Abstract
Several fly species have distinctly red‐coloured eyes, meaning that the screening pigments that provide a restricted angular sensitivity of the photoreceptors may perform poorly in the longer wavelength range. The functional reasons for the red transparency and possible negative visual effects of the spectral properties of the eye‐colouring screening pigments are discussed within the context of the photochemistry, arrestin binding and turnover of the visual pigments located in the various photoreceptor types. A phylogenetic survey of the spectral properties of the main photoreceptors of the Diptera indicates that the transition of the brown eye colour of the Nematocera and lower Brachycera to a much redder eye colour of the higher Brachycera occurred around the emergence of the Tabanidae family.

Keywords: arrestin, metarhodopsin, photoreceptors, pupil, rhodopsin
Abbreviations
- A
arrestin
- ERG
electroretinography
- kb
arrestin binding constant
- kf
rate constant of the enzymatic degradation
- kg
rate constant of rebuilding active rhodopsin
- kM
rate constant for photoconversion of Ma and Mi
- kR
rate constant for photoconversion of Ra and Ri
- I
relative photon flux
- M
metarhodopsin
- Ma
active metarhodopsin
- Ma
concentration of active metarhodopsin
- Mi
inactive metarhodopsin
- PDA
prolonged depolarizing afterpotential
- R
rhodopsin
- Ra
active rhodopsin
- Ra
concentration of active rhodopsin
- Ri
inactive rhodopsin
- R0
number of visual pigment molecules in a microvillus
- Rh
rhodopsin
- R1–6
peripheral or outer photoreceptors
- R7,8
central or inner photoreceptors
- R7p, R8p
central photoreceptors in pale rhabdomere
- R7y, R8y
central photoreceptors in yellow rhabdomere
- UV
ultraviolet
Introduction
Insect compound eyes analyse the light distribution in their environment with their photoreceptors, commonly packed in sets of eight to nine per ommatidium. In the apposition eyes of diurnal insects, the facet lens of each ommatidium focuses incident light onto the underlying photoreceptor cells, which transduce the absorbed light into an electrical signal, thus starting the visual process. To ensure that only approximately axially entering light reaches the photoreceptors, strongly light‐absorbing screening pigment cells envelop the individual ommatidia, effectively blocking off‐axis stray light. With their numerous ommatidia arranged in a more or less spherical shell, insect compound eyes thus sample almost their complete surroundings with good spatial resolution (Land & Nilsson, 2002). The spatial resolution principally depends on the size of the eye (and in turn of the animal), but can furthermore distinctly vary across the eye, especially in predatory insects. For instance, whereas the range of the interommatidial angles of the small fruitfly Drosophila is ∼2.5–7 deg (Hardie, 1985), in the also rather small robber fly, Holcocephala fusca, this range is ∼0.3–5 deg (Wardill et al. 2017).
In most insects, the screening pigments have a high density, yielding a blackish eye colour, thus forming perfect optical screens around the ommatidia; remarkably, the screening pigments of red‐eyed flies belonging to the dipteran suborder Brachycera are quite transparent in the long‐wavelength range. Consequently, they do not perform very well in preventing red stray light from entering the photoreceptors, thereby potentially degrading spatial resolution. The screening pigments’ poor absorption in the longer wavelength range is of historical interest, because in the early days, when spectral sensitivities were measured in fly eyes by electroretinography (ERG), a distinct red peak in the ERG of Calliphora blowflies was interpreted as representing a class of red‐sensitive receptors, although its participation in colour discrimination could not be demonstrated (Autrum & Stumpf, 1953). Goldsmith later demonstrated, by measuring ERGs in wild‐type and white‐eyed mutant Musca houseflies, that the red receptor was an artefact due to long‐wavelength stray light, leaking through the red‐transparent screening pigment, thus stimulating large numbers of photoreceptors (Goldsmith, 1965). Subsequent intracellular recordings of blowfly photoreceptors confirmed this view (Streck, 1972). In the main visible wavelength range, wild‐type photoreceptors were found to have angular sensitivities with an acceptance angle (i.e. the half‐width of the Gaussian approximation to the angular sensitivity characteristic) of 2.8 deg. In the long‐wavelength range, in which sensitivity is comparatively low, the acceptance angle widened to 4 deg (at 625 nm), and the angular sensitivity function had a distinct tail, which extended to large off‐axial values (Streck, 1972).
Consequently, red‐eyed flies exposed to direct sunlight experience a considerable challenge. The acceptance angle of the photoreceptors is generally much larger than the sun's angular diameter so that photoreceptors facing the sun are saturated within milliseconds. In the rest of the eye, the light entering photoreceptors from the visual surroundings that they face is several log units less intense. The screening pigments must hence be very dense, to prevent light from the sun from overwhelming the signals coding other parts of image.
A most notable example of a distinctly red‐eyed fly is the fruitfly Drosophila. Its red eyes must mean poor vision in the long‐wavelength part of the visual spectrum, which raises the question why the red screening pigments have evolved. To understand the flies’ screening pigment transparency and thus poor visual performance in the red we have to recall the organization of fly ommatidia (Hardie, 1985; Hardie & Postma, 2008). Each ommatidium contains a set of six so‐called peripheral or outer photoreceptors, R1–6, and two central or inner photoreceptors, R7 and R8 (Fig. 1 A and B). A photoreceptor's principal organelle is the rhabdomere, harbouring the visual pigment as well as the phototransduction apparatus. The rhabdomeres of the R1–6 cells extend the full length from the distal to the proximal retina, while the rhabdomeres of R7 and R8 cells form a tandem with the R7 rhabdomere sitting distal to the R8 rhabdomere. Two primary pigment cells surround the transparent pseudocone, located proximally of the facet lens, as well as the distal part of the photoreceptors. Secondary pigment cells surround the primary pigment cells and the photoreceptors, along the whole length of the ommatidia (Fig. 1 A and B).
Figure 1. Fly ommatidia, screening pigments and pupil mechanism.
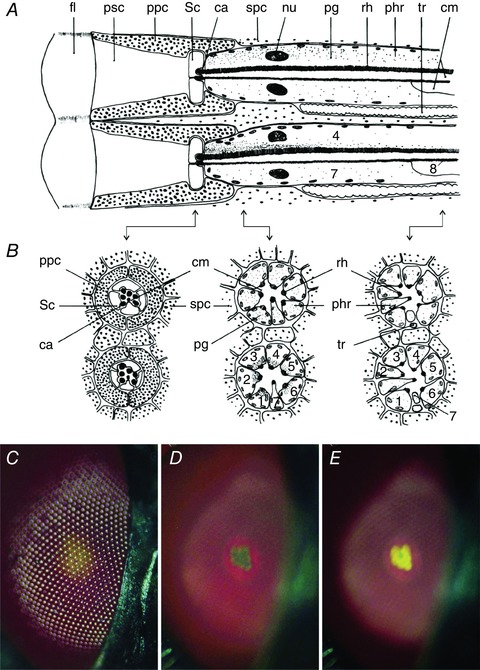
A, diagram of two fly eye ommatidia (upper: dark adapted; lower: light adapted), with each a facet lens (fl), a pseudocone (psc), two primary pigment cells (ppc), four Semper cells (Sc), seven rhabdomere caps (ca), six secondary pigment cells (spc), eight photoreceptor cells (phr) having a rhabdomere (rh) and in the cell soma a nucleus (nu) and mobile pigment granules (pg), and furthermore there is a trachea (tr) and in between the photoreceptors a central matrix (cm). B, cross‐sections of the diagram at locations indicated by the arrows. C, epi‐illumination of a light‐adapted, red‐coloured fly eye (genus unidentified) with centrally yellowish facets seen at the level of the cornea. D, the eye observed at the level of the centre of curvature with the dark‐adapted deep‐pseudopupil. E, the light‐adapted deep‐pseudopupil.
