Abstract
A photoreceptor's information capture is constrained by the structure and function of its light‐sensitive parts. Specifically, in a fly photoreceptor, this limit is set by the number of its photon sampling units (microvilli), constituting its light sensor (the rhabdomere), and the speed and recoverability of their phototransduction reactions. In this review, using an insightful constructionist viewpoint of a fly photoreceptor being an ‘imperfect’ photon counting machine, we explain how these constraints give rise to adaptive quantal information sampling in time, which maximises information in responses to salient light changes while antialiasing visual signals. Interestingly, such sampling innately determines also why photoreceptors extract more information, and more economically, from naturalistic light contrast changes than Gaussian white‐noise stimuli, and we explicate why this is so. Our main message is that stochasticity in quantal information sampling is less noise and more processing, representing an ‘evolutionary adaptation’ to generate a reliable neural estimate of the variable world.
Keywords: adaptive sampling, drosophila, information theory, photoreceptor, vision, quantum bump
Abbreviations
- GWN
Gaussian white‐noise
- NS
naturalistic stimulation
- R1–R6s
outer photoreceptors
- R7 and R8
inner photoreceptors
- TRP/TRPL
transient receptor potential/transient receptor potential like
Introduction to adaptive quantal information sampling
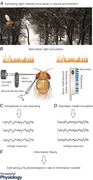
A fly photoreceptor collects information about the world by counting photons within its receptive field (Fig. 1 A). These counts (samples) are its quantum bumps – small discrete transmembrane ion fluxes, produced by single microvilli (sampling units, Fig. 1 B) in response to single photons (Hardie & Juusola, 2015). A fruit fly (Drosophila melanogaster) R1–R6 photoreceptor has ∼30,000 microvilli, each of which houses full phototransduction reactions. Collectively, the microvilli form the photoreceptor's light guide, the rhabdomere, and their quantum bumps, through stochastic size and timing variations (Fig. 1 A), integrate for each moment (time bin) its graded macroscopic response (output) to light intensity changes (input).
Figure 1. Schematic representation of adaptive quantal light information sampling by a Drosophila R1–R6 photoreceptor.
A, each R1–R6 samples photon influx by ∼30,000 microvilli, which together form its photosensitive light guide, the rhabdomere. Single‐photon responses (quantum bumps) from individual microvilli integrate a macroscopic response. B, top, each microvillus contains full phototransduction reactions, generating one quantum bump (sample) to an absorbed photon at a time; voltage and Ca2+‐dependent feedbacks regulate sample size and speed. Bottom, stochastic processes simulate bump generation. Molecular participants in microvillar phototransduction reactions: C, Ca2+‐dependent negative feedback to multiple targets; D, DAG; M, metarhodopsin; P, G protein‐PLC complex; T, TRP/TRPL channels (*, activated form). Red and green dotted arrows indicate negative and positive feedbacks, respectively, as used in the stochastically operating R1–R6 model (Song et al. 2012; Song & Juusola, 2014; Juusola et al. 2015). The gating mechanisms are yet unresolved, but these probably include production of DAG, InsP 3, proton, and physical microvilli contraction (Hardie & Franze, 2012).
Based on information theory (Shannon, 1948), information in photoreceptor output depends upon the signal‐to‐noise ratio of its frequency‐domain representation (Fig. 2). This estimate, which measures the reproducibility of the underlying quantum bump size and rate changes (Juusola & Hardie, 2001a), can be inferred from high‐quality intracellular recordings to repeated light stimulation by signal and noise analyses (Juusola et al. 1994, 2016b; Juusola & Hardie, 2001a, b ) and reproduced by stochastic simulations (Song et al. 2012; Song & Juusola, 2014, 2017; Juusola et al. 2015). Equally, this information is the difference between the output entropy and noise entropy rates (Shannon, 1948; Juusola & de Polavieja, 2003).
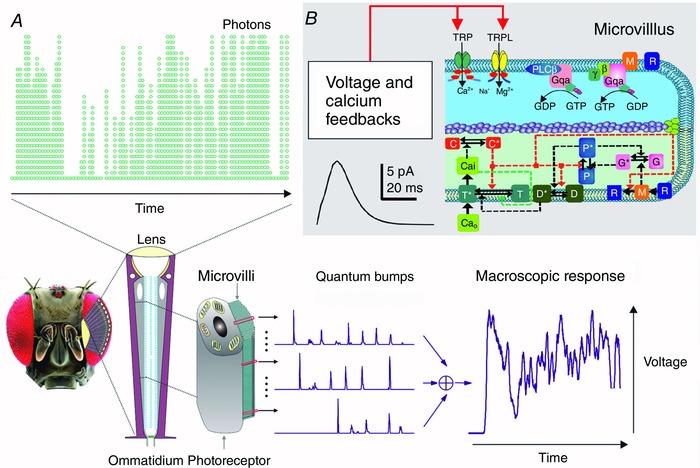
Figure 2. Photoreceptors that generate more, faster and more precise samples (quantum bumps) from the same light stimulus carry more visual information.
