Abstract
Deep-sea coral assemblages are key components of marine ecosystems that generate habitats for fish and invertebrate communities and act as marine biodiversity hot spots. Because of their life history traits, deep-sea corals are highly vulnerable to human impacts such as fishing. They are an indicator of vulnerable marine ecosystems (VMEs), therefore their conservation is essential to preserve marine biodiversity. In the Mediterranean Sea deep-sea coral habitats are associated with commercially important crustaceans, consequently their abundance has dramatically declined due to the effects of trawling. Marine spatial planning is required to ensure that the conservation of these habitats is achieved. Species distribution models were used to investigate the distribution of two critically endangered octocorals (Funiculina quadrangularis and Isidella elongata) in the central Mediterranean as a function of environmental and fisheries variables. Results show that both species exhibit species-specific habitat preferences and spatial patterns in response to environmental variables, but the impact of trawling on their distribution differed. In particular F. quadrangularis can overlap with fishing activities, whereas I. elongata occurs exclusively where fishing is low or absent. This study represents the first attempt to identify key areas for the protection of soft and compact mud VMEs in the central Mediterranean Sea.
Introduction
Deep-sea coral assemblages play a significant structural role in marine benthic ecosystems by providing essential three-dimensional habitats for fish and invertebrate communities, acting as biodiversity hot spots and contributing to the maintenance of ecosystem functioning1–3. They have long lifespans and slow growth rates (few to several mm per year4, 5, long reproductive cycles and low recruitment6). Their life history traits make deep-sea corals highly vulnerable to human-induced impacts (e.g. fishing, pollution, coastal development, invasive species and climate change). Consequently, a lack of management to preserve deep-sea corals may have permanent or irreversible effects on the entire marine ecosystem7.
Deep-sea fisheries, in particular bottom trawling, have dramatic impacts on deep-sea communities as they remove most of the habitat forming organisms from the seafloor8, 9 and alter its morphology and physical proprieties (e.g. increased resuspension of bottom sediment, which damages filter feeding organisms by siltation10, 11). The impact of fisheries on deep-sea habitats has been increasing over the last decades in response to the decline of many shelf commercial stocks11, 12. As a result the abundance of deep-sea corals has been diminishing13. The recovery of deep-sea corals from the damage produced by bottom trawling could take decades or centuries, with considerable effects on the associated fish and invertebrate communities and ecosystem biodiversity. International organisations14–16 as well as the European Marine Strategy Framework Directive17 recognized the fragility of deep-sea habitats, recommending the protection of deep-sea coral species and habitats particularly because they are indicators of vulnerable marine ecosystems (VMEs)18. Several deep-sea VMEs have been identified by the General Fisheries Commission for the Mediterranean Sea (GFCM)19, however most of these habitats still lack a comprehensive ecological characterisation, including spatial distribution maps.
Important Mediterranean deep-sea coral habitats are represented by relatively dense aggregation of scleractinian colonies of Lophelia pertusa, Madrepora oculata, Desmophyllum dianthus (creating three-dimensional habitats for a number of associated fish and invertebrates taxa), a soft-mud facies characterized by the sea pen Funiculina quandrangularis (commonly inhabited by commercially valuable crustaceans such as Parapenaeus longirostris and Nephrops norvegicus), and a compact-mud facies characterized by the so-called bamboo coral, the gorgonian Isidella elongata (associated with high densities of the red shrimps Aristeus antennatus and Aristaeomorpha foliacea 20–22). Due to the high commercial value of the associated crustaceans, the impact of fisheries, and in particular bottom trawling, on Mediterranean deep-sea coral habitats has been severe22, 23, and some local key species, such as I. elongata and F. quadrangularis have been almost entirely eradicated with drastic consequences for the associated invertebrate fauna10, 22, 24, 25. Despite the banning of trawling activities below a depth of 1000 m in the Mediterranean by the GFCM for the protection of benthic habitats26, the effectiveness of this measure is still unknown. It is certainly inadequate for the protection of several habitat-forming taxa such as I. elongata and F. quadrangularis, which are partially or exclusively found at shallower depths (see Table 1). Due to such threats adequate marine spatial planning is essential for the conservation of these important habitats in the Mediterranean Sea.
Table 1.
Information relative to the biology and fisheries of Funiculina quadrangularis and Isidella elongata.
| Species | Description | Anchoring structure | Body flexibility | Habitat type | Fisheries | References |
|---|---|---|---|---|---|---|
| Funiculina quadrangularis (Pallas, 1766) | • It is a tall, narrow sea pen, often found in large populations | • A bulb, or peduncle at the bottom of the modified axial polyp that may penetrates into the sediment down to about 50 cm | • It can lie flat under the pressure of wave of approaching gears | • It prefers soft muddy habitat at depths of between 20–2000 m | • This is considered one of the most sensitive sea pen species to fisheries as it is unable to withdraw into the sediment. | 22, 75, 83, 84 |
| • It is often found in moderately high energy environments characterised by a noticeable bottom current, necessary for procuring adequate food | ||||||
| • It can reach 2 m in height with the lower quarter embedded in the sediment and usually curved in the upper third | ||||||
| • In the northeast Atlantic and Mediterranean Sea is associated with Nephrops norvegicus fisheries | ||||||
| • It has a calcareous white axis, typically square in section | ||||||
| • Information on its longevity does not exist | ||||||
| Isidella elongata (Esper, 1788) | • This species is a near-endemic deep-water gorgonian also known as bamboo coral | • A lobed, root-like holdfast attached to small stones embedded in the surface sediment | • Not flexible due to its fan body shape | • It prefers compact mud | • In the Mediterranean Sea it is usually associated with the high-value, deep-water red shrimps Aristeus antennatus, Aristaeomorpha foliacea and Plesionika martia | 72, 85–90 |
| • This species occurs mainly in intermediate and deep waters (200–1500 m depth) on moderately flat bottoms | ||||||
| • It can reach up to 3 m in height forming large single-species stands | ||||||
| • This species characterises a facies of bathyal compact mud substrate on moderately flat bottoms (slope <5%) | ||||||
| • It is a long-lived species (75–126 years) |
The central Mediterranean Sea (Figure 1) is a very important area for deep-sea corals, such as white corals (L. pertusa and M. oculata 27, 28). However, little information is available about the large-scale spatial distribution and habitat preferences of other important species such as F. quadrangularis and I. elongata 28 now recognized as critically endangered by IUCN29. This area is one of the most important fishing grounds in the Mediterranean Sea, in particular for trawlers targeting deep-water rose shrimp (P. longirostris) and other decapods of commercial interest (A. foliacea, N. norvegicus and A. antennatus 30, 31). Investigations into the habitat requirements and spatial patterns of these exploited crustacean species are mandatory to ensure their stock management and conservation, as well as the protection of their associated VMEs.
Figure 1.

Location of the study region within the Strait of Sicily (Central Mediterranean Sea). This area corresponds to the Geographic Sub Area (GSA) 16. Trawl stations sampled during the MEDITS Survey (2008–2013) are indicated with an x. This map was created with ArcGIS version 10.3 http://www.esriitalia.it by Valentina Lauria.
Species distribution models (SDMs) have been increasingly used amongst conservation biologists, ecologists and government bodies to identify where VMEs could occur at regional and global scales and to provide insight into the environmental drivers that control their distribution32, 33. The main advantages of using such models is the ability to predict the species presence or abundance at unsurveyed locations, to understand species-environment relationships and to provide distribution maps that can be used to inform policy makers (e.g. planning of marine protected areas34–36). In this study SDMs were applied to investigate the preferential habitat (portion of potential habitat used on average over time) and fisheries impacts on F. quadrangularis and I. elongata, two critically endangered deep-sea octocorals in the central Mediterranean Sea. Species densities were extracted from a long-term dataset (covering the period 2008–2013) and modelled as a function of physical (i.e. depth, slope, rugosity, aspect), oceanographic (i.e. sea bottom temperature, sea bottom salinity and currents), and fisheries variables (i.e. average distribution of the fishing effort over time). Predictive distribution maps were produced in order to identify species-specific spatial patterns at a regional scale. This study will provide new knowledge about the habitat preferences and fishing impact on two critically endangered deep-sea octocoral species and will support future spatial conservation plans for VMEs in the Mediterranean Sea.
