Abstract
Butterflies use colour vision when searching for flowers. Unlike the trichromatic retinas of humans (blue, green and red cones; plus rods) and honeybees (ultraviolet, blue and green photoreceptors), butterfly retinas typically have six or more photoreceptor classes with distinct spectral sensitivities. The eyes of the Japanese yellow swallowtail (Papilio xuthus) contain ultraviolet, violet, blue, green, red and broad‐band receptors, with each ommatidium housing nine photoreceptor cells in one of three fixed combinations. The Papilio eye is thus a random patchwork of three types of spectrally heterogeneous ommatidia. To determine whether Papilio use all of their receptors to see colours, we measured their ability to discriminate monochromatic lights of slightly different wavelengths. We found that Papilio can detect differences as small as 1–2 nm in three wavelength regions, rivalling human performance. We then used mathematical modelling to infer which photoreceptors are involved in wavelength discrimination. Our simulation indicated that the Papilio vision is tetrachromatic, employing the ultraviolet, blue, green and red receptors. The random array of three ommatidial types is a common feature in butterflies. To address the question of how the spectrally complex eyes of butterflies evolved, we studied their developmental process. We have found that the development of butterfly eyes shares its molecular logic with that of Drosophila: the three‐way stochastic expression pattern of the transcription factor Spineless determines the fate of ommatidia, creating the random array in Papilio.

Keywords: behaviour, colour vision, compound eye, CRISPR‐Cas9, development, insect, photoreceptor, spectral sensitivity
Abbreviations
- Cas9
CRISPR‐associated protein‐9 nuclease
- CRISPR
clustered regularly interspaced short palindromic repeats
- Pros
Prospero
- R1–R9
photoreceptor number 1–9
- Sal
Spalt
- Sens
Senseless
- Ss
Spineless
- UV
ultraviolet
- Δλ
minimum discriminable wavelength difference
Introduction: the butterfly retina
Compound eyes are composed of a number of units called ommatidia. An ommatidium contains several photoreceptor cells (Fig. 1 A). The photoreceptor cells each bear visual pigment‐containing microvilli positioned towards the central axis of the ommatidium, where they together form a phototransductive rhabdom.
Figure 1. The compound eye of Papilio .
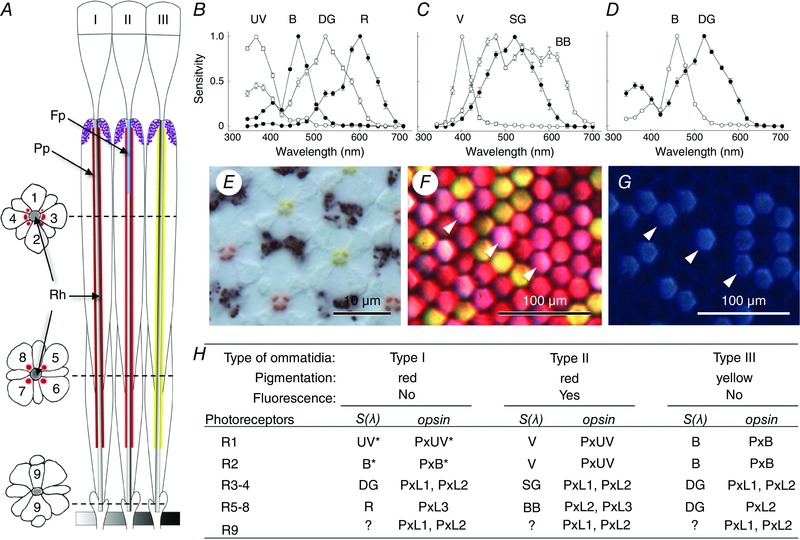
A, schematic diagram of three types of ommatidia, I, II and III. Longitudinal view with transverse sections on the left. An ommatidium contains nine photoreceptor cells (1–9) that together form the rhabdom in the centre. The rhabdom (Rh) is surrounded by red (type I and II) or yellow (type III) perirhabdomal pigments (Pp). Type II has fluorescence pigment (Fp) in the distal portion of the rhabdom. B–D, spectral sensitivities of photoreceptors contained in type I (B), type II (C) and type III (D) ommatidium. UV, ultraviolet; B, blue; DG, double‐peaked green; R, red; V, violet; SG, single‐peaked green; BB, broad‐band. E, an unstained transverse section of the ommatidia showing red and yellow pigments. F, an image of the corneal surface illuminated from the inside of the eye, showing the filtering effect of the pigments. G, UV‐induced fluorescence of the sample in F. The fluorescing (type II) ommatidia appear pink in F (arrowheads). H, summary of the characteristics of three ommatidial types with photoreceptor spectral sensitivities and the localization of visual pigment opsins (PxUV, Papilio xuthus (Px) UV; PxB, blue; PxL1–3, long wavelength‐absorbing type 1–3). Scale bars: 10 μm in E, 100 μm in F and G.
