Abstract
Deficits in the integration of motor prediction and its feedback have been reported in Parkinson’s disease. Conscious awareness of action is proposed to emerge under the integration of motor prediction and its feedback. Thus, it may lead to changes in the awareness of the authorship of action (in other words, the sense of agency) in Parkinson’s disease. We have employed both explicit and implicit measures to assess the awareness of action in Parkinson’s disease and matched controls. As an explicit measure, an action recognition task requiring explicit judgments was used. Patients showed less attribution of their movements to non-biased and angular-biased visual feedbacks. As an implicit measure, the temporal attraction between the perceived time of actions and their effects, which is known as intentional binding task, was used. While action-effect association was observed in the control group, actions were not experienced as having shifted towards their subsequent effects in the patient group. These tendencies were consistent regardless of the side of the asymmetrical motor symptoms. These results may reflect an underlying abnormality in the awareness of voluntary action in Parkinson’s disease.
Introduction
Parkinson’s disease (PD) is a progressive neurological disorder characterized by the degeneration of dopamine-producing neurons. It is the second most common neurodegenerative disorder after Alzheimer’s disease, and its incidence range from 1.5% to 2% after age 651. Motor symptoms develop in an asymmetrical order, with symptom dominance either on the right or left side2. Besides motor symptoms, patients experience various deficits in the emotional and cognitive domain, as well as in sensorimotor integration3–5.
The belief that we ourselves are responsible for our own action outcome has been referred to as the “awareness of action” or the “sense of agency”. It is one of the minimal components of self-awareness known as “minimal self”, that is “a consciousness of oneself as an immediate subject of experience”6. The concept is related to some common neurocognitive models and neural processes6. Disruptions in the awareness of action present widely among psychiatric and neurological diseases7–9. Growing evidence points to the linkage between disruptions in the awareness of action and movement disorders, where different aspects of volitional control have been focused10–14. It has been suggested that the conscious awareness of action emerges under the integration of motor prediction and its feedback in the framework of the central monitoring theory15, 16. Comparison of prediction with sensory afference will enable us to distinguish self-produced sensory information from externally caused events. In addition to this predictive process, the awareness of action is also known to be affected postdictively17, 18. That is, experiences of causation for voluntary actions are determined in part by post-action information19. Various cues in the level of perception, cognition and emotion are known to contribute to the awareness of action20.
Previous studies have approached the awareness of action by two distinct methods. One classical approach is an action recognition task requiring explicit judgments. Participants are given visual feedback of a voluntary action, which is either neutral or distorted. They are asked if their action is responsible for the given feedback, in a way that the feedback is either in concordance with their action or not21. The other approach is to measure the awareness of action implicitly as an attraction between the perceived time of actions and their effects22. This is called the intentional binding effect. Participants view a clock hand rotating over a certain period of time, and make a volitional button press at the timing of their own choosing, as in Libet’s paradigm23. The button press will be followed by a tone as its consequence. Perception of actions will be shifted towards the subsequent effects (action binding: AB), and that perception of effects will be shifted towards the preceding actions (tone binding: TB). Because such action-effect association occurs only when the actions are made voluntarily but not when they are made involuntarily, this has been suggested as being a quantitative marker for measuring awareness of action22. These two empirical methods have been repeatedly verified through many studies12, 19, 24–30, and the two approaches have been linked to the implicit “feeling of agency” and the explicit “judgement of agency”, respectively31. The lower level feeling of agency represents a step that is not conceptual upon our actions. The second level judgement of agency represents a conceptual, interpretative step of making judgement for attribution of actions. The dissociation of these two aspects has been reported both theoretically31 and experimentally32.
Given the disruption of sensorimotor integration in PD3–5, it has been questioned whether there are subsequent changes in the awareness of action in PD. Being able to experimentally verify the theoretical framework of central monitoring is one of the advantages for testing the issue in PD patients, because there have been accumulative studies on sensorimotor integration in PD. From a clinical viewpoint, PD patients manifest various psychiatric disturbances including anxiety, apathy and depression33. Patients experience constant frustration in unreliable motor execution34. Uncertainties in the activities of daily life cast a negative effect on patients’ quality of life33, 34. It would be of clinical importance to know if patients are experiencing an uncertainty in the awareness of action even for their intended actions.
A study has measured intentional binding in PD patients, comparing them when they are on and off dopaminergic medication27. PD patients on medication showed an increase in the overall amount of action-effect association compared to those off medication. However, it has not been clear whether this result following dopamine intake was driven by improvements in central sensorimotor processing of the disease or by secondary psychological changes following improvements in motor impairment. The authors of the study suggested the possibility of the former, although no direct test was performed. Motor impairment may cause altered visual feedback as a secondary effect. The knowledge of having motor disabilities may as well influence priors and beliefs of action. It is known that priors and beliefs of action influence the experience of personal causation35, 36. Additionally, there is a “self-serving” cognitive bias for underestimating the awareness of action for negative outcomes37. These may also be factors to affect the awareness of action20. It has remained unclear whether the primary components of central sensorimotor processing or secondary psychological effects from motor impairment matter the most.