Screening pigment and pupil mechanism in fly eyes
The various cells contain different screening pigments, as can be demonstrated by epi‐illumination light microscopy of a fly eye (Fig. 1 C–E). A light‐adapted eye observed at the level of the cornea displays reflections from the lattice of convex facet lenses together with a main brown‐red eye colour and a central yellowish area (Fig. 1 C). Focusing at the level of the eye's centre of curvature reveals the so‐called deep pseudopupil (Franceschini, 1975; Franceschini & Kirschfeld, 1976). This shows within the main brown‐red background colour, which is generated by reflections from pigment in the secondary pigment cells, a reddish ring, representing an image of the primary pigment cells (Fig. 1 D and E). When dark‐adapted, within the red ring a hint of yellow is seen (Fig. 1 D), which upon prolonged illumination intensifies and creates a yellow‐coloured trapezoidal pattern, identical to the pattern of the R1–6 rhabdomeres (Fig. 1 E). This phenomenon is due to yellow granules in the cell somata of the R1–6 photoreceptors, which migrate toward the rhabdomere when the photoreceptors are illuminated with bright light (Fig. 1 A and B). The assembly of pigment granules functions in each photoreceptor as a dynamic screen, which reduces the light flux propagating in the visual pigment‐containing rhabdomere, i.e. it acts in a similar way as the light‐controlling pupil of vertebrate eyes; in the dark the granules migrate away from the rhabdomere (Kirschfeld & Franceschini, 1969; Franceschini & Kirschfeld, 1976; Stavenga, 1979; Satoh et al. 2008). The spectral sensitivity of the fly's pupil mechanism corresponds to that of the photoreceptors, meaning that light absorbed by the photoreceptor's visual pigment is the principal trigger activating the pigment migration (Bernard & Stavenga, 1979; Stavenga, 1995).
Fly visual pigment photochemistry, phototransduction and photoregeneration
Blowfly, housefly and fruitfly photoreceptors express a variety of visual pigments (Fig. 2 A, Table 1). The R1–6 cells express a rhodopsin, Rh1, with main absorption band peaking at ∼490 nm. Whereas the chromophore of human rhodopsin is retinal, the chromophore of fly rhodopsin is 3‐hydroxy‐retinal (Vogt & Kirschfeld, 1984; Vogt, 1989; Seki & Vogt, 1998). After photon absorption by the rhodopsin molecule, the 11‐cis conformation of the chromophore changes into the all‐trans form, which causes a transformation of the visual pigment's opsin, resulting in the metarhodopsin state. The peak wavelength of Rh1's metarhodopsin absorption spectrum is strongly bathochromic‐shifted (i.e. toward longer wavelengths), to ∼570 nm (Hamdorf, 1979; Hardie, 1985; Salcedo et al. 1999). The created metarhodopsin triggers the phototransduction process, but this occurs only temporarily, because the active metarhodopsin molecule is rapidly inactivated by binding an arrestin molecule, similar to what occurs in the phototransduction process of human photoreceptors (Dolph et al. 1993; Alloway & Dolph, 1999). Importantly, and very different from human metarhodopsin which rapidly decays by photolysis, fly metarhodopsin is long‐term thermostable. The interesting consequence of the thermostability is that the all‐trans chromophore reisomerizes back into the 11‐cis conformation when the metarhodopsin molecule absorbs a photon, which is then followed by refolding of the opsin moiety and restoration of the native rhodopsin state. Subsequently the bound arrestin dissociates and the visual pigment returns to its active state, i.e. is ready for another phototransduction round (Yau & Hardie, 2009).
Figure 2. Pigments of a fly compound eye.

A–C, absorption spectra of visual pigments Rh1 and Rh3–6, of their rhodopsin state R and their metarhodopsin state M, calculated with template formulae (Stavenga, 2010). D, absorption spectra of sensitizing (antenna) pigment, carotenoid, pupillary pigment of the blowfly Calliphora (Stavenga & Hardie, 2011), and the red screening pigments of Drosophila (Strother & Casella, 1972) and Calliphora (Stavenga & Hardie, 2011). E, Absorption spectra of the rhodopsin (Rh1) and metarhodopsin (M1) of blowfly R1–6 photoreceptors with bound sensitizing pigment, and transmittance spectra of the pupillary pigment of Calliphora, assuming a peak absorbance of 1 log unit, and the screening pigments of Drosophila and Calliphora, assuming a peak absorbance of 3 log units. F, the spectral sensitivity of the photoreceptor cell R8y and the absorption spectrum of its visual pigment Rh6, which is modified by the carotenoid filter in R7y and a UV‐absorbing sensitizing pigment, and the transmittance spectra of the screening pigments of Drosophila and Calliphora.
Table 1.
Visual pigment peak wavelengths of Drosophila and Musca
| Drosophila | Musca | |||
|---|---|---|---|---|
| Rh | R/M [1] | R [2] | R/M [3] | R [4] |
| 1 | 486 / 566 | 478 | 486 / 566 | 490 |
| 3 | 331 / 468 | 345 | 340 / 468 | 335 |
| 4 | 355 / 470 | 375 | 363 / 470 | 430 |
| 5 | 442 / 494 | 437 | 437 / 494 | 460 |
| 6 | 515 /468 | 508 | 510 / 468 | 520 |
This highlights a major advantage of the photochemistry of fly visual pigments over that of vertebrate rhodopsins. To maintain sensitivity, human photoreceptors have to fully rely on a tortuous enzymatic process of metarhodopsin degradation, translation of the all‐trans retinal to the pigment epithelium and glia (Muller cells), where the chromophore is reisomerized and then shuttled back to the photoreceptor, where it couples to opsin to form rhodopsin again. The thermostability of fly metarhodopsin allows recovery of the native rhodopsin state by simply another photon absorption (Fig. 3).
Figure 3. Visual pigment cycle in fly photoreceptors.

Photoconversion of active rhodopsin (Ra) creates active metarhodopsin (Ma) which triggers the phototransduction process (red circle). Upon binding arrestin (A) it becomes inactive metarhodopsin (Mi). Photoconversion of Mi creates inactive rhodopsin (Ri), which upon arrestin release converts into the native rhodopsin (Ra). The light‐induced conversion processes have rate constants k R and k M, and the rate constants of arrestin binding and dissociation are k b and k d. Ri and Mi are degraded with rate constant k f, resulting in release of the chromophore (ret) from its opsin (ops), which then is further degraded. Ra is regenerated with rate constant k g.
A unique property of fly visual pigments is not only their 3‐hydroxy‐retinal chromophore, but also their strongly binding 3‐hydroxy‐retinol, the alcohol of the chromophore aldehyde, which has an absorption spectrum restricted to the ultraviolet. The latter acts as a sensitizing pigment (Fig. 2 D), because the energy of the absorbed ultraviolet photons is transferred to the chromophore, which then isomerizes as usual, thereby considerably enhancing the chance of molecular transformation by UV light (Kirschfeld et al. 1983; Stavenga, 2004b). The intimate connection of the sensitizing pigment with the visual pigment molecule causes a fine‐structured spectrum in the UV of both the rhodopsin and metarhodopsin (Fig. 2 E).