Since Karl von Frisch demonstrated that honeybees have colour vision (Frisch, 1914), researchers have extensively studied honeybees and concluded that their eyes are equipped with three classes of spectral receptors (Autrum & von Zwehl, 1964; Menzel & Backhaus, 1989; Hempel de Ibarra et al. 2014). The spectral sensitivities of these receptors peak in the ultraviolet (UV), blue and green wavelength regions, forming the physiological basis of bees’ trichromatic colour vision (Menzel & Backhaus, 1989). This eye organization is common among insects (Briscoe & Chittka, 2001; Kelber, 2006).
The eyes of butterflies are structurally similar to those of bees, but their spectral organization is considerably more complex. The Japanese yellow swallowtail butterfly, Papilio xuthus, is one of the first butterfly species in which the spectral organization of the eye was characterized in detail. Their eyes are furnished with six classes of spectral receptors: UV, violet, blue, green, red and broad‐band (Fig. 1 B–D). Combined intracellular electrophysiology, anatomy and molecular biology studies revealed that these six classes of receptors are situated in the ommatidia in three fixed combinations (Fig. 1). The spectrally heterogeneous ommatidia are distributed randomly in the hexagonal lattice of the compound eye, at least locally; some dorso‐ventral specialization exists (Arikawa, 2003).
A random array of three types of ommatidia is a common feature of butterfly eyes (Stavenga et al. 2001; Stavenga, 2002). However, photoreceptor spectral sensitivities and the number of photoreceptor classes vary considerably among species. The painted lady, Vanessa cardui, appears to have only three classes (Briscoe et al. 2003), which are similar to those of honeybees. The number of receptor classes increases to nine in the golden birdwing, Troides aeacus (Chen et al. 2013), and reaches 15 in the common bluebottle Graphium sarpedon (Chen et al. 2016b). The eyes of the small white (Pieris rapae), and the Eastern pale clouded yellow (Colias erate) have six and eight classes, respectively, the spectral sensitivities of which even differ between sexes (Marshall & Arikawa, 2014).
Colour vision of Papilio
Papilio butterflies strongly rely on vision when flower foraging. They have sophisticated colour vision that exhibits the properties of colour constancy and simultaneous colour contrast (Kelber & Pfaff, 1999; Kinoshita & Arikawa, 2014). Are they perhaps ‘hexa‐chromatic’, using all six classes of spectral receptors for colour vision? This question can be addressed by measuring their sensitivity to subtle differences in light wavelength. We humans are red–green–blue (RGB) trichromatic, and can discriminate 1–2 nm differences at around 500 and 600 nm, which correspond to the wavelength regions where the spectral sensitivities of two of the three cone photoreceptors overlap (De Valois & Jacobs, 1968). Other well‐established trichromatic systems in honeybees and hawkmoths also feature two such high‐sensitivity regions, at around 400 and 500 nm (von Helversen, 1972; Telles et al. 2014). In tetrachromatic fish, birds and turtles, three high‐sensitivity wavelength regions are found (Wright, 1972; Emmerton & Delius, 1980; Goldsmith et al. 1981; Neumeyer, 1986) (Fig. 2 A).
Figure 2. Wavelength discrimination ability.