A–C, macroscopic voltage responses of fruit fly (Drosophila), blowfly (Calliphora) and killer fly (Coenosia) R1–R6 photoreceptors, respectively, to the same repeated naturalistic light intensity time series stimulus (NS) recorded in vivo and simulated by stochastic models. The number of microvilli (sampling units) and their average quantum bump waveforms (sample size; green) and latency distributions (sample jitter; grey) from in vivo recordings were used in the corresponding stochastic models, having no free parameters. The simulated voltage responses (green) to the NS behaved as their real counterparts (black). D–F, respective signal‐to‐noise ratios (SNR) and the corresponding information transfer rates of the simulated responses follow those of the real recordings. Data are from Song et al. (2012).
Experiments and simulations about light information sampling in R1–R6 photoreceptors of different fly species, which boast different microvilli numbers and quantum bump speeds (Fig. 2 A–C), have demonstrated that the photoreceptor's signalling performance (Fig. 2 D–F) increases as a function of quantum bump production rate. Specifically, the larger, the finer and the more precise a photoreceptor's bump rate changes are, the higher its information transfer rate. This means that fast flying Calliphora and Coenosia can extract more visual information from the same natural environment than a slow flying Drosophila, in which photoreceptors, respectively, have either fewer or slower microvilli and thus produce fewer, slower and more variable quantum bumps.
Visual invariance emerging from adaptive quantal sampling
Nonetheless, the accurate behaviours of both diurnal and nocturnal insects (Esch et al. 2001; Gonzalez‐Bellido et al. 2011; Baird et al. 2015; Stürzl et al. 2016) suggest that insects perceive the world consistently, requiring their visual systems to generate highly invariable neural representations of natural images and objects over vastly (logarithmically) varying light conditions. Remarkably, this invariance is already clearly seen in both locust (Fig. 3 A and B) and fly photoreceptor (Fig. 3 C) outputs (Faivre & Juusola, 2008; Song et al. 2012; Friederich et al. 2016) and is mechanistically traceable to two central adaptations in quantal information sampling:
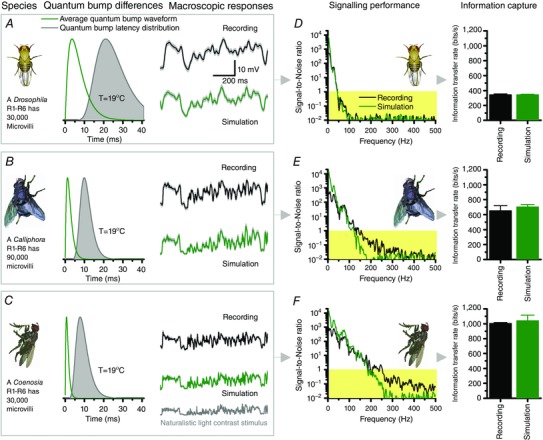
Figure 3. Quantal sampling adapts to provide invariable responses from natural contrast changes.
A, intracellularly recorded locust photoreceptor output to the same naturalistic contrast pattern has a similar waveform at dim (1,500 photons s−1; grey) and bright (1.5 × 105 photons s−1; black) stimulation, implying that the same frequency range is utilised at different illumination. B, this is confirmed by the similar power spectra of the corresponding average responses, or signals (n = 100 repetitions). The arrow highlights the up‐shift in gain with brightening. C, normalised voltage signals (n = 100 repetitions) of both real and simulated Drosophila R1–R6 photoreceptors to the same naturalistic contrast pattern at dim and bright illuminations indicate comparable invariance. D–F, with brightening naturalistic stimulation: D, quantum efficiency (photon‐to‐bump conversion probability) decreases as more of a R1–R6 photoreceptor's 30,000 microvilli becomes refractory (insets), incapable of producing quantum bumps for the next 50–500 ms after their last photon hit. E, however, with more microvilli being activated, sample rate increases (continuous line) until progressive reduction in quantum efficiency D, stabilizes their quantum bump output. Simultaneously, sample size (bump waveform) is attenuated (dashed line). F, a photoreceptor's information transfer rate follows the increase in its quantum bump rate. Together, the adapting quantum bump dynamics ensure that relative changes in voltage responses represent naturalistic light changes (contrasts) accurately, irrespective of the ambient illumination. Although contrast gain in absolute terms (voltage/unit contrast) increases with light intensity, the temporal structure of the transmitted signal remains practically invariable. Data are from Faivre & Juusola (2008) and Song et al. (2012).
First, after a microvillus generates a quantum bump, it is briefly rendered refractory (full range: 50–500 ms; Fig. 3 D, inset), during which it cannot respond to another photon (Song et al. 2012). This progressively reduces quantum efficiency (Fig. 3 D; a photoreceptor's photon‐to‐bump‐conversion probability) with increasing light intensity, ultimately saturating sample (quantum bump) rate changes (Fig. 3 E, continuous line) to a given contrast stimulus (Song et al. 2012).
Second, during intense quantum bump production in bright illumination, the increased Ca2+ and Na+ influx through transient receptor potential (TRP)/transient receptor potential like (TRPL) channels reduces cationic driving force (Song et al. 2012) and the average bump size and duration (Figs 1 B and 3 E, inset and dotted line) (Henderson et al. 2000; Juusola & Hardie, 2001a). Photoreceptor output is further smoothened by quantum bump jitter (latency distribution; Fig. 2 A, grey), caused by stochasticity in the microvillar phototransduction reactions (Henderson et al. 2000; Juusola & Hardie, 2001a; Song et al. 2012).
