See article vol. 24: 793–803
Patients with suspected acute coronary syndromes (ACS) are differentiated based on their electrocardiogram (ECG) results as follows1, 2): (1) ST-elevation myocardial infarction (STEMI): patients with acute chest pain and persistent (20 min) ST segment elevation indicating acute total coronary occlusion and generally requiring immediate reperfusion with primary percutaneous coronary intervention (PPCI) or (2) non-ST-elevation myocardial infarction (NSTEMI): patients with acute chest pain but no persistent ST-segment elevation and who may show a clinical spectrum from no symptoms at presentation to ongoing ischemia, electrical or hemodynamic instability, or cardiac arrest, indicating cardiomyocyte necrosis or less frequently, myocardial ischemia without cell loss (unstable angina).
In the therapeutic strategy for ACS, biomarkers may improve the diagnostic accuracy and risk stratification by clinical assessment based on ECG and identify subgroups of patients who would benefit from a specific therapeutic modality in the acute phase3). The new-generation cardiac troponin (cTn) assays, which reflect the presence of myocardial necrosis, provide considerably sensitive and specific diagnosis of ACS compared with less sensitive cTn and creatine kinase (CK) with its MB isoenzyme (CK-MB). The new-generation cTn assays reduce the proportion of cases with unstable angina and increase that of cases with NSTEMI in the patients with NSTE-ACS (Fig. 1). Consequently, this reclassification is believed to guide appropriate decisions of treatment. However, the long-term benefit of an invasive approach and its subsequent medical treatment in subgroups of patients reclassified from unstable angina to NSTE myocardial infarction has not been fully clarified (Fig. 1)3, 4).
Fig. 1.
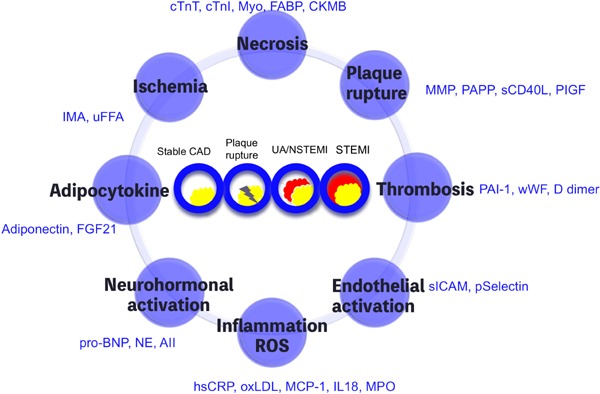
Multiple biomarker strategy for acute coronary syndrome
Biomarkers for acute coronary syndrome are subgrouped into eight categories by modifying deLomos JA, University of Texas Southwestern at Dallas. CAD: coronary artery disease; UA: unstable angina; NSTEMI: non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction.
Meanwhile, the diagnosis and acute management of STEMI are considered to be simple compared with those of NSTEMI, with the main decision of whether to perform immediate revascularization, pharmacological or mechanical, solely based on ST-segment elevation and symptoms1, 2). The prognosis of patients with STEMI who are promptly revascularized with PPCI is widely perceived to be good and is largely dependent on the efficacy of reperfusion and rescue of viable myocardial tissues. Although there is a striking decrease in acute mortality in patients with STEMI, an unacceptable rate of recurrent events still occurs after ACS including STEMI, prompting us identify biomarkers of STEMI4).
The current issue by Natsukawa et al. may provide an insight into a strategy for the diagnosis and risk stratification of STEMI5). In 49 Japanese subjects who underwent PPCI for STEMI, the area under the curve (AUC) for serum CK-MB levels was associated with serum adiponectin levels on admission and serum adiponectin levels at the acute phase. The present study may indicate that serum adiponectin levels are useful in the prediction of prognosis after PCI-treated STEMI subjects.
Adiponectin, an adipocyte-specific secretory protein, abundantly exists in the blood stream. Serum adiponectin levels paradoxically decrease in patients with visceral fat obesity and are associated with endothelial dysfunction6), presence of ischemic heart diseases7), and complexity of coronary lesion8). The current study found that serum adiponectin levels decreased from admission to after 24 h and gradually recovered to baseline levels, and Δ serum adiponectin levels at the acute phase were negatively associated with serum AUC of CK-MB levels5), suggesting that accumulation or consumption of adiponectin in the local area at the risk for myocardial necrosis may protect from myocardial damage and result in the reduction of the infarct size. It has been reported that expression of protective effects of adiponectin in the cardiovascular system requires coexistence of T-cadherin9). In a genome-wide association study based on independent cohorts, genetic variations in CDH13 gene-coding T-cadherin influence circulating adiponectin levels and cardiovascular events10–12). Thus, T-cadherin-mediated accumulation of adiponectin in the cardiovascular system may play a crucial role in cardiovascular events. Collectively, it can be suggested that accumulation or adhesion of adiponectin to the local ischemic area is critically operative via T-cadherin and the phenomenon is reflected by serial changes in serum adiponectin levels in patients with STEMI.
