Abstract
Marine ecosystems are difficult to sample quantitatively at increasing depth. Hence, few studies attempt to measure patterns of beta diversity for ecological communities in the deep sea. Here we (i) present and quantify large-scale gradients in fish community structure along depth and latitude gradients of the New Zealand EEZ, (ii) obtain rigorous quantitative estimates of these depth (50–1200 m) and latitudinal effects (29.15–50.91°S) and their interaction, and (iii) explicitly model how latitudinal beta diversity of fishes varies with depth. The sampling design was highly structured, replicated and stratified for latitude and depth, using data obtained from 345 standardised baited remote underwater stereo-video deployments. Results showed that gradients in fish community structure along depth and latitude were strong and interactive in New Zealand waters; latitudinal variation in fish communities progressively decreased with depth following an exponential decay (r 2 = 0.96), revealing increasingly similar fish communities with increasing depth. In contrast, variation in fish community structure along the depth gradient was of a similar magnitude across all of the latitudes investigated here. We conclude that an exponential decay in beta diversity vs depth exists for fish communities present in areas shallower than the New Zealand upper continental slope.
Introduction
Understanding spatial patterns of biodiversity along large-scale global gradients, such as latitude, altitude and depth, is a primary goal in ecology1–3. Such knowledge is key to the development of principles for sound environmental management4. In the terrestrial environment, latitudinal and altitudinal biodiversity gradients are well-characterised5, 6, but there are far fewer ecological studies quantifying patterns of marine biodiversity vs depth, due to logistic difficulties associated with sampling and observing marine systems, especially at increasing depths7. Most marine studies of fish diversity have been limited to narrow geographic8 or depth ranges9, or rely on museum or web-based records of species distributions that are incomplete10, 11. Some attempts at synthesizing patterns of species richness and zonation in the deep sea have been made7, 11–13, but studies of global-scale patterns of beta diversity in the deep sea are absent for the megafauna and rare for other taxa14. Exploratory field-based studies at larger scales, especially for the deep sea, tend to have sampling designs that are opportunistic or unstructured (where depth, latitude and longitude are measured as covariates)15–18 or have incommensurable sampling units (e.g., trawls of varying duration or of varying types)13, 16–20. Consequently, most studies are not directly amenable to drawing broader scientific inferences without certain caveats or the use of additional modelling or standardization techniques to account for such issues21, 22.
Early ecological studies on land suggested that observed changes in community structure with increasing latitude (i.e., latitudinal beta diversity) mirror changes seen with increases in elevation; hence generating interactions in their effects on biodiversity23, 24. As early as 1956, the hypothesis that faunal communities are wide-spread and broadly connected with large-scale patterns of oceanic circulation was put forward25. This idea was further supported by distributional studies of North Atlantic26 and southeastern Australian fishes27. Later, studies in the ocean also showed possible interactive effects of latitude and depth on fish communities; more specifically, assemblages become more similar to one another with increasing depth, reducing differences in fish communities observed along latitudinal gradients. For example, increased similarity in megafaunal assemblages with depth was reported from New England and Cape Hatteras28, 29, and similar patterns were more recently depicted for fishes21, 30–32.
Here, we describe results from a large-scale study of the biodiversity of fishes in New Zealand waters having a sampling design that was carefully structured with respect to two factors: latitude (7 locations spanning 29.15–50.91°S) and depth (7 strata spanning 50–1200 m). Spatial stratification, replication and the use of a standardized size of sampling unit ensured the validity of the study design for drawing rigorous quantitative inferences. The essential aims of our study were: (1) to partition the variation in fish community structure (beta diversity) according to latitude, depth and their interaction; (2) to rigorously estimate latitudinal beta diversity separately for each depth stratum, with estimates of its variability; and (3) to model explicitly how latitudinal beta diversity of fishes varies with depth. This study presents patterns on beta diversity resulting from the interaction between latitude and depth, while most studies in the past have focussed on presenting changes in species identities along the depth gradient only, without reference to any horizontal spatial component7, 14.
Results
From 345 video deployments (Fig. 1), a total of 242 fish taxa belonging to 166 genera in 84 families and 25 orders were identified (Supplementary Tables S1 and S2, and Supporting Video S3; see also Supplementary Figure S4 for species accumulation curves per depth stratum). The composition of fish assemblages was strongly structured by depth (i.e., from shallow to deep along nMDS axis 1, Fig. 2) and by latitude (i.e., from south to north along nMDS axis 2, Fig. 2). There was also a clear and highly statistically significant interaction in fish community structure between latitude and depth (Fig. 2 and Table 1). Fish communities became progressively more similar to one another with increasing depth, as indicated by an increased clustering of points associated with the different locations for deeper strata, especially at 900 m and 1200 m (Fig. 2). The largest component of variation was that associated with the residual (42.2% of the total), followed by the depth-by-location interaction (24%) and the main effects of depth (17.2%) and location (12.4%), respectively, while individual transects (variation among sites within the same location) accounted for comparatively little (3.8%, Table 1).
Figure 1.