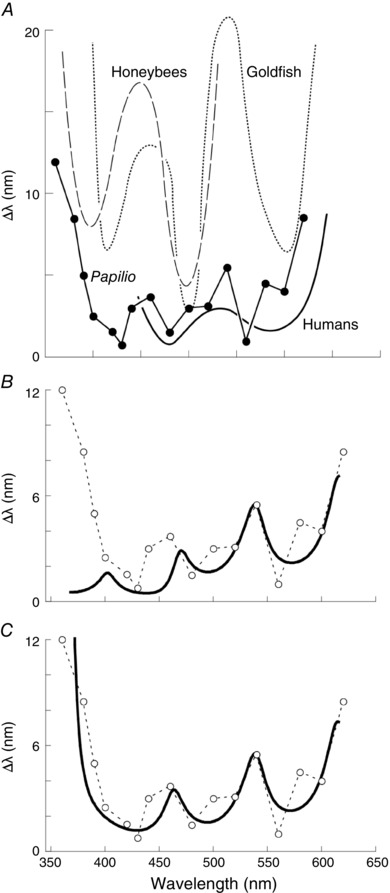
A, wavelength discrimination function of Papilio xuthus (circles) with those of other animals for comparison. Continuous curve, humans (de Valois & Jacobs, 1968); dotted curve, goldfish (Neumeyer, 1986); dashed curve, honeybee (von Helversen, 1972) B, model calculation (continuous curve) with all spectral classes except for the BB taken into account. C, model calculation with receptor classes except for the BB, V and R3–4 green receptors. Modified from Koshitaka et al. (2008).
We measured the wavelength discrimination ability in Papilio using a setup equipped with two monochromators. We first trained Papilio butterflies to associate nectar reward with a certain monochromatic light, and then presented the training light alongside another light whose wavelength was systematically varied. The trained individuals extended their proboscis toward the training light when the wavelength difference was sufficiently large, indicating that they were able to detect the difference between two lights. When the selection rate of the training light fell below 60%, we deemed that the butterflies could barely discriminate between the lights and recorded the wavelength difference as the minimum discriminable wavelength difference, Δλ, for that particular training wavelength. We measured Δλ for 18 training wavelengths, and found that the Δλ values of Papilio are comparable to those of humans: the values dip to 1–2 nm around 430, 480 and 560 nm (Fig. 2 A). Thus, the Papilio colour vision system may be tetrachromatic (Koshitaka et al. 2008). Strictly speaking, however, the Δλ function shows discrimination ability on the wavelength axis only, and therefore reveals what could be termed ‘sequential dichromacy’. For a convincing demonstration of tetrachromacy, the Papilio subjective colour space would have to be reconstructed, for example by colour mixture experiments (Backhaus et al. 1998).
Which of the six spectral receptor classes are involved in the task? We have taken a theoretical approach to tackle this question. We incorporated anatomical data about the number of spectral receptors into a receptor noise‐limited colour opponency model (Vorobyev & Osorio, 1998). The predicted wavelength discrimination function did not match at all with the behaviourally determined function when we assumed that all six receptor classes are involved (Fig. 2 B). We could obtain the best fit when we took just the UV, blue, proximal green and red receptors into account (Fig. 2 C); the violet, distal green and broad‐band receptors were excluded. The violet and broad‐band receptors are found exclusively in type II ommatidia, which may therefore not be involved in colour vision.
The distal green receptors, which are also excluded from the model, have some unique characteristics. First, this is the only class that is found in all ommatidia in the same positions (i.e. the R3 and R4 photoreceptors in the distal tier), forming a uniform hexagonal lattice (Arikawa, 2003); the distribution of other spectral receptors is random. Second, their axons terminating in the lamina are thicker and smoother than those of other photoreceptors (Takemura et al. 2005). A preliminary ‘connectome’ analysis of the Papilio lamina has revealed that the R3 and R4 axons make numerous contacts with second order neurons as expected, but not with other photoreceptors; photoreceptors other than R3 and R4 are mutually connected by synapse‐like structures in the lamina (author's unpublished observation). These interphotoreceptor connections are most likely histaminergic inhibitory synapses (Hardie, 1989), as histamine is the only neurotransmitter so far identified in insect photoreceptors. Such antagonistic synaptic interaction could provide a physiological basis of spectral opponency even at the level of photoreceptors (Chen et al. 2016b), and therefore may be crucial for colour vision. Third, the gain of the frequency–response function in distal green receptors extends to higher frequencies than in others, indicating that they are the fastest (Kawasaki et al. 2015). Taken together, this evidence points to the distal green receptor system being well‐suited for high spatial resolution vision as well as motion detection (Stewart et al. 2015). If these receptors are not involved in tetrachromatic colour vision, then the green receptors in the proximal tier of type III ommatidia would presumably provide the green colour channel (Fig. 1 H).