Consequently, with the average quantum count (Fig. 3 E, continuous line) and bump size (dotted line) adapting to mean light intensity, the resulting macroscopic photoreceptor voltage response to a given natural contrast stimulus scales to look similar at different illumination conditions (Faivre & Juusola, 2008; Song et al. 2012; Friederich et al. 2016) (Fig. 3 A–C). These adapting quantum bump dynamics thus much contribute to the divisive nonlinearity, associated with amplitude normalisation in empirical photoreceptor models (French et al. 1993; van Hateren & Snippe, 2006; Friederich et al. 2016), providing mechanistic insight into descriptive nonlinear systems identification. [Note: Divisive nonlinearity is an arbitrary but necessary mathematical operation in empirical (black‐box) photoreceptor models to compress vast light input changes into their limited output range. It makes the model output better approximate the real photoreceptor output.] But while the average light intensity increasing over a range of magnitudes progressively reduces the photoreceptor's quantum efficiency (Fig. 3 D), the macroscopic responses still count in more quantum bumps (samples) (Fig. 3 E). This increase in their sample rate changes (from the same contrast) is the main reason why an insect photoreceptor's signal‐to‐noise ratio and information transfer (Fig. 3 F) increases with brightening until saturation (Juusola et al. 1994; Juusola & Hardie, 2001a; Faivre & Juusola, 2008; Frederiksen et al. 2008; Heimonen et al. 2012; Song & Juusola, 2014), when about half of its microvilli become refractory (Song et al. 2012). [Note: in dim stimulation, a photoreceptor may count 10 quantum bumps (samples) in time‐bin1 and 2 in time‐bin2. But in brighter stimulation, time‐bin1 may have 100 and time‐bin2 20 samples. Thus, the corresponding sample rate changes would be 8 and 80 samples/time, with the brightening increasing their difference by 10‐fold. The larger the sample rate changes, the higher the entropy rate. And if reproducible (having low noise), then the larger sample rate changes have a higher signal‐to‐noise ratio and transmit more information.]
Thus, at the level of sampling, the neural code of insect photoreceptors inherently emphasises natural contrast constancy (relative light changes in the environment remain the same in different illumination conditions) (Attneave, 1954; Barlow, 1961; van Hateren, 1997) and efficiently allocates this information as invariable response waveforms within their limited output range (Atick, 1992; van Hateren, 1992b). And it does this, unavoidably, at the expense of coding the absolute light intensity.
Markedly, however, losing photons galore to refractory microvilli, when the flux of incident photons into distinct receptive fields in daylight can be 106–109 photons s−1, is not critical for good vision. As long as a Drosophila R1–R6 photoreceptor counts up ∼15,000–150,000 quantum bumps s−1, its neural estimate of local contrast changes will be reliable (of very high signal‐to‐noise ratio), with each photoreceptor in the eye providing hundreds of bits of information per second to the brain.
Functional comparisons in light information processing and representation between different insect photoreceptors suggest that perhaps all rhabdomeric photoreceptors would sample quantal light information similarly. Yet, by evolving different microvilli numbers and phototransduction speeds (Fig. 2), photoreceptors of different species have specialised visual capabilities for different life‐styles and habitats (Wong et al. 1982; van Hateren, 1992a; Juusola et al. 1994; van Steveninck & Laughlin, 1996; Anderson & Laughlin, 2000; Juusola & Hardie, 2001a; Niven et al. 2007; Faivre & Juusola, 2008; Frederiksen et al. 2008; Gonzalez‐Bellido et al. 2011; Frolov et al. 2012; Heimonen et al. 2012; Song et al. 2012; Song & Juusola, 2014; Song et al. 2016). Here, the trade‐off is that while having more microvilli increases photoreceptor output, its bandwidth, and information transfer rate for representing natural contrast changes (Song & Juusola, 2014), so does the total cost for constructing, maintaining and running this sampling machinery (Laughlin et al. 1998; Song & Juusola, 2014).
Future work is needed to test how these sampling rules and constraints apply to nocturnal moths, which have high microvillus numbers but seemingly noisy macroscopic responses (Stockl et al. 2016) and possibly inferior information transfer rates compared to diurnal insects with fewer microvilli. Moreover, an open question remains of how the refractory light information sampling dynamics of microvillar photoreceptors compare mechanistically with bleaching adaptation of vertebrate ciliary photoreceptors (Yau & Hardie, 2009).
Antialiasing through quantal adaptive sampling
Interestingly, stochastic quantal information sampling is not only an elegant light‐adaptation strategy but possibly also an evolutionary solution to the temporal aliasing problem to provide reliable neural estimates of the variable world (Juusola et al. 2015). It scatters high‐frequency information into broadband noise rather than generating the false patterns produced by regular sampling (Dippe & Wold, 1985). Thus, variable sampling times and sample sizes (quantum bump jitter and size differences) prevent distortions or artefacts, such as harmonic oscillations (Song et al. 2012), in reconstruction of macroscopic responses from the original (continuous) light patterns. And because the flies have neural superposition eyes, which provide eight independent estimates (outer R1–R6 and inner R7 and R8 photoreceptors) of local light intensity changes for each image pixel (Kirschfeld & Franceschini, 1969; Horridge & Meinertzhagen, 1970), parallel sampling by microvilli in each photoreceptor and the later synaptic pooling of their macroscopic outputs would actively cancel noise.