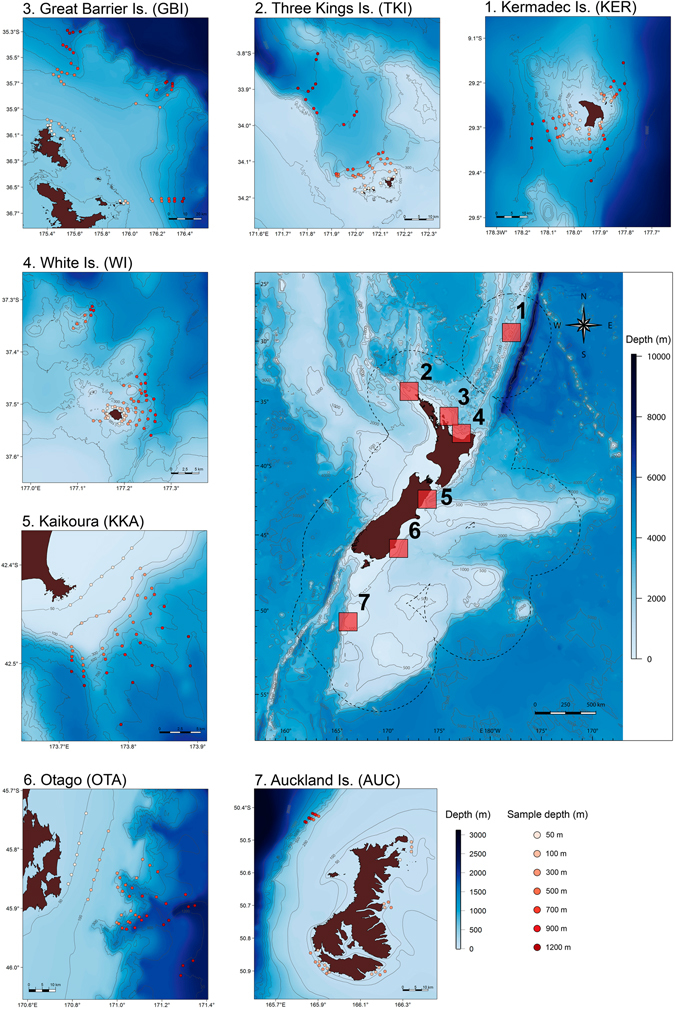
Latitudinal beta diversity sampling sites. Fishes were sampled at multiple depths (50, 100, 300, 500, 700, 900 and 1200 m) using baited remote underwater stereo-video (stereo-BRUVs) deployments at each of seven locations: Kermadec Islands, Three Kings Islands, Great Barrier Island, White Island, Kaikoura, Otago and the Auckland Islands. Map created using R85. Bathymetry source: Depth contour polyline (Hydro, 1:350 k–1:1,500 k), Land Information New Zealand, Crown Copyright Reserved. Land data source: NZ Coastlines (Topo, 1:50 k), Land Information New Zealand, Crown Copyright Reserved.
Figure 2.
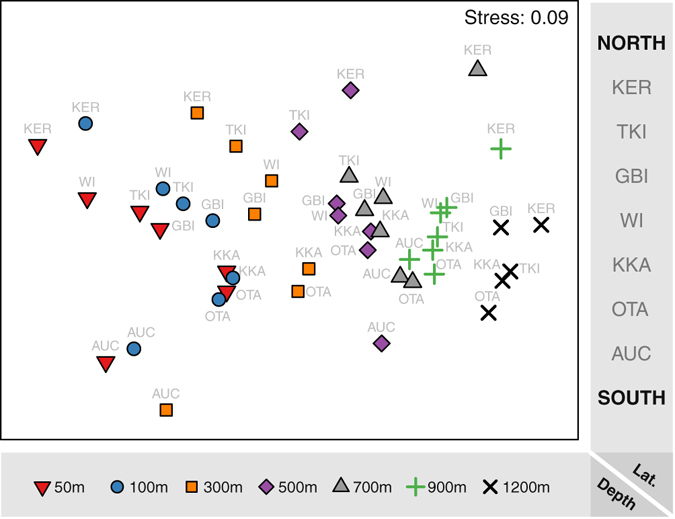
Ordination analysis of fish communities by depth and latitude. Non-metric multi-dimensional scaling (nMDS) ordination plot of Jaccard dissimilarities among fish assemblages consisting of depth-by-location averages (centroids) from a minimum of n = 6 video deployments (except for the Auckland Islands and White Island where no deployments were made at 1200 m). Each centroid is identified by a symbol to indicate the depth (50, 100, 300, 500, 700, 900 or 1200 m) and a label to indicate the location (from north to south: Kermadec Islands (KER), Three Kings Islands (TKI), Great Barrier Island (GBI), White Island (WI), Kaikoura (KKA), Otago (OTA) and the Auckland Islands (AUC)).
Table 1.
PERMANOVA partitioning of fish assemblages on the basis of Jaccard dissimilarities in response to Location (Lo, fixed, 7 levels), Depth (De, fixed, 7 levels) and Transects (Tr, random, nested within Lo) using Type I (sequential) sums of squares.
| Source | df | MS | Pseudo-F | P | Sqrt(Component of variation) | % |
|---|---|---|---|---|---|---|
| Lo | 6 | 34947 | 8.486 | 0.0001 | 25.5 | 12.4 |
| De | 6 | 45295 | 20.235 | 0.0001 | 30.0 | 17.2 |
| Tr(Lo) | 70 | 3068 | 1.386 | 0.0001 | 14.2 | 3.8 |
| Lo × De | 33 | 10806 | 4.882 | 0.0001 | 35.8 | 24.4 |
| Residual | 225 | 2200 | 47.0 | 42.2 | ||
| Total | 340 |
P-values were obtained for each term in the model using 9999 permutations under a reduced model. Estimated sizes of effects for each term in the model are shown in Jaccard units as the square-root of the pseudo multivariate component of variation. Variation attributable to each term in the model is also expressed as a percentage of the total (%). Note that 4 of the original 345 video deployments were omitted prior to analysis, as there were no fish recorded in them.
The source of the significant depth-by-location interaction arises from a decrease in latitudinal variation with increasing depth, from 50 to 1200 m (Fig. 3a). Although this relationship could be modelled with a simple linear regression (r 2 = 0.97), a more appropriate (and highly statistically significant) ecological model is an exponential decay (r 2 = 0.96, P = 0.0001, Fig. 3a); variation in the structure of fish assemblages is not expected to reach an absolute value of zero and then to become negative, as a linear model would imply. In contrast, there was no clear relationship between the estimated component of variation for depth vs latitude (r 2 = 0.12, P = 0.445, Fig. 3b). Thus, variation in fish community structure along the depth gradient was of a similar magnitude across all locations. The estimated component of variation for the residuals (i.e., variation among individual deployments within a given combination of depth-by-location) showed no consistent relationship with either depth or location (P = 0.461 and P = 0.168, respectively).
Figure 3.