Materials and Methods
Study area
Our study area is situated in the central Mediterranean Sea and includes the northern side of the Strait of Sicily between 34°59′–38°00′N and 10°59′–15°18′W (Fig. 1). This area corresponds to the Geographic Sub Area (GSA) 16 of the GFCM37 and extends for about 34 000 km2. It presents a varied seafloor morphology, including a shallow bank (Adventure Bank) in the western part (about 100 m depth) and deeper areas in the southeast (about 1800m depth; Fig. 1). It shows a complex circulation pattern that makes the Strait of Sicily a highly productive area38 and a biodiversity hotspot for bony fish, elasmobranchs and invertebrates39–41. A number of significant deep-sea coral communities are found in this region, mainly dominated by the octocorals such as bamboo coral I. elongata, tall sea pen F. quadrangularis and red coral Corallium rubrum 28. Since the early 1980s, this area has been intensively exploited by many demersal fisheries, mainly bottom trawlers, operating along the southern coast of Sicily, including the Mazara del Vallo fleet, one of the largest and most active fleets in the Mediterranean42.
Survey data
Since 1994 the area has been investigated under the Mediterranean International Trawl Survey program -MEDITS43. This survey is carried out annually in late spring/early summer, and takes place in several areas of the Mediterranean Sea using a standardised sampling methodology44. It provides a spatio-temporal dataset of fishery-independent indices relating to demersal species abundance, demographic structure and spatial distribution. In GSA16, sampling stations are fixed (Fig. 1) and replicated each year according to a stratified random sampling design based on five depth strata: 10–50 m, 51–100 m, 101–200 m, 200–500 m, 500–800 m, where the number of hauls is proportional to the area of each stratum. A total of 120 stations (haul duration = 30–60 min; trawl speed = 3 knots) were sampled for each year of our study period (2008–2013) on board the commercial trawler Sant’Anna. The gear was a bottom trawl net with a high vertical opening and 20 mm side diamond stretched mesh in the cod-end. At each trawl station the collected species were sorted, weighed, counted and measured. Species density was calculated as the number of individuals per km2 (Nkm−2) for a total of 720 trawl hauls covering the period 2008–2013. The overall percentage of occurrence (described as the number of hauls in which the species was found) for F. quadrangularis and I. elongata was calculated. Information about deep-sea octocoral biology and fisheries is provided in Table 1.
Environmental and fishery variables
For modelling, both environmental and fishery data were used as predictors of octocoral habitat suitability (Table 2; Fig. 2). Environmental variables included physical descriptors (i.e. depth, slope, rugosity and aspect) and oceanographic variables (i.e. sea bottom temperature - SBT, sea bottom salinity - SBS and currents), while for fishery impact predictor the average distribution of the fishing effort over time was used. Digital continuous maps were obtained for all the variables from existing databases, or derivate in ArcGIS (Table 2).
Table 2.
Predictors used for habitat modelling.
| Variable | Unit | Resolution | Descriptor | Type of data | Data source | Reference |
|---|---|---|---|---|---|---|
| Depth | m | 0.866 km | Bathymetry | Continuous digital map | http://www.marspec.org/ | 46 |
| Slope | degrees | 0.866 km | Typology of substrata | Derived from bathymetry data | Benthic Terrain Modeller in ArcGIS 10.3 | 47–49 |
| Rugosity | No unit | 0.866 km | Derived from bathymetry data | Benthic Terrain Modeller in ArcGIS 10.3 | 47–49 | |
| Aspect East/West and North/South | Radians | 0.866 km | Orientation of the substrata | Derived from bathymetry data | http://www.marspec.org/ | 46, 51 |
| Currents | m/s | 6.5 km | Current | Derived from model | http://marine.copernicus.eu/ | 91 |
| Sea bottom temperature | °C | 6.5 km | Temperature | Derived from model | http://marine.copernicus.eu/ | 91 |
| Sea bottom salinity | psu | 6.5 km | Salinity | Derived from model | http://marine.copernicus.eu/ | 91 |
| Fishing pressure | Number of fishing hours/year | 0.866 km | Fishery effort | VMS data | European Vessel Monitoring System | 92 |
Figure 2.
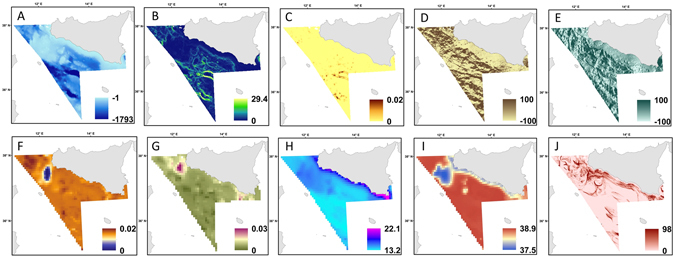
The spatial patterns of the environmental variables used to map the habitat models. These include (A) depth (m); (B) slope (degrees) values range from to 0° to 90° with low slope values corresponding to flat terrain and higher values to steeper terrain; (C) rugosity values range from 0 (no terrain variation) to 1 (complete terrain variation); (D) aspect north-south and east-west (E) scaled to 100 (radians); (F) Current north-south (m/s); (G) Current west-east (m/s); (H) sea bottom temperature (°C); (I) sea bottom salinity (PSU); (J) Average distribution of the fishing effort between 2008–2013 in terms of number of fishing hours/year. These maps were created with ArcGIS version 10.3 http://www.esriitalia.it by Valentina Lauria.
Depth (Fig. 2A) is one of the main environmental gradients that govern species spatial patterns, and in the case of corals has been shown to be a main factor that influences their distribution20, 32, 45. Aside of giving information on species bathymetry range this environmental factor can also capture the potential effect of unmeasured variables that might shape species spatial distribution. For this study bathymetry data were extracted from a re-projection of the MARSPEC database, a world ocean dataset developed for marine spatial ecology46.
Slope and rugosity provide a measure of the seabed morphology and were derived from the continuous digital map of depth using the Benthic Terrain Modeller tool in ArcGIS 10.147 (Table 2). Slope (Fig. 2B) describes the rate of change in elevation over distance. Low values of slope are associated with flat ocean bottoms or areas of sediment deposition, while higher values indicate potential rocky ledges. Rugosity (Fig. 2C) provides an indicator of the bumpiness and complexity of the seafloor and emphasises small variations in the seabed terrain. Low values of terrain variables indicate soft seabed substrata while high values indicate a rocky seabed. These parameters are largely used as predictors of species distribution when detailed information on sediment type is not available48, 49. Studies on the distribution of deep-sea corals have shown different species (i.e. sea pen Virgularia mirabilis, Funiculina quadrangularis and Pennatula phosphorea; gorgonian corals Paragorgia arborea and Primnoa resedaeformis) preference for a range of sediments and sea bottom types (from soft to hard45, some of which might be important for initial coral settlement50).
Aspect (Fig. 2D and E; Table 2) identifies the orientation of the seabed at any given location and provides information on the exposure of any given area to local and regional currents51. This seabed topographic feature is essential in shaping benthic community structure as it can influence current regimes and the flux of suspended food material50, 52. This terrain variable has been shown to be a good indicator of habitat suitability for cold waters corals as it acts as a proxy for other environmental processes53. It is measured in radians and divided into two components: North-South and East-West gradient, which vary between −1 and +1 describing the direction that the surface slope faces.
Data on currents, temperature and salinity were obtained from the Ocean General Circulation Model (OGCM) implemented for the Mediterranean Sea. Despite these data have a broader resolution than the bathymetry derived data they represent key environmental drivers of deep-sea corals habitat selection (Table 2). Monthly mean bottom values were extracted from the OGCM model corresponding to the month of the median day of the survey (see online Supplementary Information Table S1). Maps of currents are presented in two directions: eastward and northward (Fig. 2F and G). Bottom currents are an important environmental factor for the habitat selection of deep-sea corals by governing food supply, and the fouling of corals with sediment, as well as influencing larval dispersal and connectivity between suitable habitat patches54, 55.
