Abstract
Arteriosclerosis, particularly aortosclerosis, is the most critical risk factor associated with cardiovascular, cerebrovascular, and renal diseases. The pulsatile hemodynamics in the central aorta consists of blood pressure, flow, and stiffness and substantially differs from the peripheral hemodynamics in muscular arteries. Arteriosclerotic changes with age appear earlier in the elastic aorta, and age-dependent increases in central pulse pressure are more marked than those apparent from brachial pressure measurement. Central pressure can be affected by lifestyle habits, metabolic disorders, and endocrine and inflammatory diseases in a manner different from brachial pressure. Central pulse pressure widening due to aortic stiffening increases left ventricular afterload in systole and reduces coronary artery flow in diastole, predisposing aortosclerotic patients to myocardial hypertrophy and ischemia. The widened pulse pressure is also transmitted deep into low-impedance organs such as the brain and kidney, causing microvascular damage responsible for lacunar stroke and albuminuria. In addition, aortic stiffening increases aortic blood flow reversal, which can lead to retrograde embolic stroke and renal function deterioration. Central pressure has been shown to predict cardiovascular events in most previous studies and potentially serves as a surrogate marker for intervention. Quantitative and comprehensive evaluation of central hemodynamics is now available through various noninvasive pressure/flow measurement modalities. This review will focus on the clinical usefulness and mechanistic rationale of central hemodynamic measurements for cardiovascular risk management.
Keywords: Arteriosclerosis, Aorta, Stiffness, Blood pressure, Blood flow
Introduction: Historical View
Arterial blood pressure (BP) has long been recognized as an important biomarker of arterial function. More than 280 years ago, Stephen Hales compared the arterial system to a fire extinguisher with an air chamber (the so-called “Windkessel”), showing that the pulse waveform of BP (e.g., systolic upstroke and diastolic exponential decay) depends on aortic distensibility1). Until the invention of the brachial cuff ausculatory method by Riva-Rocci and Korotkoff, the BP waveform had been utilized to diagnose hypertension with arterial degeneration2). In the early 20th century, it was realized that the pulsatile BP wave is transmitted along the arterial tree from the central aorta to peripheral arteries at a speed depending on arterial stiffness3). In the mid-20th century, the reflection phenomenon of BP waves was recognized by experimental studies involving frequency domain analysis of aortic impedance4, 5). Human (invasive) studies involving time domain analysis of the intra-aortic BP waveform also confirmed that it is largely explicable by the transmission and reflection phenomena and closely associated with aortic wall stiffness6, 7). Clinical introduction of applanation tonometry for noninvasive BP recording revealed age-dependent (i.e., arteriosclerosis-induced) changes in the radial and carotid artery waveforms, leading to the proposal of the concept of the augmentation index (AIx)8). A mathematical transformation method has also been devised to generate aortic waveforms from radial waveforms and to estimate aortic BP9).
Similar to BP measurement, blood flow measurement has progressed from invasive intra-arterial recordings (e.g., through an electromagnetic flow-meter) to noninvasive duplex ultrasound or MRI recordings, which enable us to evaluate pulsatile hemodynamics in the central aorta more easily and comprehensively. With the increasing availability of these new pressure/flow measurement modalities, more attention has recently been paid to the clinical significance of central hemodynamics in cardiovascular diseases (CVDs). This review will summarize the current evidence on the potential for central hemodynamic indices to stratify cardiovascular risk and/or serve as a surrogate marker of clinical endpoints. This review will also focus on the pathophysiological mechanisms underlying the link between central hemodynamic abnormalities and end-organ damage/dysfunction and discuss future directions of clinical research.
What is Central BP?
Central BP is generally defined as BP in the ascending aorta. In a broader sense, it can refer to BP in the thoracic or abdominal aorta, representing BP exerted at the level of the heart, brain, and kidney10). Due to the phenomenon of pulse pressure (PP) amplification11), the central (systolic and pulse) pressures essentially differ from the brachial pressures. The central– brachial BP difference considerably varies among individuals12); for instance, there is an approximate difference of < 5 mmHg in some people (e.g., elderly people with a very stiff aorta), whereas it occasionally exceeds 30 mmHg in others (e.g., young people with a distensible aorta13) or during exercise14)). This difference cannot be estimated directly from conventional cuff BP measurement alone (i.e., solely from the two extremes of brachial BPs), but it can be actually estimated by adding information on the BP pulse waveform (see “How is central BP measured?” below).
The BP pulse waveform can be explained on the basis of the wave transmission and reflection phenomena along the arterial tree. In cardiac systole, the blood ejected from the left ventricle generates an incident (forward) BP wave. The incident wave then travels antegradely from the central aorta to the peripheral arteries at a speed termed “pulse wave velocity (PWV).” When the incident wave encounters high-impedance sites (e.g., high-resistance arterioles and arterial bifurcations), it is reflected and then travels retrogradely toward the central aorta at a speed similar to PWV. PWV is high (normally around 5–10 m/s) enough for the pulse to return up to the central aorta within the same cardiac cycle so that the reflected wave interferes with the incident wave to augment BP. The degree of augmentation is generally evaluated as AIx, which is the ratio of augmented pressure to PP (Fig. 115)).
Fig. 1.
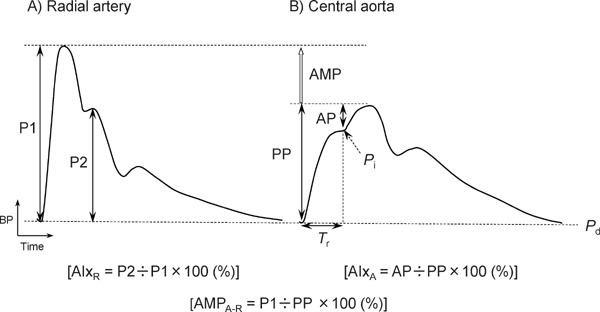
Pressure pulse waveforms of radial artery (A) and central aorta (B)
AIxA indicates aortic augmentation index; AIxR, radial augmentation index; AMP, pulse pressure amplification; AMPA-R, aorta-to-radial AMP ratio; AP, augmented pressure; BP, blood pressure; P1, early systolic peak pressure; P2, late systolic peak pressure; Pd, diastolic pressure; Pi, pressure at inflection point; PP, pulse pressure; and Tr, round-trip travel time. Reproduced from Hashimoto15).
How is Central BP Measured?
Until a quarter-century ago, aortic BP was only available in an invasive manner by inserting an intraarterial catheter-tip manometer or a fluid-filled catheter connected to a pressure transducer. Noninvasive estimation of aortic BP has become feasible since the technological development of applanation tonometry16, 17) performed on arteries running near the body surface (such as the carotid and radial arteries)8) and mathematical transformation of tonometric BP waveforms9, 18). Applanation (flattening) of arteries can be manually performed using a pencil-type probe (i.e., tonometer) or (semi)automatically performed using a hands-free tonometric system. To date, there are three major methods available for central BP estimation.
1). Estimation from Carotid BP Waveform
This method is based on the premise that the carotid waveform is deemed to be identical to the aortic waveform19). The carotid waveform is usually recorded with applanation tonometry and then calibrated by the brachial cuff pressures [i.e., by the mean arterial pressure (MAP) and diastolic BP] to derive absolute values of central BP. The rationale for calibration by brachial BPs is the observation that MAP and diastolic BP are almost constant in the elastic and conduit arteries (e.g., between the central aorta and brachial artery)20). MAP is either calculated directly from the area under the curve of the brachial (or radial) pulse waveform or estimated indirectly from an equation such as MAP = diastolic BP + 0.4 × PP21). This method has the advantage of not requiring any mathematical waveform transformation to estimate absolute central BP values. Nevertheless, it may be technically difficult in some cases to record (flatten) the carotid waveforms appropriately or reproducibly because of the anatomical characteristics of the carotid artery surrounded by soft tissues. The carotid pulse wave recordings can occasionally be affected by respiratory variation. Carotid AIx correlates well with invasively determined aortic AIx, although the former is reported to be half of the latter19).
2). Mathematical Conversion from Radial to Central BP Waveform with Transfer Function
In this method, the radial waveform is recorded with applanation tonometry and then converted to the corresponding aortic waveform using a frequency domain transfer function. There are two types of transfer function: generalized9, 18) and individualized. The former is commercially available and more widely used. Though still debated22), a generalized transfer function is considered valid for normal subjects, for patients with various diseases18, 23, 24), during exercise25), during pharmacological intervention26), and during the Valsalva maneuver18). The synthesized aortic waveform is normally calibrated with brachial diastolic BP and MAP to obtain central BP; MAP is calculated from the area under the radial waveform on the basis that the radial and brachial waveforms are deemed to be identical8). Aortic BP estimated using this method somewhat underestimates systolic BP and overestimates diastolic BP as measured directly using an invasive method27), but these errors almost entirely depend on the inaccurate calibration by the brachial cuff measurement28). The estimated aortic BP closely correlates with invasive aortic BP29). Reproducibility has been repeatedly verified in terms of aortic BP and AIx estimation30–32).