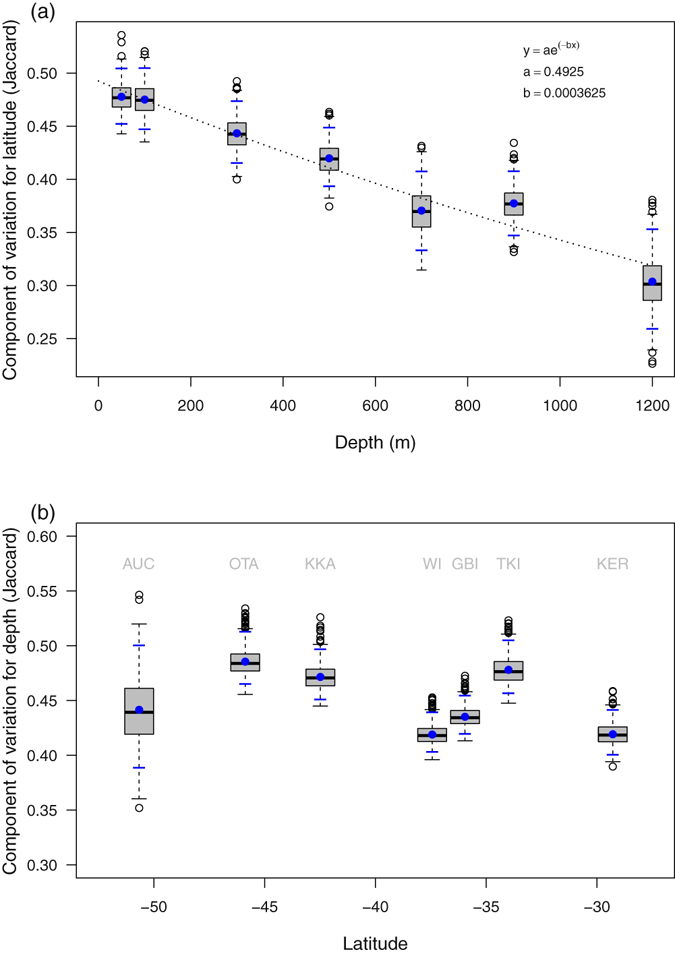
Large-scale beta diversity of fishes versus depth and latitude. Relationship between (a) component of variation within each depth stratum (a surrogate for latitudinal beta diversity) vs depth; and (b) component of variation within each location (vertical beta diversity) vs latitude, based on square-root-transformed pseudo multivariate components of variation calculated from a PERMANOVA partitioning of Jaccard dissimilarities (blue dots). Box plots in grey show distributions of values from 1000 bias-corrected separate-sample bootstraps of residuals. Blue dashes show 95% confidence intervals based on the 0.025 and 0.975 quantiles of the bias-corrected bootstrap distributions. The dotted line in (a) shows the exponential model of the decay in latitudinal beta diversity vs depth.
Furthermore, the exponential decay in latitudinal beta diversity versus depth appeared not to be driven by patterns in either mean alpha or gamma diversity alone, whose patterns along the depth gradient each varied in different ways at different locations (see Supplementary Figure S5).
Discussion
Here we have provided the first direct quantitative model of latitudinal beta diversity at large spatial scales in the ocean along a depth gradient, calculated rigorously from a carefully stratified, standardized replicated sampling design. Previous studies based on observational data were not sufficiently standardized to allow direct explicit quantification of these effects for modelling purposes21, 30, 31.
Most previous studies of beta diversity vs depth have focused specifically on how species’ identities, abundance or biomass changes from one depth stratum to another, particularly to identify patterns of zonation12, 14, 18, 33–35, or to quantify overall turnover vs depth18, 21, 32. Our study provides the first attempt to rigorously model the general increase in similarity of fish assemblages with increasing depth at broad spatial scales (i.e, along a large latitudinal span, not just along a depth gradient). This has also been called horizontal beta diversity14.
The parsimonious model we propose indicates strongly that the effects of latitude diminish exponentially with depth. This model of exponential decay might hold more generally for other marine taxa, such as benthic megafauna28, 36, molluscs37 or asteroids38, which all show a general pattern of increased similarity in large-scale comparisons with increasing depth based on unstructured observational data. More recently, a distance decay relationship between depth and Bray-Curtis similarities between samples of molluscs was found in the Atlantic Ocean14, based on previously published data37. Habitats in deep environments are more homogeneous than those in shallower environments39, 40. They are also geologically more stable, cover a greater geographic area and are biogeographically more connected than are habitats in shallower systems41, 42. These patterns contrast strongly with those found for terrestrial habitats at high elevations, which are environmentally highly variable, are smaller in area, less geologically stable and are geographically fragmented compared to low-elevation habitats43, 44. Hence, observed decreases in beta diversity vs depth in the ocean will require different mechanistic explanations to those that may be advanced to explain decreasing beta diversity vs elevation in terrestrial studies.
Our observations are consistent with the hypothesis that circulation of large-scale water masses influences species’ distributions26, 27. This hypothesis is based on the broad extent of intermediate and deep water masses45 which mirrors the large biogeographic ranges of many deep-sea species. Five waters masses are important around New Zealand46: the surface subtropical and Subantarctic Waters, the Antarctic Intermediate Water, the Deep Water and the Antarctic Bottom Water. The two surface water masses meet to form a front called the Subtropical Convergence which approximately follows the 15 °C surface isotherm in summer and the 10 °C surface isotherm in winter and the 34.7–34.8 × 10−3 surface salinity isopleth47. These two water masses have very different origins and characteristics (high vs low salinity). The complex front created by their collision creates an increased diversity of potential niches for species. In addition, the Subantarctic Front, which separates Australasian Subantarctic Water from Circumpolar Subantarctic Water, is also likely to influence the southern locations of this study. In contrast, the entire latitudinal range we sampled is bathed, at depths starting at 700–1000 m, by a unique water mass, the Antarctic Intermediate Water. Although a degree of temporal and spatial variability in the properties of Antarctic Intermediate Waters exists48, the relative homogeneity of this water mass may explain a decrease in latitudinal beta diversity of fishes. Indeed, our results showed a marked decrease in beta diversity at 700–900 m, in agreement with the expected position of this water mass.
In addition to the “water mass” hypothesis, several other potential mechanistic explanations could explain these observed patterns of beta diversity along the depth gradient. For example, energy can play an important role in structuring patterns of biodiversity49. Particulate organic carbon (POC) that sinks from shallower waters is the main source of energy for assemblages occurring at depths in the ocean >200 m, beyond the photic zone. The flux of POC is seasonal and also decreases with depth following a power law; it is estimated that less than 10% of the carbon produced in shallow waters reaches depths >2000 m50. POC availability appears to be a very important determinant in structuring communities from local to global scales14. A relatively homogeneous distribution of generalist wide-ranging species may present an optimal evolutionary strategy to optimize biological intake of scarce energy resources, yielding reduced large scale horizontal beta diversity at depth.
Another important feature of increased depth is the increasing pressure, which can have strong effects on the molecular structure of proteins; specialized physiological mechanisms are required to stabilize protein structures at deep depths51. Levels of trimethylamine oxide (TMAO) that may help to stabilize proteins under high pressure increase linearly with depth in teleosts52. Thus, TMAO content may set the depth limit for teleosts at ca. 8,500 m, where fish cells may become hyperosmotic to seawater53; it might also more generally limit the speciation of fishes with increasing depth. Although reduced species’ diversity in itself does not necessarily explain reduced beta diversity, narrow available niche space would allow a small number of successful species to become widely distributed across a spatially homogeneous and highly pressurized habitat, resulting in reduced beta diversity.