SBT data were also extracted from the OGCM model (Table 2; Fig. 2H). Sea temperature plays a key role in the habitat selection of deep-sea corals, as this environmental factor is thought to influence their calcification rates, physiology and biochemistry56. Some studies have suggested that temperature does not limit coral growth and distribution57. Data on SBS values were obtained from the OGCM model (Table 2; Fig. 2I). This variable can influence the water column stratification and small oceanographic processes (e.g. internal waves) that may affect food availability33.
The fishing pressure exerted by trawlers in the area was used to quantify the potential impact of towed fisheries. The trawling pressure was estimated using the data provided by the vessel monitoring system (VMS), the main geo-positioning device currently used to track and analyse the spatio-temporal behaviour of fishing vessels. The VMS data covering the years 2008–2013 were processed following the methodology described in58, 59 and used to assess the spatial distribution of the fishing effort in terms of number of fishing hours per year (Fig. 2J; Table 2).
Values of environmental and fisheries variables were extracted in ArcGIS per station and then used for model construction.
Model selection
All variables were tested for collinearity using the Variance Inflation Factor60 and used for habitat model construction. Histograms of species densities showed a discontinuity between the zero values and positive density data, therefore a two-part modelling approach seemed appropriate61. In the case of trawl samples, due to both trawl geometry and species behaviour, zero observations may indicate either low density or true absence, with different processes governing presence probability and density levels62. Generalised Additive Models (GAMs) were used to construct a two-part model consisting of a binomial occurrence model developed using presence-absence data (family binomial and logit link function) then modelling presence-only data (positive log-transformed densities) using a Gaussian distribution with a canonical (identity) link function. The final preferential habitat model (also known as delta model) was obtained by the multiplication of the predictions from both models. GAMs are non-parametric regression techniques63 that allow for the modelling of relationships between variables without specifying any particular form for the underlying regression function. The use of smooth functions as regressors gives GAMs greater flexibility over linear (or other parametric) types of models.
Starting from the full model that included all explanatory variables, the most parsimonious model was selected on the basis of the lowest Akaike Information Criterion (AIC), corrected for small sample size (AICc). This approach selects the model with the best balance between bias and precision and avoids problems of, for example, multiple testing among explanatory variables64. All possible models (set of candidate models that included all combinations of explanatory variables) were ranked using the difference in AICc between the top-ranked and current model (delta AICc), and by calculating the AICc weight (the scaled likelihood that each model is the best description of the data). Competing models of the best supported model were selected when having their AICc within 2 of the minimum64. In addition the relative importance of the predictor variables was also calculated by summing the AICc weights from the models that contain that variable. Model performance was measured as the proportion of the null deviance explained (Dev) or the adjusted regression coefficient (R2).
Binomial models were tested for sensitivity by using the area under the receiver operating characteristic curve (AUC)65. An AUC value of 0.5 indicates that the model performs no better than a random model, whereas a value of 1 indicates that the model is fully capable of distinguishing between occupied and unoccupied sites. AUC values of 0.7–0.9 indicate very good discrimination, while values >0.9 indicate excellent discrimination. Final models were evaluated by comparing predictions in relation to the observations with Spearman’s rank correlation test (rs) corrected for spatial autocorrelation and implemented in SAM software66, 67. The predictive power of each final model was qualitatively assessed using a range of diagnostic plots60. All modeling was carried out using the mgcv library in R v.3.2.0 software68, 69.
Model Mapping
Maps of species predictions were constructed within the raster and rgdal libraries in R70 and visualized in ArcGIS software (version 10.3). The model error, defined as the absolute difference between observed and predicted species abundance, was also used to check and illustrate model fit. The spatial distribution of the model error was scaled between 0 and 1 (with a value of 1 corresponding to the maximum possible prediction error) and mapped by interpolation using the Inverse Distance Weighting (IDW) algorithm (in ArcGIS software). This method allows predicting a value for any unmeasured location as it uses the measured values surrounding the prediction location. IDW interpolation assumes that the measured values closest to the prediction location have more influence on the predicted value than those farther away and each measured point has a local influence that diminishes with distance. It gives greater weights to points closest to the prediction location, and the weights diminish as a function of distance.
Results
The overall percentage of occurrence for F. quadrangularis and I. elongata was respectively 0.24% and 0.14%. Covariates were not collinear (Variance Inflation Factor <2), models were developed for both species and the results of the best models are summarized in Tables 3 and 4. The relative importance of predictor variables for each species is presented in Table 5. All binomial models passed the sensitivity test suggesting that models had very good discriminating ability (Tables 3 and 4). Depth was found to be an important predictor of presence in both octocoral species habitat models (binomial models only; Tables 3 and 5; Figs 3 and 4). The effect of terrain variables was not particularly pronounced for the habitat suitability of I. elongata (rugosity only found in the binomial model, Fig. 4; Tables 4 and 5), while these were important predictors for F. quadrangularis (Tables 3 and 5; Fig. 3). Aspect North-South was also an important predictor for the I. elongata habitat model, while for F. quadrangularis it was found in the positive model only (Table 5; Figs 3 and 4). Conversely, aspect East-West was not significant for any of the coral species habitat models (Tables 3 and 4), still this variable had a modest relative importance for F. quadrangularis positive models (Table 5). Our results suggest that the habitat selection of F. quadrangularis and I. elongata is related to both directions of currents (I. elongata binomial model only; Tables 4 and 5). Similarly, SBT and SBS and fishing effort were found to be important environmental factors for both species (binomials and positive models; Tables 3 and 5). Model behaviour, showing relationships between species probability of presence/abundance and environmental and fisheries variables, is presented in Figs 3 and 4. Findings by species are given below.
Table 3.
Best supported and competing models (using binomial and positive models) for Funiculina quadrangularis.
| Model | Depth | Slope | Rugosity | AspectNS | AspectEW | CurrentNS | CurrentWE | SBT | SBS | Fishing effort | ΔAIC | AICw | Adj-R2 | Dev % | rs | ROC AUC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Best model | Binomial | ##### | ##### | ##### | ##### | ##### | 0 | 0.08 | 0.16 | 15.6 | 0.29 *** | 0.77 | |||||
| Positive | ##### | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 0 | 0.07 | 0.61 | 64.9 | |||||
| Competing m1 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | 0.5 | 0.06 | 0.15 | 15.6 | — | — | ||||
| Competing m2 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | 1.13 | 0.04 | 0.15 | 15.5 | — | — | ||||
| Competing m3 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 1.39 | 0.03 | 0.15 | 15.5 | — | — | |||
| Competing m4 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | 1.78 | 0.03 | 0.15 | 15.5 | — | — | ||||
| Competing m5 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 1.89 | 0.03 | 0.15 | 16.2 | — | — | |||
| Competing m6 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | 1.93 | 0.03 | 0.15 | 15.5 | — | — | ||||
| Competing m1 | Positive | ##### | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 0.96 | 0.04 | 0.60 | 63.3 | — | — | ||
| Competing m2 | Positive | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 1.25 | 0.03 | 0.59 | 61.9 | — | — | |||
| Competing m3 | Positive | ##### | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 1.25 | 0.03 | 0.59 | 62.2 | — | — | ||
| Competing m4 | Positive | ##### | ##### | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 1.45 | 0.02 | 0.60 | 63.9 | — | — | |
| Competing m5 | Positive | ##### | ##### | ##### | ##### | ##### | ##### | 1.7 | 0.02 | 0.58 | 61.4 | — | — |
Variables included in model are indicated with the symbol #. Predictors include depth, slope, rugosity, aspectNS, aspectEW, CurrentNS, CurrentWE, sea bottom temperature (SBT), sea bottom salinity (SBS) and fishing effort. ΔAIC: delta AIC (difference in AIC between the best model and current model); AICw: Akaike’s Information Criteria (corrected) weights, values range from 0 to 1, and high values indicate strong support for a given predictor. Models were evaluated by R2-adjusted coefficient and deviance (Dev): percentage of deviance explained. Only for the binomial model the Receiver Operating Characteristic (ROC) and Area Under the Curve (AUC) were calculated. Significance value of the Spearman’s correlation coefficient (rs) (corrected for spatial autocorrelation) for the delta model is given as ***p value < 0.001, **p value < 0.01, *p value < 0.05.