The strong bathochromic‐shifted absorption spectrum of the metarhodopsin with respect to the rhodopsin makes it understandable why flies have red‐leaky screening pigment. The absorption spectrum of the screening pigments fully spans the wavelength range of the rhodopsin absorption spectrum, up to ∼600 nm (Fig. 2 D and E; fruitfly Drosophila and blowfly Calliphora), but not so well the range of the metarhodopsin absorption spectrum (Fig. 2 D and E). Because long‐wavelength light preferentially reconverts the metarhodopsin back to the native rhodopsin, red‐stray light roaming in the eyes causes ready regeneration of the rhodopsin state, so restoring the pool of molecules that provides the photoreceptor cells with their light sensitivity (Stavenga et al. 1973; Stavenga, 1995; Stavenga & Hardie, 2011).
In fact, the pigment of the mobile, pupillary granules inside the photoreceptors plays a similar role. It absorbs mostly at short‐wavelengths and thus a light‐adapted, closed pupil favours metarhodopsin conversion (Stavenga et al. 1973; Stavenga, 1995; Stavenga & Hardie, 2011). Furthermore, it is interesting to note that whereas the red screening pigments in the primary and secondary pigment cells create a sharp long‐pass filter, with little absorption only at those wavelengths where rhodopsin absorption is negligible, so to preserve a narrow angular sensitivity of the photoreceptors and thus optimal spatial resolution of the visual system, the pupil filter can be less fastidious. It plays at most a minor role in blocking stray light, since the pupillary granules are located within the photoreceptors where they affect the light flux propagating in the rhabdomere. Nevertheless, like the screening pigments, the pupil does affect the angular sensitivity, because when it closes, it decreases the photoreceptor acceptance angle, due to waveguide effects (Hardie, 1979; Smakman et al. 1984; Stavenga, 2004a).
Visual pigment cycle and turnover
Continuous light exposure of photoreceptors causes rhodopsin conversion, followed by visual pigment degradation and thus a decrease in light sensitivity, which is counteracted by a continuous cycle of restoration. As stated above, the latter occurs in vertebrates by an elaborate enzymatic process. Prolonged illumination of fly photoreceptors creates a photosteady state (also called photo‐equilibrium), where the distribution of the visual pigment molecules over the two thermostable states, rhodopsin and metarhodopsin, critically depends on their respective absorption spectra as well as the spectral content of the illumination. For instance, bright blue light rapidly creates in the fly R1–6 photoreceptors a photosteady state with a high metarhodopsin content, while red light results in a low metarhodopsin concentration (Stavenga et al. 1973; Hamdorf, 1979; Belušič et al. 2010). Nevertheless, visual pigment degradation also occurs in insect eyes. The presence of an enzymatic visual pigment cycle was demonstrated in Calliphora blowfly eyes (Schwemer, 1979). Strikingly, also this cycle involves both the photoreceptors and the pigment cells. First, the chromophore of the metarhodopsin molecules, all‐trans‐3‐hydroxy‐retinal, is liberated from the opsin part, which is further broken down. The all‐trans‐retinal is transported by retinal‐binding protein to the pigment cells, where a photoisomerase assists in the reconversion of the visual pigment chromophore by blue light to the 11‐cis conformation. Retinoid‐binding protein then translates it back to the photoreceptors where it is reinserted into native opsin, i.e. resulting in the biosynthesis of new rhodopsin (Schwemer, 1984, 1989). A similar visual pigment cycle exists also in Drosophila (Wang et al. 2010). Rhodopsin regeneration through an enzymatic cycle hence occurs universally. Vertebrate visual systems are different mainly because the metarhodopsins rapidly decay, while the metarhodopsins of invertebrates are much more thermostable (Yau & Hardie, 2009).
Curiously, the thermostability of invertebrate metarhodopsins and the possibility to regain the rhodopsin state by photoconversion has led some researchers of Drosophila photoreceptors to adhere to ‘the dogma that the visual cycle in invertebrates does not exist’ (Arshavsky, 2010; Wang et al. 2010). A possible reason for this erroneous belief may have been the observation of an extremely long‐lasting prolonged depolarizing afterpotential (PDA) that can be created in Drosophila photoreceptors (Wright & Cosens, 1977; Pak, 1995). The PDA phenomenon occurs generally in insect photoreceptors when a very bright flash converts an excessive amount of rhodopsin molecules, thus suddenly creating a large number of active metarhodopsin molecules that trigger the phototransduction process. Arresting all active metarhodopsins after the flash then takes some time, resulting in a prolonged afterpotential, the PDA. The PDA's duration strongly depends on the illumination conditions. In Drosophila photoreceptors, bright blue light results in an unabated PDA, because the number of available arrestins is less than the number of created metarhodopsin molecules, so that the surplus of active metarhodopsins continues to trigger the phototransduction process for many hours (Liu et al. 2008; Satoh et al. 2010). In blowflies, the time course of the PDA lasts at most a few minutes (Hamdorf, 1979), suggesting that here the photoreceptors contain a larger amount of arrestin molecules. Here, the time course of the PDA decay probably reflects translocation of arrestin molecules from the cytosol to the rhabdomere (Satoh et al. 2010; Stavenga & Hardie, 2011).
In early electrophysiological experiments on fruitfly eyes, distinct, long‐lasting changes in the ERG responsivity were found when bright blue light converted a majority of rhodopsin to the metarhodopsin state (Cosens & Briscoe, 1972; Pak & Lidington, 1974; Wright & Cosens, 1977). These changes, intimately connected to the PDA elicited in the photoreceptors, had a duration of many hours, which until recently has been interpreted as an inability of photoreceptors of invertebrates, unlike the vertebrate photoreceptors, to regain the original sensitivity via enzymatic means (Arshavsky, 2010; Wang et al. 2010).
It is now clear that both photochemical and enzymatic pathways can govern the recovery of sensitivity through rhodopsin regeneration. The speed of enzymatic turnover can be very different between species. For instance, metarhodopsin breakdown occurs in butterflies within minutes and rhodopsin recovery within an hour, of course dependent on temperature (Bernard, 1983; Vanhoutte & Stavenga, 2005). However, in the principal R1–6 fly photoreceptors, metarhodopsin persists for several hours in the dark, which indicates that the enzymatic cycle has a low efficiency, presumably due to a low concentration of the metarhodopsin‐degrading enzymes and/or of the other members of the enzymatic cycle. Photoreconversion of metarhodopsin via red stray light is apparently largely sufficient for maintenance of the rhodopsin content (Hardie & Postma, 2008).