The exponential decay model for beta diversity vs depth proposed here has important implications for our understanding of ecological biodiversity in the deep sea. Extrapolation of the model, although clearly speculative, suggests that the percentage of unshared species among fish assemblages becomes very small (i.e., ca. 10% unshared and 90% shared) at depths beyond 4400 m. This coincides with the abyssal sea floor – vast plains at 3,000–5,000 m depth that represents the most common benthic environment on the planet, covering 54% of the Earth’s surface54. Obviously, there are multiple factors that might come into play at depths greater than 1200 m, including influences of water masses26, 27 and surface-water productivity55, 56. However, the relative homogeneity of abyssal environments is consistent with past observations indicating that species inhabiting these habitats have broad distributional ranges25, 26, 41, 42. Indeed, as early as 1880, Henry N. Moseley, in his report of the Challenger expedition stated: “There is absolutely nothing to restrict the geographical range of animals in the deep sea. We got tired on the Challenger of dredging up the same monotonous animals wherever we went”57.
Deep-sea research, in general, and spatially-structured and replicated deep-sea data, in particular, are still far too depauperate, however, to address many fundamental hypotheses, which remain to be tested. Further research exploring wider depth ranges at other locations and latitudes is warranted to explore the generality of the exponential decay model of fish beta diversity vs depth. In addition, the ecological and evolutionary drivers of changes in beta diversity with increasing depth may be clarified in future studies by comparing and contrasting patterns in taxonomic, phylogenetic and functional beta diversity at large scales44, 58, 59.
New knowledge gained from future studies regarding the physical, historical and biological drivers of beta diversity in the ocean must be integrated with known effects of human-mediated disturbances, such as pollution, habitat modification, and fishing. Indeed, a further implication of our results is that conservation efforts will require an extra level of sophistication in shallow-water systems, which not only tend to be highly impacted by human activities, but are also the richest and show the greatest variation and turnover of marine fauna. Establishment of marine reserves at remote, deep, offshore locations may be valuable for deep-sea ecosystem functioning, but will provide little-or-no protection or compensation for degradation of habitat and rapid species loss in coastal environments. Multi-faceted large-scale assessments of the biodiversity of earth’s oceans59 will serve our ultimate aim for ecosystem knowledge, resource management and sustainability.
Methods
Locations and sampling methods
Baited remote underwater video (BRUVs) imagery was collected from 2009–2012, but always during the austral summer months, at each of seven locations: the Kermadec Islands (KER), the Three Kings Islands (TKI), Great Barrier Island (GBI), White Island (WI), Kaikoura (KKA), the Otago Peninsula (OTA) and the Auckland Islands (AUC), spanning a latitudinal range of 21° in New Zealand waters (Fig. 1 and Table 2). At each location, video samples were generally taken along each of at least n = 6 replicate transects at each of seven targeted depths: 50, 100, 300, 500, 700, 900 and 1200 m. However, logistic constraints and poor weather led to some imbalance in the study design (Table 2). A sonar was used to deploy units at the desired depth, achieving average depths (±SD) for the different target strata of 51 m (±8.6), 101 m (±9.7), 304 m (±21.5), 505 m (±24.3), 699 m (±32.6), 894 m (±40.5) and 1170 m (±41.0), respectively. The resulting dataset comprised a total of 345 deployments. Individual deployments were separated by at least 500 metres (usually by much more, see Fig. 1) and remained on the seabed for a minimum of two hours (only the first two hours of each deployment are considered in this study).
Table 2.
Range in latitude and longitude for deployments obtained at each of the seven locations and the number of baited remote underwater stereo-video (stereo-BRUVs) deployments obtained at each depth within each location.
| Location (date of sampling) | Latitude range Longitude range | Sample size | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 m | 100 m | 300 m | 500 m | 700 m | 900 m | 1200 m | Total | ||
| Kermadec Islands (KER) (05/2011) | 29.4176°S–29.1549°S | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 49 |
| 181.8354°E–182.1948°E | |||||||||
| Three Kings Islands (TKI) (03/2010) | 34.1851°S–33.8010°S | 8 | 6 | 6 | 7 | 6 | 6 | 6 | 45 |
| 171.7616°E–172.1666°E | |||||||||
| Great Barrier Island (GBI) (12/2009) | 35.2893°S–36.6235°S | 8 | 9 | 8 | 8 | 6 | 8 | 8 | 55 |
| 175.3588°E–176.3911°E | |||||||||
| White Island (WI) (03/2009) | 37.5595°S–37.3128°S | 11 | 12 | 11 | 11 | 9 | 11 | 0 | 65 |
| 177.0911°E–177.2663°E | |||||||||
| Kaikoura (KKA) (11/2010) | 42.5900°S–42.3829°S | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 49 |
| 173.7161°E–173.9983°E | |||||||||
| Otago peninsula (OTA) (02/2012) | 46.0136°S–45.7387°S | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 49 |
| 170.7819°E–171.3465°E | |||||||||
| Auckland Islands (AUC) (02/2012) | 50.9114°S–50.4181°S | 14 | 9 | 2 | 2 | 3 | 3 | 0 | 33 |
| 165.8385°E–166.3412°E | |||||||||
| Total | 62 | 57 | 48 | 49 | 45 | 49 | 35 | 345 | |
The stereo-video units consisted of two HD Sony handycams in underwater housings mounted on a base bar 0.7 m apart and inwardly converged at 8°. Bait consisted of 2 kg of chopped pilchards (Sardinops sagax) held in bait bags that were visible in the cameras’ field of view. Lighting was provided by eight blue LED lamps, each delivering a radiant flux of 340–425 mW at wavelengths of 450–465 nm. Sensors attached to the frame of the units also recorded the depth, temperature and salinity of each deployment in situ. See Zintzen et al.32 for further technical details regarding the stereo-BRUV apparatus. The use of BRUVs provided a standardized sampling methodology in situ across the full study design and across the full range of potential habitats, capturing images of a wide spectrum of fishes, not just piscivores (see Supplementary Tables 1 and 2). For further details regarding pros and cons associated with the use of BRUV methodology, see Harvey et al.60 and related refs 61, 62.