Table 4.
Best supported and competing models (using binomial and positive models) for Isidella elongata.
| Model | Depth | Slope | Rugosity | AspectNS | AspectEW | CurrentNS | CurrentWE | SBT | SBS | Fishing effort | AICw | ΔAIC | Adj-R2 | Dev % | rs | ROC AUC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Best model | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 0.13 | 0 | 0.29 | 35.3 | 0.18 | 0.89 | |||
| Positive | ##### | ##### | ##### | ##### | 0.07 | 0 | 0.25 | 29.9 | |||||||||
| Competing m1 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | 0.12 | 0.20 | 0.28 | 34.7 | — | — | ||||
| Competing m2 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 0.11 | 0.30 | 0.29 | 35.0 | — | — | ||
| Competing m3 | Binomial | ##### | ##### | ##### | ##### | ##### | ##### | ##### | 0.10 | 0.45 | 0.28 | 35.2 | — | — | |||
| Competing m4 | Binomial | #### | ##### | ##### | ##### | ##### | ##### | ##### | 0.07 | 1.21 | 0.27 | 34.2 | — | — | |||
| Competing m1 | Positive | ##### | ##### | ##### | ##### | ##### | 0.04 | 1.33 | 0.26 | 30.6 | — | — | |||||
| Competing m2 | Positive | ##### | ##### | ##### | ##### | 0.03 | 1.55 | 0.23 | 27.6 | — | — |
Variables included in model are indicated with the symbol #. Predictors include depth, slope, rugosity, aspectNS, aspectEW, CurrentNS, CurrentWE, sea bottom temperature (SBT), sea bottom salinity (SBS) and fishing effort. ΔAIC: delta AIC (difference in AIC between the best model and current model); AICw: Akaike’s Information Criteria (corrected) weights, values range from 0 to 1, and high values indicate strong support for a given predictor. Models were evaluated by R2-adjusted coefficient and deviance (Dev): percentage of deviance explained. Only for the binomial model the Receiver Operating Characteristic (ROC) and Area Under the Curve (AUC) were calculated. Significance value of the Spearman’s correlation coefficient (rs) (corrected for spatial autocorrelation) for the delta model is given as ***p value < 0.001, **p value < 0.01, *p value < 0.05.
Table 5.
Relative importance of predictor variables (calculated as the sum AIC weights from models that contain that variable within the 95% confidence interval) for Funiculina quadrangularis and Isidella elongata.
| Species | Model | Depth | Slope | Rugosity | AspectNS | AspectEW | CurrentNS | CurrentWE | SBT | SBS | Fishing effort | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Funiculina quadrangularis | Binomial | Importance | 1.00 | 1.00 | 0.28 | 0.29 | 0.50 | 0.47 | 0.83 | 0.29 | 0.91 | 0.90 |
| N containing models | 113 | 111 | 43 | 48 | 59 | 64 | 75 | 50 | 81 | 92 | ||
| Positive | Importance | 0.59 | 0.69 | 0.84 | 0.66 | 0.66 | 1.00 | 0.48 | 0.90 | 0.68 | 0.94 | |
| N containing models | 121 | 83 | 117 | 78 | 106 | 156 | 72 | 111 | 89 | 115 | ||
| Isidella elongata | Binomial | Importance | 1.00 | 0.27 | 0.83 | 0.72 | 0.46 | 0.99 | 0.65 | 1.00 | 0.91 | 0.15 |
| N containing models | 61 | 24 | 39 | 34 | 31 | 57 | 39 | 61 | 43 | 23 | ||
| Positive | Importance | 0.36 | 0.34 | 0.35 | 0.99 | 0.28 | 0.37 | 0.28 | 0.77 | 0.99 | 0.70 | |
| N containing models | 109 | 109 | 102 | 229 | 97 | 108 | 96 | 140 | 232 | 124 |
Figure 3.
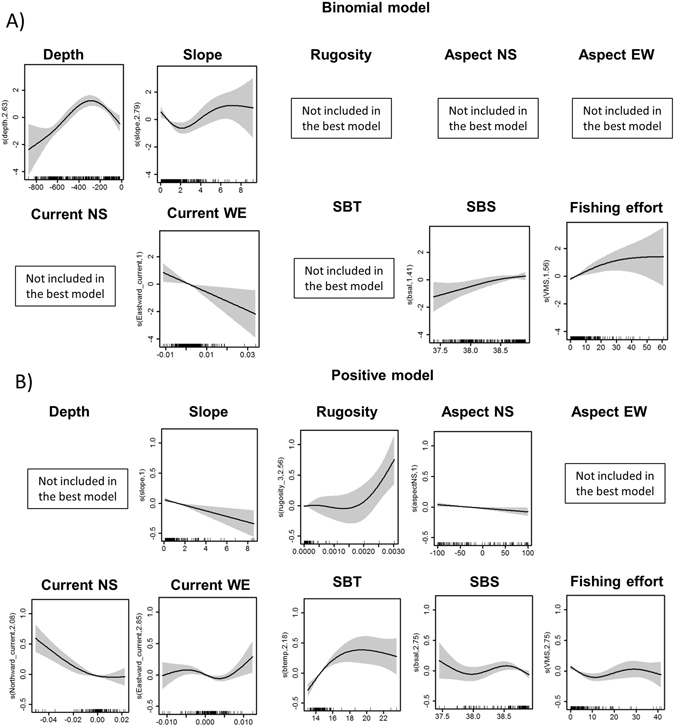
Partial GAM plots for the best binomial and positive models for Funiculina quadrangularis. Each plot represents the response variable shape, independent of the other variables, in relation to the probability of the species occurrence (A) and abundance (B) in the multivariate model. The ranges of environmental variables are represented on the x-axis and the probability of occurrence of the species is represented on the y-axis (logit scale). The zero value indicates mean model estimates, while the y-axis is a relative scale where the effect of different values of the predictors on the response variable is shown. The degree of smoothing is indicated in the y-axis label. Confidence intervals (95%) around the response curve are shaded in grey.
Figure 4.
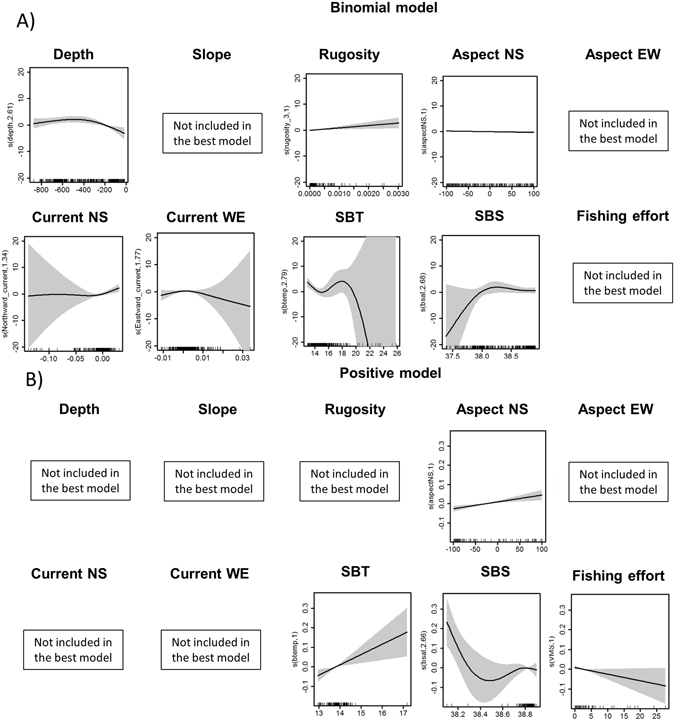
Partial GAM plots for the best binomial and positive models for Isidella elongata. Each plot represents the response variable shape, independent of the other variables, in relation to the probability of the species occurrence (A) and abundance (B) in the multivariate model. The ranges of environmental variables are represented on the x-axis and the probability of occurrence of the species is represented on the y-axis (logit scale). The zero value indicates mean model estimates, while the y-axis is a relative scale where the effect of different values of the predictors on the response variable is shown. The degree of smoothing is indicated in the y-axis label. Confidence intervals (95%) around the response curve are shaded in grey.