Comparative aspects
How much can we generalize our findings on the eye and colour vision of Papilio to other butterfly species? Perhaps not very much. An ommatidium of the small cabbage white, Pieris rapae, also contains nine photoreceptors forming a two‐tiered rhabdom as in Papilio. However, the photoreceptor spectral sensitivities are quite different. Six classes of receptors are found in female Pieris eyes: UV, violet, blue, green, red and deep‐red. Males lack the violet receptors, which are instead double‐peaked blue receptors due to the filtering effect of male‐specific fluorescence in a subset of ommatidia (Marshall & Arikawa, 2014). Localization of the various photoreceptor types in the ommatidia revealed that all those in the proximal tier are either red or dark‐red receptors, and green receptors are found only as R3 and R4 in the distal tier. We have assumed that the R3–4 green receptor system is used for motion rather than colour vision in Papilio. If this were also the case in Pieris, then its colour vision would lack a green channel. This seems improbable, because green is one of the most common colours in nature. The pathways of visual information processing may therefore be quite different among species, which should reflect their ecology and evolution.
Because honeybees are ‘blind’ to red light (Autrum & von Zwehl, 1964), the existence of red receptors in other insect eyes was a rather controversial topic in the past (Swihart & Gordon, 1971; Bernard, 1979; Matic, 1983). Accumulated evidence, however, indicates that red receptors are quite common in butterflies (Briscoe & Chittka, 2001). An extreme case is the females of the Eastern pale clouded yellow butterfly, Colias erate, which have three distinct ‘red’ receptors with peak sensitivities at 600, 620 and 640 nm (Ogawa et al. 2013). The female‐specific red receptor system might be an adaptation for finding the best quality leaves on which to lay eggs (Kelber, 1999). It would be interesting to ask whether and how sexual selection has played a role in creating sexually dimorphic visual systems.
Studies on the evolution of colour vision have perhaps over‐relied on visual pigment analysis. One might jump to conclude that a species has colour vision if it has two or more genes encoding visual pigment opsins. However, what is essential for colour vision is to have photoreceptors with different spectral sensitivities, not multiple visual pigments. This point has to be stressed because different spectral receptors often share a single visual pigment, but with differing spectral filters (Ogawa et al. 2013; McCulloch et al. 2016). It is therefore possible to have colour vision in an animal that has only one visual pigment opsin (Zaccardi et al. 2006). Conversely, the existence of two or more opsins does not constitute proof of colour vision. These opsins would have to be differentially expressed in photoreceptors whose visual fields at least partially overlap, and which form the input to appropriate neural circuitry, for colours to be perceived.
In addition to the detailed analyses of the visual systems of butterflies, we have been conducting an extensive survey of spectral sensitivity among various insect orders including Coleoptera, Hemiptera, Odonata, Thysanoptera, Lepidoptera, Diptera and Hymenoptera (author's unpublished observation). The method we employ is electroretinography (ERG), which is a straightforward protocol to record sensitivity of the eye as a whole. We have found rather similar results across nocturnal moths: their sensitivity typically peaks in the UV and green wavelength regions (White et al. 1983; Telles et al. 2014). Even with the rather crude ERG method, the variability observed in butterflies is remarkable (Eguchi et al. 1984). Inter‐order comparison is necessary to understand how flower‐visiting butterflies acquired sophisticated colour vision, while comparison among butterflies should reveal more recent evolutionary events that shaped this sensory system. Additionally, comparing between nocturnal and diurnal lepidopterans may provide a clue to understand how the colour vision of flower visitors has adapted to extreme light environments.
Formation of the retinal mosaic
Uncovering the developmental processes by which the retinal mosaic is formed may yield valuable insights into the evolution of colour vision. A random array of spectrally heterogeneous ommatidia is a common feature of many insect species including Drosophila. In contrast to butterfly eyes that are composed of three types of ommatidia, each bearing nine photoreceptor cells, Drosophila eyes have only two types of ommatidia, each bearing eight photoreceptor cells (at least in the main part of the eye).
The increased number of photoreceptors per ommatidium in butterflies may serve to make their eyes spectrally richer. What is the evolutionary history of the photoreceptor number? Is one receptor duplicated in butterflies, or one missing in Drosophila? Alternatively, they could have completely different origins. Comparative anatomy has suggested that a photoreceptor for colour vision in Drosophila may be duplicated in butterflies (Friedrich et al. 2011).