In support of this view, stochastic modelling implies that intrinsic noise – caused by the quantum bump variations – seems to degrade the fly photoreceptor output less than what was thought before (Lillywhite & Laughlin, 1979; Laughlin & Lillywhite, 1982), maximally ∼5–10% (Song et al. 2012; Song & Juusola, 2014; Juusola et al. 2015). The concerted action of many thousands of microvilli in photon sampling reduces intrinsic noise as their quantum bumps add up the macroscopic response. Moreover, global (intracellular calcium and membrane voltage) feedbacks (Fig. 1 B), which carry memory of the past events, reduce noise by adapting the bump sizes to the ongoing light stimulation. This accounts for ∼10% improvement in the rate of information transfer, in comparison to sampling the bumps randomly from the same distribution (Song et al. 2012). Intrinsic noise is almost certainly further reduced when the parallel macroscopic responses of photoreceptors, which view the same point in space, are pooled in convergent synaptic transmission to interneurons (Zheng et al. 2006).
Here, the key realisation is that integration of variable samples, which are much briefer than the world structure they encode in time, increases information and reduces noise, improving the robustness, reliability and accuracy of the resulting neural estimates (see also: Galton, 1907; Heimonen et al. 2006; Padmanabhan & Urban, 2010). Conversely, filtering responses downstream, which occurs by a voltage‐sensitive cell membrane (Hardie, 1991; Weckstrom et al. 1991; Vähäsöyrinki et al. 2006), can reshape and smoothen photoreceptor output but not increase its information (data processing theorem) (Shannon, 1948; Juusola & de Polavieja, 2003; Abou Tayoun et al. 2011).
Armed with this essential mechanistic knowledge about fly photoreceptors’ stochastic refractory photon sampling, we next briefly consider why and how this makes encoding inefficient for Gaussian white‐noise (GWN) stimulation but sensitised to salient natural world features.
Gaussian white‐noise stimulation does not fully test photoreceptor performance
In electronic systems, GWN stimulus, which maximises information within its bandwidth and variance, is regularly used for testing information transmission capacity. However, for neural systems, such as photoreceptors, which employ adaptive quantal information sampling, GWN fails to test their true signalling performance (Rieke et al. 1995; Juusola & de Polavieja, 2003; Song & Juusola, 2014).
This disparity has less to do with the used GWN's absolute energy, phase distribution or photon content, but primarily depends upon how a photoreceptor samples photons (Song & Juusola, 2014). Therefore, a bright GWN stimulus with a mean intensity that is 100‐fold higher than the mean intensity of a naturalistic stimulus would still yield lower information transfer estimates in a photoreceptor. This is because stochastic photon sampling by finite refractory microvilli populations makes a photoreceptor to encode different stimulus statistics differently, with different efficiencies and costs (Fig. 4). Specifically, longer dark contrasts, which characterize naturalistic stimuli, help to recover more refractory microvilli than equally bright stimuli without these features, improving neural information while lowering its metabolic costs (Song & Juusola, 2014). Photoreceptor output to natural/naturalistic stimulation thus typically entails larger phasic quantum bump rate changes, while maintaining a lower mean level of depolarization (costing less) than corresponding responses to GWN (Juusola & de Polavieja, 2003; Song & Juusola, 2014). Conversely, GWN experiments underestimate a photoreceptor's information transmission capacity, while often overestimating its normal energy consumption (Song & Juusola, 2014), making the subsequent neuro‐economics estimates and their cross‐species comparisons unrealistic.
Figure 4. Fly photoreceptors encode Gaussian white‐noise (GWN) and naturalistic stimuli (NS) differently, with different efficiencies and costs.
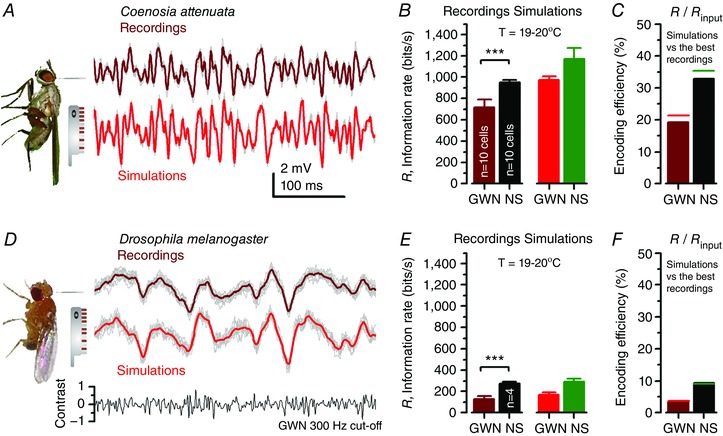
Both fast‐flying Coenosia and slow‐flying Drosophila photoreceptors have 30,000 microvilli, but those of Coenosia sample light changes and recover from them faster, resulting in higher information capture. A, voltage responses of a Coenosia R1–R6 photoreceptor (brown) and respective stochastic model simulations (red) to unit‐contrast GWN stimulation with 300 Hz cut‐off; light level: ∼106 photons s−1. B, information transfer of recorded and simulated Coenosia R1–R6 voltage responses to GWN stimulus and naturalistic stimulation (NS; see Fig. 2); these cells capture ∼20% less information from the GWN than NS. C, overall, Coenosia R1–R6s encoded ∼30% of information in NS; performing ∼1.7 times more efficiently than with 300 Hz GWN. D, Drosophila R1–R6 voltage output (brown) and respective stochastic model simulations (red) to the same unit‐contrast GWN stimulation as in A. E, information transfer rates of recorded and simulated Drosophila R1–R6 voltage responses to GWN (D) and NS; these cells capture ∼60% less information from the GWN than NS. Moreover, Drosophila R1–R6s encode both the stimuli less efficiently than Coenosia R1–R6s. F, Drosophila photoreceptors encoded NS ∼2.5 times more efficiently than 300 Hz GWN. In every cell, NS evoked higher information transfer. Here simulated Coenosia photoreceptor output carries proportionally more information than the average recordings because it is based on the best GWN and NS recordings. Simulations lack recording noise and muscle activity, which reduce information in recordings, and the intracellular pupil mechanism. Data are from Song & Juusola (2014).