3). Direct Estimation from Radial BP Waveform without Using Transfer Function
It is empirically recognized that aortic systolic (maximum) BP is equal to the late systolic (second) peak (or “shoulder”) of radial BP33). Based on this recognition, the radial waveform is calibrated with brachial systolic and diastolic BPs to determine radial late systolic BP, which corresponds to aortic systolic BP. Although this method cannot generate the aortic waveform itself, it can offer the advantage of directly estimating aortic systolic BP and PP without applying a complicated waveform transformation. Radial AIx is usually calculated as the ratio of late systolic to early systolic BP peak amplitude (Fig. 1)34). This method has been validated using invasive studies35, 36), and the estimated radial AIx and late systolic pressure are reported to be reproducible37). Though only rare (< 5%), aortic BP may be unavailable because of an undetectable radial second peak (e.g., under conditions of marked tachycardia or vasodilation).
In addition, several devices have been newly developed and are currently available for noninvasive central BP recordings. Some of them adopt brachial cuff-based pulse volume plethysmography in place of applanation tonometry38–41); therefore, they are portable and capable of measuring ambulatory 24-h central BP. The N-point moving average method has also been proposed for the estimation of the aortic BP peak from the radial BP waveform42). These novel methodologies can provide more detailed information on central BP (e.g., circadian variation); further validation studies are required for general use in terms of accuracy, reproducibility, and prognostic significance.
Factors Affecting Central BP
1). Age (Arteriosclerosis)
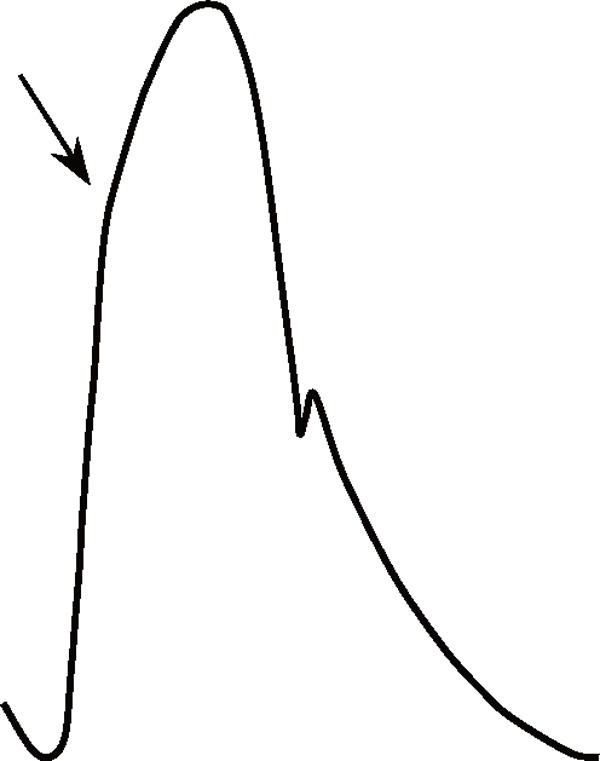
Age is the most potent accelerating factor associated with arteriosclerosis, and it strongly influences aortic BP waveforms (Table 1). With advancing age, elastic fibers in the aortic medial layer gradually undergo degeneration and are replaced with stiffer collagenous fibers, and the resultant decrease in the medial elastin/collagen ratio causes progressive stiffening of the aortic wall and reduction in aortic compliance43, 44). Such a pathological process is usually referred to as arteriosclerosis. Hypertension accelerates this process because of the fatiguing effects of excessive cyclic stress on elastic fibers43). Aortic stiffening increases aortic PWV and shortens the return time of the reflected BP wave and thus increases the augmented pressure. Moreover, as aortic distensibility decreases with age, systolic upstroke and diastolic decay of aortic BP become steeper. All these changes are responsible for a marked widening of aortic PP with age (Table 1). It should be noted here that compared with central elastic arteries, peripheral muscular arteries undergo little arteriosclerotic change45); therefore, age-dependent PP change is more remarkable in the former46).
Table 1. Comparisons of aortic pulse waveform characteristics between subjects with and without aortosclerosis.
| Aortosclerosis | (−) | (+) |
|---|---|---|
| Aortic pressure waveform* | 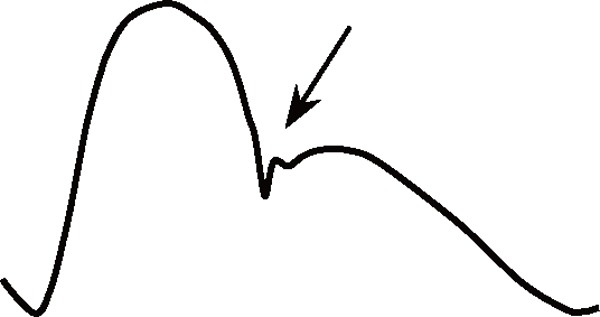 |
 |
| Incident pressure wave | Low | High |
| Augmented pressure | Low | High |
| Pulse pressure | Narrow | Wide |
| Pulse wave velocity | Slow | Fast |
| Round-trip travel time | Long | Short |
| Augmentation index | Normal | High |
| Diastolic pressure decay | Slow | Fast |
| Pulse pressure amplification | Large | Small |
Arrows indicate the arrival of reflected waves.
Epidemiological studies have shown progressive linear increases in aortic augmented pressure and reflected wave magnitude with age47, 48). Aortic AIx increases until the sixth decade but reaches a plateau or even decreases thereafter47, 49), probably because both the numerator (augmented pressure) and denominator (PP) increase linearly with age but with different intercepts on the pressure axis50).
2). Gender and Height
In general, women show a higher AIx than men51). This is mainly attributed to their difference in body height52), which relates to the path length between the central aorta and peripheral reflecting sites and thus determines the timing of wave reflection. However, the association between gender and AIx can be observed even independently of body height, suggesting an intrinsic gender difference in arterial function53). Given a higher AIx in women, gender difference in aortic PP is less obvious than that in brachial PP54).
3). Heart Rate and Ejection Duration
AIx increases as the heart rate decreases55). This is because a longer ejection duration resulting from a lower heart rate causes a wider incident wave to overlap with a reflected wave, with the round-trip travel time of these two waves being little affected by the heart rate. It can also be a reason why nonvasodilating beta-blocker administration increases AIx56).
4). Lifestyle (Smoking, Alcohol, Caffeine, and Sodium Intake)
Smoking of cigarettes or cigars increases aortic AIx and PWV acutely and chronically57, 58). Chronic smokers had a higher aortic (but a similar brachial) systolic BP than nonsmokers57). Smoking-induced AIx increase correlates with the change in the plasma cotinine level59). Even short-term smoking cessation reduces AIx60).
Alcohol intake has divergent effects on AIx; acute ingestion reduces aortic systolic BP and AIx, while chronic excessive consumption elevates these in men61). The effect of chronic alcohol consumption on aortic BP may differ by gender; heavier alcohol intake is associated with a higher AIx in healthy young men62) but not in postmenopausal women63).
Caffeine affects peripheral wave reflection to elevate aortic BP to a greater extent than that apparent from brachial BP64). Caffeinated but not decaffeinated coffee ingestion raises aortic AIx and PWV65). Black and green tea can also elevate AIx66). The combination of smoking and caffeine intake produces a synergistic detrimental effect on aortic PWV and AIx67).
Excessive sodium intake increases aortic stiffness and wave reflection, while sodium restriction improves them effectively68, 69).
5). Diabetes, Hypercholesterolemia, and Obesity
Inconsistent results have been reported with regard to AIx in diabetes. Pediatric patients with type 1 diabetes are reported to have an increased aortic AIx70), while adult patients with type 2 diabetes can have an increased71), normal72), or even reduced73) AIx despite widened PP. The mechanism underlying these apparently conflicting observations is still debated, but some patients with type 2 diabetes may have compensatory hyperinsulinemia (in response to insulin resistance), which could induce peripheral vasodilation and diminish wave reflection, even with a stiffened aorta. It may also involve central (abdominal) obesity, which is known to reduce AIx73). Aortic AIx in patients with hypercholesterolemia is reported to be higher than74) or similar to75) that in subjects with normocholesterolemia. Metabolic syndrome may not be associated with AIx in treated hypertension76). Taking these reports together, metabolic disorders can have inconsistent effects on AIx and thus peripheral wave reflection, in contrast to a relatively consistent (increasing) effect on PWV and arterial stiffness77). It should be of note, however, that aortic BP elevation can often be observed in these disorders even without any increase in wave reflection, namely through an increase in the incident wave height.
Arterial stiffness and wave reflection may be influenced not only by glucose or lipid metabolism but also by mineral metabolism abnormalities. For instance, higher serum phosphorus levels are shown to be associated with increases in PWV and AIx even among the general population with normal kidney function78).