The aim of our study was to determine whether there is a change in the awareness of action in PD patients, and to detect whether the change is driven primarily by deficits in central sensorimotor processing or secondary by motor impairment. We have tested two different explicit and implicit measures, based on the dissociations between explicit and implicit aspects in awareness of action. More specifically, we focused on the asymmetrical motor symptoms in PD, conducting measures with the hands of both the more affected side and the less affected side of motor symptoms. If the awareness of action changes in PD would be more related to the deficits in primary components of central sensorimotor processing, which have been shown to be evident prior to the appearance of motor signs38, 39, the results between the two sides would not differ. If the awareness of action changes in PD would be related to the secondary effects from motor impairment, there could be a difference in the results between the two sides. This asymmetrical comparison will be one of the advantages for investigating the awareness of action in PD, compared to other pathologies, that volitional control of action would be affected in a symmetrical manner.
Results
Demographic and clinical characteristics
Demographic and clinical variables are presented in Table 1. The average Unified Parkinson’s Disease Rating Scale (UPDRS) right/left subscale of the more-affected side was 10.2 (SD: 4.6), and of the less-affected side was 6.3 (SD: 3.7). There was a significant difference between the scores of the two sides [t(18) = 4.69, p < 0.001].
Table 1.
Demographic and clinical variables.
| Parkinson’s disease patients (n = 19) | Controls (n = 25) | Analysis | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | p | |
| Demographic scores | |||||
| Age (years) | 66.0 | 6.2 | 64.9 | 2.9 | 0.49 |
| Years of education | 13.7 | 2.2 | 13.9 | 2.2 | 0.79 |
| Total daily LED (mg/day) | 369.5 | 276.5 | — | — | — |
| Clinical scores | |||||
| BDI | 14.7 | 8.8 | 9.3 | 6.5 | 0.02 |
| Apathy Scale | 13.9 | 6.5 | 11.7 | 5.1 | 0.20 |
| General Self Efficacy Scale | 75.7 | 16.1 | 81.6 | 11.7 | 0.17 |
| Cognistat | 96.5 | 4.6 | 98.4 | 4.2 | 0.16 |
| FAB | 15.1 | 2.3 | 15.3 | 1.7 | 0.77 |
| UPDRS motor subscale | 23.2 | 9.4 | — | — | — |
Statistics of demographic and clinical variables in PD and control groups are shown.
Experiment 1- implicit task
Baseline condition
To determine whether there was a difference in time perception between groups, the results of the baseline condition were analyzed. Perception of action alone when the action was made by right and left hand did not differ in controls [t(24) = 1.26, p = 0.22]. Perception of action alone when the action was made by the more and the less affected hand did not differ in patients [t(18) = 0.33, p = 0.75]. Therefore, the average value of the perception of action when made by both hands was used in the following analysis. The perception of action alone between patients and controls did not differ [t(42) = 0.40, p = 0.69]. The perception of tone alone between patients and controls also did not differ [t(42) = 0.09, p = 0.93]. The average number of unsuccessful trials in baseline condition was 0.05 per block in patients, and 0 per block in controls.
Agency condition
In controls, a positive shift in the perceived time of action was observed in the agency condition compared to the baseline condition (AB) [right: 64.2 (SD: 119.4) ms, t(24) = 2.69, p = 0.013; left: 78.3 (SD: 117.7) ms, t(24) = 3.32, p = 0.003]. At the same time, a negative shift in the perceived time of tone was observed in the agency condition compared to the baseline condition (TB) [right: −113.1 (SD: 155.5) ms, t(24) = 3.64, p = 0.001; left: −114.5 (SD: 171.3) ms, t(24) = 3.34, p = 0.003].
In PD patients, on the other hand, this shift of perceived time of action was not observed in the agency condition compared to the baseline condition (AB) [less affected side: 1.0 (SD: 71.6) ms, t(18) = 0.06, p = 0.95; more affected side: −5.1 (SD: 43.8) ms, t(18) = 0.50, p = 0.62]. As for tone, a negative shift in the perceived time was observed in the agency condition compared to the baseline condition (TB) [less affected side: −113.9 (SD: 115.0) ms, t(18) = 4.32, p < 0.001; more affected side: −161.6 (SD: 131.2) ms, t(18) = 5.37, p < 0.001] (Fig. 1).
Figure 1.
