Abstract
Endothelial dysfunction is one of the hallmarks of atherogenesis, and correlates with many cardiovascular risk factors. One of the features of endothelial dysfunction is the loss of nitric oxide (NO) bioavailability, resulting in derangements in the vasodilatory response of the vessel wall. Flow mediated dilatation (FMD) of the brachial artery is an accepted method for non-invasive assessment of systemic endothelial function. FMD is examined extensively in the context of cardiovascular research, and has been utilised as a routine assessment in large cohorts such as the Framingham Heart Study, Young Finns Study, and Gutenberg Heart Study. However, FMD is less known in the context of vascular surgery research, despite the similarities between the underpinning disease mechanisms. This review will provide a summary of FMD in terms of its history of development and the conduct of the test in research settings. It will further highlight the key literature of FMD as a biomarker for vascular surgeons, particularly in the context of abdominal aortic aneurysms and lower limb peripheral arterial disease.
Keywords: Flow mediated dilatation, Biomarkers, Abdominal aortic aneurysm, Peripheral arterial disease
Introduction
The vascular endothelium is the predominant regulator of vascular homeostasis, governing the processes of vasoconstriction and vasodilation, thrombogenesis, fibrinolysis, smooth muscle cell proliferation, and cell adhesion1). Calcification, stenosis and atherosclerotic plaques can attenuate the effectiveness of these functions, bringing about endothelial dysfunction in regulatory behaviour and impacting the overall cardiovascular health of an individual2). Extensive literature suggests endothelial dysfunction as an early hallmark in the development of atherosclerosis3), and indeed the ability of endothelial dysfunction to reflect atherosclerotic disease severity, and as a predictor of future cardiovasulcar events3, 4).
A recognised non-invasive assessment of endothelial function is flow mediated dilatation (FMD) of a main conduit artery, such as the brachial artery. The FMD measurement capitalises on the reaction of the vascular endothelium when subjected to shear stress. The typical response in a healthy individual is an observable dilation5, 6). Early studies found low shear stress to be correlated with atherosclerotic plaque burden, resulting in uneven stiffness across various regions of artery7, 8). It was subsequently observed that external stimuli, such as compression of the artery from the skin surface, could result in the same provocation of the vasodilatory response that high sheer stress from blood flow induced, resulting in the development of FMD as a measure of responsiveness of the vascular endothelium.
The measurement of FMD has been widely adopted in cardiovascular research, and is reproducible in the setting of clinical trials9, 10). FMD has been shown to associate with other markers that are relevant in the context of cardiovascular disease, such as serum levels of docosahexaenoic acid (a polyunsaturated fatty acid)11). It has also been examined in the context of paediatric populations who may be at risk of subsequent atherosclerotic disease12). Acceptance of FMD as an adjunctive marker for cardiovascular risk prediction is evident by its inclusion in large prospective cohort studies such as the Framingham Heart study13), Young Finns studies14), and the Gutenberg Heart Study15). In Japan, a population known for its long life expectancy, the measurement of FMD has been adopted into routine clinical practice, where the Government reimburses the fee for FMD tests performed for clinical practice16).
The aim of this review is to provide a summary of the FMD test in terms of its history of development, conduct and analysis of the test, and its relevance as a potential biomarker in vascular surgery research.
Historic Perspective
The methods of FMD measurement in humans first emerged in the literature in 1989 through the seminal work of Anderson et al.17), and was subsequently applied into the clinical research setting by Celermajer et al. in 19926). Although it is feasible to measure FMD in different conduit arteries, such as the radial, brachial, or femoral artery. Brachial arteries are typically preferred due to the ease of the procedure. The brachial artery is relatively superficial and large enough for clear imaging, and enables the application of a blood pressure cuff in the forearm with ease.
During the early phase of adoption of this technique, many variations emerged in the methodology leading to significant repeatability and comparison issues between studies. Emergence of standard timing protocol began the process of standardising the method18). Initially, analysis of recorded sonograms was labour intensive and subject largely to observer interpretation. However, at the turn of the millennium the introduction of automated edge detection software offered a faster and more reproducible method of analysis19). Subsequently the technique was further standardised to place the occlusion cuff on the forearm which further improved the reproducibility of FMD values were observed between distal and proximal placements on the upper limb20).
Conduct of the FMD Procedure18, 21)
The FMD procedure should be performed in a dimly lit quiet room, with the patient in the supine position. ECG tabs can be applied to enable ECG gated acquisition of images. The brachial artery of the right arm is identified by ultrasound and a baseline measurement of lumen diameter obtained. To ensure the stability and fixed position of the ultrasound probe during recording, it is beneficial to hold the ultrasound probe in place using a stereotactic probe holding device. Different variations of the stereotactic fixation device exist. It is simple to incorporate a customised silicon mould of a ultrasound probe holder to a stereotactic clamp with a central magnetic base (UK RS online, Cat# 213-0670). This enables magnetic fixation of the clamp against a metallic table surface which serves as an arm rest.