6). Other Related Diseases
An elevation in AIx is observed in hypothyroidism79) and hyperparathyroidism80), both of which represent the endocrine disorders considered responsible for hypertension. In obstructive sleep apnea syndrome, aortic (but not peripheral) BP and AIx can be elevated, particularly in the early morning81). Aortic BP may decrease in response to nasal continuous positive airway pressure treatment even without parallel changes in peripheral BP82). In patients with Kawasaki disease who develop coronary artery lesions, wave reflection indices are elevated and the aortic waveforms resemble those observed in the elderly83). Primary inflammatory diseases, including rheumatoid arthritis, may be independently associated with increased central BP and AIx84), although it remains controversial whether systemic inflammation in primary hypertension and chronic kidney disease (CKD) is a cause or consequence of aortic stiffness85). Systemic sclerosis, which often involves microvascular lesions, is associated with enhanced peripheral wave reflection despite normal aortic stiffness86). Sickle cell disease with the hemoglobin SS genotype is associated with lower arterial stiffness and wave reflections owing to higher nitric oxide availability87).
Is Central BP Useful for Risk Stratification of CVD?
1). Prospective Studies
So far, a number of prospective observational cohort studies have been conducted to investigate the predictive ability of central BP indices for CVD events. The results are summarized in Table 288–106) and have been discussed elsewhere in our previous review107). These study cohorts can be classified according to the underlying diseases, e.g., patients with renal disease, patients with coronary heart disease, and the general population. As a whole, most (but not all) of currently available data indicate that central BP indices (such as central PP, AIx, and PP amplification) can predict CVD events more precisely than and/or independently of brachial BP, particularly in high-risk populations (Table 2; for more details, please refer to the previous review107)).
Table 2. Prospective observational studies on predictive value of central blood pressure parameters for cardiovascular events.
| First Author | Subjects | N | Male (%) | Age (years)* | Brachial BP (mmHg)† | Follow-up (years)* | Recording/Estimation | Endpoint | Event rate‡ | Central BP parameter | Adjusted RR | Lower 95%CI | Upper 95%CI | Central vs. brachial |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| London88) | ESRD | 180 | 60 | 54 | 156/83 | 4.3 | Carotid | All-cause mortality CV mortality |
89.7 51.3 |
AIx (/10%) AIx (/10%) |
1.51 1.48 |
1.23 1.16 |
1.86 1.90 |
Sup, Ind Ind |
| Safar89) | ESRD | 180 | 59 | 54 | 156/83 | 4.3 | Carotid | All-cause mortality | 89.7 | PP (/25 mmHg) AMP (/16%) |
1.40 0.50 |
1.10 0.30 |
1.80 0.80 |
Sup Sup |
| Covic90) | ESRD | 92 | 54 | 43 | 129/84 | 5.1 | Radial | All-cause mortality | 32.1 | AIx (> 24.6 vs. < 12.0%) | 0.64 | 0.13 | 3.15 | n/a |
| Briet91) | CKD | 180 | 74 | 60 | 134/74 | 3.1 | Carotid | Hemodialysis | 73.5 | PP (/10 mmHg) | 1.24 | 1.03 | 1.49 | n/a |
| Verbeke92) | Renal Tx | 512 | 59 | 53 | 135/80 | 5.0 | Radial | CV events | 37.1 | AugP (/8.6 mmHg) | 1.49 | 1.22 | 1.81 | Ind |
| Ueda93) | CAD | 103 | 78 | 63 | (139/72) | 0.5 | Intraaortic | Coronary restenosis | 699.0 | AIx (/10%) Tr (/-10 ms) |
1.70 1.34 |
1.16 1.10 |
2.48 1.63 |
n/a n/a |
| Weber94) | CAD | 262 | 71 | 66 | 132/78 | 2.0 | Radial | CV events | 116.0 | AIx@Hr75 (/1%) | 1.04 | 1.01 | 1.07 | Sup |
| Chirinos95) | CAD | 297 | 100 | 64 | 136/70 | 3.2 | Intraaortic | CV events | 132.3 | AugP (/10 mmHg) AIx (/10%) |
1.19 1.28 |
1.06 1.09 |
1.34 1.50 |
Sup Sup |
| Jankowski96) | CAD | 1109 | 74 | 58 | (135/72) | 4.4 | Intraaortic | CV events | 50.5 | PP (/18.2 mmHg) | 1.28 | 1.10 | 1.49 | Sup |
| Weber97) | CAD | 520 | 100 | 64 | 133/80 | 4.1 | Radial | CV events | 80.1 | AIx (/10%) AIx@Hr75 (/10%) |
1.15 1.25 |
1.01 1.06 |
1.32 1.47 |
Ind Ind |
| Roman98) | General | 2403 | 35 | 63 | 132/75 | 4.8 | Radial | CV events | 27.7 | PP (/10 mmHg) | 1.15 | 1.07 | 1.24 | Sup |
| Roman99) | General | 2405 | 35 | 63 | 132/75 | 5.6 | Radial | CV events | 25.5 | PP (≥ 50 vs. ≤ 31 mmHg) | 1.69 | 1.20 | 2.39 | Sup |
| Pini100) | General | 398 | 45 | 73 | 145/84 | 8.0 | Carotid | CV events | 38.3 | PP (/10 mmHg) | 1.23 | 1.10 | 1.37 | Sup |
| Leone101) | General | 3337 | 39 | 73 | 145/82 | 3.6 | Nomogram | Coronary events | 10.6 | PP (/14.6 mmHg) | 1.39 | 1.17 | 1.60 | n/a |
| Benetos102) | General | 125151 | 58 | 40 | 132/81 | 12.1 | Nomogram | All-cause mortality CV mortality |
2.6 0.5 |
PP (/7.8 mmHg) PP (/7.8 mmHg) |
1.18 1.21 |
1.12 1.15 |
1.25 1.28 |
Sup Sup |
| Wang103) | General | 1272 | 53 | 52 | 139/88 | 10.8 | Carotid | CV mortality | 2.7 | PP (/10 mmHg) | 1.26 | 1.02 | 1.56 | Sup |
| Wang104) | General | 1272 | 53 | 52 | 139/88 | 15.0 | Carotid | CV mortality | 3.3 | Pb (/6 mmHg) | 1.59 | 1.21 | 2.09 | Sup, Ind |
| Huang105) | General | 1014 | 54 | 52 | 139/89 | 15.0 | Carotid | All-cause mortality | 13.2 | PP (/1 mmHg) | 1.16 | 1.01 | 1.32 | Sup |
| Mitchell106) | General | 2232 | 42 | 63 | 127/74 | 7.8 | Carotid | CV events | 8.7 | PP (/1 mmHg) AIx (/1%) |
1.00 0.91 |
0.99 0.77 |
1.01 1.07 |
n/a n/a |
AIx: augmentation index; AIx@Hr75: AIx adjusted for heart rate of 75 bpm; AMP: pulse amplification; AugP: augmented pressure; BP: blood pressure; CAD: coronary artery disease (or suspect); CI: confidence interval; CKD: chronic kidney disease; CV: cardiovascular; ESRD: end-stage renal disease; Ind: predictive independency of central BP parameters from brachial BP; n/a: data not available; Pb: reflected wave amplitude; PP: pulse pressure; RR: relative risk; Sup: predictive superiority of central BP parameters over brachial BP; Tr: travel time; Tx: transplantation.
Mean or median.
Except for aortic BP in parentheses.
Per 1000 person-year (by estimate). Reproduced from Hashimoto et al.107).
2). Cut-Off Value
The definition of the cut-off values for central BP has not yet been established. While the normal upper limits of brachial (casual) BP are usually defined as 140/90 mmHg, it is difficult to set corresponding thresholds for central BP because of the PP amplification, which considerably varies among individuals according to age and BP levels12). Based on a distribution approach from an epidemiological cross-sectional database, brachial BP values of 140/90 mmHg correspond to central BP values of ∼125/90 mmHg108). Based on a prognostic outcome-driven approach from a prospective database, central BP of 130/90 mmHg has been proposed as a diagnostic threshold value109). Clearly, the validity of these cut-off values needs to be confirmed by further investigation.
Does Central BP Serve as a Surrogate Marker for Intervention?
Brachial BP has long been recognized as a useful marker for the management of CVD as well as hypertension. For introducing central BP measurement into routine clinical practice, the most critical issue is to determine whether central BP is superior to brachial BP in evaluating therapeutic effects and predicting their consequences.
It is known that nitroglycerine has a stronger hypotensive effect on central BP than that apparent from brachial BP measurement110). This is attributable to a reduction in wave reflection resulting from the peripheral vasodilating action of nitroglycerine. The opposite effects are observed for nonvasodilating β-blockers, which lower central BP to a lesser extent than brachial BP111). So far, different effects of various antihypertensive drug classes on central BP have been demonstrated by several randomized multicenter clinical trials. For instance, the CAFÉ substudy56) of the ASCOT trial112) showed that individuals assigned to the amlodipine/perindopril group had a 4.3 mmHg lower central systolic BP than those assigned to the atenolol/thiazide group, despite a similar brachial BP. The EXPLOR study113) demonstrated that an amlodipine/valsartan combination decreases central BP and AIx more effectively than an amlodipine/atenolol combination. In addition, the PARAMETER study has recently shown that sacubitril/valsartan (LCZ696), an angiotensin receptor neprilysin inhibitor, is more potent than olmesartan in reducing central BP114).