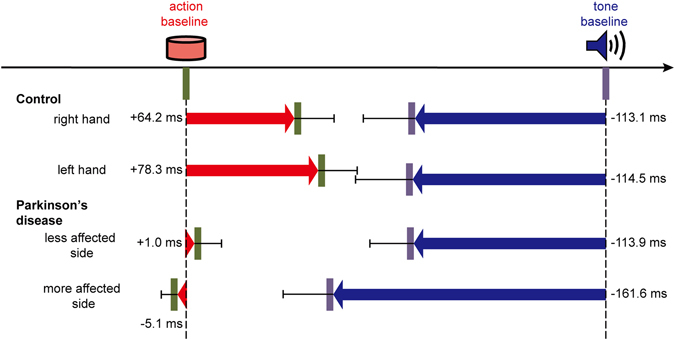
Perceived times of actions and tones in controls and in PD group. Red arrows represent perceived timing of actions, and blue arrows represent perceived timing of tones in agency condition compared to baseline condition. Bars represent standard errors.
AB was analyzed by mixed-effect ANOVA, with group (patients versus controls) as between-subject factor, and hand (more-affected versus less-affected in patients, left versus right in controls) as within-subject factor. This revealed a main effect of group [F(1,42) = 6.60, p = 0.01]. Neither an interaction of group × hand [F(1,42) = 1.44, p = 0.24], nor a main effect of hand [F(1,42) = 0.23, p = 0.64] was observed. TB was also analyzed by mixed-effect ANOVA, with group (patients versus controls) as between-subject factor and hand (more-affected versus less-affected in patients, left versus right in controls) as within-subject factor. This revealed no interaction of group × hand [F(1,42) = 3.45, p = 0.07], main effect of group [F(1,42) = 0.31, p = 0.58], or main effect of hand [F(1,42) = 3.87, p = 0.06]. The average number of unsuccessful trials in agency condition was 0.07 per block in patients, and 0.03 per block in controls.
No significant correlation was found by Spearman’s rank correlation analysis between patients’ scores of the two conditions (AB and TB averaged across hands) and age, Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores, total daily levodopa equivalent dose (LED), Beck Depression Inventory (BDI), Apathy Scale, General Self Efficacy Scale and Neurobehavioral Cognitive Status Examination (Cognistat) total scores. No significant correlation was also found between controls’ scores of the two conditions (AB and TB averaged across hands) and age, BDI, Apathy Scale, General Self Efficacy Scale and Cognistat total scores. The statistical values for correlation are shown in Supplementary Table S1.
Experiment 2- explicit task
In both patients and controls, the most “yes” responses were given in the neutral condition, with the percentage of “yes” responses decreasing as the angular or temporal biases increased. Angular biases condition was analyzed by mixed-effect ANOVA, with group (patients versus controls) as between-subject factor, and event (gap of 0°, 5°, 10°, 15°, or 20°) and hand (more-affected versus less-affected in patients, left versus right in controls) as within-subject factor (Fig. 2). There was a main effect of group [F(1,41) = 4.31, p = 0.04] and a main effect of event [F(2.9,118.1) = 120.7, p < 0.001]. There were no significant interactions of event × group × hand [F(2.3,93.5) = 0.82, p = 0.46], event × group [F(2.9,118.1) = 0.34, p = 0.79], group × hand [F(1,41) = 0.89, p = 0.35], event × hand [F(2.3,93.5) = 0.60, p = 0.57] or main effect of hand [F(1,41) = 0.48, p = 0.49]. Temporal biases condition was also analyzed by mixed-effect ANOVA, with group (patients versus controls) as between-subject factor, and event (gap of 0, 50, 100, 150, 200, 300, 400, or 500 ms) and hand (more-affected versus less-affected hand in patients, left versus right hand in controls) as within-subject factor. There was a main effect of event [F(3.4,138.9) = 117.9, p < 0.001]. There were no significant interactions of event × group × hand [F(4.6,187.3) = 0.49, p = 0.77], event × group [F(3.4,138.9) = 1.16, p = 0.33], group × hand [F(1,41) = 1.36, p = 0.25], event × hand [F(4.6,187.3) = 1.01, p = 0.41], main effect of group [F(1,41) = 0.24, p = 0.63], or main effect of hand [F(1,41) = 0.08, p = 0.78]. There was no unsuccessful trial in the two groups.
Figure 2.
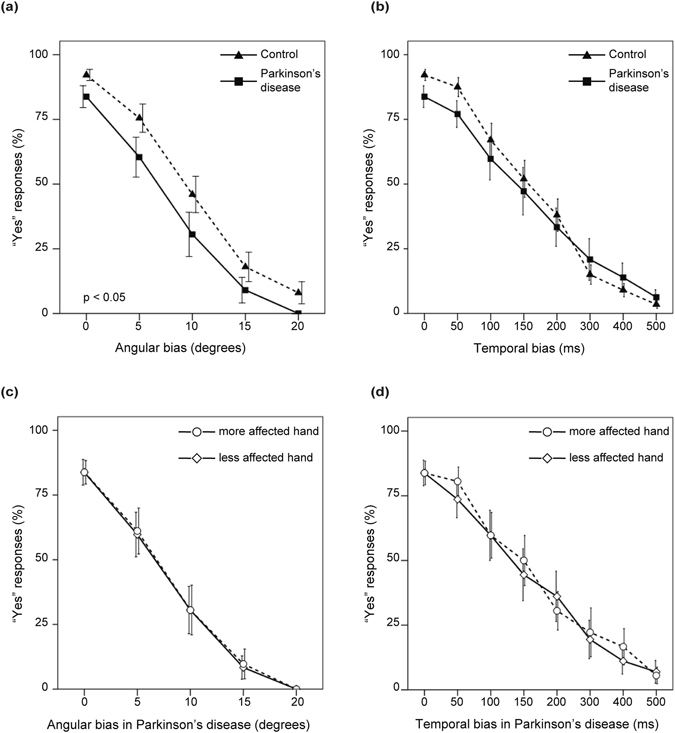
“Yes” responses for judgements in relation to the movements on screen with their actual movements. Bars represent standard errors.