Deleterious effects of red‐transparent screening pigment on the R1–6 photoreceptors
An important point to consider now is that the stray light not only can photoreconvert metarhodopsin molecules, but can also be absorbed by rhodopsins, as demonstrated by the artefactually encountered red‐receptor and the tail in the angular sensitivity functions of the R1–6 fly photoreceptors when tested with red light stimuli (Streck, 1972). Although the spectral overlap of the absorption spectrum of the R1–6 rhodopsin and the transmittance spectrum of the screening pigment is minor (Fig. 2 E), red stray light will inevitably create a noisy background, degrading the photoreceptor's contrast signal, especially in red‐eyed flies exposed to bright sunlight. This must have a negative effect on spatial resolution and signal quality, opposing the benefit of sensitivity gained by rhodopsin photoregeneration. Although it is generally assumed that the signal‐degrading effect in normal environmental conditions must be negligible, it has not yet been unequivocally demonstrated. Flies are well known for their excellent visual capacities, and several studies have shown optimization of signal‐to‐noise in fly photoreceptors (e.g. Burton et al. 2001; Juusola & Hardie, 2001; Niven et al. 2007; Song et al. 2016). We may therefore expect that flies do not suffer from noise contamination and contrast loss of their visual signals by stray light, but explicit experimental studies demonstrating that indeed fly vision is improved by red‐transparent screening pigments have not yet been undertaken.
If the function of the red screening pigments of flies is indeed to assist the photoconversion of metarhodopsin, it will only work when the metarhodopsin spectrum is bathochromically shifted and well separated from its rhodopsin spectrum. This is, however, only the case for rhodopsins with absorption peak wavelength below 500 nm (Stavenga, 1992, 1995). The main rhodopsins of virtually all insects apart from higher Diptera are green‐absorbing, with peak wavelength above 500 nm (Table 2), and their metarhodopsins absorb maximally in the blue, i.e. have a hypsochromic‐shifted (i.e. toward shorter wavelengths) absorption spectrum. Virtually all insect eyes therefore have very dense pigments with broad‐wavelength absorption spectra, because red‐transparent screening pigments will result in red stray light, preferentially converting the green rhodopsin instead of the blue‐peaking metarhodopsin, thus severely reducing sensitivity (Stavenga & Hardie, 2011). This immediately raises another question, also needing further study, namely whether the red‐transparency of the screening pigments of red‐eyed flies acts beneficially for all photoreceptors. We will further discuss this below.
Table 2.
Peak wavelengths (λmax) of the main photoreceptors and eye colour of Diptera
| Family | Genus, species | λmax (nm) | SP | Sex | Region | Eye colour | Method | Ref |
|---|---|---|---|---|---|---|---|---|
| Culicidae | Aedes aegypti | 523 | F | D,V | B | E | 1 | |
| Culicidae | Aedes albopictus | 515 | + | M | D | B | E | 2 |
| Psychodidae | Lutzomyia longipalpis | 520 | F | B | E | 3 | ||
| Psychodidae | Lutzomyia longipalpis | 546 | M | B | E | 3 | ||
| Simuliidae | Simulium sp. | 430 | + | M | D | B | I, M | 4 |
| Keroplatidae | Arachnocampa luminosa | 540 | M | B | E | 5 | ||
| Bibionidae | Bibio sp. | 440 | + | M | D | B | E | 6 |
| Bibionidae | Bibio marci | 520 | M | V | B | E | 7 | |
| Bibionidae | Bibio marci | 440 | M | D | B | E | 7 | |
| Bibionidae | Bibio marci | 520 | F | D,V | B | E | 7 | |
| Tabanidae | Tabanus bromius | 525 | F | D,V | B | I | 2 | |
| Tabanidae | Tabanus bromius | 525 | M | V | B | I | 2 | |
| Tabanidae | Tabanus bromius | 440 | + | M | D | B | I | 2 |
| Tabanidae | Haematopota sp. | 530 | B | 6 | ||||
| Stratiomyidae | Hermetia illucens | 504 | M,F | D | B | I | 8 | |
| Stratiomyidae | Hermetia illucens | 440 | + | M,F | V | B | I | 8 |
| * | — | |||||||
| Dolichopodidae | Condylostylus japonicus | 480 | + | M,F | D,V | B | E | 9 |
| Syrphidae | Allograpta obliqua | 480 | F,M | D | RB | P | 10 | |
| Syrphidae | Allograpta obliqua | 455 | F | V | RB | P | 10 | |
| Syrphidae | Eristalis arbustorum | 450 | F | D | RB | P | 10 | |
| Syrphidae | Eristalis tenax | 450 | + | F, M | RB | I, P, M | 11, 12 | |
| Syrphidae | Syrphus balteatus | 450 | M | V | RB | M | 13 | |
| Syrphidae | Syrphus balteatus | 490 | M | D | RB | M | 13 | |
| Syrphidae | Syrphus balteatus | 450 | F | D,V | RB | M | 13 | |
| Syrphidae | Syrphus sp. | 455 | M | V | RB | P | 10 | |
| Syrphidae | Toxomerus marginatus | 450 | M,F | V | RB | P | 10 | |
| Glossinidae | Glossina morsitans | 500 | + | RB | I | 14 | ||
| Calliphoridae | Lucilia (Phaenicia) sericata | 480 | + | V | RB | I | 15 | |
| Calliphoridae | Calliphora vicina | 490 | + | F, M | RB | E, I, P | 10, 16 | |
| Muscidae | Musca domestica | 490 | + | RB | I | 17 | ||
| Anthomyiidae | Delia radicum | 490 | RB | E | 18 | |||
| Tephritidae | Dacus oleae | 490 | RB | E | 19 | |||
| Diopsidae | Cyrtodiopsis dalmanni | 490 | + | RB | E | 20 | ||
| Chloropidae | Chlorops sp. | 480 | F, M | E | RB | P | 10 | |
| Drosophilidae | Drosophila melanogaster | 480 | + | F, M | V | R | P, M | 10, 21 |
| Ephydridae | Dimecoenia spinose | 480 | M | D | RB | P | 10 |
SP, sensitizing pigment; +: presence. Sex: F, female; M, male. Region: D, dorsal; E, equatorial; V, ventral. Eye colour: B, black‐brown; RB, red‐brown; R, red. Method applied for determining the photoreceptor sensitivity spectra: E, electroretinography (ERG); I, intracellular recordings; P, pupillary recordings. For the visual pigments: M, microspectrophotometry (MSP). Ref, references: 1, Muir et al. 1992; 2, G. Belušič, in prep.; 3, Mellor et al. 1996; 4, Kirschfeld & Vogt, 1986; 5, Meyer‐Rochow & Eguchi, 1984; 6, Kirschfeld, 1986; 7, Burkhardt & De la Motte, 1972; 8, Oonincx et al. 2016; 9, Stavenga et al. 2017; 10, Bernard & Stavenga, 1979; 11, Horridge et al. 1975; 12, Stavenga, 1976; 13, Stavenga, 1979; 14, Hardie et al. 1989; 15, McCann & Arnett, 1972; 16, Paul et al. 1986; 17, Hardie, 1986; 18, Brown & Anderson, 1996; 19, Remund et al. 1981; 20, Burkhardt, 1972; 21, Salcedo et al. 1999; The asterisk corresponds to the one in Fig. 5.
Visual and screening pigments of the central photoreceptors
Whereas the ommatidia of fly eyes are homogeneous concerning the R1–6 photoreceptors, which all express rhodopsin Rh1, absorbing maximally in the blue‐green, at ∼490 nm (Fig. 2 A, Table 1), the ommatidia are heterogeneous concerning the properties of the R7 and R8 photoreceptors. As was demonstrated in the housefly Musca, in two‐thirds of the ommatidia, the R7 rhabdomeres contain, in addition to their visual pigment, a blue‐absorbing carotenoid pigment (Fig. 2 D), causing a yellow colour of their rhabdomeres when observed in transmitted light (Kirschfeld et al. 1978); the central photoreceptors hence are called R7y and R8y. The other one‐third of the ommatidia have pale central rhabdomeres, and thus these photoreceptors are called R7p and R8p. R7p and R7y express the UV‐absorbing Rh3 and Rh4, while R8p and R8y express the blue‐absorbing Rh5 and blue‐green‐absorbing Rh6, respectively (Fig. 2 B and C, Table 1).