Supra-systolic pressure is applied to the forearm via a blood pressure cuff for 5 minutes to achieve blood flow occlusion. The diameter of the brachial artery is recorded at specific time points during flow occlusion and after the release of the blood pressure cuff to assess flow mediated changes in diameter. Subsequent computer analysis allows for the calculation of a percentage increase in lumen diameter during FMD.
Off-line analysis of flow mediated diameter changes can be performed using commercially available software, such as Vascular Research Tool (Medical Imaging Applications LLC, USA). The percentage change in flow mediated dilatation or constriction can be calculated by: (Δbrachial artery diameter before and after flow stimuli/brachial artery diameter at baseline).
FMD can be affected by diurnal rhythm, medications, and sympathetic stimuli such as caffeine, nicotine, and exercise. Therefore every attempt should be made to minimise these effects. For example, changes in medications should be recorded and accounted for; participants should abstain from tobacco, coffee and tea for at least 24 hours before the procedure; allow a period of rest in the quite room before undertaking the FMD measurement; perform the test at around the same time if serial measurements are taken from one person. It is also important for each research lab to conduct quality assurance exercises to ensure reproducibility of the analysis.
Next Generation Device for the Measurement of FMD
The conduct of FMD can be challenging. It requires dedicated training of the operator, adherence to standard operating protocols for the acquisition and analysis of the test, and quality assurance exercise to minimise intra-observer variation. Additional hardware modifications (such as a customised stereotactic holder for the ultrasound probe) are also recommended. Slight variations in the conduct of FMD tests can exist between different centers, which means it may be difficult to compare the FMD values between different studies. Despite the extensive literature on FMD in research settings, the lack of a standardised international test procedure affects the ability for FMD to be applied as a clinical test.
A semi-automated device for the FMD test (UNEXEF, UNEX, Nagoya, Japan) overcomes these challenges and has been commercially available in Japan since 2007. The UNEXEF device provides automated vessel wall tracking and real time FMD/FMC analyses, which in term reduces operator related variations. Many investigators in Japan have adopted this device for their investigations on FMD in the context cardiovascular research22–24). This device has received FDA approval (K131973) and is currently subject to the CE Mark approval process.
Mechanisms of Flow Mediated Changes in Conduit Artery
Flow Mediated Dilatation
The fundamental principle of FMD is to measure the bioavailability of nitric oxide (NO), the primary vasodilator in the human cardiovascular system25). The mechanism for NO-dependent vasodilation is through the enzyme endothelial Nitric Oxide Synthase (eNOS) which facilitates the production of NO from L-Arginine. NO diffuses through the tissue layers to the smooth muscle to initiate the production of cyclic GMP through Guanylate Cyclase, resulting in vasodilation26).
Nitroglycerine Mediated Dilatation
Nitroglycerine mediated dilatation (NMD) is regularly performed as part of the FMD test as a control procedure21). Pharmacological supplementation of NO (via the administration of nitroglycerine) triggers the NO mediated response downstream of its receptor (guanylyl cyclase) within the vascular smooth muscle cells (VSMC). In the presence of normal NO bioavailability, FMD may still be impaired if there is derangement in the VSMC receptor pathway. NMD is therefore often conducted as part of the FMD protocol as a positive control for FMD. For the assessment of NMD, 400 µg of glyceryl trinitrate can be administered sublingually at the end of the FMD protocol, followed by further recording of the brachial artery diameter to record the maximal dilatation achieved.
Flow Mediated Constriction
The process of occluding the brachial artery induces a low flow state, and can lead to constriction of the artery called flow mediated constriction (FMC)27). This is thought to reflect the basal vasoconstrictive tone conferred by the release of endothelin28). Brachial artery FMC is reported as apparent in only 40% of individuals, but when it does occur it can be associated with both a delayed and blunted FMD response29).
Time Course of the Flow Mediated Response
The temporal relationship of the flow mediated responses are illustrated in Fig. 1. Following baseline vessel lumen diameter measurements (FMB) the occlusion cuff is inflated for a period of five minutes; during which time the vessel may constrict (FMC). FMC is denoted by the minimum arterial diameter observed before the blood pressure cuff deflation (typically taken within 60 seconds of cuff deflation). After cuff deflation, brachial artery lumen diameter is continuously measured. FMD is typically defined by the maximal diameter recorded during the three minutes after cuff deflation.
Fig. 1.