The introduction of new biomarkers has undoubtedly moved our approach several steps forward, but it also given rise to novel issues that need to be addressed by future research. Future analysis of serum and local dynamics of adiponectin at the acute phase of ACS may uncover pathophysiological perceptions in terms of accumulation or adhesion property of adiponectin, and large clinical trials may provide more persuading evidences showing that serial changes in adiponectin are useful in the prediction of prognosis after PCI-treated STEMI subjects.
References
- 1). Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol C, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J: 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J, 2016; 37: 267-315 [DOI] [PubMed] [Google Scholar]
- 2). O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr., Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 2013; 61: e78-140 [DOI] [PubMed] [Google Scholar]
- 3). Filippatos G, Farmakis D, Parissis J: Novel biomarkers in acute coronary syndromes: new molecules, new concepts, but what about new treatment strategies? J Am Coll Cardiol, 2014; 63: 1654-1656 [DOI] [PubMed] [Google Scholar]
- 4). O'Malley RG, Bonaca MP, Scirica BM, Murphy SA, Jarolim P, Sabatine MS, Braunwald E, Morrow DA: Prognostic Performance of Multiple Biomarkers in Patients With Non–ST-Segment Elevation Acute Coronary Syndrome. J Am Coll Cardiol, 2014; 63: 1644-1653 [DOI] [PubMed] [Google Scholar]
- 5). Natsukawa T, Maeda N, Fukuda S, Yamaoka M, Fujishima Y, Nagao H, Sato F, Nishizawa H, Sawano H, Hayashi Y, Funahashi T, Kai T, Shimomura I: Significant association of serum adiponectin and creatine kinase-MB levels in ST-segment elevation myocardial infarction. J Atheroscler Thromb, 2017; 24: 793-803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, Ueda S, Shimomura I, Funahashi T, Matsuzawa Y: Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab, 2003; 88: 3236-3240 [DOI] [PubMed] [Google Scholar]
- 7). Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB: Plasma adiponectin levels and risk of myocardial infarction in men. JAMA, 2004; 291: 1730-1737 [DOI] [PubMed] [Google Scholar]
- 8). Otsuka F, Sugiyama S, Kojima S, Maruyoshi H, Funahashi T, Matsui K, Sakamoto T, Yoshimura M, Kimura K, Umemura S, Ogawa H: Plasma adiponectin levels are associated with coronary lesion complexity in men with coronary artery disease. J Am Coll Cardiol, 2006; 48: 1155-1162 [DOI] [PubMed] [Google Scholar]
- 9). Matsuda K, Fujishima Y, Maeda N, Mori T, Hirata A, Sekimoto R, Tsushima Y, Masuda S, Yamaoka M, Inoue K, Nishizawa H, Kita S, Ranscht B, Funahashi T, Shimomura I: Positive feedback regulation between adiponectin and T-cadherin impacts adiponectin levels in tissue and plasma of male mice. Endocrinology, 2015; 156: 934-946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Chung CM, Lin TH, Chen JW, Leu HB, Yang HC, Ho HY, Ting CT, Sheu SH, Tsai WC, Chen JH, Lin SJ, Chen YT, Pan WH: A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes, 2011; 60: 2417-2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Kitamoto A, Kitamoto T, Nakamura T, Matsuo T, Nakata Y, Hyogo H, Ochi H, Kamohara S, Miyatake N, Kotani K, Mineo I, Wada J, Ogawa Y, Yoneda M, Nakajima A, Funahashi T, Miyazaki S, Tokunaga K, Masuzaki H, Ueno T, Chayama K, Hamaguchi K, Yamada K, Hanafusa T, Oikawa S, Sakata T, Tanaka K, Matsuzawa Y, Hotta K. CDH13 Polymorphisms are Associated with Adiponectin Levels and Metabolic Syndrome Traits Independently of Visceral Fat Mass. J Atheroscler Thromb. 2016; 23: 309-319 [DOI] [PubMed] [Google Scholar]
- 12). Gotoda T. Another Paradox Regarding Adiponectin Revisited. J Atheroscler Thromb. 2016; 23: 292-294 [DOI] [PubMed] [Google Scholar]