Central BP can serve as a therapeutic surrogate marker for the prevention of major CVD events. Data from the abovementioned CAFÉ study indicate that the modest intergroup difference in central BP, which is undetectable by conventional brachial BP measurement, contributed to the better cardiovascular outcome in the amlodipine group observed in the ASCOT study112). In addition, compared with brachial BP, central BP can better predict the regression of target organ damage (e.g., left ventricular hypertrophy115) and carotid artery wall hypertrophy116)) during antihypertensive treatment. Such regression, which likely results from vasolidator-induced reduction in peripheral wave reflection117, 118), could be closely linked to a reduction in CVD events. These lines of evidence suggest a potential benefit of central BP-guided antihypertensive therapy for CVD management.
At present, only limited data are available regarding the therapeutic impact of medication other than antihypertensive drugs (e.g., statin and antidiabetic drugs). In the CAFÉ-LLA substudy119), atorvastatin therapy sufficient to reduce CVD events in the ASCOT trial112) had no influence on central BP or AIx. In contrast, in a placebo-controlled study120), atorvastatin but not the placebo induced significant reductions in central BP, AIx and PWV. Linagliptin (a dipeptidyl peptidase-4 inhibitor) did not affect central BP and AIx in a placebo-controlled study of patients with diabetes121). Metformin (but not the placebo) reduces central BP and AIx in patients with nonalcoholic fatty liver disease122) and in young women with polycystic ovary syndrome123). Tetrahydrobiopterin, a nitric oxide synthase cofactor, can lower central AIx but not PWV in patients with CKD124). Anti-inflammatory treatment may reduce PWV and/or AIx in some patients with primary inflammatory disorders125). Obviously, further investigation is required.
Pathophysiological Relations of Central Hemodynamics to Arteriosclerotic Diseases: Hypothetical View and Future Directions
As the central aorta stiffens, peripheral endorgans undergo progressive degeneration in the vascular structure and gradual deterioration in the physiological function, which can lead eventually to the sudden occurrence of symptomatic CVD. There is substantial evidence showing that aortic PWV predicts major cardiovascular events, including myocardial infarction, heart failure, stroke, and renal failure126, 127). Until quite recently, however, relatively little was known about the underlying mechanisms by which aortic stiffening causes target organ damage or dysfunction. Results of the latest studies indicate that the central hemodynamics, including aortic BP and flow, plays a pivotal role in the causal mechanisms.
Aortic pulsatile flow is an important constituent of central hemodynamics. The flow waveform differs from the pressure waveform and greatly varies among different aortic sites128). According to previous studies based on Doppler ultrasound, the flow waveform in the proximal descending aorta is biphasic (to-and-fro) comprising systolic forward and diastolic reverse components, and the reverse/forward flow ratio linearly increases with aortic stiffening129). Such an increase in aortic flow reversal could alter the blood flow distribution to various organs and thus directly relate to the pathophysiological mechanisms of arteriosclerotic CVD (see below). The bidirectional (i.e., antegrade and retrograde) nature of aortic flow seems quite intriguing from a physiological view, given its potential relevance to aortic BP composed of the two waves going in opposite directions (i.e., forward and reflected waves). The PP amplification (i.e., pressure gradient) and aortic/peripheral PWV ratio (i.e., stiffness gradient) also seem to be significant determinants of pulsatile flow production130–132).
1). Stroke
Cerebrovascular disease can be broadly divided into two categories: microvascular and macrovascular stroke (Table 3). Cerebral microvascular diseases include lacunar infarction, white matter hyper-intensity lesions, and intracerebral hemorrhage (including microbleeds), which are often observed in the regions perfused by deep perforating arteries. Several previous studies have associated these microvascular lesions with elevations in aortic PWV and BP133–137). The underlying mechanisms are likely that 1) the small perforating arteries are directly branched from the large cerebral arteries and 2) BP and flow pulsations are prone to deeply transmitting into the cerebral microvasculature on account of its low impedance properties132, 138). It is thought that with aortic stiffening, BP pulsation in large arteries becomes greater; thus, higher pulsatile stress is imposed on the fragile microvascular walls, which results in microvascular injury and eventually causes ischemic or hemorrhagic stroke.
Table 3. Different characteristics of micro- and macrovascular stroke.
| Stroke types | Microvascular | Macrovascular |
|---|---|---|
| Designations |
|
|
| Main causes | Lipohyalinosis |
|
| Vascular territories | Small deep perforating arteries | Cerebral/carotid/vertebral arteries |
| Association with aortic stiffness | + | + |
| Suggested central hemodynamic mechanisms |
|
|
In contrast to microvascular stroke, macrovascular stroke usually occurs in the regions perfused by large cortical arteries and could be fatal in some cases. Studies have demonstrated that aortic PWV is an independent predictor of fatal stroke139). The underlying hemodynamic mechanisms linking aortic stiffness to macrovascular stroke may include 1) cerebral thromboembolism due to plaque rupture in the carotid/cerebral arteries and 2) cardiogenic embolism due to atrial fibrillation, both of which are triggered by widened central PP and/or enhanced peripheral wave reflection140, 141). In addition, another mechanism has recently been proposed: 3) retrograde plaque embolism due to the abovementioned aortic flow reversal129). In particular, the term “retrograde embolism” means that atherosclerotic mobile plaques are detached from the aortic arch or descending aortic walls and then retrogradely delivered through the supra-aortic (i.e., carotid and subclavian) arteries up to cerebral arteries to cause “cryptogenic” stroke. This type of stroke can indeed occur142) because diastolic reverse flow in the descending aorta directly contributes to forward flow into the supra-aortic arteries. In addition, the risk of the retrograde embolism may be heightened by aortic stiffening because the stiffer the aorta is, the greater the aortic flow reversal becomes129). The rationale for this retrograde embolism also includes the facts that 1) cryptogenic stroke can occur without any embolic sources in the heart or ascending aorta and 2) mobile plaques are more often seen in the descending aorta than in the ascending aorta143).
2). CKD
CKD is defined as the presence of (micro)albuminuria and/or a reduced glomerular filtration rate (GFR). Both albuminuria and GFR reduction are often preceded by aortic stiffening, but they can predict future CVD events independently of each other144). This indicates the existence of at least partially different etiologies underlying these distinct entities. Recent investigation does suggest different mediating influences of central hemodynamic (i.e., BP and flow) abnormalities on these aorto-renal associations.
The kidney has a unique structure and function. The renal arteries branch off from the abdominal aorta into intrarenal smaller arteries, leading to tiny afferent arterioles and fragile glomerular capillaries. Intraglomerular pressure is as high as 60/40 mmHg even under normal conditions, reflecting the low impedance properties of the renal vasculature. In case of aortosclerosis, the central PP widens, and then, the high BP pulsation is transmitted down to the glomerular capillaries. This most likely results in intraglomerular hypertension and endothelial injury, leading to albuminuria132). In fact, central PP and PWV have been shown to be independent predictors of the urinary albumin/creatinine ratio131, 145).
Aortic stiffness is also known to be associated with GFR decline146, 147). The GFR decline may result from such glomerular injury, as mentioned above, but another possibility has recently been raised that aortic flow abnormality is a primary cause of renal dysfunction accompanying aortosclerosis. As mentioned earlier, aortic stiffening increases flow reversal in the thoracic descending aorta and thus reduces forward flow toward the suprarenal abdominal aorta129). This reduction in suprarenal aortic flow (also caused by an impaired Windkessel function) could reduce blood inflow into the renal arteries (i.e., renal blood flow) and thereby deteriorate GFR148). This flow mechanism could also explain why renal function decreases with age in the general population (i.e., even in the absence of albuminuria).
3). Heart Disease
For over a half century, numerous studies have shown an important etiological role of central hemodynamics in cardiac diseases. The details have been extensively reviewed previously43, 149). In brief, an elevation in central BP, which directly reflects left ventricular afterload, is primarily responsible for left ventricular hypertrophy150, 151), causing various cardiac dysfunctions (e.g., heart failure and arrhythmia). Hastening of exponential decay of central BP during diastole, which is accelerated by aortic stiffening, reduces the diastolic inflow from the ascending aorta to the coronary arteries and thereby predisposes hypertensive patients to myocardial ischemia152). The risk of myocardial ischemia could be further heightened by the presence of atherosclerotic coronary artery stenosis153) and/or left ventricular hypertrophy with increased oxygen demand154). Obviously, more studies are needed to investigate the crucial link between aortic and coronary pulsatile flow.