Image analysis
Analysis of video footage was performed following Zintzen, et al.32. The maximum number of individuals (MaxN) of the same species appearing within five meters of the unit in a video frame was used as a conservative estimate of the relative abundance of fish seen from each two-hour stereo-BRUV deployment63, 64. At the beginning and completion of the field work, the stereo-BRUVs were calibrated following procedures outlined in Harvey and Shortis65 and Shortis and Harvey66 using Cal software (v1.32, www.seagis.com.au). Calibration was also done at the completion of fieldwork to ensure that the positions of stereo units remained fixed over the course of all deployments. Importantly, stereo (bi-camera) systems ensure a standardized field of view (volume ~24.6 m3) for rigorous estimation of relative abundance and composition across the study design, as well as 3D perception for observing behaviour and movements for an extensive variety of fishes, alive and in situ 63.
Fish identification
Accurate species identification made solely from camera images is often difficult, particularly when the fish fauna is poorly known taxonomically. In our study, identification errors have been greatly reduced through (i) obtaining very high definition digital images from top quality BRUV equipment; (ii) using forward-facing BRUV system cameras to provide taxonomically essential lateral images of fishes (cf. downward-facing views, commonly used for benthos in deep sea systems); (iii) large fish traps deployed at the same time, depth range, and location as the video systems, to capture voucher specimens for identification and registration into the Te Papa National Fish Collection and used to underpin and inform most of the video identifications; (iv) all laboratory and most video identifications being carried out by world experts, who were preparing new descriptions and keys for all 1262 known fishes known within the EEZ67; (v) difficult identifications were made conservatively; fishes not identifiable to species were named to their lowest taxonomic level possible. As a result, 191 out of the 242 taxa recorded (79%) were identified to species level. Analyses done using only fish identified to species level did not change the fundamental results and inferences.
Multivariate partitioning of variation in community structure (beta diversity)
To quantify biodiversity along depth (or altitude), direct measures of spatial variation based on standardized units must be obtained and compared for different depths (or altitudes). Given an appropriately structured and replicated sampling design, a multivariate partitioning of the variation in community structure (i.e., beta diversity)68 due to large-scale factors (such as depth, latitude and their interaction) can be quantified explicitly69, 70, and the relative sizes of these components of variation can be directly compared71, 72. This general and new approach has never been applied before to any large-scale study of beta diversity from a structured sampling design in either marine or terrestrial systems and is described in the following sections.
Statistical analysis
Although counts of each fish species (MaxN) were available, we focused on presence/absence (i.e., compositional) information only. All analyses were based on the Jaccard dissimilarity measure73), which is directly interpretable as the proportion of unshared species between two sampling units out of the total number of species present in both sampling units. It is also a measure of beta diversity74 and can optionally be expressed as a percentage (i.e., ×100). The matrix correlation between the dissimilarity matrix based on Jaccard vs that based on Bray-Curtis75, a measure widely used in ecology that includes relative abundances76, was very large (Spearman’s rho = 0.987).
To visualise patterns of dissimilarities among fish communities across the study design, non-metric multidimensional scaling ordination (nMDS)77 was done on Jaccard dissimilarities calculated on averages for each species within each combination of depth-by-location (n ≥ 6 deployments per cell). The full set of data at the replicate level was analysed using permutational multivariate analysis of variance (PERMANOVA69, 78) according to the following three factors: Location (fixed with 7 levels), Depth (fixed with 7 levels) and Transect (random, nested in Location, with varying numbers of transects per Location).
Although the design was unbalanced (Table 2), multivariate analogues to traditional univariate expectations of mean squares can be used to construct correct pseudo F-ratios (i.e., where the numerator and denominator have the same expectation under a true null hypothesis) and to obtain appropriate tests by permutation for each term in the model, using the software PERMANOVA+70, an add-on to PRIMER v779. These analyses were done using Type I (sequential) sums of squares and P-values were obtained using 9999 permutations under a reduced model80, 81. Results were not affected in any way by changing the order of terms in the sequential analysis.
Components of variation for each term in the model were calculated using ANOVA estimators, obtained by setting mean squares equal to their expectations and solving for the parameters of interest82. When calculated from a PERMANOVA model, these should generally be thought of as “pseudo” multivariate components of variation70, as they do not include estimation of covariances, and they can be based on a dissimilarity measure of choice (in our case, Jaccard). These components of variation are direct measures of beta diversity74, and are additive in the sequential PERMANOVA model, so can also be expressed as a percentage of the total variation across the study design. Furthermore, when these components are transformed by taking square roots, they are then in the units of the original dissimilarity measure (e.g., Jaccard units), and provide a direct dissimilarity-based multivariate analogue to a standard deviation. Thus, for example, a square-root-transformed component of variation with a value of 20 for the factor of Latitude would mean that the standard deviation of a given location centroid from the overall centroid is approximately 20 Jaccard units (i.e., 20% unshared species).
A statistically significant location-by-depth interaction (see Results) indicated that latitudinal variation in fish community structure differed for different depth strata. Multivariate pseudo variance components, as measures of beta diversity for a given spatial scale, can be calculated along one or more environmental gradients (e.g., see mission statement V4(b) depicted in Fig. 4 of Anderson et al.74). Thus, a component of variation for latitude was estimated separately within each depth stratum, and vice versa. Bootstrap resampling algorithms (including appropriate bias corrections) can be implemented to provide empirical confidence intervals around such estimates for statistical inference72. Empirical 95% confidence intervals for each estimated component were obtained from 1000 bias-corrected separate-sample bootstraps of residuals83, 84. These analyses were done using purpose-built R code85. A general function in R (“comp.var”) for estimating pseudo multivariate components of variation for one factor (factor A) within each level of another factor (factor B) on the basis of the Jaccard measure, including bootstrap confidence intervals with empirical bias-correction, is provided as Supporting Information (Supplementary Method S6).
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
The MV Tranquil Image crew, N. Furley, G. Gibbs and S. Kelly, helped to organize all of the fieldwork using baited underwater video units. C. Struthers, R. Crech’riou, A. Smith, C. Bedford, O. Hannaford, J. Williams, J. Barker, C. Struthers and K. Rodgers also contributed to the sampling effort. E. Myers contributed fish identifications and counts from stereo-BRUVs imagery during an internship at Te Papa. P. McMillan (Macrouridae), P. Last and C. Duffy (sharks), and D. Smith (Synaphobranchidae) helped with certain species’ identifications from video footage. The stereo measurements from the video footage were made by Mr T. Bond. This work was supported by a Royal Society of New Zealand Marsden grant (MAU0713), Te Papa Collection Development Programme (AP3126) and FRST/NIWA Marine Biodiversity and Biosecurity OBI (contract COIX0502).