We approached this question using three marker proteins that are involved in ommatidial development in Drosophila: Prospero (Pros), Senseless (Sens) and Spalt (Sal). Of the eight photoreceptors in a Drosophila ommatidium, six (R1–6) are peripheral photoreceptors with identical broad‐band spectral sensitivity. The other two are central photoreceptors (R7–8) whose spectral sensitivities differ between ommatidia: the sensitivities of R7 and R8 peak at UV and green, respectively, in a subset of ommatidia (called yellow type), while in the rest (pale type), R7 is violet sensitive and R8 is blue sensitive. In the pupal eye disc of Drosophila, Pros is expressed in R7 precursor cells in all ommatidia regardless of type. Sens is expressed in R8, and Sal is expressed in both R7 and R8 precursor cells (Cook et al. 2003). We localized these proteins in the pupal eye of Papilio using antibodies specific to Papilio homologues. The anti‐Pros antibody labelled two cells per ommatidium, indicating that two R7‐like cells exist in the Papilio eye (Fig. 3 A). The anti‐Sens labelled only one cell (not shown), and the anti‐Sal labelled both the Pros‐positive and Sens‐positive cells (not shown): there is only one R8‐like cell in Papilio (Perry et al. 2016).
Figure 3. Formation of retinal mosaic in Papilio .
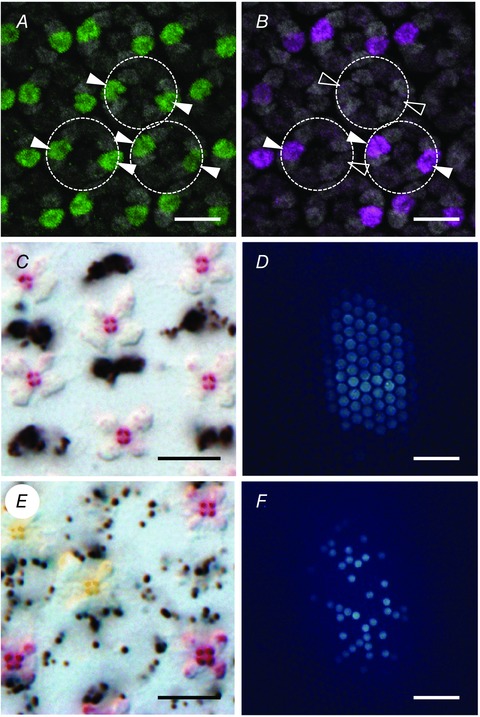
A, a Papilio eye disc isolated from 5 day pupa, stained with anti‐Papilio Prospero. Filled arrowheads indicate the labelled cells. B, same as A, labelled with anti‐Papilio Spineless. Filled and non‐filled arrowheads indicate the labelled and non‐labelled cells, respectively, both of which are Prospero‐positive. C, transverse section of the retina of spineless(−) Papilio. D, UV‐induced fluorescence in the eye of a spineless(−) Papilio. E and F, transverse section of the retina (E) and UV‐induced fluorescence (F) of a control (a pigment gene yellow knocked out by CRISPR‐Cas9) individual. Scale bars: 10 μm in A, B, C and E, 100 μm in D and F. Modified from Perry et al. (2016).
The random array of the yellow‐type and pale‐type ommatidia in Drosophila is determined by the stochastic expression of Spineless (Ss), a transcription factor required for the proper formation of legs and antennae. Ommatidia containing one Ss‐positive cell (R7 precursor) are the yellow type (Wernet et al. 2006). Immunohistochemical localization of Ss in pupal eyes of Papilio reveals that Ss protein is expressed in a subset of the Pros‐positive cells in three patterns: two, one, or zero positive cells per ommatidium (Fig. 3 B).
It seems likely that these alternative Ss expression patterns reflect the three types of ommatidia in Papilio. We confirmed the relationship between the Ss expression pattern and ommatidial type by knocking out the spineless gene using a gene‐editing technique CRISPR‐Cas9. All of the ommatidia in the Ss‐knockout individuals had red perirhabdomal pigment (Fig. 3 C), and emitted strong fluorescence under UV epi‐illumination (Fig. 3 D), which are characteristic features of type II ommatidia (Fig. 1 H) (Perry et al. 2016). Figure 3 D and E shows the perirhabdomal and fluorescence pigments in control animals.