The carotenoid in the y‐type ommatidia strongly modifies the spectral sensitivity of both the R7y and R8y photoreceptors. Notably the spectral sensitivity band of R8y is narrowed and its peak is shifted, from the blue‐green maximum of its Rh6 visual pigment, at 520 nm, into the green, at 540 nm (Fig. 2 F; Hardie, 1985). Quite curiously, however, the spectral sensitivity of the Musca R8y photoreceptors also has a fine‐structure in the UV, demonstrating the additional action of a sensitizing pigment (Hardie & Kirschfeld, 1983). Musca and Drosophila have similar photoreceptor expression patterns, but the visual pigment absorption spectra and hence the photoreceptor spectral sensitivities differ slightly (Hardie, 1985; see Table 1). Because the R7y rhabdomeres of Drosophila also contain carotenoid pigment (Feiler et al. 1992), the green‐sensitive R8y probably has a similar spectral sensitivity as shown in Fig. 2 F, but due to the shorter rhabdomere length of Drosophila photoreceptors, the induced spectral shift may be less than that in Musca.
Red‐screening pigment and the central photoreceptors
It must be considered quite worrisome that the spectral overlap of the absorption spectrum of the Rh6 rhodopsin and the transmittance spectrum of the screening pigment is significant (Fig. 2 F), and therefore in bright sunlight the absorption of red stray light by the green rhodopsin will no longer be minor. This will especially be the case for the very red‐eyed Drosophila (Fig. 2 F), and therefore it is interesting to note that the absorption peak wavelengths of the Rh6 rhodopsins of Musca and Drosophila are around 520 nm and 510 nm, respectively. The shift of Drosophila’s Rh6 absorption spectrum to shorter wavelengths may have been driven by the much less‐dense screening pigment of the fruitfly compared to the housefly.
The metarhodopsin states of the visual pigments of the R7 and R8 photoreceptors all have a main absorption band peaking in the blue to blue‐green wavelength range (Fig. 2 B and C, Table 1). The absorption spectra of the metarhodopsins of Rh3, Rh4 and Rh5 are strongly bathochromic‐shifted with respect to the rhodopsin spectra, and a long‐wavelength transparent screening pigment could then in principle be beneficial. However, photoconversion of the metarhodopsins by stray light will be negligible, because their absorption spectrum is restricted to the blue wavelength range.
On the other hand, the green‐absorbing Rh6 rhodopsin of the R8y photoreceptors has a hypsochromic‐shifted metarhodopsin, like the other green‐peaking insect rhodopsins. Red stray light hence will cause a progressive decrease in the Rh6 rhodopsin content, and thus the beneficial effect of photoregeneration of Rh1 rhodopsin in the R1–6 cells is inevitably connected to a distinctly deleterious effect on the R8y receptors. Possibly to maintain the sensitivity of the green R8 photoreceptors, rhodopsin turnover is very high, quite different from the situation in the R1–6 cells, which may be related to the demonstrated thermal instability of the green visual pigment's metarhodopsin (Salcedo et al. 1999).
Visual pigment, arrestin and the turnover cycle
The visual pigment composition in the photoreceptors depends on the light conditions, the concentration of arrestin molecules, and the components of the enzymatic turnover cycle. As shown in Fig. 3, both rhodopsin and metarhodopsin can exist in an active and inactive state. The light‐induced signal is proportional to the concentration of active rhodopsin, R a, and the rate constant k R for creation of active metarhodopsin, Ma, the trigger of the phototransduction process; k R is proportional to the illumination intensity. The lifetime of Ma is determined by the concentration of arrestin, A, and the arrestin binding constant, k b. Fly photoreceptors express two arrestin types, but arrestin2 is the dominant form that blocks Ma (Dolph et al. 1993; Hardie & Postma, 2008). Under normal light conditions, the rate constant k M of the photochemical reconversion of Ma into Ra is negligible with respect to k b A, the rate constant of Ma decay (A is the concentration of free arrestin). This is completely different for the long‐lived inactive forms, Mi and Ri. Prolonged illumination will cause a photosteady state, where the concentration ratio of rhodopsin and metarhodopsin depends on the ratio of the rate constants k R and k M, which contain the absorption spectra of the visual pigment states and the illumination spectrum (for a formal treatise of fly visual pigment photochemistry and the arrestin cycle, see Stavenga et al. 2000, and Stavenga & Hardie, 2011).
The distribution of the visual pigment molecules over the four active and inactive states crucially depends on the rate constant k f of the enzymatic degradation of the inactive states Mi and Ri and the rate constant k g of rebuilding native, active rhodopsin Ra (Fig. 3). Two special examples are shown in Fig. 4.
Figure 4. Visual pigments Rh1 and Rh6 in the photosteady state in a rhabdomeric microvillus with total R 0 = 1000 visual pigment molecules, assuming different amounts of arrestin and enzymatic rate constants, as a function of light intensity (logI; see text).

A‐‐C, population in the photosteady state of the different visual pigment states, Ra, Ri, Ma and Mi, representative for Drosophila R1–6 photoreceptors, where the number of arrestin molecules in a microvillus is A 0 = 370 of which arrestin is unbound, and turnover can be considered negligible. Illumination with broad‐band white light creates a photosteady state with 800 rhodopsin and 200 metarhodopsin molecules. D–F, population of the visual pigment states representative for a green receptor with 1000 arrestin molecules per microvillus, and assuming time constants of degradation and regeneration k f –1 = 3 min and k g –1 = 10 min.
In the case of Drosophila R1–6 photoreceptors (Fig. 4 A–C), each of the 30,000 rhabdomeric microvilli contains about R 0 = 1000 visual pigment molecules Rh1 and A 0 = 370 arrestin molecules, with k b = 0.54 s−1 molecule−1 (Hardie & Postma 2008; Liu et al. 2008). A broad‐band illumination is assumed, which causes a photosteady state of the visual pigment with 80% rhodopsin and 20% in the metarhodopsin state. With negligible degradation, i.e. k f = 0, the concentration of active metarhodopsin increases linearly with the light intensity up to about logI = 0, where I is the relative photon flux normalized so that at logI = 0 the sum of the photoconversion rate constants k R + k M = 1 s−1, that is, the time constant for creating a photosteady state is 1 s. At logI = 0 (very bright daylight) in each microvillus about one active metarhodopsin molecule then exists. At higher intensities, metarhodopsin inactivation can no longer keep up with the creation of new active metarhodopsins, so that Ma accumulates, leading to saturation (or even blinding) of the photoreceptor. Note, however, that the light‐controlling effect of the pupil has not been incorporated in Fig. 4 A–C; in Musca photoreceptors, the reduction in light flux will stretch the high‐intensity part of the graphs with about 0.8 log unit, the estimated extreme density of the pupil (Stavenga, 2004b).