Conclusions
The quantitative assessment of central pulsatile hemodynamics has now been enabled by noninvasive tonometry/ultrasound and pulse waveform analysis. Central hemodynamic indices have some advantages in terms of 1) the technical facility for repeated measurements, 2) the immediate response to antihypertensive treatment, and 3) the predictive ability of therapeutic consequences (e.g., preventive effects on CVD). Recent international guidelines or recommendations for vascular biomarkers have also endorsed their usefulness155, 156). In the future, for wider application to clinical practice, further investigation is needed to determine the normal reference values, develop handy apparatuses with high precision and dedicated analytical programs, and verify the epidemiological evidence and mechanistic rationale for general use in CVD risk management.
Conflict of Interest
The author belonged to an endowment department supported with unrestricted grants from Omron Healthcare and MSD.
References
- 1). Hales S: Statistical Esseys: Haemastaticks. History of Medical Series, Hafner Publishing; (reproduced 1964), New York, 1733 [Google Scholar]
- 2). Mahomed FA: The etiology of Bright's disease and the prealbuminuric stage. Med Chir Trans, 1874; 57: 197-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Bramwell JC, Hill AV: Velocity of transmission of the pulse wave. Lancet, 1922; 891-892 [Google Scholar]
- 4). O'Rourke MF, Taylor MG: Input impedance of the systemic circulation. Circ Res, 1967; 20: 365-380 [DOI] [PubMed] [Google Scholar]
- 5). O'Rourke MF: The arterial pulse in health and disease. Am Heart J, 1971; 82: 687-702 [DOI] [PubMed] [Google Scholar]
- 6). O'Rourke MF, Avolio AP: Pulsatile flow and pressure in human systemic arteries. Studies in man and in a multibranched model of the human systemic arterial tree. Circ Res, 1980; 46: 363-372 [DOI] [PubMed] [Google Scholar]
- 7). Murgo JP, Westerhof N, Giolma JP, Altobelli SA: Aortic input impedance in normal man: relationship to pressure wave forms. Circulation, 1980; 62: 105-116 [DOI] [PubMed] [Google Scholar]
- 8). Kelly R, Hayward C, Avolio A, O'Rourke M: Noninvasive determination of age-related changes in the human arterial pulse. Circulation, 1989; 80: 1652-1659 [DOI] [PubMed] [Google Scholar]
- 9). Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP: An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J, 1993; 14: 160-167 [DOI] [PubMed] [Google Scholar]
- 10). Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, et al. : 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J, 2007; 28: 1462-1536 [DOI] [PubMed] [Google Scholar]
- 11). Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H: Role of pulse pressure amplification in arterial hypertension: experts' opinion and review of the data. Hypertension, 2009; 54: 375-383 [DOI] [PubMed] [Google Scholar]
- 12). Herbert A, Cruickshank JK, Laurent S, Boutouyrie P, Reference Values for Arterial Measurements C : Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J, 2014; 35: 3122-3133 [DOI] [PubMed] [Google Scholar]
- 13). Mahmud A, Feely J: Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens, 2003; 16: 229-232 [DOI] [PubMed] [Google Scholar]
- 14). Rowell LB, Brengelmann GL, Blackmon JR, Bruce RA, Murray JA: Disparities between aortic and peripheral pulse pressures induced by upright exercise and vasomotor changes in man. Circulation, 1968; 37: 954-964 [DOI] [PubMed] [Google Scholar]
- 15). Hashimoto J: Pulse wave velocity and pulse wave analysis. Curr Rev Clin Pathol, 2013; 151: 27-37 (Japanese) [Google Scholar]
- 16). Pressman GL, Newgard PM: A Transducer for the continuous external measurement of arterial blood pressure. IEEE Trans Biomed Eng, 1963; 10: 73-81 [DOI] [PubMed] [Google Scholar]
- 17). Drzewiecki GM, Melbin J, Noordergraaf A: Arterial tonometry: review and analysis. J Biomech, 1983; 16: 141-152 [DOI] [PubMed] [Google Scholar]
- 18). Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA: Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation, 1997; 95: 1827-1836 [DOI] [PubMed] [Google Scholar]
- 19). Chen CH, Ting CT, Nussbacher A, Nevo E, Kass DA, Pak P, Wang SP, Chang MS, Yin FC: Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension, 1996; 27: 168-175 [DOI] [PubMed] [Google Scholar]
- 20). Pauca AL, Wallenhaupt SL, Kon ND, Tucker WY: Does radial artery pressure accurately reflect aortic pressure? Chest, 1992; 102: 1193-1198 [DOI] [PubMed] [Google Scholar]
- 21). Mahieu D, Kips J, Rietzschel ER, De Buyzere ML, Verbeke F, Gillebert TC, De Backer GG, De Bacquer D, Verdonck P, Van Bortel LM, Segers P: Noninvasive assessment of central and peripheral arterial pressure (waveforms): implications of calibration methods. J Hypertens, 2010; 28: 300-305 [DOI] [PubMed] [Google Scholar]
- 22). Hope SA, Meredith IT, Cameron JD: Arterial transfer functions and the reconstruction of central aortic waveforms: myths, controversies and misconceptions. J Hypertens, 2008; 26: 4-7 [DOI] [PubMed] [Google Scholar]
- 23). Westerhof BE, Guelen I, Stok WJ, Lasance HA, Ascoop CA, Wesseling KH, Westerhof N, Bos WJ, Stergiopulos N, Spaan JA: Individualization of transfer function in estimation of central aortic pressure from the peripheral pulse is not required in patients at rest. J Appl Physiol, 2008; 105: 1858-1863 [DOI] [PubMed] [Google Scholar]
- 24). Payne RA, Lilitkarntakul P, Dhaun N, Melville V, Asai T, Goddard J, Webb DJ: Renal dysfunction does not affect the peripheral-to-central arterial pressure transfer function. Hypertension, 2010; 56: 1083-1088 [DOI] [PubMed] [Google Scholar]
- 25). Sharman JE, Lim R, Qasem AM, Coombes JS, Burgess MI, Franco J, Garrahy P, Wilkinson IB, Marwick TH: Validation of a generalized transfer function to noninvasively derive central blood pressure during exercise. Hypertension, 2006; 47: 1203-1208 [DOI] [PubMed] [Google Scholar]
- 26). Pauca AL, O'Rourke MF, Kon ND: Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension, 2001; 38: 932-937 [DOI] [PubMed] [Google Scholar]
- 27). Cloud GC, Rajkumar C, Kooner J, Cooke J, Bulpitt CJ: Estimation of central aortic pressure by SphygmoCor requires intra-arterial peripheral pressures. Clin Sci, 2003; 105: 219-225 [DOI] [PubMed] [Google Scholar]
- 28). Smulyan H, Siddiqui DS, Carlson RJ, London GM, Safar ME: Clinical utility of aortic pulses and pressures calculated from applanated radial-artery pulses. Hypertension, 2003; 42: 150-155 [DOI] [PubMed] [Google Scholar]
- 29). Ding FH, Fan WX, Zhang RY, Zhang Q, Li Y, Wang JG: Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am J Hypertens, 2011; 24: 1306-1311 [DOI] [PubMed] [Google Scholar]
- 30). Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ: Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens, 1998; 16: 2079-2084 [DOI] [PubMed] [Google Scholar]
- 31). Siebenhofer A, Kemp C, Sutton A, Williams B: The reproducibility of central aortic blood pressure measurements in healthy subjects using applanation tonometry and sphygmocardiography. J Hum Hypertens, 1999; 13: 625-629 [DOI] [PubMed] [Google Scholar]
- 32). Filipovsky J, Svobodova V, Pecen L: Reproducibility of radial pulse wave analysis in healthy subjects. J Hypertens, 2000; 18: 1033-1040 [DOI] [PubMed] [Google Scholar]
- 33). Pauca AL, Kon ND, O'Rourke MF: The second peak of the radial artery pressure wave represents aortic systolic pressure in hypertensive and elderly patients. Br J Anaesth, 2004; 92: 651-657 [DOI] [PubMed] [Google Scholar]
- 34). Hashimoto J, Watabe D, Hatanaka R, Hanasawa T, Metoki H, Asayama K, Ohkubo T, Totsune K, Imai Y: Enhanced radial late systolic pressure augmentation in hypertensive patients with left ventricular hypertrophy. Am J Hypertens, 2006; 19: 27-32 [DOI] [PubMed] [Google Scholar]
- 35). Hickson SS, Butlin M, Mir FA, Graggaber J, Cheriyan J, Khan F, Grace AA, Yasmin, Cockcroft JR, Wilkinson IB, McEniery CM: The accuracy of central SBP determined from the second systolic peak of the peripheral pressure waveform. J Hypertens, 2009; 27: 1784-1788 [DOI] [PubMed] [Google Scholar]
- 36). Takazawa K, Kobayashi H, Kojima I, Aizawa A, Kinoh M, Sugo Y, Shimizu M, Miyawaki Y, Tanaka N, Yamashina A, Avolio A: Estimation of central aortic systolic pressure using late systolic inflection of radial artery pulse and its application to vasodilator therapy. J Hypertens, 2012; 30: 908-916 [DOI] [PubMed] [Google Scholar]
- 37). Funada J, Takata Y, Hashida H, Matsumoto Y, Sato S, Hiasa G, Inoue K, Higaki J, Okayama H: Dysfunctional central hemodynamic regulation after daily meal intake in metabolic syndrome. Atherosclerosis, 2010; 210: 268-273 [DOI] [PubMed] [Google Scholar]