What genetic mechanism controls the stochastic expression of Ss, and how does it work? These questions are particularly important in understanding why random ommatidial arrangements are common among insects. A clue may come from long‐legged flies (Dolichopodidae) where the distribution of heterogeneous ommatidia is not necessarily random (M. Perry and C. Desplan, personal communication) (Stavenga et al. 2017). Another challenging question arises from the observation that the fluorescing ommatidia in Ss‐knockout Papilio appear to be perfect type II ommatidia with all their features intact: visual pigment expression pattern, red perirhabdomal pigment, fluorescence pigment, and maybe the projection pattern in the optic lobe. These features characterizing ommatidial type identity must therefore be determined downstream of Ss, but how this is accomplished is presently completely unknown.
Future directions
We have applied a broad spectrum of experimental and theoretical techniques to study butterfly colour vision, and we intend to continue in the same vein. Recent innovations in genomics allow us to investigate evolutionary questions through even more ambitious comparative studies. The CRISPR‐Cas9 technique, which appears to be effective in many non‐model insects (Li et al. 2015; Chen et al. 2016a; Li et al. 2016; Markert et al. 2016), can be applied to elucidate not only developmental processes but also the neural basis of behaviour. Even the most classical anatomy is enjoying a renaissance thanks to a technique called serial block‐face scanning electron microscopy (Denk & Horstmann, 2004). This allows us to obtain thousands of sequential images from which the detailed anatomy of individual neurons and the circuits they form can be ascertained, in a way that was simply not feasible using traditional transmission electron microscopy (Helmstaedter et al. 2013).
Additional information
Competing interests
None declared.
Funding
This work is supported by research grants to the author from the Japan Society for the Promotion of Science (JSPS; nos 26650117 and 26251036) and from the National Agriculture and Food Research Organization (NARO) under the Cross‐ministerial Strategic Innovation Promotion Program (SIP) ‘Technologies for creating next generation agriculture, forestry, and fisheries’ (not numbered).
Acknowledgements
I am grateful to past and present members of my laboratory for participating in the experiments described here. I thank Dr Finlay Stewart of SOKENDAI‐Hayama for critical reading of the manuscript and editing the English.
Biography
Kentaro Arikawa graduated from Jiyu‐Gakuen College Tokyo (natural science) and Sophia University graduate school Tokyo, (behavioural biology). As a first year graduate student, he found butterflies detect light by their genitals, and analysed the mechanism and function of this unique photoreceptive system for his PhD study. After being a biology professor at Yokohama City University for more than 20 years, he moved to SOKENDAI in 2006. He also served as a visiting fellow at the Australian National University (neurobiology), a research student at Mitsubishi‐Kasei Institute of Life Science, a research fellow at NIH (visual science) and a researcher at JST‐PRESTO.

This review was presented at the symposium “Phototransduction and synaptic transmission” which took place at the Phototransduction UK workshop, Sheffield, 31 August – 2 September 2016.
This is an Editor's Choice article from the 15 August 2017 issue.
References
- Arikawa K (2003). Spectral organization of the eye of a butterfly, Papilio . J Comp Physiol A 189, 791–800. [DOI] [PubMed] [Google Scholar]
- Autrum H & von Zwehl V (1964). Die spektrale Empfindlichkeit einzelner Sehzellen des Bienenauges. Z Vergl Physiol 48, 357–384. [Google Scholar]
- Backhaus WGK, Kliegl R. & Werner JS. (eds) (1998). Color Vision: Perspectives from Different Disciplines. Walter de Gruyter, Berlin, New York. [Google Scholar]
- Bernard GD (1979). Red‐absorbing visual pigment of butterflies. Science 203, 1125–1127. [DOI] [PubMed] [Google Scholar]