Funiculina quadrangularis
This species has a wide bathymetric range in our study area but it is preferentially associated with a depth range of 200–400 m (binomial model only; Tables 3 and 5; Fig. 3). The shape of the smoother of slope shows a linear negative relationship in the positive model, while this negative effect is not marked in the binomial model (non-linear relationship; Tables 3 and 5; Fig. 3). A positive non-linear relationship is found with rugosity (positive model only), however the probability of abundance of F. quadrangularis is mainly associated with lower values of this terrain variable (Fig. 3). The habitat preference of this species seems to be negatively correlated with North-South aspect and North-South current direction (linear relationship for aspect and non-linear for current in the positive model), while a negative relationship is shown for a West-East current direction (linear in the binomial model and non-linear in the positive; Fig. 3). Our results suggest that this species prefers bottom temperatures of 14–16 °C (positive model only) and bottom salinity values of around 38.5 PSU (Fig. 3). The effect of fishing effort was not consistent between the two models, in particular a positive non-linear relationship was found in the binomial model, while a negative non-linear relationship with fishing effort was found in the positive model suggesting that higher abundances are associated with low values of fishing activity (between 0 and 10 hours/year; Fig. 3).
Isidella elongata
This species is found at depths between 200–800 m, with higher presence above 400 m depth (binomial model only; Tables 4 and 5; Fig. 4). The shape of the smoothers for rugosity (binomial model only) and North-South aspect (positive model only) suggests that the probability of presence and abundance of this species increases with higher values of these terrain variables (Table 5; Fig. 4). The bamboo coral seems to prefer areas characterised by greater current (binomial model only, North-South direction), while it reaches its optimum in correspondence of low speed of the West-East current (Fig. 4). Our results suggest that this species is associated with habitats where sea bottom temperature values range is 13–14 °C and bottom salinity values are around 38.7–38.9 PSU (Fig. 4). A negative linear relationship is shown with fishing effort, suggesting a rapid decline of I. elongata abundance when fishing activity increases (Table 5; Fig. 4).
Species distribution maps
The maps of model predictions of F. quadrangularis and I. elongata showed species-specific distribution patterns in response to diverse habitat requirements and are presented in Fig. 5. These revealed that F. quadrangularis prefers the shallow waters of the continental shelf and coastal areas (Fig. 5A), mostly corresponding to the Adventure bank and south-eastern coast of Sicily (Fig. 1). In contrast I. elongata seems to prefer deeper waters (Fig. 5B). The distribution patterns of these two octocorals species is rather different (Fig. 5) probably because their sensitivity to impact of fishing activities is diverse (see Fig. 2J and Figs 3 and 4). Model errors were calculated as the absolute difference between observed and predicted species abundance and mapped for the study area. In general, higher model uncertainty corresponded to areas of higher predictions (zones where species were caught regularly; Fig. 5).
Figure 5.

(A) Funiculina quadrangularis and (B) Isidella elongata in the Strait of Sicily. Predicted population densities from delta model (Nkm−2; top figures) representing preferential habitat, and associated prediction error (down Figures; 0 and 1 correspond to the minimum and maximum possible errors, respectively). These maps were created with ArcGIS version 10.3 http://www.esriitalia.it by Valentina Lauria.
Discussion
This study enhances our understanding of the habitat requirements of and fishing impacts on two critically endangered deep-sea octocorals and provides the basis for the conservation of VMEs in the central Mediterranean Sea. Depth was an important factor influencing the habitat preferences of both octocoral species in the Strait of Sicily (Tables 3 and 5). The probability of presence of F. quadrangularis was higher at depths between 200–400 m, despite this species having a wider distribution range (20–2000 m; Table 1; Fig. 3). For I. elongata the probability of abundance was higher above 400 m depth (although this species favors depths between 200–800 m; Fig. 4). Our results are probably a regional characteristic of the Strait of Sicily, as generally both species can be found in deeper waters (Table 1). However, these results are partially limited by the fact that the MEDITS survey only reaches a maximum depth of 800 m, so it is difficult to know whether these species could be found at greater depths in this area. Our findings confirm other studies that have shown different depth preferences at a regional scale, for example in some parts of the Mediterranean Sea (Gulf of Lion) F. quadrangularis has been observed at an average depth of 239 m22, while in the North Atlantic it can be found at a depth range of 100–1200 m20, 45. Similarly I. elongata has been observed at different depths ranging from 680–750 m in the central and western Mediterranean Sea71, 72.
In the Strait of Sicily F. quadrangularis prefers areas identified by flat terrain that usually are characterised by soft bottoms (probability of abundance associated with low values of slope and rugosity; Tables 3 and 5; Fig. 3B), while the effect of these terrain variables was less marked on the habitat selection of I. elongata (Tables 3 and 5; Fig. 4B). This suggests that flat terrain soft-bottoms are not a limiting factor for I. elongata habitat selection. These results confirm the strong affinity previously observed between the distribution of pennatulaceans and fine sediments such as silt and clay20, 73. Isidella elongata appears to be mainly associated with compact slope muds74 even though it is also found on the sloping seafloor of marine canyons; probably because these areas cannot be reached by bottom-trawling and represent a refuge area for this vulnerable species22.
In our study the spatial distribution of F. quadrangularis seems to be negatively correlated to the seabed orientation (North-South direction) and currents while these relationships were positive for I. elongata (Figs 3 and 4; Table 5). Other studies on the spatial distribution of deep-sea corals have suggested the importance of seabed orientation and its exposure to currents50, 53, 55. In particular, deep-sea corals mounds were found to be more abundant in the central Mediterranean Sea associated with higher currents and consequently an increased nutrient supply53. In the Strait of Sicily I. elongata prefers substrata characterised by stronger currents (North-South direction; Fig. 4) probably due to local enrichment of food availability and low sediment rates72. In contrast, the spatial distribution of F. quadrangularis is associated with less exposed areas, as this species seems to prefer more stable environmental conditions73, 74. The possibility that the effect of currents on these species distribution patterns might vary locally cannot be ruled out; however, because of the broader resolution of the data used in this study (6.5 km) this relationship might not be fully explained in our model results.
The relationship with sea bottom temperature identified a species-specific optimum temperature range for the two octocorals in the Strait of Sicily. The probability of increased abundance of F. quadrangularis is higher at temperatures between 14–16 °C, while I. elongata seems to prefer temperatures between 13–14 °C (Figs 3 and 4; Table 5). In contrast, bottom salinity in the Strait of Sicily did not seem to constrain the spatial distribution of both species, despite an optimum range (38–38.5 PSU) being identified (Figs 3 and 4; Table 5). This specific preference for certain temperature and salinity ranges has been observed for other deep-sea coral species (such as L. pertusa and other sea pens in the North Atlantic33, 46, 75), which may be interpreted as a regional characteristic of the central Mediterranean Sea. Nonetheless, inference on the occurrence of local-scale effects in the study area is not allowed by current data limitation (resolution of 6.5 km), and further investigation at finer spatial resolution will be required.
Despite both F. quadrangularis and I. elongata are associated to fishing grounds of commercially valuable crustaceans (i.e. P. longirostris, N. norvegicus, A. antennatus and A. foliacea 20–22) the effect of fisheries on their probability of presence/abundance is quite different (Figs 3 and 4). In particular our results and predictive maps (Figs 2J and 5) suggest that while F. quadrangularis can be found in areas that are exploited by fisheries (positive curvilinear relationship between the probability of presence of F. quadrangularis and fishing effort; Fig. 3A), I. elongata is only present in areas where the fishing effort is low or absent (negative relationship between the probability of abundance and fishing effort). These species-specific responses to fishing impacts could be explained by their different body shapes (F. quadrangularis is pen-shaped while I. elongata is fan-shaped) and their structures for anchoring to the substrata. Funiculina quadrangularis attaches itself to the seafloor by using a bulb or peduncle that penetrates the sediment down to about 50 cm providing flexibility to the colony that can temporarily lie flat under the passage of fishing gear. Conversely I. elongata has a rooted holdfast embedded in the surface sediment and, due to its branched fan shape, a lower flexibility and a higher risk of being snagged by fishing gears (Table 1). Our results are supported by other findings where, contrary to expectations, sea pens were able to re-establish themselves in the sediments after fishing activities76–78, while the spatial distribution of I. elongata was strongly negatively correlated with the presence of bottom trawling suggesting that the optimal habitat of this species in the Mediterranean Sea has been significantly reduced72, 79.