- 38). Lowe A, Harrison W, El-Aklouk E, Ruygrok P, Al-Jumaily AM: Non-invasive model-based estimation of aortic pulse pressure using suprasystolic brachial pressure waveforms. J Biomech, 2009; 42: 2111-2115 [DOI] [PubMed] [Google Scholar]
- 39). Horvath IG, Nemeth A, Lenkey Z, Alessandri N, Tufano F, Kis P, Gaszner B, Cziraki A: Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens, 2010; 28: 2068-2075 [DOI] [PubMed] [Google Scholar]
- 40). Ageenkova OA, Purygina MA: Central aortic blood pressure, augmentation index, and reflected wave transit time: reproducibility and repeatability of data obtained by oscillometry. Vasc Health Risk Manag, 2011; 7: 649-656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, Kropf J, Eber B: Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension, 2011; 58: 825-832 [DOI] [PubMed] [Google Scholar]
- 42). Williams B, Lacy PS, Yan P, Hwee CN, Liang C, Ting CM: Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an N-point moving average method. J Am Coll Cardiol, 2011; 57: 951-961 [DOI] [PubMed] [Google Scholar]
- 43). O'Rourke MF, Hashimoto J: Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol, 2007; 50: 1-13 [DOI] [PubMed] [Google Scholar]
- 44). Hashimoto J, O'Rourke MF: Physical factors in arterial ageing. In: Oxford Textbook of Geriatric Medicine, ed by Michel JP, Beattie BL, Martin FC, Walston J, Oxford University Press, London, 2018. (In press) [Google Scholar]
- 45). Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O'Rourke MF: Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation, 1985; 71: 202-210 [DOI] [PubMed] [Google Scholar]
- 46). McEniery CM, Wilkinson IB, Avolio AP: Age, hypertension and arterial function. Clin Exp Pharmacol Physiol, 2007; 34: 665-671 [DOI] [PubMed] [Google Scholar]
- 47). McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR: Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol, 2005; 46: 1753-1760 [DOI] [PubMed] [Google Scholar]
- 48). Namasivayam M, McDonnell BJ, McEniery CM, O'Rourke MF: Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension, 2009; 53: 979-985 [DOI] [PubMed] [Google Scholar]
- 49). Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D: Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension, 2004; 43: 1239-1245 [DOI] [PubMed] [Google Scholar]
- 50). Namasivayam M, Adji A, O'Rourke MF: Aortic augmentation index and aging: mathematical resolution of a physiological dilemma? Hypertension, 2010; 56: e9-10 [DOI] [PubMed] [Google Scholar]
- 51). Hayward CS, Kelly RP: Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol, 1997; 30: 1863-1871 [DOI] [PubMed] [Google Scholar]
- 52). Smulyan H, Marchais SJ, Pannier B, Guerin AP, Safar ME, London GM: Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol, 1998; 31: 1103-1109 [DOI] [PubMed] [Google Scholar]
- 53). Gatzka CD, Kingwell BA, Cameron JD, Berry KL, Liang YL, Dewar EM, Reid CM, Jennings GL, Dart AM: Gender differences in the timing of arterial wave reflection beyond differences in body height. J Hypertens, 2001; 19: 2197-2203 [DOI] [PubMed] [Google Scholar]
- 54). Stoner L, Faulkner J, Westrupp N, Lambrick D: Sexual differences in central arterial wave reflection are evident in prepubescent children. J Hypertens, 2015; 33: 304-307 [DOI] [PubMed] [Google Scholar]
- 55). Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ: The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol, 2000; 525 Pt 1: 263-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M: Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation, 2006; 113: 1213-1225 [DOI] [PubMed] [Google Scholar]
- 57). Mahmud A, Feely J: Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension, 2003; 41: 183-187 [DOI] [PubMed] [Google Scholar]
- 58). Vlachopoulos C, Alexopoulos N, Panagiotakos D, O'Rourke MF, Stefanadis C: Cigar smoking has an acute detrimental effect on arterial stiffness. Am J Hypertens, 2004; 17: 299-303 [DOI] [PubMed] [Google Scholar]
- 59). Lemogoum D, Van Bortel L, Leeman M, Degaute JP, van de Borne P: Ethnic differences in arterial stiffness and wave reflections after cigarette smoking. J Hypertens, 2006; 24: 683-689 [DOI] [PubMed] [Google Scholar]
- 60). Rehill N, Beck CR, Yeo KR, Yeo WW: The effect of chronic tobacco smoking on arterial stiffness. Br J Clin Pharmacol, 2006; 61: 767-773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Mahmud A, Feely J: Divergent effect of acute and chronic alcohol on arterial stiffness. Am J Hypertens, 2002; 15: 240-243 [DOI] [PubMed] [Google Scholar]
- 62). van Trijp MJ, Beulens JW, Bos WJ, Uiterwaal CS, Grobbee DE, Hendriks HF, Bots ML: Alcohol consumption and augmentation index in healthy young men: the ARYA study. Am J Hypertens, 2005; 18: 792-796 [DOI] [PubMed] [Google Scholar]
- 63). Sierksma A, Lebrun CE, van der Schouw YT, Grobbee DE, Lamberts SW, Hendriks HF, Bots ML: Alcohol consumption in relation to aortic stiffness and aortic wave reflections: a cross-sectional study in healthy postmenopausal women. Arterioscler Thromb Vac Biol, 2004; 24: 342-348 [DOI] [PubMed] [Google Scholar]
- 64). Vlachopoulos C, Hirata K, O'Rourke MF: Pressure-altering agents affect central aortic pressures more than is apparent from upper limb measurements in hypertensive patients: the role of arterial wave reflections. Hypertension, 2001; 38: 1456-1460 [DOI] [PubMed] [Google Scholar]
- 65). Mahmud A, Feely J: Acute effect of caffeine on arterial stiffness and aortic pressure waveform. Hypertension, 2001; 38: 227-231 [DOI] [PubMed] [Google Scholar]
- 66). Vlachopoulos C, Alexopoulos N, Dima I, Aznaouridis K, Andreadou I, Stefanadis C: Acute effect of black and green tea on aortic stiffness and wave reflections. J Am Coll Nutr, 2006; 25: 216-223 [DOI] [PubMed] [Google Scholar]
- 67). Vlachopoulos C, Kosmopoulou F, Panagiotakos D, Ioakeimidis N, Alexopoulos N, Pitsavos C, Stefanadis C: Smoking and caffeine have a synergistic detrimental effect on aortic stiffness and wave reflections. J Am Coll Cardiol, 2004; 44: 1911-1917 [DOI] [PubMed] [Google Scholar]
- 68). Seals DR, Tanaka H, Clevenger CM, Monahan KD, Reiling MJ, Hiatt WR, Davy KP, DeSouza CA: Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol, 2001; 38: 506-513 [DOI] [PubMed] [Google Scholar]
- 69). Gates PE, Tanaka H, Hiatt WR, Seals DR: Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension, 2004; 44: 35-41 [DOI] [PubMed] [Google Scholar]
- 70). Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C, Schwartz RF, Atkinson M, Shuster JJ, Pierce GL, Silverstein JH: Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care, 2004; 27: 2911-2917 [DOI] [PubMed] [Google Scholar]
- 71). Schram MT, Henry RM, van Dijk RA, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Westerhof N, Stehouwer CD: Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension, 2004; 43: 176-181 [DOI] [PubMed] [Google Scholar]
- 72). Lacy PS, O'Brien DG, Stanley AG, Dewar MM, Swales PP, Williams B: Increased pulse wave velocity is not associated with elevated augmentation index in patients with diabetes. J Hypertens, 2004; 22: 1937-1944 [DOI] [PubMed] [Google Scholar]
- 73). Maple-Brown LJ, Piers LS, O'Rourke MF, Celermajer DS, O'Dea K: Central obesity is associated with reduced peripheral wave reflection in Indigenous Australians irrespective of diabetes status. J Hypertens, 2005; 23: 1403-1407 [DOI] [PubMed] [Google Scholar]
- 74). Wilkinson IB, Prasad K, Hall IR, Thomas A, MacCallum H, Webb DJ, Frenneaux MP, Cockcroft JR: Increased central pulse pressure and augmentation index in subjects with hypercholesterolemia. J Am Coll Cardiol, 2002; 39: 1005-1011 [DOI] [PubMed] [Google Scholar]
- 75). Saba PS, Roman MJ, Longhini C, Scorzoni D, Pini R, Devereux RB, Ganau A: Carotid intimal-medial thickness and stiffness are not affected by hypercholesterolemia in uncomplicated essential hypertension. Arterioscler Thromb Vac Biol, 1999; 19: 2788-2794 [DOI] [PubMed] [Google Scholar]
- 76). Protogerou AD, Blacher J, Aslangul E, Le Jeunne C, Lekakis J, Mavrikakis M, Safar ME: Gender influence on metabolic syndrome's effects on arterial stiffness and pressure wave reflections in treated hypertensive subjects. Atherosclerosis, 2007; 193: 151-158 [DOI] [PubMed] [Google Scholar]
- 77). Morigami H, Morioka T, Yamazaki Y, Imamura S, Numaguchi R, Asada M, Motoyama K, Mori K, Fukumoto S, Shoji T, Emoto M, Inaba M: Visceral adiposity is preferentially associated with vascular stiffness rather than thickness in men with type 2 diabetes. J Atheroscler Thromb, 2016; 23: 1067-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Wang J, Wang F, Dong S, Zeng Q, Zhang L: Levels of serum phosphorus and cardiovascular surrogate markers. J Atheroscler Thromb, 2016; 23: 95-104 [DOI] [PubMed] [Google Scholar]
