012;308:804–811. doi: 10.1001/jama.2012.8439. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Goldberg JI. Hypertriglyceridemia-induced pancreatitis created by oral estrogen and in vitro fertilization ovulation induction. Journal of Clinical Lipidology. 2008;281:63–66. doi: 10.1016/j.jacl.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We declare that the data is available.