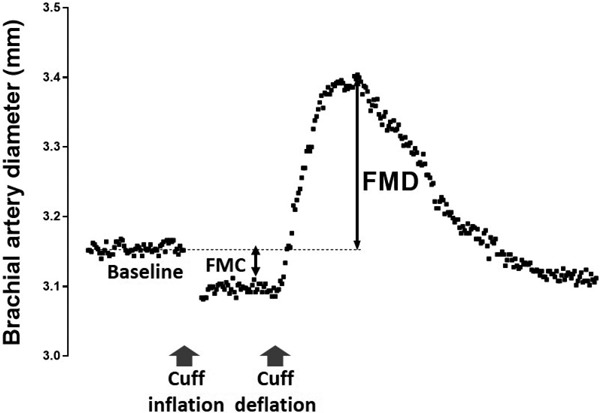
Graphical illustration of the time course of FMC and FMD
FMD as a Biomarker in the Context of Abdominal Aortic Aneurysms
The earliest study to examine FMD as a research test in human AAAs was reported in 2002, by Knipp et al.30). FMD and NMD were measured in 30 patients with AAA, peripheral arterial disease (PAD), or healthy volunteers (HVs). Subjects with AAA exhibited the worst FMD response among the three groups. A diminished NMD was also observed in the AAA group. Patient with PAD had diminished FMD response compared to HVs, but not in their NMD responses. Around the same time, Gokce et al. examined the association between pre-operative FMD and the occurrence of peri-operative complications after major vascular surgery (including 24 patients undergoing AAA repair). They found that patients who suffered postoperative consequences had a lower FMD pre-operatively31).
Akamatsu et al. provided two subsequent reports regarding FMD in AAA patients. The first study examined FMD in a cohort of AAA and PAD patients (n = 30 and 27 respectively) compared to HVs. HVs had significantly higher FMD than patients with AAA or PAD. No difference in NMD was observed between patients and HVs. (Of note the age of HVs was also significantly younger in this study)32). In their other study, FMD and NMD was measured in patients with PAD or AAA/thoracic aortic aneurysm (n = 26 and 47 respectively). During the follow up period (47 ± 13 months), 18 cardiovascular events were recorded. FMD and NMD were significantly lower in subjects with events. In Cox regression, lower FMD or NMD independently predicted future cardiovascular events33).
Medina et al. was the first to report the correlation between AAA size and FMD34). In this cross sectional study of 30 AAA patients (mean AAA diameter 43 mm), an inverse correlation was observed between aneurysm diameter and FMD. They further observed an inverse correlation between FMD and plasma CRP levels, while a positive correlation between AAA diameter and plasma CRP levels was seen. Medina concluded that the increased levels of inflammation have an effect on the bioavailability of NO. Further to these observations, Sung et al. examined FMD in 78 subjects (HVs n = 15, small AAAs n = 27, or large AAAs n = 36). The AAA patients demonstrated significantly lower vasodilation than the HVs, as well as a significant difference between the AAA size groups. In addition, fewer circulating endothelial progenitor cells (EPCs) were present in AAA patients than in HVs. EPCs functions (proliferation, adhesion, migration, tube formation etc) were also significantly impaired in AAA patients35).
The Oxford Abdominal Aortic Aneurysm Study was the first to systemically examine FMD during the progression of AAA in humans. In this largest cohort of AAA patients with FMD measurement (n = 162), there was a significant inverse correlation between FMD and the diameter of AAAs (median diameter = 50 mm). Eighty-eight of the participants with small to moderate size AAAs (30–55 mm) under surveillance were re-assessed at 12 months (median duration of follow-up was 365 days). FMD deteriorated significantly during the surveillance period. Furthermore, there was a significant inverse correlation between FMD (at baseline) and AAA growth in the following 12 months, particularly in the subgroup of AAAs with diameter of 40–55 mm36).
FMD as a Biomarker in the Context of PAD
There are few reports of FMD as a biomarker in the context of peripheral arterial disease. In 2007, Loffredo et al. reported that patients with PAD (n = 20) had significantly lower FMD compared with matched control subjects without PAD (n = 40)37). The same group subsequently utilised FMD as a surrogate marker for their investigation on the effect of propionyl-l-carnitine (an anti-oxidant) infusion in patients with PAD (n = 50, vs 50 controls)38). Maldonado et al. examined FMD in a cohort of 50 patients with PAD (claudication n = 30, critical limb ischaemia n = 20) and 30 healthy individuals. They found that FMD was significantly lower in PAD patients compared to HVs, but the severity of PAD (ie claudication vs critical ischaemia) did not affect FMD39). Subsequently, Allan et al. compared the brachial artery FMD with peripheral artery tonometry (PAT) derived reactive hyperaemia index (RHI) in 26 PAD patients with claudication and 25 HVs. Patients with PAD had a significantly lower FMD than healthy subjects, but there was no difference in the RHIs between the two groups. Further, there was no correlation between the FMD and RHI in either group. They concluded that PAT is not a sensitive measure of endothelial function for PAD patients, and FMD should be the choice of measurement in this context40).