Conclusions
This study has important implications for the conservation of soft- and compact-mud VMEs in the central Mediterranean Sea as it represents the first attempt to identify key areas (Fig. 5). This enclosed basin has a unique marine fauna that includes several endemic species of deep-sea corals80. While some form of fisheries regulation has been proposed by the European Commission81 for these important deep-water ecosystems, its application is still ineffective in the Mediterranean, leaving the majority of its VMEs unprotected.
Deep-sea ecosystems are under-represented in the Marine Protected Areas of the Mediterranean Sea as the majority of these areas are coastal82. Following the recommendations of the GFCM (2006) for the establishment of fisheries-restricted areas and the protection of VMEs, some guidelines have been applied in the Mediterranean Sea26. However, they are only effective for certain species and regions (i.e. Lophelia Reefs of Santa Maria di Leuca; the area of cold hydrocarbon seeps off the Nile Delta; and the Eratosthenes Seamount). Another issue for the conservation of VMEs in the Mediterranean Sea is that the ban on trawling is only effective for species that are present below depths of 1000 m, leaving ecologically important deep-sea VMEs occurring at shallower depths unprotected. This includes the coral gardens formed by I. elongata, F. quadrangularis and other habitat-forming organisms such as crinoids and brachiopods83. The majority of the trawl fisheries in the Mediterranean deep waters targets highly prized crustaceans, such as rose and red shrimps and Norway lobsters, with significant impacts on non-target species, such as deep-sea corals. So far, in the Mediterranean Sea no specific measures have been taken to detect or map VMEs or to monitor the impacts caused by bottom fisheries83. Our results could be useful in implementing spatial conservation plans, informing the GFCM’s deep-sea fisheries, and protecting critically endangered deep-sea octocoral species, as well as their related VMEs, in the Mediterranean Sea. Further investigations into the habitat suitability for and spatial distribution of Mediterranean deep-sea corals will constitute a key requisite for informing policy makers and guaranteeing that fishing activities are compatible with conservation plans and objectives.
Electronic supplementary material
Acknowledgements
This work was funded by the European Commission under the program “Scientific evidence to inform management plans for fisheries resource in the context of Common Fishery Policy, environmental and economic policies – MIPAAF”. The contribution of SP was supported by CERES (Climate Change and European Aquatic Resources, grant n. 678193, Horizon 2020 programme). We thank all the technical staff of CNR-IAMC UOS of Mazara del Vallo (Italy) involved in data collection and processing. We also thank Dr. Kate de la Haye for her valuable comments.
Author Contributions
V.L., G.G. and M.G. conceived the ideas and designed the methodology; V.L., D.M., G.M. and T.R. collected the data; V.L. analysed the data; V.L. and M.G. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08386-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Piraino S, Fanelli G, Boero F. Variability of species’ roles in marine communities: change of paradigms for conservation priorities. Mar. Biol. 2002;140:1067–1074. doi: 10.1007/s00227-001-0769-2. [DOI] [Google Scholar]
- 2.Bongiorni L, et al. Deep-water scleractinian corals promote higher biodiversity in deep-sea meiofaunal assemblages along continental margins. Biol. consrvation. 2010;143:1687–1700. doi: 10.1016/j.biocon.2010.04.009. [DOI] [Google Scholar]
- 3.Baillon S, Hamel JF, Wareham VE, Mercier A. Deep cold-water corals as nurseries for fish larvae. Front. Ecol. Environ. 2012;10:351–356. doi: 10.1890/120022. [DOI] [Google Scholar]
- 4.Andrews A, Stone RP, Lundstrom CC, Devogelaere AP. Growth rate and age determination of bamboo corals from the northeastern Pacific Ocean using refined 210Pb dating. Mar. Ecol. Prog. Ser. 2009;397:173–185. doi: 10.3354/meps08193. [DOI] [Google Scholar]
- 5.Andrews, A. et al. Investigations of age and growth for three deep-sea corals from the Davidson Seamount off central California. Cold-Water Corals Ecosyst. 1021–1038, doi:10.1007/3-540-27673-4_51 (2005).
- 6.Lacharité, M. & Metaxas, A. Early Life History of Deep-Water Gorgonian Corals May Limit Their Abundance. PLoS One8 (2013). [DOI] [PMC free article] [PubMed]
- 7.Tsounis G, et al. The Exploitation and Conservation of Precious Corals. Oceanogr. Mar. Biol. - an Annu. Rev. 2010;48:161–212. [Google Scholar]
- 8.Pham CK, et al. Deep-water longline fishing has reduced impact on Vulnerable Marine Ecosystems. Sci. Rep. 2014;4:4837. doi: 10.1038/srep04837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puig P, et al. Ploughing the deep sea floor. Nature. 2012;489:286–289. doi: 10.1038/nature11410. [DOI] [PubMed] [Google Scholar]
- 10.Maynou F, Cartes JE. Effects of trawling on fish and invertebrates from deep-sea coral facies of Isidella elongata in the western Mediterranean. J. Mar. Biol. Assoc. United Kingdom. 2012;92:1501–1507. doi: 10.1017/S0025315411001603. [DOI] [Google Scholar]
- 11.Clark MR, et al. The impacts of deep-sea fisheries on benthic communities: A review. ICES J. Mar. Sci. 2016;73:i51–i69. doi: 10.1093/icesjms/fsv123. [DOI] [Google Scholar]
- 12.Watson R, Morato T. Fishing down the deep: Accounting for within-species changes in depth of fishing. Fish. Res. 2013;140:63–65. doi: 10.1016/j.fishres.2012.12.004. [DOI] [Google Scholar]
- 13.Clark, M. R., Bowden, D. A., Baird, S. J. & Stewart, R. Effects of fishing on the benthic biodiversity of seamounts of the ‘Graveyard’ complex, northern Chatham Rise. (2010).
- 14.UNEP Deep-Sea Biodiversity and Ecosystems: A scoping report on their socio-economy, management and governance. (2007).
- 15.OSPAR. ase Reports for the OSPAR List of Threatened and/or Declining Species and Habitats. Biological Diversity and Ecosystems. 2008 No. ISBN: 358 978-1-905859-97-. (2008).
- 16.OSPAR. OSPAR Recommendation 2010/8 on furthering the protection and restoration of Lophelia pertusa reefs in the OSPAR Maritime Area. (2010).
- 17.MSFD 2008/56/EC. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy.
- 18.FAO. International Guidelines for the Management of Deep-sea Fisheries in the High Seas 73 (2009).
- 19.Thompson, A., Sanders, J., Tandstad, M., Carocci, F. & Fuller, M. Vulnerable Marine Ecosystems: Processes and Practices in the High Seas. (2016).
- 20.Murillo FJ, Durán Muñoz P, Altuna A, Serrano A. Distribution of deep-water corals of the Flemish Cap, Flemish Pass, and the Grand Banks of Newfoundland (Northwest Atlantic Ocean): Interaction with fishing activities. ICES J. Mar. Sci. 2011;68:319–332. doi: 10.1093/icesjms/fsq071. [DOI] [Google Scholar]
- 21.Bellan-Santini, D., Bellan, G., Bittar, G., Harmelin, J. & Pergent, G. Handbook for interpreting types of marine habitat for the selection of sites to be included in the national inventories of natural sites of conservation interest. (2002).
- 22.Fabri M, et al. Megafauna of vulnerable marine ecosystems in French mediterranean submarine canyons: Spatial distribution and anthropogenic impacts. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014;104:184–207. doi: 10.1016/j.dsr2.2013.06.016. [DOI] [Google Scholar]
- 23.European Commission. Sensitive and essential fish habitats in the Mediterranean Sea (2006).