- Briscoe AD, Bernard GD, Szeto AS, Nagy LM & White RH (2003). Not all butterfly eyes are created equal: rhodopsin absorption spectra, molecular identification and localization of UV‐ blue‐ and green‐sensitive rhodopsin encoding mRNA in the retina of Vanessa cardui . J Comp Neurol 458, 334–349. [DOI] [PubMed] [Google Scholar]
- Briscoe AD & Chittka L (2001). The evolution of color vision in insects. Ann Rev Entomol 46, 471–510. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang G, Zhu Y‐N, Xiang H & Wang W (2016a). Advances and perspectives in the application of CRISPR/Cas9 in insects. Zool Res 37, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P‐J, Arikawa K & Yang E‐C (2013). Diversity of the photoreceptors and spectral opponency in the compound eye of the Golden Birdwing, Troides aeacus formosanus . PLoS One 8, e62240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P‐J, Awata H, Matsushita A, Yang E‐C & Arikawa K (2016b). Extreme spectral richness in the eye of the common bluebottle butterfly, Graphium sarpedon . Front Ecol Evol 4, 1–12. [Google Scholar]
- Cook T, Pichaud F, Sonneville R, Papatsenko D & Desplan C (2003). Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila . Dev Cell 4, 853–864. [DOI] [PubMed] [Google Scholar]
- Denk W & Horstmann H (2004). Serial block‐face scanning electron microscopy to reconstruct three‐dimensional tissue nanostructure. PLoS Biol 2, e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R & Jacobs G (1968). Primate color vision. Science 162, 533–540. [DOI] [PubMed] [Google Scholar]
- Eguchi E, Nemoto A, Meyer‐Rochow VB & Ohba N (1984). A comparative study of spectral sensitivity curves in 3 diurnal and 8 nocturnal species of Japanese fireflies. J Insect Physiol 30, 607–612. [Google Scholar]
- Emmerton J & Delius JD (1980). Wavelength discrimination in the ‘visible’ and ultraviolet spectrum by pigeons. J Comp Physiol A 141, 47–52. [Google Scholar]
- Friedrich M, Wood EJ & Wu M (2011). Developmental evolution of the insect retina: insights from standardized numbering of homologous photoreceptors. J Exp Zool B Mol Dev Evol 316, 484–499. [DOI] [PubMed] [Google Scholar]
- Frisch Kv (1914). Der Farbensinn und Formensinn der Biene. Zool Jb Physiol 37, 1–238. [Google Scholar]
- Goldsmith TH, Collins JS & Perlman DL (1981). A wavelength discrimination function for the hummingbird Archilochus alexandri . J Comp Physiol A 143, 103–110. [Google Scholar]
- Hardie RC (1989). A histamine‐activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature 339, 704–706. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS & Denk W (2013). Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174. [DOI] [PubMed] [Google Scholar]
- Hempel de Ibarra N, Vorobyev M & Menzel R (2014). Mechanisms, functions and ecology of colour vision in the honeybee. J Comp Physiol A 200, 411–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Kinoshita M, Weckstrom M & Arikawa K (2015). Difference in dynamic properties of photoreceptors in a butterfly, Papilio xuthus: possible segregation of motion and color processing. J Comp Physiol A 201, 1115–1123. [DOI] [PubMed] [Google Scholar]
- Kelber A (1999). Ovipositing butterflies use a red receptor to see green. J Exp Biol 202, 2619–2630. [DOI] [PubMed] [Google Scholar]
- Kelber A (2006). Invertebrate colour vision In Invertebrate Vision, ed. Warrant E. & Nilsson DE, pp. 250–290. Cambridge University Press, Cambridge. [Google Scholar]
- Kelber A & Pfaff M (1999). True colour vision in the orchard butterfly, Papilio aegeus . Naturwissenschaften 86, 221–224. [Google Scholar]
- Kinoshita M & Arikawa K (2014). Color and polarization vision in foraging Papilio . J Comp Physiol A 200, 513–526. [DOI] [PubMed] [Google Scholar]
- Koshitaka H, Kinoshita M, Vorobyev M & Arikawa K (2008). Tetrachromacy in a butterfly that has eight varieties of spectral receptors. Proc R Soc B 275, 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Fan D, Zhang W, Liu G, Zhang L, Zhao L, Fang X, Chen L, Dong Y, Chen Y, Ding Y, Zhao R, Feng M, Zhu Y, Feng Y, Jiang X, Zhu D, Xiang H, Feng X, Li S, Wang J, Zhang G, Kronforst MR & Wang W (2015). Outbred genome sequencing and CRISPR/Cas9 gene editing in butterflies. Nat Commun 6, 8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X‐Y, Liu G‐C, Sheng W‐J, Dong Z‐W, Chen L, Zhao R‐P & Wang W (2016). Genome editing in the butterfly type‐species Papilio machaon . Insect Sci DOI:10.1111/1744-7917.12421. [DOI] [PubMed] [Google Scholar]