A very different case, a green receptor with Rh6, is treated in Fig. 4 D–F. Each microvillus contains again 1000 visual pigment molecules, and a broad‐band illumination is assumed to cause a photosteady state with 30% rhodopsin and 70% metarhodopsin molecules. To prevent an endless PDA now 1000 arrestin molecules per microvillus are assumed, and degradation of the inactive Mi and Ri and regeneration of active Ra occurs with time constants 3 and 10 min, respectively (see Stavenga & Hardie, 2011). At low light intensities, the active metarhodopsin content then increases again linearly, but at higher light intensities the total number of visual pigment molecules (R 0; Fig. 4 D) decreases, because the rhodopsin decay is faster than the regeneration; in the vertebrate literature this is known as bleaching. The concomitant effect is that the number of active metarhodopsin molecules is maintained within bounds, i.e. less than one per microvillus, and this number stabilizes at light intensities above logI = 0 (Fig. 4 F). It also means that the photoreceptor remains within the physiological working range at very high intensities without being blinded. Further cases, with details how to calculate the concentrations of the different visual pigment states as a function of light intensities, have been discussed by Stavenga & Hardie (2011).
Visual and screening pigments in various Diptera
The Diptera (true flies) are divided into the suborders Nematocera (e.g. midges, mosquitoes) and Brachycera; the latter is subdivided into the lower Brachycera (e.g. horse flies, soldier flies, robber flies) and higher Brachycera (e.g. flower flies, fruitflies, houseflies) (Marshall, 2006). Several studies have shown that in many fly species belonging to the Brachycera, the majority of photoreceptors have a peak sensitivity at a wavelength < 500 nm, in the blue‐green wavelength range, at variance with the Nematocera, which have a majority of green‐sensitive photoreceptors, with peak wavelength around or above 530 nm. Table 2 lists the dipteran species investigated so far, together with the peak wavelengths of the main photoreceptors as determined by electro‐ or optophysiological recordings, or via spectrophotometry of the visual pigments, together with the eye colour as visually observed. The rhodopsins of the main photoreceptors of species belonging to the Nematocera have a peak wavelength ∼530 nm. This is also the case for the horse flies and soldier flies. The main photoreceptors R1–6 of the higher brachyceran species, at least the hoverflies, houseflies, blowflies and fruitflies, however, have peak wavelengths hypsochromic‐shifted to the blue‐green. Combining the peak wavelength of the major class of photoreceptors with the phylogeny of the dipteran families shows that the transition of the main photoreceptors’ peak wavelength from above to below 500 nm has occurred around the time of separation of the Tabanidae (horse flies) and the Stratiomyidae (soldier flies) from the other Brachyceran families (Fig. 5). In some nematoceran and lower brachyceran species, the main photoreceptors in the male dorsal area have peak sensitivities in the blue wavelength range, distinctly different from the main green receptors in the ventral retina (Similium, Bibio, Tabanus). Presumably the dorsal short‐wavelength receptors serve for spotting potential female partners against the blue sky. Funnily, the situation in soldier flies is the opposite: in both the male and female, the main class of photoreceptors is dorsally green sensitive and ventrally blue sensitive (Table 2).
Figure 5. Phylogeny of Diptera and peak wavelengths of the main class of photoreceptors.

The asterisk indicates the event where the rhodopsin absorption spectrum of the main photoreceptors (R1–6) has become bathochromic shifted from > 500 nm to < 500 nm. The tree was created with phyloT, which is based on NCBI taxonomy, and visualized with iTOL (Letunic & Bork, 2016).
The screening pigments of the Nematocera and lower Brachycera have a dense, dark‐brown to black colour, but those of the higher brachycerans are rather reddish‐brown (Table 2). The close correlation between the spectral properties of the main class of photoreceptors and the screening pigments strongly suggests an intimate, functional relationship. As argued above, we nevertheless may have to be cautious in concluding that the red‐transparency is a clever, beneficial solution to cheaply regenerate visual pigment. Quite possibly this is a tightrope strategy, a balancing act between optimization of the function of the R1–6 receptors and restricted degradation of the function of the R8y receptors.
An indication of the subtle tuning of the screening pigment properties may be gleaned from the difference in the pigments of the primary and secondary pigment cells. As can be deduced from Fig. 1, the primary and secondary pigment cells have a red and brown colour, respectively. Microspectrophotometry studies on squash preparations of blowfly (Calliphora) eyes revealed different types of pigment granules, red and brown, due to different redox states of xanthommatin pigment (Langer, 1967, 1975). The concentration of redder pigment in the primary pigment cells may be functionally significant, because the increased red transparency means a higher efficiency for reconversion of Rh1 metarhodopsin into rhodopsin. Incident red light entering an ommatidium only slightly off‐axis will traverse the primary pigment cells and hence only causes a slightly increased acceptance angle. Incident light entering at larger angles must be largely blocked by the pigment in the secondary pigment cells, which has a broader absorption band than the pigment in the primary pigment cells.
As a final remark, we may note that the intimate relationship between screening pigment and the photochemical properties of the visual pigments can be recognized also in a number of special cases of insect compound eyes. Striking examples are the regionally specialized dorsal eyes of dragonflies, owlflies, mayflies, male horse flies, and even of the nematoceran simuliids and bibionids (Table 2), where screening pigments only guard the rhodopsins of purely UV‐ or blue‐sensitive photoreceptors and not the bathochromic‐shifted metarhodopsins (for a review, Stavenga, 2002).
Additional information
Competing interests
None declared.
Author contributions
All authors discussed the paper content, contributed to the writing and approved the final version of the manuscript, and all those who qualify for authorship are listed.
Funding
This research was supported by the AFOSR/EOARD (grant FA9550‐15‐1‐0068 to D.S., M.W. and G.B.) and ARRS (grant P3‐0333 to G.B.).
Acknowledgements
We thank Profs Roger Hardie and Simon Laughlin for critical comments and most valuable suggestions.
Biographies
Doekele Stavenga (left), emeritus professor in Biophysics at the University of Groningen, the Netherlands, studies insect vision using optical methods. Closely related to vision, he also investigates the optics of flowers and animal coloration, of butterfly wing scales, bird feathers, beetle elytra and spider scales, applying microspectrophotometry, a newly developed imaging scatterometer and optical modelling. He enjoys a long‐term collaboration with the team of Kentaro Arikawa (Japan).

Ric Wehling is an amateur entomologist associated with Eglin Air Force Base, Florida.
Gregor Belušič (right) is an assistant professor of Animal Physiology at the University of Ljubljana, Slovenia. When not teaching, he studies the detection of colour and polarization in the insect retina with electrophysiological recordings and anatomical methods.