For example, at 25°C, a typical Drosophila R1–R6 photoreceptor would sample 282 bits s−1 from 200 Hz band‐limited GWN, costing 1.31 × 107 ATP molecules bit−1. However, from a rich naturalistic contrast stimulus of equal mean brightness, the same photoreceptor would sample 455 bits s−1 with the price of 1.15 × 107 ATP molecules bit−1 (Song & Juusola, 2014).
Adaptive quantal sampling enhances salient stimuli
Importantly, adaptive quantal sampling gives a fly photoreceptor an innate capacity to enhance stimulus salience. Adaptation that follows the phototransduction cannot increase the photoreceptor's information transfer rate (Juusola & de Polavieja, 2003). However, if the receptor adapts during the process of sampling, it can accentuate quantum bump rate changes to new (surprising) stimuli, increasing information transfer rate transiently.
Naturalistic stimulation includes longer dark contrasts that are not present in GWN stimulation. The effect of short‐term adaptation to these events was first quantified by showing how Calliphora R1–R6s’ information transfer changes during three identical naturalistic stimulus sequences (Fig. 5 A; marked 1, 2 and 3) that followed a 1 s dark period (Juusola & de Polavieja, 2003). In repetitive stimulation, the responses to the 1st sequence (green) were always larger (Fig. 5 B) and carried more information (Fig. 5 C) than the responses to the 2nd and 3rd sequences. Thus, the signalling precision of fly photoreceptors was higher at transitions from dark to bright light and then reduced with adaptation to a lower voltage response.
Figure 5. Quantal adaptive sampling innately accentuates salient contrast changes, boosting their information content.
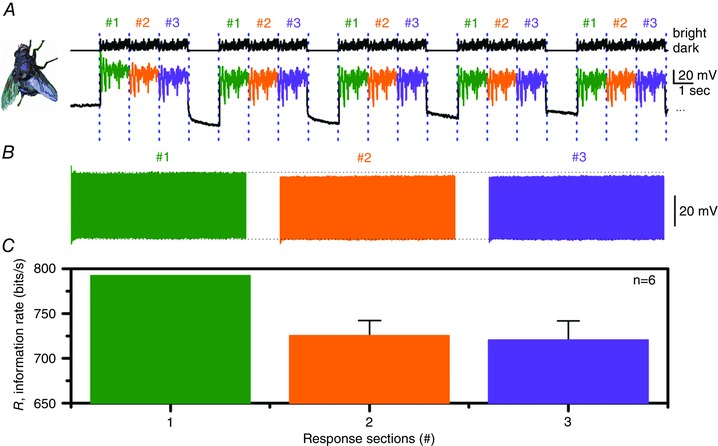
A, upper traces, a bright light stimulus consisting of 3 identical naturalistic intensity sequences, each lasting 1 s and numbered 1, 2 and 3, followed by a 1 s‐long dark period is repeated 1000 times. Lower traces, a typical Calliphora R1–R6 photoreceptor voltage response to this stimulus. B, the photoreceptor responses for these three groups are separated and grouped retaining the timing order. Notice that the responses to the first naturalistic stimulus sequence are slightly larger than the responses to the second and third stimulus sequences. C, the average information transfer rate of the responses during the three stimulus sequences. Voltage responses to the 1st stimulus sequence carry more information than those to the 2nd and 3rd sequences. This behaviour was consistent in all the recordings (n = 6) giving the first second of responses on average 9.5% higher information transfer rates. Data are from Juusola & de Polavieja (2003).
Further recordings and photoreceptor model simulations have since demonstrated that the first larger response to a bright step simply contains more quantum bumps, and thus has a higher signal‐to‐noise ratio than subsequent responses, for which fewer microvilli are activated, with more microvilli becoming refractory (Juusola & de Polavieja, 2003; Song et al. 2012; Song & Juusola, 2014). Similarly, the first negative voltage response to a dark contrast step will be larger (Juusola, 1993) because more microvilli will be refractory, generating fewer quantum bumps than the subsequent responses. Accordingly, the photoreceptors’ information transfer is higher at large dark‐to‐bright or bright‐to‐dark contrast transitions and decreases afterwards in correlation with the adaptation to the stimulus (Juusola & de Polavieja, 2003; Zheng et al. 2006, 2009).