No significant correlation was found by Spearman’s rank correlation analysis between patients’ total number of “yes” responses in the two conditions (with angular and temporal biases) and age, UPDRS motor scores, total LED, BDI, Apathy Scale, General Self Efficacy Scale and Cognistat total scores. Significant correlation was also not found between controls’ total number of “yes” responses of the conditions and age, BDI, Apathy Scale, General Self Efficacy Scale and Cognistat total scores. The statistical values for correlation are shown in Supplementary Table S1.
Discussion
Our study investigated the awareness of volitional action in PD through explicit and implicit measures. Both explicit and implicit measures were assessed, because differences between the two aspects have been pointed out32, 40. In the implicit task, intentional binding effects were confirmed in our control group. In the PD group, AB was not observed while TB was observed. One plausible cause for this could be the change of time perception in PD patients. However, the perception of action alone and tone alone in the baseline condition did not differ between patients and controls, suggesting that time perception itself was preserved in our patient group. Another cause could be the problem of attention in patients. Regarding this point, TB did not differ between controls and the PD group, suggesting that it did not simply stem from impaired allocation of attention to action and tone.
In the intentional binding paradigm, different explanations for AB and TB have been proposed17. It has been suggested that AB, but not TB, results from cue integration, where the sensorimotor system combines information from various sources to integrate prediction and action consequences28. As uncertainty about the action effect increases, action binding will be reduced28. Because patients are nervous about their unreliable motor execution34, these uncertainties for action outcomes might have led to the diminishment of AB, together with the reported deficits in sensorimotor integration3–5. TB is explained as being driven by a different mechanism, in line with perceptual latencies28, 41. This may be the reason for the different results for AB and TB in PD patients.
It has also been shown that AB depends on postdictive as well as predictive processes17. The contents of the observed outcome influence the agent retrospectively for ownership of the effect18. Intentional binding is known to change according to the contents of the effects; action-effect association will be weakened for negative outcome variance19, 29. Action is known to be linked to affective valences42. PD patients experience negative feelings such as fluctuations in personal competence, low self-confidence and a sense of shame due to their unwilling psychical symptoms, which are further connected to social withdrawals34. Actions made by the limb with stronger motor impairment may therefore lead to negative valences such as less confidence, resulting in weakened action-effect association. Priors and beliefs of action are other factors to influence personal authorship35, 36. A prior knowledge about the side of stronger motor impairment may lead to change in awareness of action. Because there was no significant difference in AB between the more and the less affected side of the hand in PD, central sensorimotor processing could have mattered more than these psychological effects following motor impairment. The results of the implicit task indicate that PD patients experience a change of action awareness in the non-conceptual level, with strong involvement of the sensorimotor domain.
In the explicit task, PD patients showed less attribution of the given feedback to themselves compared to the control group in the non-biased and angular biased conditions. Here, attribution of movements in the more and the less affected hand also did not differ. PD patients largely depend on external cues for compensation. Providing external visual cues will improve motor impairments such as bradykinesia43. It has also been reported that, when patients cannot see their movement hand, they undershoot the movement targets44. Although visual and kinesthetic inputs play roles in sensory feedback, patients largely rely on visual cues. In our experiment, participants’ actual movements of their own hands were concealed, making it difficult for the patients to predict spatial trajectory of movements in angular biased condition. In contrast, kinesthetic inputs should be sufficient to predict the consequence of movement initiation in temporal biased condition. This may have led to different results in angular and temporal conditions. Another explanation for the dissociation between angular and temporal conditions might also be possible. Because PD patients usually experience a time lag of their own movement as part of bradykinesia, and the lag is in a single direction, namely, a delay, compensation could have occurred, or it may have been easier for patients to predict action consequence in temporal condition than in angular condition. These results of the explicit task indicate that PD patients also experience a change in the conceptual judgement level for attribution of action.
It has been suggested through neurophysiological observations that changes of sensorimotor integration in PD do not take place at the peripheral level but depend on central processing of sensory input5. Studies show that non-motor symptoms in PD originate from bilateral pathological changes of widespread regions of the brain, starting from an ascending involvement of the lower brain stem nuclei, as well as the cerebral cortex45. Non-motor symptoms in PD can precede the occurrence of motor features, both clinically and pathologically46, 47. It was shown that abnormal motor- and cognition-related brain networks activity was evident bilaterally in hemiparkinsonian patients before clinical onset on the opposite body side39. Diverse brain regions play roles to form the awareness of action25. The observed changes might be related to the widespread pathological progression in PD, although further investigation would be needed.