Subsequent to these, Heinen et al. examined a cohort of 40 subjects in four distinct groups: symptomatic superficial femoral artery (SFA) PAD (n = 10), symptomatic below knee (BK) PAD (n = 10), age matched controls without PAD (n = 10) and healthy volunteers (n = 10). Both brachial and SFA FMD was recorded in all groups at baseline, and then again in the SFA PAD group following balloon angioplasty. In addition, intimal media thickness at the site of FMD measurement was also recorded. In patients with SFA or BK PAD, both brachial artery and SFA FMDs were significantly worse than those of the HVs'. FMD correlated with intima/media or plaque thickness at the site of FMD measurement. Progressive deterioration of FMD was further observed across the segment of SFA disease (pre-, intra-, post-stenosis)41).
A recent report by Iwamoto et al. examined FMD in 100 subjects (30 patients with PAD, 30 patients with Buerger's disease, age and sex matched subjects without PAD (n = 30) or without Buerger's disease (n = 20). FMD was worse in patients with PAD compared to the non-PAD subjects. Interestingly, they did not observe differences in FMD between patients with Buerger's disease and control subjects42).
Surgical Intervention and the Effect on FMD
In the broader cardiovascular literature, it is known that the derangement in FMD is a reversible process3). Medications commonly used for cardiovascular risk prevention, such as angiotensin converting enzyme inhibitors and statins, can improve endothelial function43–46). However, very few studies have examined the reversibility of FMD in the context of vascular surgery intervention. These are summarised below.
In 2008, Husmann et al. reported a randomised controlled study of 33 patients of chronic PAD due for femoral-popliteal intervention. Patients were randomised to receive either endovascular revascularisation plus best medical therapy (EV+BMT, n = 17) or best medical therapy only (BMT, n = 16). Baseline FMD did not different between the two groups. FMD significantly improved in the EV+BMT group at 4 weeks after procedure, but remained unchanged in the BMT only group47). Subsequently, Unal et al. observed significant improvement of brachial FMD in 54 patients four weeks after femoro-popliteal bypass grafting for SFA PAD48). In another study, Jacomella et al. examined the FMDs in 24 hypertensive patients with renal artery stenosis before and one day after renal artery stenosis (RAS) angioplasty. In this cohort, FMD significantly improved after RAS angioplasty, while NMD remained unchanged49). In the aforementioned study by Heinen et al., they also observe significant improvement of SFA FMD within 24 hours of SFA balloon angioplasty to the level observed in the pre-stenotic SFA segment41). The OxAAA study was the first to examine the effect of AAA surgery on brachial artery FMD. FMD was measured in 50 patients before and 8–12 weeks after AAA surgery (endovascular repair, n = 28; open surgical repair, n = 22). FMD was significantly improved by AAA surgery, irrespective of the type of surgery performed50).
These studies are summarised in Table 1 and 2.
Table 1. Studies which examined FMD in the context of abdominal aortic aneurysm in humans.
| Author | Year | Context | Measure | N | Main findings |
|---|---|---|---|---|---|
| Knipp30) | 2002 | * AAA #PAD ×HV |
FMD NMD |
* 11 #9 +10 |
FMD and NMD are both lower in patients with AAA, and although FMD in patients with PAD is not distinguishable from HVs, NMD is significantly decreased, possibly pointing to systemic processes involved in endothelial dysfunction. |
| Gokce31) | 2002 | * AAA #PAD |
FMD NMD |
* 24 #100 |
Preoperative measurements of FMD were taken in a cohort of patients undergoing vascular interventions. Patients who suffered perioperative complications had worse preoperative FMD compared to those who didn't. No difference observed in NMD between the two groups. |
| Akamatsu32) | 2007 | * AAA #PAD |
FMD NMD |
* 30 #27 |
In patients with AAA or PAD, there was a correlation between NMD and FMD. HVs had a significantly higher FMD than patients (although the age of HVs was also significantly lower). There was no significant difference in NMD between subjects and HVs. |
| Akamatsu33) | 2010 | * AAA/TAA #PAD |
FMD NMD |
* 47 #26 |
During the follow up period (47 ± 13 months), 18 cardiovascular events were recorded. FMD and NMD were significantly lower in subjects with events. In Cox regression, lower FMD or NMD independently predicted future cardiovascular events. |
| Medina34) | 2010 | AAA | FMD | 30 | AAA diameter is inversely correlated with FMD. FMD was also inversely correlated with plasma CRP levels, while AAA diameter was positively correlated with plasma CRP levels. |
| Sung35) | 2013 | * AAA +HV |
FMD | * 63 +15 |
FMD differed between patients with large, small, and no AAAs. FMD was inversely correlated with AAA diameter. Fewer circulating endothelial progenitor cells (EPCs) were present in AAA patients than in controls. On multivariate analysis, CFUs and circulating EPCs (CD34+/KDR+) were independently, inversely correlated to AAA diameter. EPCs function (proliferation, adhesion, migration, tube formation etc) were significantly impaired in AAA patients. |
| Lee36) | 2017 | AAA | FMD NMD FMC |
162 | FMD is inversely correlated with the size of AAAs. FMD deteriorates during the natural progression of AAA in an individual. FMD is inversely correlated with the future 12-month growth of AAAs. Surgical repair of AAA (open or endovascular) leads to significant improvements in FMD at 8–12 weeks. No association between NMD and FMC with AAA size or growth. |
Table 2. Studies which examined FMD in the context of peripheral arterial disease in humans.