Thus, not only does adaptive quantal sampling lead to robust encoding of natural light changes over the full dynamic range of environmental light intensities (Fig. 3) (Faivre & Juusola, 2008; Song et al. 2012, 2016; Friederich et al. 2016; Juusola et al. 2016a), it also enhances novel or surprising stimuli, which generate the largest quantum bump rate changes (increments or decrements) with respect to the ongoing average (Juusola & de Polavieja, 2003; Song et al. 2012; Song & Juusola, 2014) (Fig. 5). Remarkably, further analyses have shown that while accentuating saliency, adaptive quantal photon sampling also improves the allocation of information in naturalistic stimulation on the photoreceptors’ limited bandwidth and amplitude range (van Hateren, 1997; Zheng et al. 2009; Song et al. 2012; Juusola et al. 2016a). As the output frequency distribution flattens (or whitens) while its amplitude distribution becomes Gaussian, every symbol (voltage value) of a message (macroscopic voltage response) would be transmitted equally often (Shannon, 1948).
Discussion
In this review, we have presented a basic account of how a fly photoreceptor samples light information in time, and why this improves vision. For clarity, the focus was upon stochastic adaptive photon sampling to highlight its fundamental role in generating reliable macroscopic responses to environmental light contrast changes. This meant that the primary sampling process was considered in isolation.
While the description given is accurate, the reality is more complex and in perpetual motion, and we know that optimal visual information sampling, at least in Drosophila, further involves elaborate photomechanical adaptations and self‐motion (body, head and eye movements), which prevent retinal images from fading during fast adaptation (Juusola et al. 2016a). In fact, during photon sampling, light input intensity is regulated by two photomechanical processes inside photoreceptors. Slower screening pigment migration (intracellular pupil, 1–10 s) (Franceschini & Kirschfeld, 1971, 1976) and much faster autonomous microsaccadic photoreceptor contractions (0.01–1 s) (Hardie & Franze, 2012; Juusola et al. 2016a) dynamically reduce photon flux into the rhabdomere, shifting and narrowing its receptive field (Juusola et al. 2016a). In addition, downstream, in the photoreceptor axons, asymmetric synaptic and gap‐junctional inputs from the network differentiate individual R1–R6 outputs (Shaw, 1984; Shaw et al. 1989; Zheng et al. 2006, 2009; Nikolaev et al. 2009; Rivera‐Alba et al. 2011; Wardill et al. 2012; Dau et al. 2016).
How all these factors contribute to spatiotemporal encoding of the visual world and perception, providing Drosophila hyperacute vision, is analysed in detail in (Juusola et al. 2016a) and is beyond the scope of this review.
Additional information
Competing interests
The authors declare no conflict of interest.
Author contributions
M.J. and Z.S. wrote the paper. M.J. and Z.S. approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the open research fund of the State Key Laboratory of Cognitive Neuroscience and Learning (M.J.), NSFC project (30810103906: M.J.), Jane and Aatos Erkko Foundation (M.J.), The Leverhulme Trust grant (RPG‐2012‐567: M.J.) and the BBSRC grants (BB/F012071/1, BB/D001900/1 and BB/H013849/1: M.J.). EPSRC‐funded 2020 Science fellowship (EP/I017909/1: S.Z.) provided the major funding for the ‘1st UK Phototransduction and Synaptic Transmission’ workshop.
Acknowledgements
We thank Uwe Friederich for some illustrations.
Biographies
Mikko Juusola (left) is Professor at the University of Sheffield, UK, and Visiting Professor at Beijing Normal University, China. His background is in neurophysiology and biophysics.

Zhuoyi Song (right) is senior researcher at the University of Sheffield. Her background is in systems engineering. Visual information processing in insect photoreceptors and interneurones is one of their major research interests. Using intracellular in vivo electrophysiology, information theory and mathematical modelling, their work has contributed to the elucidation of biophysical mechanisms that govern visual information sampling and processing in microvillar photoreceptors.
This review was presented at the symposium “Phototransduction and synaptic transmission” which took place at the Phototransduction UK workshop, Sheffield, 31 August – 2 September 2016.