A limitation of this study is that patients participated in the experiment while under regular dopaminergic medication. This could be a confounding factor for the results. However, total daily LED and the performances of the two experiments (bindings in Experiment 1 and total number of “yes” responses in Experiment 2) did not show significant correlation. In addition, it has been shown that intakes of dopamine strengthen the causal association of action and its effects27. Regarding these points, our results can be interpreted such that the patients showed less causal action-effect association even though medication worked in the opposite direction. Succeeding studies excluding the effects of medication would be needed for further interpretations. The small sample size of both PD patients and healthy controls is another limitation of this study. Employing only two of the representative measures among various means of assessing the awareness of action can be another limitation. Examinations by various means reflecting the volitional control of action are needed for further discussion.
In summary, we investigated the awareness of voluntary action in PD through implicit and explicit tasks. In both tasks, PD patients showed different patterns of reaction from controls, suggesting change in the subjective awareness of action in PD. These tendencies were consistent regardless of the side of asymmetrical motor symptoms, supporting a linkage to primary deficits in central sensorimotor processing rather than to secondary effects from motor impairment. Further investigations will be required to examine the underlying neurobiological basis.
Methods
Participants
Nineteen patients with PD (eight females) and twenty-five normal control subjects (thirteen females) participated in the study. Patients were recruited from Kyoto University Hospital and met the UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria. Patients were excluded if they had a cognitive decline that prevented them from providing informed consent. The Hoehn and Yahr stages of the patients were 1–3. Eighteen patients were being treated with daily dopaminergic medications. Patients were on their daily medication at the time of the experiment. The total daily levodopa equivalent dose (LED) was calculated48. Sixteen patients had left dominant onset of the disease, while three patients had right dominant onset. Motor symptoms were assessed with the UPDRS Part 3 motor subscale. The UPDRS right and left subscales were calculated by combining the scores of the UPDRS part 3 items 20–26 in each of the right and left limbs. The more-affected and the less-affected sides of motor symptoms were decided by comparing these scores, as right symptom dominant (right > left) and left symptom dominant (left > right). None of the controls had a known neurological or psychiatric history. All participants in both groups were right-handed according to the Edinburg Inventory49. BDI50, Apathy Scale51, General Self Efficacy Scale52, 53, Cognistat54, and Frontal Assessment Battery (FAB)55 were assessed in both groups. Written informed consent was obtained from each participant. This study was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee and was carried out in accordance with The Code of Ethics of the World Medical Association.
Experiment 1- implicit task
Procedures
The sequence of events from a previous study22, known as intentional binding task, was employed. The task consisted of four categories of conditions: 1) agency action, 2) agency tone, 3) baseline action, and 4) baseline tone. In the agency action and agency tone conditions, participants performed a voluntary action. In each condition, a blank screen was first presented, followed by a picture of a clock-face and clock-hand. The clock-hand was 12 mm long, and it rotated clockwise for a full rotation in 2,560 ms. The clock-face was marked with 12 conventional interval positions (5, 10, 15, etc.). Initial positions of the clock-hand were chosen randomly from the 12 positions. The clock-hand remained stationary at the initial position for 500 ms, and then began to rotate. Participants performed a key press at a time of their own choosing during clock-hand rotation. They were instructed to avoid responding at a pre-decided clock position, or during the first half-rotation of the clock-hand. Each key press triggered a tone after a fixed period of 250 ms. The tone duration was 100 ms, and the frequency was 1000 Hz. In the agency action condition, participants were asked to report the perceived onset time of their voluntary key press as judged by the perceived position of the clock-hand. Similarly, in the agency tone condition, participants were asked to report the perceived onset time of the triggered tone. In the baseline action condition, participants performed a voluntary key press at the time of their own choosing, but this did not yield a tone. Participants reported the perceived onset time of their voluntary key press. In the baseline tone condition, participants did not press a key but instead waited for a tone to be delivered, judging the onset time at which they heard the tone. Because the performance of patients could be disturbed by motor symptoms, trials that were missed or not conducted successfully were repeated. The first 24 trials conducted successfully with the participants’ intended button press were brought to analysis. After completing the task with one hand, participants conducted the task with the other hand. The order of right and left hand was counterbalanced across the participants. In order to keep participants naïve to the purpose of the experiment, the implicit task was conducted first, followed by the explicit task. Details of the task procedure are described elsewhere32.
Data analysis
The perceived time of action or tone in each trial was compared with the actual onset time. The mean estimation for actions and tones in the baseline condition was subtracted from that in the agency condition. These shifts served as measures of “action binding (AB)” and “tone binding (TB)”, respectively.