- 79). Owen PJ, Rajiv C, Vinereanu D, Mathew T, Fraser AG, Lazarus JH: Subclinical hypothyroidism, arterial stiffness, and myocardial reserve. J Clin Endocrinol Metab, 2006; 91: 2126-2132 [DOI] [PubMed] [Google Scholar]
- 80). Smith JC, Page MD, John R, Wheeler MH, Cockcroft JR, Scanlon MF, Davies JS: Augmentation of central arterial pressure in mild primary hyperparathyroidism. J Clin Endocrinol Metab, 2000; 85: 3515-3519 [DOI] [PubMed] [Google Scholar]
- 81). Phillips C, Hedner J, Berend N, Grunstein R: Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep, 2005; 28: 604-609 [DOI] [PubMed] [Google Scholar]
- 82). Phillips CL, Yee B, Yang Q, Villaneuva AT, Hedner J, Berend N, Grunstein R: Effects of continuous positive airway pressure treatment and withdrawal in patients with obstructive sleep apnea on arterial stiffness and central BP. Chest, 2008; 134: 94-100 [DOI] [PubMed] [Google Scholar]
- 83). Senzaki H, Chen CH, Ishido H, Masutani S, Matsunaga T, Taketazu M, Kobayashi T, Sasaki N, Kyo S, Yokote Y: Arterial hemodynamics in patients after Kawasaki disease. Circulation, 2005; 111: 2119-2125 [DOI] [PubMed] [Google Scholar]
- 84). Klocke R, Cockcroft JR, Taylor GJ, Hall IR, Blake DR: Arterial stiffness and central blood pressure, as determined by pulse wave analysis, in rheumatoid arthritis. Ann Rheum Dis, 2003; 62: 414-418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85). Hashimoto J, O'Rourke MF: Inflammation and arterial stiffness in chronic kidney disease: cause or consequence? Am J Hypertens, 2017; 30: 350-352 [DOI] [PubMed] [Google Scholar]
- 86). Bartoloni E, Pucci G, Cannarile F, Battista F, Alunno A, Giuliani M, Cafaro G, Gerli R, Schillaci G: Central hemodynamics and arterial stiffness in systemic sclerosis. Hypertension, 2016; 68: 1504-1511 [DOI] [PubMed] [Google Scholar]
- 87). Lemogoum D, Van Bortel L, Najem B, Dzudie A, Teutcha C, Madu E, Leeman M, Degaute JP, van de Borne P: Arterial stiffness and wave reflections in patients with sickle cell disease. Hypertension, 2004; 44: 924-929 [DOI] [PubMed] [Google Scholar]
- 88). London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME: Arterial wave reflections and survival in end-stage renal failure. Hypertension, 2001; 38: 434-438 [DOI] [PubMed] [Google Scholar]
- 89). Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc'h PM, London GM: Central pulse pressure and mortality in end-stage renal disease. Hypertension, 2002; 39: 735-738 [DOI] [PubMed] [Google Scholar]
- 90). Covic A, Mardare N, Gusbeth-Tatomir P, Prisada O, Sascau R, Goldsmith DJ: Arterial wave reflections and mortality in haemodialysis patients—only relevant in elderly, cardiovascularly compromised? Nephrol Dial Transplant, 2006; 21: 2859-2866 [DOI] [PubMed] [Google Scholar]
- 91). Briet M, Collin C, Karras A, Laurent S, Bozec E, Jacquot C, Stengel B, Houillier P, Froissart M, Boutouyrie P: Arterial remodeling associates with CKD progression. J Am Soc Nephrol, 2011; 22: 967-974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Verbeke F, Marechal C, Van Laecke S, Van Biesen W, Devuyst O, Van Bortel LM, Jadoul M, Vanholder R: Aortic stiffness and central wave reflections predict outcome in renal transplant recipients. Hypertension, 2011; 58: 833-838 [DOI] [PubMed] [Google Scholar]
- 93). Ueda H, Hayashi T, Tsumura K, Yoshimaru K, Nakayama Y, Yoshikawa J: The timing of the reflected wave in the ascending aortic pressure predicts restenosis after coronary stent placement. Hypertens Res, 2004; 27: 535-540 [DOI] [PubMed] [Google Scholar]
- 94). Weber T, Auer J, O'Rourke M F, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B: Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J, 2005; 26: 2657-2663 [DOI] [PubMed] [Google Scholar]
- 95). Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ: Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension, 2005; 45: 980-985 [DOI] [PubMed] [Google Scholar]
- 96). Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, Kloch-Badelek M, Wilinski J, Curylo AM, Dudek D: Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension, 2008; 51: 848-855 [DOI] [PubMed] [Google Scholar]
- 97). Weber T, O'Rourke MF, Lassnig E, Porodko M, Ammer M, Rammer M, Eber B: Pulse waveform characteristics predict cardiovascular events and mortality in patients undergoing coronary angiography. J Hypertens, 2010; 28: 797-805 [DOI] [PubMed] [Google Scholar]
- 98). Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV: Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension, 2007; 50: 197-203 [DOI] [PubMed] [Google Scholar]
- 99). Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV: High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol, 2009; 54: 1730-1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100). Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, Masotti G, Roman MJ: Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol, 2008; 51: 2432-2439 [DOI] [PubMed] [Google Scholar]
- 101). Leone N, Ducimetiere P, Gariepy J, Courbon D, Tzourio C, Dartigues JF, Ritchie K, Alperovitch A, Amouyel P, Safar ME, Zureik M: Distension of the carotid artery and risk of coronary events: the three-city study. Arterioscler Thromb Vac Biol, 2008; 28: 1392-1397 [DOI] [PubMed] [Google Scholar]
- 102). Benetos A, Thomas F, Joly L, Blacher J, Pannier B, Labat C, Salvi P, Smulyan H, Safar ME: Pulse pressure amplification a mechanical biomarker of cardiovascular risk. J Am Coll Cardiol, 2010; 55: 1032-1037 [DOI] [PubMed] [Google Scholar]
- 103). Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH: Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens, 2009; 27: 461-467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104). Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH: Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension, 2010; 55: 799-805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105). Huang CM, Wang KL, Cheng HM, Chuang SY, Sung SH, Yu WC, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH: Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens, 2011; 29: 454-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106). Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ: Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation, 2010; 121: 505-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107). Hashimoto J, Ito S: Central blood pressure and prediction of cardiovascular events. Curr Hypertens Rev, 2012; 8: 108-113 [Google Scholar]
- 108). McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, Cockcroft JR, Wilkinson IB: Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension, 2008; 51: 1476-1482 [DOI] [PubMed] [Google Scholar]
- 109). Cheng HM, Chuang SY, Sung SH, Yu WC, Pearson A, Lakatta EG, Pan WH, Chen CH: Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long-term cardiovascular risks. J Am Coll Cardiol, 2013; 62: 1780-1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110). Kelly RP, Gibbs HH, O'Rourke MF, Daley JE, Mang K, Morgan JJ, Avolio AP: Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery. Eur Heart J, 1990; 11: 138-144 [DOI] [PubMed] [Google Scholar]
- 111). Morgan T, Lauri J, Bertram D, Anderson A: Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens, 2004; 17: 118-123 [DOI] [PubMed] [Google Scholar]
- 112). Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J: Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOTBPLA): a multicentre randomised controlled trial. Lancet, 2005; 366: 895-906 [DOI] [PubMed] [Google Scholar]
- 113). Boutouyrie P, Achouba A, Trunet P, Laurent S: Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipineatenolol combination: the EXPLOR study. Hypertension, 2010; 55: 1314-1322 [DOI] [PubMed] [Google Scholar]
- 114). Williams B, Cockcroft JR, Kario K, Zappe DH, Brunel PC, Wang Q, Guo W: Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: the PARAMETER study. Hypertension, 2017; 69: 411-420 [DOI] [PubMed] [Google Scholar]
- 115). Hashimoto J, Imai Y, O'Rourke MF: Indices of pulse wave analysis are better predictors of left ventricular mass reduction than cuff pressure. Am J Hypertens, 2007; 20: 378-384 [DOI] [PubMed] [Google Scholar]
- 116). Boutouyrie P, Bussy C, Hayoz D, Hengstler J, Dartois N, Laloux B, Brunner H, Laurent S: Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation, 2000; 101: 2601-2606 [DOI] [PubMed] [Google Scholar]