Author Contributions
V.Z. performed sample collection with the essential technical support of E.S.H. and financial backing from C.D.R. and M.J.A. The video footage was reviewed and analysed by V.Z., who also organized and prepared the figures, tables and supplementary videos. R code for statistical analysis was contributed by M.J.A. The initial draft of this manuscript was written by V.Z. and M.J.A. All of the authors discussed the results and contributed to the writing of the final draft of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08427-7
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gaston KJ. Global patterns in biodiversity. Nature. 2000;405:220–227. doi: 10.1038/35012228. [DOI] [PubMed] [Google Scholar]
- 2.Rosenzweig, M. L. Species diversity in space and time. (Cambridge University Press, 1995).
- 3.Soininen J, Lennon JJ, Hillebrand H. A multivariate analysis of beta diversity across organisms and environments. Ecology. 2007;88:2830–2838. doi: 10.1890/06-1730.1. [DOI] [PubMed] [Google Scholar]
- 4.Levin SA. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. doi: 10.2307/1941447. [DOI] [Google Scholar]
- 5.Hillebrand H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004;163:192–211. doi: 10.1086/381004. [DOI] [PubMed] [Google Scholar]
- 6.Rahbek C. The elevational gradient of species richness: a uniform pattern? Ecography. 1995;18:200–205. doi: 10.1111/j.1600-0587.1995.tb00341.x. [DOI] [Google Scholar]
- 7.Rex, M. A. & Etter, R. J. Deep-sea biodiversity - pattern and scale. (Harvard University Press, 2010).
- 8.Lorance P, Souissi S, Uiblein F. Point, alpha and beta diversity of carnivorous fish along a depth gradient. Aquat. Living Resour. 2002;15:263–271. doi: 10.1016/S0990-7440(02)01189-0. [DOI] [Google Scholar]
- 9.Magnussen E. Demersal fish assemblages of Faroe Bank: species composition, distribution, biomass spectrum and diversity. Mar. Ecol. Prog. Ser. 2002;238:211–225. doi: 10.3354/meps238211. [DOI] [Google Scholar]
- 10.Mora C, Tittensor DP, Myers RA. The completeness of taxonomic inventories for describing the global diversity and distribution of marine fishes. Proc. R. Soc. Lond. B. 2008;275:149–155. doi: 10.1098/rspb.2007.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens GC. Extending Rapoport’s rule to Pacific marine fishes. J. Biogeogr. 1996;23:149–154. doi: 10.1046/j.1365-2699.1996.00977.x. [DOI] [Google Scholar]
- 12.Carney RS. Zonation of deep biota on continental margins. Oceanogr. Mar. Biol. Annu. Rev. 2005;43:211–278. [Google Scholar]
- 13.Kendall VJ, Haedrich RL. Species richness in Atlantic deep-sea fishes assessed in terms of the mid-domain effect and Rapoport’s rule. Deep-Sea Res. Part I. 2006;53:506–515. doi: 10.1016/j.dsr.2005.12.005. [DOI] [Google Scholar]
- 14.McClain CR, Rex MA. Toward a Conceptual Understanding of β-Diversity in the Deep-Sea Benthos. Annu. Rev. Ecol. Evol. Syst. 2015;46:623–642. doi: 10.1146/annurev-ecolsys-120213-091640. [DOI] [Google Scholar]
- 15.Zintzen V, Anderson MJ, Roberts CD, Diebel CE. Increasing variation in taxonomic distinctness reveals clusters of specialists in the deep sea. Ecography. 2011;34:306–317. doi: 10.1111/j.1600-0587.2010.06546.x. [DOI] [Google Scholar]
- 16.Leathwick JR, Elith J, Francis MP, Hastie T, Taylor P. Variation in demersal fish species richness in the oceans surrounding New Zealand: an analysis using boosted regression trees. Mar. Ecol. Prog. Ser. 2006;321:267–281. doi: 10.3354/meps321267. [DOI] [Google Scholar]
- 17.McClatchie S, et al. Demersal fish community diversity off New Zealand: Is it related to depth, latitude and regional surface phytoplankton? Deep-Sea Res. Part I. 1997;44:647–667. doi: 10.1016/S0967-0637(96)00096-9. [DOI] [Google Scholar]
- 18.Wei C-L, Rowe GT, Haedrich RL, Boland GS. Long-Term Observations of Epibenthic Fish Zonation in the Deep Northern Gulf of Mexico. PLoS One. 2012;7:e46707. doi: 10.1371/journal.pone.0046707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tolimieri N. Patterns in species richness, species density, and evenness in groundfish assemblages on the continental slope of the US Pacific coast. Environ. Biol. Fishes. 2007;78:241–256. doi: 10.1007/s10641-006-9093-5. [DOI] [Google Scholar]
- 20.Gaertner, J. C. et al. Large-scale diversity of slope fishes: pattern inconsistency between multiple diversity indices. PLoS One8, doi:e6675310.1371/journal.pone.0066753 (2013). [DOI] [PMC free article] [PubMed]
- 21.Anderson MJ, Tolimieri N, Millar RB. Beta diversity of demersal fish assemblages in the north-eastern Pacific: interactions of latitude and depth. PLoS One. 2013;8:e57918. doi: 10.1371/journal.pone.0057918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jouffre D, et al. Estimating EAF indicators from scientific trawl surveys: theoretical and practical concerns. ICES J. Mar. Sci. 2010;67:796–806. doi: 10.1093/icesjms/fsp285. [DOI] [Google Scholar]
- 23.Janzen DH. Why mountain passes are higher in the tropics. Am. Nat. 1967;112:233–249. doi: 10.1086/282487. [DOI] [Google Scholar]
- 24.Pielou, E. C. Biogeograhy. (John Wiley & Sons, 1979).