| Author | Year | Context | Measure | N | Main findings |
|---|---|---|---|---|---|
| Loffredo37) | 2007 | * PAD +No-PAD |
FMD | * 20 +40 |
Patient with PAD had lower FMD compared to matched control subjects without PAD |
| Husmann47) | 2008 | PAD | FMD | 33 | Patients who underwent femoral-popliteal revascularisation demonstrated significantly improved FMD |
| Maldonado39) | 2009 | * PAD +HV |
FMD | * 50 +30 |
FMD is lower in patients demonstrating either claudication or critical limb ischaemia when comparted to HVs No difference in FMD between claudicants or critical limb ischaemia |
| Unal48) | 2011 | PAD | FMD | 54 | Patients presenting with lower limb PAD due to femero-popliteal obstruction demonstrated significant improvements in FMD following femoro-popliteal bypass grafting |
| Jacomella49) | 2012 | PAD | FMD | 24 | Hypertensive patients presenting with renal artery stenosis produced significantly improved FMD responses following percutaneous transluminal renal artery angioplasty |
| Allan40) | 2013 | * PAD +HV |
FMD PAT |
* 26 +25 |
When compared to healthy volunteers; PAD patients demonstrated significant reductions in FMD but not in peripheral artery tonometry (PAT) measurements of reactive hyperaemia |
| Heinen41) | 2015 | * PAD #No PAD +HV |
FMD | * 20 #10 +10 |
In patients with SFA or BK PAD, both brachial artery and SFA FMDs were significantly worse than those of the HVs'. FMD correlated with intima/media or plaque thickness at the site of FMD measurement. Progressive deterioration of FMD was further observed across the segment of SFA disease (pre-, intra-, poststenosis). Significant improvement of SFA FMD within 24 hours of SFA balloon angioplasty to the level observed in pre-stenotic SFA segment |
| Iwamoto42) | 2016 | * PAD #No PAD +Buerger 's ^No Buerger's |
FMD | * 30 #30 +20 ^20 |
FMD was worse in patients with PAD compared to the non-PAD subjects. No differences in FMD between patients with Buerger's disease and control (no Buerger's) subjects |
Flow Mediated Constriction as a Biomarker of Disease
There are few published reports regarding FMC in cardiovascular research. After Gori et al. first described the method of FMC measurement in radial arteries of patients with cardiovascular risk factors, this technique was adopted by Weissgerber et al. who further described FMC measurement in the brachial arteries51). Spiro et al. were the first to examine brachial FMC in the context of coronary artery disease (CAD)52). In patients with symptomatic CAD (n = 86), FMC was greater in those with unstable coronary disease compared with those with stable angina53). Norioka et al. reported the largest series on FMC to date. A total of 188 participants (140 smokers and 48 non-smokers) were studied. A significant correlation between body mass index and FMC was observed in male participants only54). In the OxAAA study, 96 patients with AAAs underwent FMC measurement at the same time as the FMD measurement. In contrast to the findings on FMD (summarised above), FMC did not correlated with AAA size or growth, and remains unchanged during AAA surveillance and after surgery50).
Conclusion
This review highlights the literature on FMD as a biomarker in the context of abdominal aortic aneurysms and peripheral arterial disease. Although limited in numbers, these studies provide important evidence to implicate endothelial dysfunction as an underpinning mechanism of the pathologies that confront vascular surgeons. FMD should be considered as a worthy biomarker to be assessed in future research by vascular surgeons.
Acknowledgements
We thank the support from the following: University of Oxford, Medical Sciences Division Medical Research Fund; Nuffield Department of Surgical Sciences, University of Oxford; National Institute of Health Research (NIHR) Oxford Biomedical Research Centre; Academy of Medical Science, UK.