References
- Alloway PG & Dolph PJ (1999). A role for the light‐dependent phosphorylation of visual arrestin. Proc Natl Acad Sci USA 96, 6072–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky VY (2010). Vision: the retinoid cycle in Drosophila . Curr Biol 20, R96–R98. [DOI] [PubMed] [Google Scholar]
- Autrum H & Stumpf H (1953). Elektrophysiologische Untersuchungen über das Farbensehen von Calliphora . Zeitschr Vergl Physiol 35, 71–104. [Google Scholar]
- Belušič G, Pirih P & Stavenga DG (2010). Photoreceptor responses of fruitflies with normal and reduced arrestin content studied by simultaneous measurements of visual pigment fluorescence and ERG. J Comp Physiol A 196, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard GD (1983). Dark‐processes following photoconversion of butterfly rhodopsins. Biophys Struct Mech 9, 227–286. [Google Scholar]
- Bernard GD & Stavenga DG (1979). Spectral sensitivities of retinular cells measured in intact, living flies by an optical method. J Comp Physiol A 134, 95–107. [Google Scholar]
- Brown P & Anderson M (1996). Spectral sensitivity of the compound eye of the cabbage root fly, Delia radicum (Diptera: Anthomyiidae). Bull Entomol Res 86, 337–342. [Google Scholar]
- Burkhardt D (1972). Electrophysiological studies on the compound eye of a stalked‐eye fly, Cyrtodiopsis dalmanni (Diopsidae, Diptera). J Comp Physiol 81, 203–214. [Google Scholar]
- Burkhardt D & De la Motte I (1972). Electrophysiological studies on the eyes of Diptera, Mecoptera and Hymenoptera In Information Processing in the Visual Systems of Anthropods, ed. Wehner R, pp. 137–145. Springer, Berlin. [Google Scholar]
- Burton BG, Tatler BW & Laughlin SB (2001). Variations in photoreceptor response dynamics across the fly retina. J Neurophysiol 86, 950–960. [DOI] [PubMed] [Google Scholar]
- Cosens D & Briscoe D (1972). A switch phenomenon in the compound eye of the white‐eyed mutant of Drosophila melanogaster . J Insect Physiol 18, 627–632. [Google Scholar]
- Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M & Zuker CS (1993). Arrestin function in inactivation of G protein‐coupled receptor rhodopsin in vivo. Science 260, 1910–1916. [DOI] [PubMed] [Google Scholar]
- Feiler R, Bjornson R, Kirschfeld K, Mismer D, Rubin GM, Smith DP, Socolich M & Zuker CS (1992). Ectopic expression of ultraviolet‐rhodopsins in the blue photoreceptor cells of Drosophila: visual physiology and photochemistry of transgenic animals. J Neurosci 12, 3862–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini N (1975). Sampling of the visual environment by the compound eye of the fly: fundamentals and applications In Photoreceptor Optics, ed. Snyder AW. & Menzel R, pp. 98–125. Springer, Berlin. [Google Scholar]
- Franceschini N & Kirschfeld K (1976). Le contrôle automatique du flux lumineux dans l'oeil composé‚ des Diptères. Propriétés spectrales, statiques et dynamiques du mécanisme. Biol Cybern 21, 181–203. [Google Scholar]
- Goldsmith TH (1965). Do flies have a red receptor. J Gen Physiol 49, 265–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdorf K (1979). The physiology of invertebrate visual pigments In Handbook of Sensory Physiology, vol. VII/6A, ed. Autrum H, pp. 145–224. Springer, Berlin. [Google Scholar]
- Hardie RC (1979). Electrophysiological analysis of the fly retina. I. Comparative properties of R1‐6 and R7 and R8. J Comp Physiol A 129, 19–33. [Google Scholar]
- Hardie RC (1985). Functional organization of the fly retina In Progress in Sensory Physiology, vol. 5, ed. Ottoson D, pp. 1–79. Springer, Berlin. [Google Scholar]
- Hardie RC (1986). The photoreceptor array of the dipteran retina. Trends Neurosci 9, 419–423. [Google Scholar]
- Hardie RC & Kirschfeld K (1983). Ultraviolet sensitivity of fly photoreceptors R7 and R8: evidence for a sensitising function. Biophys Struct Mech 9, 171–180. [Google Scholar]
- Hardie RC & Postma M (2008). Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates In The Senses: A Comprehensive Reference, vol 1, Vision I, ed. Basbaum AI, pp. 77–130. Academic Press, San Diego. [Google Scholar]
- Hardie R, Vogt K & Rudolph A (1989). The compound eye of the tsetse fly (Glossina morsitans morsitans and Glossina palpalis palpalis). J Insect Physiol 35, 423–431. [Google Scholar]
- Horridge GA, Mimura K & Tsukahara Y (1975). Fly photoreceptors. II. Spectral and polarized light sensitivity in the drone fly Eristalis . Proc R Soc Lond B 190, 225–237. [DOI] [PubMed] [Google Scholar]
- Juusola M & Hardie RC (2001). Light adaptation in Drosophila photoreceptors: I. Response dynamics and signaling efficiency at 25 degrees C. J Gen Physiol 117, 3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschfeld K (1986). Activation of visual pigment: chromophore structure and function In The Molecular Mechanism of Photoreception, ed. Stieve H, pp. 31–49. Springer, Berlin, Heidelberg, New York. [Google Scholar]
- Kirschfeld K & Franceschini N (1969). Ein Mechanismus zur Steuerung des Lichtflusses in den Rhabdomeren des Komplexauges von Musca. Kybernetik 6, 13–22. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K & Vogt K (1986). Does retinol serve a sensitizing function in insect photoreceptors? Vision Res 26, 1771–1777. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K, Feiler R & Franceschini N (1978). A photostable pigment within the rhabdomere of fly photoreceptors no. 7. J Comp Physiol 125, 275–284. [Google Scholar]
- Kirschfeld K, Feiler R, Hardie RC, Vogt K & Franceschini N (1983). The sensitizing pigment in fly photoreceptors. Biophys Struct Mech 10, 81–92. [Google Scholar]
- Land MF & Nilsson D‐E (2002). Animal Eyes. Oxford University Press, Oxford. [Google Scholar]
- Langer H (1967). Über die Pigmentgranula im Facettenauge von Calliphora erythrocephala . Zeitschr Vergl Physiol 55, 354–377. [Google Scholar]
- Langer H (1975). Properties of screening pigments in insect eyes In Photoreceptor Optics, ed. Snyder AW. & Menzel R, pp. 429–455. Springer, Berlin. [Google Scholar]
- Letunic I & Bork P (2016). Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44, W242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Satoh AK, Postma M, Huang J, Ready DF & Hardie RC (2008). Ca2+‐dependent metarhodopsin inactivation mediated by calmodulin and NINAC myosin III. Neuron 59, 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA (2006) Insects: Their Natural History and Diversity. Firefly Books, Buffalo, NY, USA. [Google Scholar]
- McCann GD & Arnett DW (1972). Spectral and polarization sensitivity of the dipteran visual system. J Gen Physiol 59, 534–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor HE, Hamilton JG & Anderson M (1996). Spectral sensitivity in the eyes of male and female Lutzomyia longipalpis sandflies. Med Vet Entomol 10, 371–374. [DOI] [PubMed] [Google Scholar]
- Meyer‐Rochow VB & Eguchi E (1984). Thoughts on the possible function and origin of bioluminescence in the New Zealand glowworm Arachnocampa luminosa (Diptera: Keroplatidae), based on electrophysiological recordings of spectral responses from the eyes of male adults. N Z Entomol 8, 111–119. [Google Scholar]
- Muir LE, Thorne MJ & Kay BH (1992). Aedes aegypti (Diptera: Culicidae) vision: spectral sensitivity and other perceptual parameters of the female eye. J Med Entomol 29, 278–281. [DOI] [PubMed] [Google Scholar]