References
- Abou Tayoun AN, Li XF, Chu B, Hardie RC, Juusola M & Dolph PJ (2011). The Drosophila SK channel (dSK) contributes to photoreceptor performance by mediating sensitivity control at the first visual network. J Neurosci 31, 13897–13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC & Laughlin SB (2000). Photoreceptor performance and the co‐ordination of achromatic and chromatic inputs in the fly visual system. Vision Res 40, 13–31. [DOI] [PubMed] [Google Scholar]
- Atick JJ (1992). Could information‐theory provide an ecological theory of sensory processing. Network 3, 213–251. [DOI] [PubMed] [Google Scholar]
- Attneave F (1954). Some informational aspects of visual perception. Psychol Rev 61, 183–193. [DOI] [PubMed] [Google Scholar]
- Baird E, Fernandez DC, Wcislo WT & Warrant EJ (2015). Flight control and landing precision in the nocturnal bee Megalopta is robust to large changes in light intensity. Front Physiol 6, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB (1961). Possible principles underlying the transformation of sensory messages In Sensory Communication, ed. Rosenblith WA. MIT Press, Cambridge, MA, USA. [Google Scholar]
- Dau A, Friederich U, Dongre S, Li X, Bollepalli MK, Hardie RC & Juusola M (2016). Evidence for dynamic network regulation of Drosophila photoreceptor function from mutants lacking the neurotransmitter histamine. Front Neural Circuits 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippe MAZ & Wold EH (1985). Antialiasing through stochastic sampling. Comput Graphics 19, 69–78. [Google Scholar]
- Esch HE, Zhang SW, Srinivasan MV & Tautz J (2001). Honeybee dances communicate distances measured by optic flow. Nature 411, 581–583. [DOI] [PubMed] [Google Scholar]
- Faivre O & Juusola M (2008). Visual coding in locust photoreceptors. PLoS One 3, e2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini N & Kirschfeld K (1971). Phenomena of pseudopupil in the compound eye of Drosophila . Kybernetik 9, 159–182. [DOI] [PubMed] [Google Scholar]
- Franceschini N & Kirschfeld K (1976). Automatic‐control of light flux in compound eye of Diptera – spectral, statical, and dynamical properties of mechanism. Biol Cybern 21, 181–203. [Google Scholar]
- Frederiksen R, Wcislo WT & Warrant EJ (2008). Visual reliability and information rate in the retina of a nocturnal bee. Curr Biol 18, 349–353. [DOI] [PubMed] [Google Scholar]
- French AS, Korenberg MJ, Jarvilehto M, Kouvalainen E, Juusola M & Weckstrom M (1993). The dynamic nonlinear behavior of fly photoreceptors evoked by a wide‐range of light intensities. Biophys J 65, 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich U, Billings SA, Hardie RC, Juusola M & Coca D (2016). Fly photoreceptors encode phase congruency. PLoS One 11, e0157993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov R, Immonen EV, Vahasoyrinki M & Weckstrom M (2012). Postembryonic developmental changes in photoreceptors of the stick insect Carausius morosus enhance the shift to an adult nocturnal life‐style. J Neurosci 32, 16821–16831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton F (1907). Vox populi. Nature 75, 450–451. [Google Scholar]
- Gonzalez‐Bellido PT, Wardill TJ & Juusola M (2011). Compound eyes and retinal information processing in miniature dipteran species match their specific ecological demands. Proc Natl Acad Sci USA 108, 4224–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC (1991). Voltage‐sensitive potassium channels in Drosophila photoreceptors. J Neurosci 11, 3079–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC & Franze K (2012). Photomechanical responses in Drosophila photoreceptors. Science 338, 260–263. [DOI] [PubMed] [Google Scholar]
- Hardie RC & Juusola M (2015). Phototransduction in Drosophila . Curr Opin Neurobiol 34C, 37–45. [DOI] [PubMed] [Google Scholar]
- Heimonen K, Immonen EV, Frolov RV, Salmela I, Juusola M, Vahasoyrinki M & Weckstrom M (2012). Signal coding in cockroach photoreceptors is tuned to dim environments. J Neurophysiol 108, 2641–2652. [DOI] [PubMed] [Google Scholar]
- Heimonen K, Salmela I, Kontiokari P & Weckstrom M (2006). Large functional variability in cockroach photoreceptors: optimization to low light levels. J Neurosci 26, 13454–13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SR, Reuss H & Hardie RC (2000). Single photon responses in Drosophila photoreceptors and their regulation by Ca2+ . J Physiol 524, 179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horridge GA & Meinertzhagen IA (1970). Accuracy of patterns of connexions of first‐order and second‐order neurons of visual system of Calliphora . Proc R Soc Lond B Biol Sci 175, 69–82. [DOI] [PubMed] [Google Scholar]
- Juusola M (1993). Linear and nonlinear contrast coding in light‐adapted blowfly photoreceptors. J Comp Physiol A 172, 511–521. [Google Scholar]
- Juusola M, Dau A, Song Z, Solanki N, Rien D, Jaciuch D, Dongre S, Blanchard F, de Polavieja GG, Hardie RC & Takalo J (2016a). Microsaccadic information sampling provides Drosophila hyperacute vision. bioRxiv doi: 10.1101/083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M, Dau A, Zheng L & Rien D (2016b). Electrophysiological method for recording intracellular voltage responses of Drosophila photoreceptors and interneurons to light stimuli in vivo. J Vis Exp, e54142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M & de Polavieja GG (2003). The rate of information transfer of naturalistic stimulation by graded potentials. J Gen Physiol 122, 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M & Hardie RC (2001a). Light adaptation in Drosophila photoreceptors: I. Response dynamics and signaling efficiency at 25 degrees C. J Gen Physiol 117, 3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M & Hardie RC (2001b). Light adaptation in Drosophila photoreceptors: II. Rising temperature increases the bandwidth of reliable signaling. J Gen Physiol 117, 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M, Kouvalainen E, Jarvilehto M & Weckstrom M (1994). Contrast gain, signal‐to‐noise ratio, and linearity in light‐adapted blowfly photoreceptors. J Gen Physiol 104, 593–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M, Song Z & Hardie RC (2015). Phototransduction biophysics In Encyclopedia of Computational Neuroscience, ed. Jaeger D. & Jung R, pp. 2359–2376. Springer, New York. [Google Scholar]