List of Abbreviations
- FMD
Flow Mediated Dilatation
- FMC
Flow Mediated Constriction
- NMD
Nitro-glycerine Mediated Dilatation
- NO
Nitric Oxide
- eNOS
endothelial Nitric Oxide Synthase
- GMP
Guanosine Mono Phosphate
- PAD
Peripheral Arterial Disease
- L-FMC
Low — Flow Mediated Constriction
- AAA
Abdominal Aortic Aneurysm
- CRP
C-Reactive Protein
- EPC
Endothelial Progenitor Cells
- SFA
Superficial Femoral Artery
- BMI
Body Mass Index
References
- 1). Lüscher TF, Barton M: Biology of the endothelium. Clin Cardiol, 1997; 20: II-3-10. [PubMed] [Google Scholar]
- 2). Herrmann J, Lerman A: The endothelium: dysfunction and beyond. J Nucl Cardiol, 2001; 8: 197-206 [DOI] [PubMed] [Google Scholar]
- 3). Deanfield JE, Halcox JP, Rabelink TJ: Endothelial Function and Dysfunction. 2007 [DOI] [PubMed] [Google Scholar]
- 4). Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A: The assessment of endothelial function: from research into clinical practice. Circulation, 2012; 126: 753-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Alam TA, Seifalian AM, Baker D: A review of methods currently used for assessment of in vivo endothelial function. Eur J Vasc Endovasc Surg, 2005; 29: 269-276 [DOI] [PubMed] [Google Scholar]
- 6). Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE: Noninvasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet, 1992; 340: 1111-1115 [DOI] [PubMed] [Google Scholar]
- 7). Davies PF: Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med, 2009; 6: 16-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Ku DN, Giddens DP, Zarins CK, Glagov S: Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis, 1985; 5: 293-302 [DOI] [PubMed] [Google Scholar]
- 9). Charakida M, de Groot E, Loukogeorgakis SP, Khan T, Luscher T, Kastelein JJ, Gasser T, Deanfield JE: Variability and reproducibility of flow-mediated dilatation in a multicentre clinical trial. Eur Heart J, 2013; 34: 3501-3507 [DOI] [PubMed] [Google Scholar]
- 10). Greyling A, van Mil AC, Zock PL, Green DJ, Ghiadoni L, Thijssen DH: Adherence to guidelines strongly improves reproducibility of brachial artery flow-mediated dilation. Atherosclerosis, 2016; 248: 196-202 [DOI] [PubMed] [Google Scholar]
- 11). Yagi S, Aihara K, Fukuda D, Takashima A, Hara T, Hotchi J, Ise T, Yamaguchi K, Tobiume T, Iwase T, Yamada H, Soeki T, Wakatsuki T, Shimabukuro M, Akaike M, Sata M: Effects of docosahexaenoic Acid on the endothelial function in patients with coronary artery disease. J Atheroscler Thromb, 2015; 22: 447-454 [DOI] [PubMed] [Google Scholar]
- 12). Ciccone MM, Faienza MF, Altomare M, Nacci C, Montagnani M, Valente F, Cortese F, Gesualdo M, Zito A, Mancarella R, Leogrande D, Viola D, Scicchitano P, Giordano P: Endothelial and Metabolic Function Interactions in Overweight/Obese Children. J Atheroscler Thromb, 2016; 23: 950-959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr., Lehman BT, Fan S, Osypiuk E, Vita JA: Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation, 2004; 109: 613-619 [DOI] [PubMed] [Google Scholar]
- 14). Juonala M, Viikari JS, Laitinen T, Marniemi J, Helenius H, Ronnemaa T, Raitakari OT: Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the cardiovascular risk in young Finns study. Circulation, 2004; 110: 2918-2923 [DOI] [PubMed] [Google Scholar]
- 15). Schnabel RB, Schulz A, Wild PS, Sinning CR, Wilde S, Eleftheriadis M, Herkenhoff S, Zeller T, Lubos E, Lackner KJ, Warnholtz A, Gori T, Blankenberg S, Munzel T: Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardiovasc Imaging, 2011; 4: 371-380 [DOI] [PubMed] [Google Scholar]
- 16). Ministry of Health, Labour and Welfare: Outline of FY 2012 Revision of Medical Fee. 2017; http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryouhoken/iryouhoken15/index.html (in Japanese)
- 17). Anderson EA, Mark AL: Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation, 1989; 79: 93-100 [DOI] [PubMed] [Google Scholar]
- 18). Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension, 2010; 55: 1075-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Preik M, Lauer T, Heiss C, Tabery S, Strauer BE, Kelm M: Automated ultrasonic measurement of human arteries for the determination of endothelial function. Ultraschall Med, 2000; 21: 195-198 [DOI] [PubMed] [Google Scholar]