- McCulloch KJ, Osorio D & Briscoe AD (2016). Sexual dimorphism in the compound eye of Heliconius erato: a nymphalid butterfly with at least five spectral classes of photoreceptor. J Exp Biol 219, 2377–2387. [DOI] [PubMed] [Google Scholar]
- Markert MJ, Zhang Y, Enuameh MS, Reppert SM, Wolfe SA & Merlin C (2016). Genomic access to monarch migration using TALEN and CRISPR/Cas9‐mediated targeted mutagenesis. G3 (Bethesda) 6, 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J & Arikawa K (2014). Unconventional colour vision. Curr Biol 24, R1150–1154. [DOI] [PubMed] [Google Scholar]
- Matic T (1983). Electrical inhibition in the retina of the butterfly Papilio. I. Four spectral types of photoreceptors. J Comp Physiol A 152, 169–182. [Google Scholar]
- Menzel R & Backhaus W (1989). Color vision in honey bees: Phenomena and physiological mechanisms In Facets of Vision, ed. Stavenga DG. & Hardie RC, pp. 281–297. Springer‐Verlag, Berlin, Heidelberg, New York, London, Paris, Tokyo. [Google Scholar]
- Neumeyer C (1986). Wavelength discrimination in the goldfish. J Comp Physiol A 158, 203–213. [Google Scholar]
- Ogawa Y, Kinoshita M, Stavenga DG & Arikawa K (2013). Sex‐specific retinal pigmentation results in sexually dimorphic long‐wavelength‐sensitive photoreceptors in the Eastern Pale Clouded Yellow butterfly, Colias erate . J Exp Biol 216, 1916–1923. [DOI] [PubMed] [Google Scholar]
- Perry M, Kinoshita M, Saldi G, Huo L, Arikawa K & Desplan C (2016). Molecular logic behind the three‐way stochastic choices that expand butterfly colour vision. Nature 535, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavenga DG (2002). Reflections on colourful ommatidia of butterfly eyes. J Exp Biol 205, 1077–1085. [DOI] [PubMed] [Google Scholar]
- Stavenga DG, Kinoshita M, Yang EC & Arikawa K (2001). Retinal regionalization and heterogeneity of butterfly eyes. Naturwissenschaften 88, 477–481. [DOI] [PubMed] [Google Scholar]
- Stavenga DG, Meglič A, Pirih P, Koshitaka H, Arikawa K, Wehling MF & Belušič G (2017). Photoreceptor spectral tuning by colorful, multilayered facet lenses in long‐legged fly eyes (Dolichopodidae). J Comp Physiol A 203, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart FJ, Kinoshita M & Arikawa K (2015). The butterfly Papilio xuthus detects visual motion using chromatic contrast. Biol Lett 11, 20150687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swihart SL & Gordon WC (1971). Red photoreceptor in butterflies. Nature 231, 126–127. [DOI] [PubMed] [Google Scholar]
- Takemura S, Kinoshita M & Arikawa K (2005). Photoreceptor projection reveals heterogeneity of lamina cartridges in the visual system of the Japanese yellow swallowtail butterfly, Papilio xuthus . J Comp Neurol 483, 341–350. [DOI] [PubMed] [Google Scholar]
- Telles FJ, Lind O, Henze MJ, Rodriguez‐Girones MA, Goyret J & Kelber A (2014). Out of the blue: the spectral sensitivity of hummingbird hawkmoths. J Comp Physiol A 200, 537–546. [DOI] [PubMed] [Google Scholar]
- von Helversen O (1972). Zur spektralen Unterschiedsempfindlichkeit der Honigbiene. J Comp Physiol A 80, 439–472. [Google Scholar]
- Vorobyev M & Osorio D (1998). Receptor noise as a determinant of colour thresholds. Proc R Soc B 265, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I & Desplan C (2006). Stochastic spineless expression creates the retinal mosaic for colour vision. Nature 440, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RH, Banister MJ & Bennett RR (1983). Spectral sensitivity of screening pigment migration in the compound eye of Manduca sexta . J Comp Physiol A 153, 59–66. [Google Scholar]
- Wright AA (1972). Psychometric and psychophysical hue discrimination functions for the pigeon. Vision Res 12, 1447–1464. [DOI] [PubMed] [Google Scholar]
- Zaccardi G, Kelber A, Sison‐Mangus MP & Briscoe AD (2006). Color discrimination in the red range with only one long‐wavelength sensitive opsin. J Exp Biol 209, 1944–1955. [DOI] [PubMed] [Google Scholar]