AB and TB, respectively, were examined by mixed-effect ANOVA, with group (patients versus controls) as between-subject factor, and hand (more-affected versus less-affected hand in patients, left versus right hand in controls) as within-subject factor. The more-affected hand in patients was matched to the left hand in controls, and the less-affected hand in patients was matched to the right hand in controls for the ANOVA analysis, as there were more patients with left dominant onset than with right dominant onset and prognosis of the disease.
Experiment 2- explicit task
Procedures
A simplified task from a previous study21 was employed. Participants were asked to hold a joystick that was connected to a computer. A black cover was placed over the joystick so that the participants could not see their actual movement. Instead, an image of an electronically constructed virtual hand was presented to the participants on a computer screen. Participants were instructed that “their hand” would appear on the computer screen. Participants made voluntary movements of the joystick, while a program synthesized images of a virtual hand holding a joystick and the virtual hand moved according to the position that was actually held by the participants. The movement of the joystick was presented on the screen with an intrinsic delay of 16 ms. In each trial, after a blank screen, an image of a virtual hand was presented for 10 sec, during which participants were asked to move the joystick according to their own choosing. Movement could be executed in four directions (right, left, back, and forth). A yes-or-no question of correspondence was made after participants stopped their movement. Participants were asked a yes-or-no question as follows: “Did the movement you saw on the screen correspond to the movement you made with your hand?” Participant’s hand was back in the home position when answering the question.
The task consisted of three categories of conditions: 1) neutral, 2) with angular biases, and 3) with temporal biases. In the neutral condition, the virtual hand moved exactly according to the movements the participants made with the joystick. In the angular biases condition, a given angular value (5°, 10°, 15°, or 20°) was introduced, and in the temporal biases condition, a given time delay (50, 100, 150, 200, 300, 400, or 500 ms) was introduced as a gap between the movements of the virtual hand and the joystick. Trials with angular biases and trials with temporal biases were run four times for each type of gap. Neutral trials were run twelve times. The order of presentation of all trials was randomized for each subject. Before running the experiment, participants performed a practice session. Missed trials were repeated. After completing the task with one hand, participants conducted the task with the other hand. The order of right and left hand was counterbalanced across participants.
There could potentially be two types of errors in the reaction: “yes” responses for trials with biases, and “no” responses for neutral trials. For data analysis, “yes” responses were focused upon, reflecting the participants’ ability to recognize the movement as their own. The experimental setups are illustrated in Fig. 3.
Figure 3.
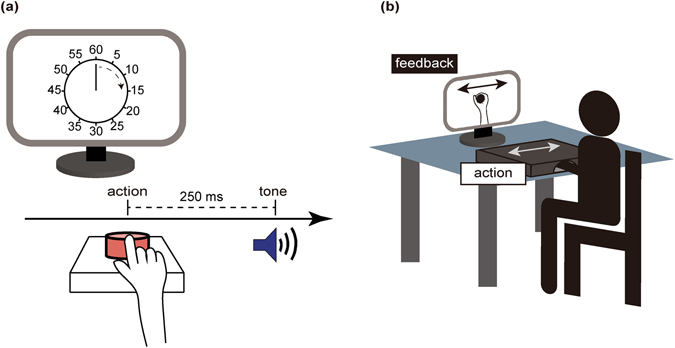
Illustration for experimental setups and protocols for (a) Experiment 1 and (b) Experiment 2.
Data analysis
One patient was excluded from the analysis due to a technical failure in presenting the task. The percentage of ‘yes’ responses in the angular biases condition was analyzed by mixed-effect ANOVA, with group (patients versus controls) as between-subject factor, and event (gap of 0°, 5°, 10°, 15°, or 20°) and hand (more-affected versus less-affected in patients, left versus right in controls) as within-subject factor. Next, the percentage of ‘yes’ responses in the temporal biases condition was analyzed by mixed-effect ANOVA, with group (patients versus controls) as between-subject factor, and event (delay of 0, 50, 100, 150, 200, 300, 400, or 500 ms) and hand (more-affected versus less-affected in patients, left versus right in controls) as within-subject factor. Degrees of freedom were adjusted by Greenhouse-Geisser correction. The more-affected hand in patients was matched to the left hand in controls, and the less-affected hand in patients was matched to the right hand in controls in the following analysis, for the same reason as in implicit task.
Electronic supplementary material
Acknowledgements
This work was supported by MEXT KAKENHI [grant numbers 24243061, 23120009, 16H01504, 16H06572]; JSPS KAKENHI [grant number 23680045]; Health and Labour Science Research Grant for Comprehensive Research on Disability Health and Welfare (H25-seishin-jitsuyouka-ippan-001) from the Ministry of Health, Labour and Welfare Japan; Grant for research and development of technology for enhancing functional recovery of elderly and disabled people based on non-invasive brain imaging and robotic assistive devices from the National Institute of Information and Communications Technology; and the Takeda Science Foundation. The authors wish to extend their gratitude to Takeshi Kaneko for critique of the study design, Takashi Tsukiura, Paeksoon Park, Shikiho Dote and Ami Tabata for cooperation, and most of all, to the participants of the study.