- 25.Bruun AF. The abyssal fauna: its ecology, distribution and origin. Nature. 1956;177:1105–1108. doi: 10.1038/1771105a0. [DOI] [Google Scholar]
- 26.Koslow JA. Community structure in North-Atlantic deep-sea fishes. Prog. Oceanogr. 1993;31:321–338. doi: 10.1016/0079-6611(93)90005-X. [DOI] [Google Scholar]
- 27.Koslow JA, Bulman CM, Lyle JM. The mid-slope demersal fish community off southeastern Australia. Deep-Sea Res. Part I. 1994;41:113–141. doi: 10.1016/0967-0637(94)90029-9. [DOI] [Google Scholar]
- 28.Hecker B. Variation in megafaunal assemblages on the continental-margin south of New-England. Deep-Sea Res. Part I. 1990;37:37–57. doi: 10.1016/0198-0149(90)90028-T. [DOI] [Google Scholar]
- 29.Hecker B. Unusual megafaunal assemblages on the continental slope off Cape Hatteras. Deep-Sea Res. Part II. 1994;41:809–834. doi: 10.1016/0967-0645(94)90050-7. [DOI] [Google Scholar]
- 30.Bergstad OA, Menezes GMM, Hoines AS, Gordon JDM, Galbraith JK. Patterns of distribution of deepwater demersal fishes of the North Atlantic mid-ocean ridge, continental slopes, islands and seamounts. Deep-Sea Res. Part I. 2012;61:74–83. doi: 10.1016/j.dsr.2011.12.002. [DOI] [Google Scholar]
- 31.Tolimieri N, Levin PS. Assemblage structure of eastern pacific groundfishes on the US continental slope in relation to physical and environmental variables. Trans. Am. Fish. Soc. 2006;135:317–332. doi: 10.1577/T05-092.1. [DOI] [Google Scholar]
- 32.Zintzen V, et al. Diversity and composition of demersal fishes along a depth gradient assessed by baited remote underwater stereo-video. PLoS One. 2012;7:1–14. doi: 10.1371/journal.pone.0048522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blake JA, Grassle JF. Benthic community structure on the U.S. South Atlantic slope off the Carolinas: spatial heterogeneity in a current-dominated system. Deep-Sea Res. Part II. 1994;41:835–874. doi: 10.1016/0967-0645(94)90051-5. [DOI] [Google Scholar]
- 34.Howell KL, Billett DSM, Tyler PA. Depth-related distribution and abundance of seastars (Echinodermata: Asteroidea) in the Porcupine Seabight and Porcupine Abyssal Plain, NE Atlantic. Deep-Sea Res. Part I. 2002;49:1901–1920. doi: 10.1016/S0967-0637(02)00090-0. [DOI] [Google Scholar]
- 35.Olabarria C. Patterns of bathymetric zonation of bivalves in the Porcupine Seabight and adjacent Abyssal Plain, NE Atlantic. Deep-Sea Res. Part I. 2005;52:15–31. doi: 10.1016/j.dsr.2004.09.005. [DOI] [Google Scholar]
- 36.Haedrich RL, Rowe GT, Polloni PT. The megabenthic fauna in the deep-sea South of New-England, USA. Mar. Biol. 1980;57:165–179. doi: 10.1007/BF00390735. [DOI] [Google Scholar]
- 37.McClain CR, Stegen JC, Hurlbert AH. Dispersal, environmental niches and oceanic-scale turnover in deep-sea bivalves. Proc. R. Soc. Lond. B. 2012;279:1993–2002. doi: 10.1098/rspb.2011.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price ARG, Keeling MJ, O’Callaghan CJ. Ocean-scale patterns of ‘biodiversity’ of Atlantic asteroids determined from taxonomic distinctness and other measures. Biol. J. Linn. Soc. 1999;66:187–203. [Google Scholar]
- 39.Labeyrie LD, Duplessy JC, Blanc PL. variations in mode of formation and temperature of oceanic deep waters over the past 125,000 years. Nature. 1987;327:477–482. doi: 10.1038/327477a0. [DOI] [Google Scholar]
- 40.Martin PA, et al. Quaternary deep sea temperature histories derived from benthic foraminiferal Mg/Ca. Earth Planet Sc. Lett. 2002;198:193–209. doi: 10.1016/S0012-821X(02)00472-7. [DOI] [Google Scholar]
- 41.Ekman, S. Zoogeography of the sea. (Sidgwick and Jackson, 1953).
- 42.Howes GJ. Biogeography of gadoid fishes. J. Biogeogr. 1991;18:595–622. doi: 10.2307/2845542. [DOI] [Google Scholar]
- 43.Lindenmayer, D. B. & Fischer, J. Habitat fragmentation and landscape change: an ecological and conservation synthesis. (Island Press, 2006).
- 44.Swenson NG, Anglada-Cordero P, Barone JA. Deterministic tropical tree community turnover: evidence from patterns of functional beta diversity along an elevational gradient. Proc. R. Soc. Lond. B. 2011;278:877–884. doi: 10.1098/rspb.2010.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sverdrup, H. U., Johnson, M. W. & Fleming, R. H. The Oceans: Their physics, chemistry, and general biology. Vol. 7 (Prentice-Hall New York, 1942).
- 46.Heath RA. A review of the physical oceanography of the seas around New Zealand - 1982. N. Z. J. Mar. Freshwat. Res. 1985;19:79–124. doi: 10.1080/00288330.1985.9516077. [DOI] [Google Scholar]
- 47.Garner D. The sub-tropical convergence in New Zealand surface waters. N. Z. J. Geol. Geophys. 1959;2:315–337. doi: 10.1080/00288306.1959.10417650. [DOI] [Google Scholar]
- 48.Stanton BR. Antarctic Intermediate Water variability in the northern New Zealand region. N. Z. J. Mar. Freshwat. Res. 2002;36:645–654. doi: 10.1080/00288330.2002.9517120. [DOI] [Google Scholar]
- 49.Evans KL, Warren PH, Gaston KJ. Species-energy relationships at the macroecological scale: a review of the mechanisms. Biol. Rev. Camb. Philos. Soc. 2005;80:1–25. doi: 10.1017/S1464793104006517. [DOI] [PubMed] [Google Scholar]
- 50.Francois, R., Honjo, S., Krishfield, R. & Manganini, S. Factors controlling the flux of organic carbon to the bathypelagic zone of the ocean. Global Biogeochem. Cy. 16 (2002).