- 20). Peretz A, Leotta DF, Sullivan JH, Trenga CA, Sands FN, Aulet MR, Paun M, Gill EA, Kaufman JD: Flow mediated dilation of the brachial artery: an investigation of methods requiring further standardization. BMC Cardiovasc Disord, 2007; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Leeson P, Thorne S, Donald A, Mullen M, Clarkson P, Deanfield J: Non-invasive measurement of endothelial function: effect on brachial artery dilatation of graded endothelial dependent and independent stimuli. Heart, 1997; 78: 22-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Kajikawa M, Maruhashi T, Hida E, Iwamoto Y, Matsumoto T, Iwamoto A, Oda N, Kishimoto S, Matsui S, Hidaka T, Kihara Y, Chayama K, Goto C, Aibara Y, Nakashima A, Noma K, Higashi Y: Combination of Flow-Mediated Vasodilation and Nitroglycerine-Induced Vasodilation Is More Effective for Prediction of Cardiovascular Events. Hypertension, 2016; 67: 1045-1052 [DOI] [PubMed] [Google Scholar]
- 23). Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Tomiyama H, Takase B, Yamashina A, Higashi Y: Relationship between flow-mediated vasodilation and cardiovascular risk factors in a large community-based study. Heart, 2013; 99: 1837-1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Tomiyama H, Kohro T, Higashi Y, Takase B, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Ikeda H, Yamashina A: Reliability of measurement of endothelial function across multiple institutions and establishment of reference values in Japanese. Atherosclerosis, 2015; 242: 433-442 [DOI] [PubMed] [Google Scholar]
- 25). Furchgott RF: The 1996 Albert Lasker Medical Research Awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. Jama, 1996; 276: 1186-1188 [PubMed] [Google Scholar]
- 26). Crabtree MJ, Channon KM: Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide, 2011; 25: 81-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD: Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol, 2008; 51: 1953-1958 [DOI] [PubMed] [Google Scholar]
- 28). Gori T, Parker JD, Münzel T: Flow-mediated constriction: further insight into a new measure of vascular function. Eur Heart J, 2011; 32: 784-787 [DOI] [PubMed] [Google Scholar]
- 29). Irace C, Tripolino C, Scavelli FB, Carallo C, Gnasso A: Brachial Low-Flow-Mediated Constriction is Associated with Delayed Brachial Flow-Mediated Dilation. J Atheroscler Thromb, 2016; 23: 355-363 [DOI] [PubMed] [Google Scholar]
- 30). Knipp BS, Peterson DA, Rajagopalan S, Kehrer C, Ford JW, D'Alecy LG, Whitesall SE, Eagleton MJ, Wakefield TW, Henke PK, Jacobs LA, Greenfield LJ, Stanley JC, Upchurch GR, Jr.: Impaired vasoreactivity despite an increase in plasma nitrite in patients with abdominal aortic aneurysms. J Vasc Surg, 2002; 35: 363-367 [DOI] [PubMed] [Google Scholar]
- 31). Gokce N, Keaney JF, Jr., Hunter LM, Watkins MT, Menzoian JO, Vita JA: Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation, 2002; 105: 1567-1572 [DOI] [PubMed] [Google Scholar]
- 32). Akamatsu D, Satou A, Watanabe T, Hashizume E, Gotou H, Satomi S: Smooth muscle dysfunction in patients older than 54 years of age with objective evidence of arteriosclerosis. Eur J Vasc Endovasc Surg, 2007; 34: 639-645 [DOI] [PubMed] [Google Scholar]
- 33). Akamatsu D, Sato A, Goto H, Watanabe T, Hashimoto M, Shimizu T, Sugawara H, Sato H, Nakano Y, Miura T, Zukeran T, Serizawa F, Hamada Y, Tsuchida K, Tsuji I, Satomi S: Nitroglycerin-mediated vasodilatation of the brachial artery may predict long-term cardiovascular events irrespective of the presence of atherosclerotic disease. J Atheroscler Thromb, 2010; 17: 1266-1274 [DOI] [PubMed] [Google Scholar]
- 34). Medina F, de Haro J, Florez A, Acin F: Relationship between endothelial dependent vasodilation and size of abdominal aortic aneurysms. Ann Vasc Surg, 2010; 24: 752-757 [DOI] [PubMed] [Google Scholar]
- 35). Sung SH, Wu TC, Chen JS, Chen YH, Huang PH, Lin SJ, Shih CC, Chen JW: Reduced number and impaired function of circulating endothelial progenitor cells in patients with abdominal aortic aneurysm. Int J Cardiol, 2013; 168: 1070-1077 [DOI] [PubMed] [Google Scholar]
- 36). Lee R, Bellamkonda K, Jones A, Killough N, Woodgate F, Williams M, Cassimjee I, Handa A: Flow Mediated Dilatation and Progression of Abdominal Aortic Aneurysms. Eur J Vasc Endovasc Surg, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Loffredo L, Marcoccia A, Pignatelli P, Andreozzi P, Borgia MC, Cangemi R, Chiarotti F, Violi F: Oxidative-stressmediated arterial dysfunction in patients with peripheral arterial disease. Eur Heart J, 2007; 28: 608-612 [DOI] [PubMed] [Google Scholar]
- 38). Loffredo L, Carnevale R, Cangemi R, Angelico F, Augelletti T, Di Santo S, Calabrese CM, Della Volpe L, Pignatelli P, Perri L, Basili S, Violi F: NOX2 up-regulation is associated with artery dysfunction in patients with peripheral artery disease. Int J Cardiol, 2013; 165: 184-192 [DOI] [PubMed] [Google Scholar]