Author Contributions
N.S., K.T. and H.T. conceived the study and participated in its design. N.S., H.Y., N.S., R.T. and T.M. took part in coordination. N.S. carried out the experiments. N.S. and S.S. participated in the data analysis. N.S. drafted the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-08482-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elbaz A, Tranchant C. Epidemiologic studies of environmental exposures in Parkinson’s disease. J. Neurol. Sci. 2007;262:37–44. doi: 10.1016/j.jns.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 3.Moore AP. Impaired sensorimotor integration in parkinsonism and dyskinesia: a role for corollary discharges? J. Neurol. Neurosurg. Psychiatry. 1987;50:544–552. doi: 10.1136/jnnp.50.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis GN, Byblow WD. Altered sensorimotor integration in Parkinson’s disease. Brain. 2002;125:2089–2099. doi: 10.1093/brain/awf200. [DOI] [PubMed] [Google Scholar]
- 5.Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov. Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher S. Philosophical conceptions of the self: implications for cognitive science. Trends Cogn. Sci. 2000;4:14–21. doi: 10.1016/S1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- 7.Synofzik M, Vosgerau G, Newen A. I move, therefore I am: a new theoretical framework to investigate agency and ownership. Conscious. Cogn. 2008;17:411–424. doi: 10.1016/j.concog.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Moore JW, Dickinson A, Fletcher PC. Sense of agency, associative learning, and schizotypy. Conscious. Cogn. 2011;20:792–800. doi: 10.1016/j.concog.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garbarini F, et al. Abnormal Sense of Agency in Patients with Schizophrenia: Evidence from Bimanual Coupling Paradigm. Front. Behav. Neurosci. 2016;10:43. doi: 10.3389/fnbeh.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Agata F, et al. The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum. 2011;10:600–610. doi: 10.1007/s12311-011-0276-z. [DOI] [PubMed] [Google Scholar]
- 11.Kranick SM, et al. Action-effect binding is decreased in motor conversion disorder: implications for sense of agency. Mov. Disord. 2013;28:1110–1116. doi: 10.1002/mds.25408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolpe N, et al. The medial frontal-prefrontal network for altered awareness and control of action in corticobasal syndrome. Brain. 2014;137:208–220. doi: 10.1093/brain/awt302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pazzaglia M, Galli G. Loss of agency in apraxia. Front. Hum. Neurosci. 2014;8:751. doi: 10.3389/fnhum.2014.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricciardi, L. et al. Acting without being in control: Exploring volition in Parkinson’s disease with impulsive compulsive behaviours. Parkinsonism Relat. Disord. (2017). [DOI] [PubMed]
- 15.Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000;355:1771–1788. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blakemore SJ, Wolpert DM, Frith CD. Abnormalities in the awareness of action. Trends Cogn. Sci. 2002;6:237–242. doi: 10.1016/S1364-6613(02)01907-1. [DOI] [PubMed] [Google Scholar]
- 17.Moore J, Haggard P. Awareness of action: Inference and prediction. Conscious. Cogn. 2008;17:136–144. doi: 10.1016/j.concog.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Wegner DM. The mind’s best trick: how we experience conscious will. Trends Cogn. Sci. 2003;7:65–69. doi: 10.1016/S1364-6613(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 19.Takahata K, et al. It’s not my fault: postdictive modulation of intentional binding by monetary gains and losses. PLoS One. 2012;7:e53421. doi: 10.1371/journal.pone.0053421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentsch A, Synofzik M. Affective coding: the emotional dimension of agency. Front. Hum. Neurosci. 2014;8:608. doi: 10.3389/fnhum.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franck N, et al. Defective recognition of one’s own actions in patients with schizophrenia. Am. J. Psychiatry. 2001;158:454–459. doi: 10.1176/appi.ajp.158.3.454. [DOI] [PubMed] [Google Scholar]
- 22.Haggard P, Clark S, Kalogeras J. Voluntary action and conscious awareness. Nat. Neurosci. 2002;5:382–385. doi: 10.1038/nn827. [DOI] [PubMed] [Google Scholar]
- 23.Libet B, Gleason CA, Wright EW, Pearl DK. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential) The unconscious initiation of a freely voluntary act. Brain. 1983;106(Pt 3):623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- 24.Farrer C, et al. The angular gyrus computes action awareness representations. Cereb. Cortex. 2008;18:254–261. doi: 10.1093/cercor/bhm050. [DOI] [PubMed] [Google Scholar]