- Niven JE, Anderson JC & Laughlin SB (2007). Fly photoreceptors demonstrate energy‐information trade‐offs in neural coding. PLoS Biol 5, e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oonincx D, Volk N, Diehl J, van Loon J & Belušič G (2016). Photoreceptor spectral sensitivity of the compound eyes of black soldier fly (Hermetia illucens) informing the design of LED‐based illumination to enhance indoor reproduction. J Insect Physiol 95, 133–139. [DOI] [PubMed] [Google Scholar]
- Pak WL (1995). Drosophila in vision research. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 36, 2340–2357. [PubMed] [Google Scholar]
- Pak WL & Lidington KJ (1974). Fast electrical potential from a long‐lived, long‐wavelength photoproduct of fly visual pigment. J Gen Physiol 63, 740–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Steiner A & Gemperlein R (1986). Spectral sensitivity of Calliphora erythrocephala and other insect species studied with Fourier Interferometric Stimulation (FIS). J Comp Physiol A 158, 669–680. [Google Scholar]
- Remund U, Economopoulos A, Boller E, Agee H & Davis J (1981). Fruit fly quality monitoring: the spectral sensitivity of field‐collected and laboratory‐reared olive flies, Dacus oleae Gmel. (Diptera, Tephritidae). J Swiss Entomol Soc 54, 221–227. [Google Scholar]
- Salcedo E, Huber A, Henrich S, Chadwell LV, Chou WH, Paulsen R & Britt SG (1999). Blue‐ and green‐absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell‐specific Rh5 and Rh6 rhodopsins. J Neurosci 19, 10716–10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh AK, Li BX, Xia H & Ready DF (2008). Calcium‐activated Myosin V closes the Drosophila pupil. Curr Biol 18, 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh AK, Xia H, Yan L, Hardie RC & Ready DF (2010). Arrestin translocation is stoichiometric to rhodopsin isomerization and accelerated by phototransduction in Drosophila photoreceptors. Neuron 67, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwemer J (1979). Molekulare Grundlagen der Photorezeption bei der Schmeissfliege Calliphora erythrocephala Meig. Habilitationsschrift, Bochum. [Google Scholar]
- Schwemer J (1984). Renewal of visual pigment in photoreceptors of the blowfly. J Comp Physiol A 154, 535–547. [Google Scholar]
- Schwemer J (1989). Visual pigments of compound eyes ‐ structure, photochemistry, and regeneration In Facets of Vision, ed. Stavenga DG. & Hardie RC, pp. 112–133. Springer, Berlin. [Google Scholar]
- Seki T & Vogt K (1998). Evolutionary aspects of the diversity of visual pigment chromophores in the class Insecta. Comp Biochem Physiol B 119, 53–64. [Google Scholar]
- Smakman JG, van Hateren JH & Stavenga DG (1984). Angular sensitivity of blowfly photoreceptors: intracellular measurements and wave‐optical predictions. J Comp Physiol A 155, 239–247. [Google Scholar]
- Song Z, Zhou Y & Juusola M (2016). Random photon absorption model elucidates how early gain control in fly photoreceptors arises from quantal sampling. Front Comput Neurosci 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga DG (1976). Fly visual pigments. Difference in visual pigments of blowfly and dronefly peripheral retinula cells. J Comp Physiol 111, 137–152. [Google Scholar]
- Stavenga DG (1979). Visual pigment processes and prolonged pupillary responses in insect photoreceptor cells. Biophys Struct Mech 5, 175–185. [DOI] [PubMed] [Google Scholar]
- Stavenga DG (1992). Eye regionalization and spectral tuning of retinal pigments in insects. Trends Neurosci 15, 213–218. [DOI] [PubMed] [Google Scholar]
- Stavenga DG (1995). Insect retinal pigments: spectral characteristics and physiological functions. Prog Retin Eye Res 15, 231–259. [Google Scholar]
- Stavenga DG (2002). Colour in the eyes of insects. J Comp Physiol A 188, 337–348. [DOI] [PubMed] [Google Scholar]
- Stavenga DG (2004a). Angular and spectral sensitivity of fly photoreceptors. III. Dependence on the pupil mechanism in the blowfly Calliphora . J Comp Physiol A 190, 115–129. [DOI] [PubMed] [Google Scholar]
- Stavenga DG (2004b). Visual acuity of fly photoreceptors in natural conditions – dependence on UV sensitizing pigment and light‐controlling pupil. J Exp Biol 207, 1703–1713. [DOI] [PubMed] [Google Scholar]
- Stavenga DG (2010). On visual pigment templates and the spectral shape of invertebrate rhodopsins and metarhodopsins. J Comp Physiol A 196, 869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga DG & Hardie RC (2011). Metarhodopsin control by arrestin, light‐filtering screening pigments, and visual pigment turnover in invertebrate photoreceptors. J Comp Physiol A 197, 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga DG, Meglič A, Pirih P, Koshitaka H, Arikawa K, Wehling MF & Belušič G (2017) Photoreceptor spectral tuning by colorful, multilayered facet lenses in long‐legged fly eyes (Dolichopodidae). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 203, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga DG, Oberwinkler J & Postma M (2000). Modeling primary visual processes in insect photoreceptors InHandbook of Biological Physics, vol. 3, Molecular Mechanisms in Visual Transduction ed. Stavenga DG, DeGrip WJ. & Pugh EN, Jr, pp. 527–574. Elsevier, Amsterdam. [Google Scholar]
- Stavenga DG, Zantema A & Kuiper JW (1973). Rhodopsin processes and the function of the pupil mechanism in flies In Biochemistry and Physiology of Visual Pigments, ed. Langer H, pp. 175–180. Springer, Berlin. [Google Scholar]
- Streck P (1972). Der Einfluss des Schirmpigments auf das Sehfeld einzelner Sehzellen der Fliege Calliphora erythrocephala Meig. Z Vergl Physiol 76, 372–402. [Google Scholar]
- Strother GK & Casella AJ (1972). Microspectrophotometry of arthropod visual screening pigments. J Gen Physiol 59, 616–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte KJA & Stavenga DG (2005). Visual pigment spectra of the comma butterfly, Polygonia c‐album, derived from in vivo epi‐illumination. J Comp Physiol A 191, 461–473. [DOI] [PubMed] [Google Scholar]
- Vogt K (1989). Distribution of insect visual chromophores: functional and phylogenetic aspects In Facets of Vision, ed. Stavenga DG. & Hardie RC, pp. 134–151. Springer, Berlin. [Google Scholar]
- Vogt K & Kirschfeld K (1984). Chemical identity of the chromophores of fly visual pigment. Naturwiss 71, 211–213. [Google Scholar]
- Wang XY, Wang T, Jiao YC, von Lintig J & Montell C (2010). Requirement for an enzymatic visual cycle in Drosophila . Curr Biol 20, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardill TJ, Fabian ST, Pettigrew AC, Stavenga DG, Nordström K & Gonzalez‐Bellido PT (2017) A novel interception strategy in a miniature robber fly with extreme visual acuity. Curr Biol 27, 854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R & Cosens D (1977). Blue‐adaptation and orange‐adaptation in white‐eyed Drosophila: Evidence that the prolonged afterpotential is correlated with the amount of M580 in R1−6. J Comp Physiol 113, 105–128. [Google Scholar]
- Yamaguchi S, Desplan C & Heisenberg M (2010). Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila . Proc Natl Acad Sci USA 107, 5634–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K & Hardie RC (2009). Phototransduction motifs and variations. Cell 139, 246–264. [DOI] [PMC free article] [PubMed] [Google Scholar]