- 39). Maldonado FJ, Miralles Jde H, Aguilar EM, Gonzalez AF, Garcia JR, Garcia FA: Relationship between noninvasively measured endothelial function and peripheral arterial disease. Angiology, 2009; 60: 725-731 [DOI] [PubMed] [Google Scholar]
- 40). Allan RB, Delaney CL,, Miller MD, Spark JI: A comparison of flow-mediated dilatation and peripheral artery tonometry for measurement of endothelial function in healthy individuals and patients with peripheral arterial disease. Eur J Vasc Endovasc Surg, 2013; 45: 263-269 [DOI] [PubMed] [Google Scholar]
- 41). Heinen Y, Stegemann E, Sansone R, Benedens K, Wagstaff R, Balzer J, Rassaf T, Lauer T, Kelm M, Heiss C: Local Association Between Endothelial Dysfunction and Intimal Hyperplasia: Relevance in Peripheral Artery Disease. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Iwamoto A, Kajikawa M, Maruhashi T, Iwamoto Y, Oda N, Kishimoto S, Matsui S, Kihara Y, Chayama K, Goto C, Noma K, Aibara Y, Nakashima A, Higashi Y: Vascular Function and Intima-media Thickness of a Leg Artery in Peripheral Artery Disease: A Comparison of Buerger Disease and Atherosclerotic Peripheral Artery Disease. J Atheroscler Thromb, 2016; 23: 1261-1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Mancini GB, Hartigan PM, Bates ER, Sedlis SP, Maron DJ, Spertus JA, Berman DS, Kostuk WJ, Shaw LJ, Weintraub WS, Teo KK, Dada M, Chaitman BR, O'Rourke RA, Boden WE: Angiographic disease progression and residual risk of cardiovascular events while on optimal medical therapy: observations from the COURAGE Trial. Circ Cardiovasc Interv, 2011; 4: 545-552 [DOI] [PubMed] [Google Scholar]
- 44). Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW: Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med, 1995; 332: 481-487 [DOI] [PubMed] [Google Scholar]
- 45). Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P: The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med, 1995; 332: 488-493 [DOI] [PubMed] [Google Scholar]
- 46). Mori H, Maeda A, Wakabayashi K, Sato T, Sasai M, Tashiro K, Iso Y, Ebato M, Suzuki H: The Effect of Cilostazol on Endothelial Function as Assessed by Flow-Mediated Dilation in Patients with Coronary Artery Disease. J Atheroscler Thromb, 2016; 23: 1168-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Husmann M, Dorffler-Melly J, Kalka C, Diehm N, Baumgartner I, Silvestro A: Successful lower extremity angioplasty improves brachial artery flow-mediated dilation in patients with peripheral arterial disease. J Vasc Surg, 2008; 48: 1211-1216 [DOI] [PubMed] [Google Scholar]
- 48). Unal O, Karatepe O, Ugurlucan M, Koc B, Filizcan U, Aksoy M: Effects of lower extremity revascularization on the endothelial functions measured with noninvasive brachial artery flow-mediated dilatation. Ann Vasc Surg, 2011; 25: 969-974 [DOI] [PubMed] [Google Scholar]
- 49). Jacomella V, Husmann M, Thalhammer C, Uike K, Pfammatter T, Amann-Vesti B: Impact of endovascular treatment of atherosclerotic renal artery stenosis on endothelial function and arterial blood pressure. Int Angiol, 2012; 31: 70-76 [PubMed] [Google Scholar]
- 50). Lee R, Bellamkonda K, Jones A, Killough N, Woodgate F, Williams M, Cassimjee I, Handa A: Flow Mediated Dilatation and the Progression of Abdominal Aortic Aneurysms. European Journal of Vascular and Endovascular Surgery, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Weissgerber TL, Davies GA, Tschakovsky ME: Low flow-mediated constriction occurs in the radial but not the brachial artery in healthy pregnant and nonpregnant women. J Appl Physiol (1985), 2010; 108: 1097-1105 [DOI] [PubMed] [Google Scholar]
- 52). Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C: Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des, 2012; 18: 1519-1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Spiro JR, Digby JE, Ghimire G, Mason M, Mitchell AG, Ilsley C, Donald A, Dalby MC, Kharbanda RK: Brachial artery low-flow-mediated constriction is increased early after coronary intervention and reduces during recovery after acute coronary syndrome: characterization of a recently described index of vascular function. Eur Heart J, 2011; 32: 856-866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Norioka N, Takemoto Y, Kobayashi M, Makuuchi A, Yoshikawa J, Yamazaki Y, Kamiyama Y, Shuto T, Yoshiyama M: Low-flow mediated constriction incorporated indices as indicators of cardiovascular risk in smokers. Atherosclerosis, 2016; 251: 132-138 [DOI] [PubMed] [Google Scholar]