- Kirschfeld K & Franceschini N (1969). A mechanism for directing flow of light in rhabdomeres of complex eyes in Musca . Kybernetik 6, 13–22. [DOI] [PubMed] [Google Scholar]
- Laughlin SB & Lillywhite PG (1982). Intrinsic noise in locust photoreceptors. J Physiol 332, 25–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, van Steveninck RRD & Anderson JC (1998). The metabolic cost of neural information. Nat Neurosci 1, 36–41. [DOI] [PubMed] [Google Scholar]
- Lillywhite PG & Laughlin SB (1979). Transducer noise in a photoreceptor. Nature 277, 569–572. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, Zheng L, Wardill TJ, O'Kane CJ, de Polavieja GG & Juusola M (2009). Network adaptation improves temporal representation of naturalistic stimuli in Drosophila eye: II mechanisms. PLoS One 4, e4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven JE, Anderson JC & Laughlin SB (2007). Fly photoreceptors demonstrate energy‐information trade‐offs in neural coding. PLoS Biol 5, 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan K & Urban NN (2010). Intrinsic biophysical diversity decorrelates neuronal firing while increasing information content. Nat Neurosci 13, 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Bodnar DA & Bialek W (1995). Naturalistic stimuli increase the rate and efficiency of information transmission by primary auditory afferents. Proc Biol Sci 262, 259–265. [DOI] [PubMed] [Google Scholar]
- Rivera‐Alba M, Vitaladevuni SN, Mischenko Y, Lu ZY, Takemura SY, Scheffer L, Meinertzhagen IA, Chklovskii DB & de Polavieja GG (2011). Wiring economy and volume exclusion determine neuronal placement in the Drosophila brain. Curr Biol 21, 2000–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE (1948). A mathematical theory of communication. AT&T Tech J 27, 379–423. [Google Scholar]
- Shaw SR (1984). Early visual processing in insects. J Exp Biol 112, 225–251. [DOI] [PubMed] [Google Scholar]
- Shaw SR, Frohlich A & Meinertzhagen IA (1989). Direct connections between the R7/8 and R1‐6 photoreceptor subsystems in the dipteran visual‐system. Cell Tissue Res 257, 295–302. [DOI] [PubMed] [Google Scholar]
- Song Z & Juusola M (2014). Refractory sampling links efficiency and costs of sensory encoding to stimulus statistics. J Neurosci 34, 7216–7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z & Juusola M (2017). A biomimetic fly photoreceptor model educidates how stochastic adaptive quantal sampling provides a large dynamic range. J Physiol 595, 5439–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Postma M, Billings SA, Coca D, Hardie RC & Juusola M (2012). Stochastic, adaptive sampling of information by microvilli in fly photoreceptors. Curr Biol 22, 1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Zhou Y & Juusola M (2016). Random photon absorption model elucidates how early gain control in fly photoreceptors arises from quantal sampling. Front Comput Neurosci 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockl AL, O'Carroll DC & Warrant EJ (2016). Neural summation in the hawkmoth visual system extends the limits of vision in dim light. Curr Biol 26, 821–826. [DOI] [PubMed] [Google Scholar]
- Stürzl W, Zeil J, Boeddeker N & Hemmi JM (2016). How wasps acquire and use views for homing. Curr Biol 26, 470–482. [DOI] [PubMed] [Google Scholar]
- Vähäsöyrinki M, Niven JE, Hardie RC, Weckström M & Juusola M (2006). Robustness of neural coding in Drosophila photoreceptors in the absence of slow delayed rectifier K+ channels. J Neurosci 26, 2652–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hateren JH (1992a). Theoretical predictions of spatiotemporal receptive‐fields of fly LMCs, and experimental validation. J Comp Physiol A 171, 157–170. [Google Scholar]
- van Hateren JH (1992b). A theory of maximizing sensory information. Biol Cybern 68, 23–29. [DOI] [PubMed] [Google Scholar]
- van Hateren JH (1997). Processing of natural time series of intensities by the visual system of the blowfly. Vision Res 37, 3407–3416. [DOI] [PubMed] [Google Scholar]
- van Hateren JH & Snippe HP (2006). Phototransduction in primate cones and blowfly photoreceptors: different mechanisms, different algorithms, similar response. J Comp Physiol A 192, 187–197. [DOI] [PubMed] [Google Scholar]
- van Steveninck RRD & Laughlin SB (1996). The rate of information transfer at graded‐potential synapses. Nature 379, 642–645. [Google Scholar]
- Wardill TJ, List O, Li XF, Dongre S, McCulloch M, Ting CY, O'Kane CJ, Tang SM, Lee CH, Hardie RC & Juusola M (2012). Multiple spectral inputs improve motion discrimination in the Drosophila visual system. Science 336, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckstrom M, Hardie RC & Laughlin SB (1991). Voltage‐activated potassium channels in blowfly photoreceptors and their role in light adaptation. J Physiol 440, 635–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Knight BW & Dodge FA (1982). Adapting bump model for ventral photoreceptors of Limulus . J Gen Physiol 79, 1089–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau KW & Hardie RC (2009). Phototransduction motifs and variations. Cell 139, 246–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, de Polavieja GG, Wolfram V, Asyali MH, Hardie RC & Juusola M (2006). Feedback network controls photoreceptor output at the layer of first visual synapses in Drosophila . J Gen Physiol 127, 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Nikolaev A, Wardill TJ, O'Kane CJ, de Polavieja GG & Juusola M (2009). Network adaptation improves temporal representation of naturalistic stimuli in Drosophila eye: I dynamics. PLoS One 4, e4307. [DOI] [PMC free article] [PubMed] [Google Scholar]