- 117). Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF: Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens, 2008; 26: 1017-1024 [DOI] [PubMed] [Google Scholar]
- 118). Hashimoto J, Ito S: Some mechanical aspects of arterial aging: physiological overview based on pulse wave analysis. Ther Adv Cardiovasc Dis, 2009; 3: 367-378 [DOI] [PubMed] [Google Scholar]
- 119). Williams B, Lacy PS, Cruickshank JK, Collier D, Hughes AD, Stanton A, Thom S, Thurston H: Impact of statin therapy on central aortic pressures and hemodynamics: principal results of the Conduit Artery Function Evaluation-Lipid-Lowering Arm (CAFE-LLA) Study. Circulation, 2009; 119: 53-61 [DOI] [PubMed] [Google Scholar]
- 120). Kanaki AI, Sarafidis PA, Georgianos PI, Kanavos K, Tziolas IM, Zebekakis PE, Lasaridis AN: Effects of low-dose atorvastatin on arterial stiffness and central aortic pressure augmentation in patients with hypertension and hypercholesterolemia. Am J Hypertens, 2013; 26: 608-616 [DOI] [PubMed] [Google Scholar]
- 121). de Boer SA, Heerspink HJ, Juarez Orozco LE, van Roon AM, Kamphuisen PW, Smit AJ, Slart RH, Lefrandt JD, Mulder DJ: Effect of linagliptin on pulse wave velocity in early type 2 diabetes (RELEASE): a randomized, double-blind, controlled 26-week trial. Diabetes Obes Metab, 2017; [DOI] [PubMed] [Google Scholar]
- 122). Sofer E, Boaz M, Matas Z, Mashavi M, Shargorodsky M: Treatment with insulin sensitizer metformin improves arterial properties, metabolic parameters, and liver function in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled trial. Metabolism, 2011; 60: 1278-1284 [DOI] [PubMed] [Google Scholar]
- 123). Agarwal N, Rice SP, Bolusani H, Luzio SD, Dunseath G, Ludgate M, Rees DA: Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab, 2010; 95: 722-730 [DOI] [PubMed] [Google Scholar]
- 124). Park J, Liao P, Sher S, Lyles RH, Deveaux DD, Quyyumi AA: Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol, 2015; 308: R208-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125). Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA: Inflammation and arterial stiffness in humans. Atherosclerosis, 2014; 237: 381-390 [DOI] [PubMed] [Google Scholar]
- 126). Vlachopoulos C, Aznaouridis K, Stefanadis C: Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol, 2010; 55: 1318-1327 [DOI] [PubMed] [Google Scholar]
- 127). Hashimoto J, O'Rourke MF: Is arterial stiffness better than blood pressure in predicting cardiovascular risk? Curr Cardiovasc Risk Rep, 2008; 2: 133-140 [Google Scholar]
- 128). Mills CJ, Gabe IT, Gault JH, Mason DT, Ross J, Jr., Braunwald E, Shillingford JP: Pressure-flow relationships and vascular impedance in man. Cardiovasc Res, 1970; 4: 405-417 [DOI] [PubMed] [Google Scholar]
- 129). Hashimoto J, Ito S: Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension, 2013; 62: 542-549 [DOI] [PubMed] [Google Scholar]
- 130). Hashimoto J, Ito S: Pulse pressure amplification, arterial stiffness, and peripheral wave reflection determine pulsatile flow waveform of the femoral artery. Hypertension, 2010; 56: 926-933 [DOI] [PubMed] [Google Scholar]
- 131). Hashimoto J, Ito S: Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension, 2011; 58: 839-846 [DOI] [PubMed] [Google Scholar]
- 132). Hashimoto J: Central hemodynamics and target organ damage in hypertension. Tohoku J Exp Med, 2014; 233: 1-8 [DOI] [PubMed] [Google Scholar]
- 133). Hashimoto J, Aikawa T, Imai Y: Large artery stiffening as a link between cerebral lacunar infarction and renal albuminuria. Am J Hypertens, 2008; 21: 1304-1309 [DOI] [PubMed] [Google Scholar]
- 134). Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW: Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension, 2008; 52: 1120-1126 [DOI] [PubMed] [Google Scholar]
- 135). Ochi N, Kohara K, Tabara Y, Nagai T, Kido T, Uetani E, Ochi M, Igase M, Miki T: Association of central systolic blood pressure with intracerebral small vessel disease in Japanese. Am J Hypertens, 2010; 23: 889-894 [DOI] [PubMed] [Google Scholar]
- 136). Shrestha I, Takahashi T, Nomura E, Ohtsuki T, Ohshita T, Ueno H, Kohriyama T, Matsumoto M: Association between central systolic blood pressure, white matter lesions in cerebral MRI and carotid atherosclerosis. Hypertens Res, 2009; 32: 869-874 [DOI] [PubMed] [Google Scholar]
- 137). Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ: Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility — Reykjavik study. Brain, 2011; 134: 3398-3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138). O'Rourke MF, Safar ME: Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension, 2005; 46: 200-204 [DOI] [PubMed] [Google Scholar]
- 139). Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, Boutouyrie P: Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke, 2003; 34: 1203-1206 [DOI] [PubMed] [Google Scholar]
- 140). Lovett JK, Howard SC, Rothwell PM: Pulse pressure is independently associated with carotid plaque ulceration. J Hypertens, 2003; 21: 1669-1676 [DOI] [PubMed] [Google Scholar]
- 141). Shaikh AY, Wang N, Yin X, Larson MG, Vasan RS, Hamburg NM, Magnani JW, Ellinor PT, Lubitz SA, Mitchell GF, Benjamin EJ, McManus DD: Relations of arterial stiffness and brachial flow-mediated dilation with new-onset atrial fibrillation: the Framingham Heart Study. Hypertension, 2016; 68: 590-596 [DOI] [PubMed] [Google Scholar]
- 142). Harloff A, Strecker C, Dudler P, Nussbaumer A, Frydrychowicz A, Olschewski M, Bock J, Stalder AF, Stroh AL, Weiller C, Hennig J, Markl M: Retrograde embolism from the descending aorta: visualization by multidirectional 3D velocity mapping in cryptogenic stroke. Stroke, 2009; 40: 1505-1508 [DOI] [PubMed] [Google Scholar]
- 143). Kronzon I, Tunick PA: Aortic atherosclerotic disease and stroke. Circulation, 2006; 114: 63-75 [DOI] [PubMed] [Google Scholar]
- 144). Levin A, Stevens PE: Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int, 2014; 85: 49-61 [DOI] [PubMed] [Google Scholar]
- 145). Temmar M, Jankowski P, Peltier M, Mouquet V, Debicka-Dabrowska D, Hamida F, Kawecka-Jaszcz K, Safar ME: Intraaortic pulse pressure amplification in subjects at high coronary risk. Hypertension, 2010; 55: 327-332 [DOI] [PubMed] [Google Scholar]
- 146). Ford ML, Tomlinson LA, Chapman TP, Rajkumar C, Holt SG: Aortic stiffness is independently associated with rate of renal function decline in chronic kidney disease stages 3 and 4. Hypertension, 2010; 55: 1110-1115 [DOI] [PubMed] [Google Scholar]
- 147). Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, Franco OH, Dehghan A: Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol, 2015; 10: 2190-2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148). Hashimoto J, Ito S: Aortic blood flow reversal determines renal function: potential explanation for renal dysfunction caused by aortic stiffening in hypertension. Hypertension, 2015; 66: 61-67 [DOI] [PubMed] [Google Scholar]
- 149). Chirinos JA, Segers P: Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension, 2010; 56: 563-570 [DOI] [PubMed] [Google Scholar]
- 150). Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS: Association of central versus brachial blood pressure with target-organ damage: systematic review and meta-analysis. Hypertension, 2016; 67: 183-190 [DOI] [PubMed] [Google Scholar]
- 151). Hashimoto J, Nichols WW, O'Rourke MF, Imai Y: Association between wasted pressure effort and left ventricular hypertrophy in hypertension: influence of arterial wave reflection. Am J Hypertens, 2008; 21: 329-333 [DOI] [PubMed] [Google Scholar]
- 152). Hashimoto J, Ito S: Central diastolic pressure decay mediates the relationship between aortic stiffness and myocardial viability: potential implications for aortosclerosis-induced myocardial ischemia. J Hypertens, 2017. (In press) [DOI] [PubMed] [Google Scholar]
- 153). Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B: Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation, 2004; 109: 184-189 [DOI] [PubMed] [Google Scholar]
- 154). Hoffman JI, Buckberg GD: The myocardial oxygen supply:demand index revisited. J Am Heart Assoc, 2014; 3: e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155). Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, Lekakis J, Mikhailidis DP, Naka KK, Protogerou AD, Rizzoni D, et al. : The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis, 2015; 241: 507-532 [DOI] [PubMed] [Google Scholar]
- 156). Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T: Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension, 2015; 66: 698-722 [DOI] [PMC free article] [PubMed] [Google Scholar]