- 24.Cartes JE, Maynou F, Fanelli E, Papiol V, Lloris D. Long-term changes in the composition and diversity of deep-slope megabenthos and trophic webs off Catalonia (western Mediterranean): Are trends related to climatic oscillations? Prog. Oceanogr. 2009;82:32–46. doi: 10.1016/j.pocean.2009.03.003. [DOI] [Google Scholar]
- 25.Mastrototaro F, et al. Isidella elongata (Cnidaria: Alcyonacea) facies in the western Mediterranean Sea: visual surveys and descriptions of its ecological role. Eur. Zool. J. 2017;84:209–225. [Google Scholar]
- 26.FAO. The State of Mediterranean and Black Sea Fisheries. General Fisheries Commission for the Mediterranean (2016).
- 27.Schembri PJ, Dimech M, Camilleri M, Page R. Living deep-water Lophelia and Madrepora corals in Maltese waters (Strait of Sicily, Mediterranean Sea) Cah. Biol. Mar. 2007;48:77–83. [Google Scholar]
- 28.Freiwald A, Beuck L, Rüggeberg A, Taviani M, Hebbeln D. The White Coral Community in the Central Mediterranean Sea Revealed by ROV Surveys. Oceanography. 2009;22:58–74. doi: 10.5670/oceanog.2009.06. [DOI] [Google Scholar]
- 29.Salvati, E., Bo, M., Rondinini, C., Battistoni, A. & Teofili, C. Lista Rossa IUCN dei coralli Italiani. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare (2014).
- 30.Fortibuoni T, et al. Nursery and Spawning Areas of Deep-water Rose Shrimp, Parapenaeus longirostris (Decapoda: Penaeidae), in the Strait of Sicily (Central Mediterranean Sea) J. Crustac. Biol. 2010;30:167–174. doi: 10.1651/09-3167.1. [DOI] [Google Scholar]
- 31.Spanò N, Porporato E, Ragonese S. Spatial distribution of decapoda in the Strait of Sicily (central Mediterranean sea) based on a trawl survey. Crustaceana. 2013;86:139–157. doi: 10.1163/15685403-00003143. [DOI] [Google Scholar]
- 32.Davies, A. J. & Guinotte, J. M. Global habitat suitability for framework-forming cold-water corals. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 33.Davies AJ, Wisshak M, Orr JC, Murray Roberts J. Predicting suitable habitat for the cold-water coral Lophelia pertusa (Scleractinia) Deep. Res. Part I Oceanogr. Res. Pap. 2008;55:1048–1062. doi: 10.1016/j.dsr.2008.04.010. [DOI] [Google Scholar]
- 34.Guisan, A. et al. Predicting species distributions for conservation decisions. Ecol. Lett. 1424–1435, doi:10.1111/ele.12189 (2013). [DOI] [PMC free article] [PubMed]
- 35.Brown CJ, Smith SJ, Lawton P, Anderson JT. Benthic habitat mapping: A review of progress towards improved understanding of the spatial ecology of the seafloor using acoustic techniques. Estuar. Coast. Shelf Sci. 2011;92:502–520. doi: 10.1016/j.ecss.2011.02.007. [DOI] [Google Scholar]
- 36.Lauria V, Gristina M, Attrill MJ, Fiorentino F, Garofalo G. Predictive habitat suitability models to aid conservation of elasmobranch diversity in the central Mediterranean Sea. Sci. Rep. 2015;5:13245. doi: 10.1038/srep13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.GCFM. Resolution 31/2007/2: On the Establishment of Geographical Sub-Areas in the GFCM Area. at http://www.gfcm.org/figis/pdf/gfcm/topic/16162/en?title=GFCM-Geographical Sub-Areas (2007).
- 38.Agostini VN, Bakun A. Ocean Triads in the Mediterranean Sea: physical mechanisms potentially structuring reproductive habitat suitability (example application to European anchovy, Engraulis encrasicolus) Fish. Oceanogr. 2002;11:128–142. doi: 10.1046/j.1365-2419.2002.00201.x. [DOI] [Google Scholar]
- 39.Garofalo G, Fortibuoni T, Gristina M, Sinopoli M, Fiorentino F. Persistence and co-occurrence of demersal nurseries in the Strait of Sicily (central Mediterranean): Implications for fishery management. J. Sea Res. 2011;66:29–38. doi: 10.1016/j.seares.2011.04.008. [DOI] [Google Scholar]
- 40.Gristina M, et al. The role of juveniles in structuring demersal assemblages in trawled fishing grounds. Estuar. Coast. Shelf Sci. 2013;133:78–87. doi: 10.1016/j.ecss.2013.08.014. [DOI] [Google Scholar]
- 41.Ragonese S, Vitale S, Dimech M, Mazzola S. Abundances of demersal sharks and chimaera from 1994–2009 scientific surveys in the central Mediterranean Sea. PLoS One. 2013;8:e74865. doi: 10.1371/journal.pone.0074865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiorentino, F., Garofalo, G., Gristina, G., Gancitano, S. & Norrito, G. In Report of the Med Sud Med Expert Consultation on Spatial Distribution of Demersal Resources in the Straits of Sicily 50–66. At http://www.faomedsudmed.org/pdf/publications/TD2/TD2.pdf (2004).
- 43.Bertrand J, de Sola L, Papaconstantinou C, Relini G, Souplet A. The general specifications of the MEDITS surveys. Sci. Mar. 2002;66:9–17. doi: 10.3989/scimar.2002.66s2169. [DOI] [Google Scholar]
- 44.Anonymous. MEDITS-Handbook. Version n. 7, 2013, MEDITS Working Groups, pp 120 (2013).
- 45.Greathead C, et al. Environmental requirements for three sea pen species: relevance to distribution and conservation. ICES J. Mar. Sci. 2014;72:576–586. doi: 10.1093/icesjms/fsu129. [DOI] [Google Scholar]
- 46.Sbrocco, E. & Barber, P. MARSPEC: Ocean climate layers for marine spatial ecology. Ecology979, doi:10.1890/12-1358.1 (2013).
- 47.Wright, D. J. et al. ArcGIS Benthic Terrain Modeler (BTM), v. 3.0 (2012).
- 48.Pittman SJ, Christensen JD, Caldow C, Menza C, Monaco ME. Predictive mapping of fish species richness across shallow-water seascapes in the Caribbean. Ecol. Modell. 2007;204:9–21. doi: 10.1016/j.ecolmodel.2006.12.017. [DOI] [Google Scholar]
- 49.Pittman, S. J. & Brown, K. a. Multi-scale approach for predicting fish species distributions across coral reef seascapes. PLoS One6 (2011). [DOI] [PMC free article] [PubMed]
- 50.Tong R, Purser A, Unnithan V, Guinan J. Multivariate statistical analysis of distribution of deep-water gorgonian corals in relation to seabed topography on the norwegian margin. PLoS One. 2012;7:1–13. doi: 10.1371/journal.pone.0043534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson, M. F. J., O’Connell, B., Brown, C., Guinan, J. C. & Grehan, A. J. Multiscale Terrain Analysis of Multibeam Bathymetry Data for Habitat Mapping on the Continental Slope. Marine Geodesy 30 (2007).
- 52.Guinan J, Grehan A, Dolan M, Brown C. Quantifying relationships between video observations of cold water coral cover and seafloor features in Rockall Trough, west of Ireland. Mar. Ecol. Prog. Ser. 2009;375:125–138. doi: 10.3354/meps07739. [DOI] [Google Scholar]
- 53.Savini, A., Vertino, A., Marchese, F., Beuck, L. & Freiwald, A. Mapping cold-water coral habitats at different scales within the Northern Ionian Sea (central Mediterranean): An assessment of coral coverage and associated vulnerability. PLoS One9 (2014). [DOI] [PMC free article] [PubMed]
- 54.White, M., Mohn, C., Stigter, H. & Mottram, G. In (eds Freiwald, A. & Robert, J. m.) 503–514 (2005).