- 25.David N, Newen A, Vogeley K. The “sense of agency” and its underlying cognitive and neural mechanisms. Conscious. Cogn. 2008;17:523–534. doi: 10.1016/j.concog.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Farrer C, et al. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003;18:324–333. doi: 10.1016/S1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 27.Moore JW, et al. Dopaminergic medication boosts action-effect binding in Parkinson’s disease. Neuropsychologia. 2010;48:1125–1132. doi: 10.1016/j.neuropsychologia.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Wolpe N, Haggard P, Siebner HR, Rowe JB. Cue integration and the perception of action in intentional binding. Exp. Brain Res. 2013;229:467–474. doi: 10.1007/s00221-013-3419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshie M, Haggard P. Negative emotional outcomes attenuate sense of agency over voluntary actions. Curr. Biol. 2013;23:2028–2032. doi: 10.1016/j.cub.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Haggard P. Sense of agency in the human brain. Nat. Rev. Neurosci. 2017;18:196–207. doi: 10.1038/nrn.2017.14. [DOI] [PubMed] [Google Scholar]
- 31.Synofzik M, Vosgerau G. Beyond the comparator model. Conscious. Cogn. 2012;21:1–3. doi: 10.1016/j.concog.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Saito N, Takahata K, Murai T, Takahashi H. Discrepancy between explicit judgement of agency and implicit feeling of agency: Implications for sense of agency and its disorders. Conscious. Cogn. 2015;37:1–7. doi: 10.1016/j.concog.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Barone P, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 2009;24:1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 34.Caap-Ahlgren M, Lannerheim L. Older Swedish women’s experiences of living with symptoms related to Parkinson’s disease. J. Adv. Nurs. 2002;39:87–95. doi: 10.1046/j.1365-2648.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- 35.Wegner DM, Wheatley T. Apparent mental causation. Sources of the experience of will. Am. Psychol. 1999;54:480–492. doi: 10.1037//0003-066x.54.7.480. [DOI] [PubMed] [Google Scholar]
- 36.Aarts H, Custers R, Wegner DM. On the inference of personal authorship: enhancing experienced agency by priming effect information. Conscious. Cogn. 2005;14:439–458. doi: 10.1016/j.concog.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Bandura A. Self-Efficacy Mechanism in Human Agency. Am. Psychol. 1982;37:122–147. doi: 10.1037/0003-066X.37.2.122. [DOI] [Google Scholar]
- 38.Godau J, Hussl A, Lolekha P, Stoessl AJ, Seppi K. Neuroimaging: current role in detecting pre-motor Parkinson’s disease. Mov. Disord. 2012;27:634–643. doi: 10.1002/mds.24976. [DOI] [PubMed] [Google Scholar]
- 39.Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson’s disease. J. Neurosci. 2010;30:1049–1056. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Synofzik M, Vosgerau G, Newen A. Beyond the comparator model: a multifactorial two-step account of agency. Conscious. Cogn. 2008;17:219–239. doi: 10.1016/j.concog.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Waszak F, Cardoso-Leite P, Hughes G. Action effect anticipation: neurophysiological basis and functional consequences. Neurosci. Biobehav. Rev. 2012;36:943–959. doi: 10.1016/j.neubiorev.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Fantoni C, Rigutti S, Gerbino W. Bodily action penetrates affective perception. PeerJ. 2016;4:e1677. doi: 10.7717/peerj.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson’s disease normalization strategies and underlying mechanisms. Brain. 1996;119:551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- 44.Klockgether T, Dichgans J. Visual control of arm movement in Parkinson’s disease. Mov. Disord. 1994;9:48–56. doi: 10.1002/mds.870090108. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 46.O’Sullivan SS, et al. Nonmotor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov. Disord. 2008;23:101–106. doi: 10.1002/mds.21813. [DOI] [PubMed] [Google Scholar]
- 47.Pont-Sunyer C, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study) Mov. Disord. 2015;30:229–237. doi: 10.1002/mds.26077. [DOI] [PubMed] [Google Scholar]
- 48.Tomlinson CL, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010;25:2649–2653. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 49.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 50.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 51.Starkstein SE, Fedoroff JP, Price TR, Leiguarda R, Robinson RG. Apathy following cerebrovascular lesions. Stroke. 1993;24:1625–1630. doi: 10.1161/01.STR.24.11.1625. [DOI] [PubMed] [Google Scholar]
- 52.Sherer M, Adams CH. Construct-Validation of the Self-Efficacy Scale. Psychol. Rep. 1983;53:899–902. doi: 10.2466/pr0.1983.53.3.899. [DOI] [Google Scholar]
- 53.Narita K, et al. A Japanese Version of the Generalized Self-Efficacy Scale - Scale Utility from the Life-Span Perspective. Japanese Journal of Educational Psychology. 1995;43:306–314. doi: 10.5926/jjep1953.43.3_306. [DOI] [Google Scholar]
- 54.Kiernan RJ, Mueller J, Langston JW, Van Dyke C. The Neurobehavioral Cognitive Status Examination: a brief but quantitative approach to cognitive assessment. Ann. Intern. Med. 1987;107:481–485. doi: 10.7326/0003-4819-107-4-481. [DOI] [PubMed] [Google Scholar]
- 55.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