- 51.Yancey PH, Fyfe-Johnson AL, Kelly RH, Walker VP, Aunon MT. Trimethylamine oxide counteracts effects of hydrostatic pressure on proteins of deep-sea teleosts. J. Exp. Zool. 2001;289:172–176. doi: 10.1002/1097-010X(20010215)289:3<172::AID-JEZ3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 52.Samerotte AL, Drazen JC, Brand GL, Seibel BA, Yancey PH. Correlation of trimethylamine oxide and habitat depth within and among species of teleost fish: An analysis of causation. Physiol. Biochem. Zool. 2007;80:197–208. doi: 10.1086/510566. [DOI] [PubMed] [Google Scholar]
- 53.Yancey PH, Gerringer ME, Drazen JC, Rowden AA, Jamieson A. Marine fish may be biochemically constrained from inhabiting the deepest ocean depths. Proc. Natl. Acad. Sci. USA. 2014;111:4461–4465. doi: 10.1073/pnas.1322003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gage, J. D. & Tyler, P. A. Deep-sea biology: a natural history of organisms at the deep-sea floor. (Cambridge University Press, 1991).
- 55.Merrett NR. A zone of faunal change in assemblages of abyssal demersal fish in the eastern North Atlantic: A response to seasonality in production? Biological Oceanography. 1987;5:137–151. [Google Scholar]
- 56.Linley T, et al. Bait attending fishes of the abyssal zone and hadal boundary: Community structure, functional groups and species distribution in the Kermadec, New Hebrides and Mariana trenches. Deep-Sea Res. Part I. 2017;121:38–53. doi: 10.1016/j.dsr.2016.12.009. [DOI] [Google Scholar]
- 57.Moseley HN. Deep-sea dredging and life in the deep sea. Nature. 1880;21:543–547. doi: 10.1038/021543a0. [DOI] [Google Scholar]
- 58.Stegen JC, Hurlbert AH. Inferring ecological processes from taxonomic, phylogenetic and functional trait β-diversity. PLoS One. 2011;6:e20906. doi: 10.1371/journal.pone.0020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tolimieri N, Shelton AO, Feist BE, Simon V. Can we increase our confidence about the locations of biodiversity ‘hotspots’ by using multiple diversity indices? Ecosphere. 2015;6:1–13. doi: 10.1890/ES14-00363.1. [DOI] [Google Scholar]
- 60.Harvey ES, Cappo M, Butler JJ, Hall N, Kendrick GA. Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Mar. Ecol. Prog. Ser. 2007;350:245–254. doi: 10.3354/meps07192. [DOI] [Google Scholar]
- 61.Dorman SR, Harvey ES, Newman SJ. Bait effects in sampling coral reef fish assemblages with stereo-BRUVs. PLoS One. 2012;7:e41538. doi: 10.1371/journal.pone.0041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hardinge J, Harvey ES, Saunders BJ, Newman SJ. A little bait goes a long way: the influence of bait quantity on a temperate fish assemblage sampled using stereo-BRUVs. J. Exp. Mar. Biol. Ecol. 2013;449:250–260. doi: 10.1016/j.jembe.2013.09.018. [DOI] [Google Scholar]
- 63.Cappo, M., Harvey, E. S. & Shortis, M. In Proceedings of the Australian Society for Fish Biology Workshop. (eds Furlani, D. & Beumer, J.P.) 101–114 (Australian Society for Fish Biology).
- 64.Priede IG, Bagley PM, Smith A, Creasey S, Merrett NR. Scavenging deep demersal fishes of the Porcupine Seabight, north-east Atlantic: observations by baited camera, trap and trawl. J. Mar. Biol. Assoc. U.K. 1994;74:481–498. doi: 10.1017/S0025315400047615. [DOI] [Google Scholar]
- 65.Harvey ES, Shortis MR. A system for stereo-video measurement of sub-tidal organisms. Mar. Technol. Soc. J. 1995;29:10–22. [Google Scholar]
- 66.Shortis MR, Harvey ES. Design and calibration of an underwater stereo-video system for the monitoring of marine fauna populations. Int. Arch. Photogramm. Remote Sens. 1998;32:792–799. [Google Scholar]
- 67.Roberts, C., Stewart, A. L. & Struthers, C. D. The Fishes of New Zealand. (Te Papa Press, 2015).
- 68.Anderson MJ, Ellingsen KE, McArdle BH. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006;9:683–693. doi: 10.1111/j.1461-0248.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- 69.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001;26:32–46. [Google Scholar]
- 70.Anderson, M. J., Gorley, R. N. & Clarke, K. R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. (PRIMER-E Ltd, 2008).
- 71.Anderson MJ, et al. Relationships between taxonomic resolution and spatial scales of multivariate variation. J. Anim. Ecol. 2005;74:636–646. doi: 10.1111/j.1365-2656.2005.00959.x. [DOI] [Google Scholar]
- 72.Terlizzi A, Anderson MJ, Fraschetti S, Benedetti-Cecchi L. Scales of spatial variation in Mediterranean subtidal sessile assemblages at different depths. Mar. Ecol. Prog. Ser. 2007;332:25–39. doi: 10.3354/meps332025. [DOI] [Google Scholar]
- 73.Jaccard P. Contribution au problème de l’immigration post-glaciaire de la flore alpine. Bull. Soc. Vaud. Sci. Nat. 1900;36:87–130. [Google Scholar]
- 74.Anderson MJ, et al. Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol. Lett. 2011;14:19–28. doi: 10.1111/j.1461-0248.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- 75.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957;27:325–349. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 76.Clarke KR, Somerfield PJ, Chapman MG. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J. Exp. Mar. Biol. Ecol. 2006;330:55–80. doi: 10.1016/j.jembe.2005.12.017. [DOI] [Google Scholar]
- 77.Kruskal, J. B. & Wish, M. Multidimensional scaling. (Sage Publications, 1978).
- 78.McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. doi: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2. [DOI] [Google Scholar]
- 79.Clarke, K. R. & Gorley, R. N. PRIMER v7: User manual/Tutorial. (PRIMER-E Ltd, 2015).
- 80.Anderson MJ, ter Braak CJF. Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003;73:85–113. doi: 10.1080/00949650215733. [DOI] [Google Scholar]
- 81.Freedman D, Lane D. A nonstochastic interpretation of reported significance levels. J. Bus. Econ. Statist. 1983;1:292–298. [Google Scholar]
- 82.Searle, S. R., Casella, G. & McCulloch, C. E. Variance components. (John Wiley & Sons, 1992).
- 83.Davison, A. C. & Hinkley, D. V. Bootstrap methods and their application. (Cambridge University Press, 1997).
- 84.Manly, B. F. J. Randomization, bootstrap and Monte Carlo methods in biology. 3rd edition edn, (Chapman and Hall, 2007).
- 85.R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