- 55.Davies AJ, et al. Downwelling and deep- water bottom currents as food supply mechanisms to the cold-water coral Lophelia pertusa (Scleractinia) at the Mingulay Reef complex. Limnol. Oceanogr. 2009;54:620–629. doi: 10.4319/lo.2009.54.2.0620. [DOI] [Google Scholar]
- 56.Guinotte J. Climate change and deep-sea corals. J. Mar. Educ. 2005;21:48–49. [Google Scholar]
- 57.Dullo WC, Flögel S, Rüggeberg A. Cold-water coral growth in relation to the hydrography of the Celtic and Nordic European continental margin. Mar. Ecol. Prog. Ser. 2008;371:165–176. doi: 10.3354/meps07623. [DOI] [Google Scholar]
- 58.Russo T, et al. SMART: a spatially explicit bio-economic model for assessing and managing demersal fisheries, with an application to italian trawlers in the strait of sicily. PLoS One. 2014;9:e86222. doi: 10.1371/journal.pone.0086222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo T, Parisi A, Cataudella S. New insights in interpolating fishing tracks from VMS data for different metiers. Fish. Res. 2011;108:184–194. doi: 10.1016/j.fishres.2010.12.020. [DOI] [Google Scholar]
- 60.Zuur, A. F., Ieno, E. N. & Smith, G. M. Analysing Ecological Data. (Springer-Verlag, 2007).
- 61.Barry SC, Welsh AH. Generalized additi v e modelling and zero inflated count data. Ecol. Modell. 2002;157:179–188. doi: 10.1016/S0304-3800(02)00194-1. [DOI] [Google Scholar]
- 62.Martin T, et al. Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol. Lett. 2005;8:1235–1246. doi: 10.1111/j.1461-0248.2005.00826.x. [DOI] [PubMed] [Google Scholar]
- 63.Hastie, T. & Tibshirani, R. Generalized Additive Models. (Chapman & Hall/CRC, 1990). At ISBN 978-0-412-34390-2.
- 64.Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference: A Practical Information-Theoretic Approach. (Springer-Verlag, 2002).
- 65.Elith J, Graham C, Anderson R, Dudık M, Ferrier S. Novel methods improve prediction of species’ distributions from occurrence data. Ecography (Cop.). 2006;29:129–151. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- 66.Dutilleul P. Modifying the t test for assessing the correlation between two spatial processeso. Biometrics. 1993;49:305–314. doi: 10.2307/2532625. [DOI] [Google Scholar]
- 67.Rangel T, Diniz-Filho J, Bini L. SAM: a comprehensive application for Spatial Analysis in Macroecology. Ecography (Cop.). 2010;33:46–50. doi: 10.1111/j.1600-0587.2009.06299.x. [DOI] [Google Scholar]
- 68.R Development Core Team. R: A language and environment for statistical computing. At http://www.r-project.org/ (2013).
- 69.Wood, S. N. Generalized Additive Models: an introduction with R. (2006).
- 70.Hijmans, R. J. Introduction to the’ raster’ package (version 2.1–25). 1–26 (2013).
- 71.Mytilineou C, Smith C, Anastasopoulou A, Papadopoulou K, Christidis G. New cold-water coral occurrences in the Eastern Ionian Sea: Results from experimental long line fishing. Deep. Res. Part I Oceanogr. Res. Pap. 2014;99:146–157. [Google Scholar]
- 72.Cartes JE, LoIacono C, Mamouridis V, López-Pérez C, Rodríguez P. Geomorphological, trophic and human influences on the bamboo coral Isidella elongata assemblages in the deep Mediterranean: To what extent does Isidella form habitat for fish and invertebrates? Deep. Res. Part I Oceanogr. Res. Pap. 2013;76:52–65. doi: 10.1016/j.dsr.2013.01.006. [DOI] [Google Scholar]
- 73.Buhl-Mortensen L, Mortensen PB, Armsworthy S, Jackson D. Field observations of Flabellum spp. and laboratory study of the behavior and respiration of Flabellum alabastrum. Bull. Mar. Sci. 2007;81:543–552. [Google Scholar]
- 74.Oceana. Montanas submarinas de las Islas Baleares: Canal de Mallorca 2011. Propuesta de proteccion para Ausıas March, Emile Baudot y Ses Olives (2011).
- 75.Greathead CF, Donnan DW, Mair JM, Saunders GR. The sea pens Virgularia mirabilis, Pennatula phosphorea and Funiculina quadrangularis: distribution and conservation issues in Scottish waters. J. Mar. Biol. Assoc. UK. 2007;87:1095–1103. doi: 10.1017/S0025315407056238. [DOI] [Google Scholar]
- 76.Pierdomenico M, Martorelli E, Dominguez-Carrió C, Gili JM, Chiocci FL. Seafloor characterization and benthic megafaunal distribution of an active submarine canyon and surrounding sectors: The case of Gioia Canyon (Southern Tyrrhenian Sea) J. Mar. Syst. 2016;157:101–117. doi: 10.1016/j.jmarsys.2016.01.005. [DOI] [Google Scholar]
- 77.Bryan T, Metaxas A. Distribution of deep-water corals along the North American continental margins: relation- ships with environmental factors. Deep Sea Res. I. 2006;53:1865–1879. doi: 10.1016/j.dsr.2006.09.006. [DOI] [Google Scholar]
- 78.Eno NC, et al. Effects of crustacean traps on benthic fauna. ICES J. Mar. Sci. 2001;58:11–20. doi: 10.1006/jmsc.2000.0984. [DOI] [Google Scholar]
- 79.D’Onghia G, Mastrototaro F, Matarrese A, Politou C, Mytilineou C. Biodiversity of the upper slope demersal community in the eastern Mediterranean: preliminary comparison between two areas with and without trawl fishing. J. Northwest. Atl. Fish Sci. 2003;31:263–273. [Google Scholar]
- 80.Danovaro, R. et al. Deep-sea biodiversity in the Mediterranean Sea: The known, the unknown, and the unknowable. PLoS One5 (2010). [DOI] [PMC free article] [PubMed]
- 81.Bo M, et al. Deep Coral Oases in the South Tyrrhenian Sea. PLoS One. 2012;7:e49870. doi: 10.1371/journal.pone.0049870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdulla, A., Gomei, M., Maison, E. & Piante, C. Status of Marine Protected Areas in the Mediterranean Sea (2008).
- 83.Rogers, A. D. & Gianni, M. The Implementation of UNGA Resolutions. 61/105 and 64/72 in the management of Deep-Sea fisheries on the high Seas. Report prepared for the deep-sea conservation coalition (2010).
- 84.Martinelli M, et al. Towed underwater television towards the quantification of Norway lobster, squat lobsters and sea pens in the Adriatic Sea. Acta Adriat. 2013;54:3–12. [Google Scholar]
- 85.Krieger KJ, Wing BL. Megafauna associations with deep water corals (primnoa spp.) in the Gulf of Alaska. Hydrobiologia. 2002;471:83–90. doi: 10.1023/A:1016597119297. [DOI] [Google Scholar]
- 86.Risk MJ, Heikoop JM, Snow MG, Beukens R. Lifespans and growth patterns of two deep-sea corals: Primnoa resedaeformis and Desmophyllum cristagalli. Hydrobiologia. 2002;471:125–131. doi: 10.1023/A:1016557405185. [DOI] [Google Scholar]
- 87.Roark EB, Guilderson TP, Dunbar RB, Fallon SJ, Mucciarone DA. Extreme longevity in proteinaceous deep-sea corals. Proc. Natl. Acad. Sci. USA. 2008;106:5204–5208. doi: 10.1073/pnas.0810875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maynou F, Sarda F, Tudela S, Demestre M. Management strategies for red shrimp (Aristeus antennatus) fisheries in the Catalan Sea (NW Mediterranean) based on bioeconomic simulation analysis. Aquat. Living Resour. 2006;19:161–171. doi: 10.1051/alr:2006014. [DOI] [Google Scholar]
- 89.Peres JM. The Mediterranean benthos. Oceanogr. Mar. Biol. - an Annu. Rev. 1967;5:449–533. [Google Scholar]
- 90.Bellan-Santini, D. in (ed. Moraitou- Apostolopoulou, M., Kiortsis, V.) 1019–1048 (1985).
- 91.Copernicus Marine Environment Monitoring Service. at http://marine.copernicus.eu/documents/QUID/CMEMS-MED-QUID-006-009.pdf.
- 92.European Commission. Council Regulation (EC) No. 2371/2002 of 20 December 2002 on the conservation and sustainable exploitation of fisheries resources under the Common Fisheries Policy (2002).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


