Abstract
Aim: Poor sleep has been shown to be associated with the development of cardiovascular risk factors, such as obesity, in both adults and children. This study aimed to investigate the relationship between sleep duration and arterial stiffness indices in Japanese children and early adolescents.
Methods: The data on 102 students (56 males, 46 females; mean age, 11.9 ± 1.8 years) were analyzed. As non-invasive arterial stiffness parameters, the cardio-ankle vascular index (CAVI) and heart-ankle pulse wave velocity (haPWV) were evaluated. Their students' sleep habits (bedtime and wake times on a usual weekday) were investigated using questionnaires, and based on these, their sleep durations were calculated.
Results: The CAVI values in the males and females were 4.8 ± 0.9 and 4.7 ± 0.9 (arbitrary unit), respectively. haPWV values in the males and females were 5.5 ± 0.6 and 5.4 ± 0.6 m/s, respectively. Sleep duration in the males, but not in the females, was negatively correlated with CAVI (r = −0.356) and haPWV (r = −0.356), suggesting that students with short sleep duration could have increased arterial stiffness. After adjusting for confounders, such as age, sex, systolic blood pressure, heart rate, adiposity, and physical fitness, the correlation of sleep duration with CAVI, but not with haPWV, was still significant (partial r = −0.253, p < 0.05).
Conclusion: Our findings suggest that shorter sleep duration influences arterial stiffening even in childhood.
Keywords: Primary prevention, Adolescent, Vascular stiffness, Cardiovascular risk
Introduction
Childhood cardiovascular (CV) risk factors, such as obesity, dyslipidemia, low physical fitness, and high blood pressure (BP), precede arteriosclerotic changes1–6) and lead to increases in CV event risks and mortality rate in early adulthood7, 8). In Japan, the rate of overweight/obesity among children at present is higher than that in the 1980s9), and further increases in the percentage of individuals with CV risk factors are expected. Primary prevention in early life is thus all the more important.
Inadequate sleep is associated with both CV risk factors10, 11) and CV events in adults12). Several studies have reported that children and adolescents with sleep problems, including short sleep duration, had more CV risk factors, such as overweight/obesity and high BP, than children without sleep problems10, 13–15). Given that arteriosclerosis progression is aggravated by these CV risk factors, we postulate that arteriosclerotic changes may occur in short sleepers, even in children and/or adolescents.
Brachial–ankle pulse wave velocity (PWV) is an indicator of systemic arterial stiffness and is shown to be related to CV events and mortality16). Although relationships between PWV and CV risk factors in children have been reported5, 17, 18), little is known about the influences of an individual's daily sleep habit on arterial stiffness. In addition, given that the PWV value depends on BP levels during the measurement time17) and that poor and/or inadequate sleep affects BP increase and body-weight gain10, 13, 14), we need to clarify the relationships between BP-independent arterial stiffness indicators and daily sleep habits in children and adolescents.
In this study, we investigated the relationship between sleep duration and arterial stiffness in Japanese children by measuring the heart-ankle PWV (haPWV) and the cardio-ankle vascular index (CAVI)19–23), which is a modified parameter of arterial stiffness and is independent of BP levels.
Methods
Participants
The study participants were Japanese students who were fourth (15 males; 14 females) and sixth (18 males; 11 females) graders in elementary school and second year students (23 males; 21 females) of junior high school (corresponding to eight grade in the U.S.). These children and adolescents participated in the Improvement of Fitness in the Hokkaido Children Project, which was designed to identify interference factors for physical fitness, especially the influences of cold and snowfall environments, and to promote healthy growth and development in children and adolescents in Hokkaido, Japan. This research project started in October 2010 and continued until June 2013. Its study protocol was approved by the Research Ethics Committee of Hokkaido University of Education. Written informed consent was obtained from a parent or guardian of all participants.
Arterial stiffness and other physiological variables of 148 students were measured during the period from late April to early May 2012. The following criteria was applied in the present analysis: (1) the resting heart rate during arterial stiffness measurement was < 100 beats/min; (2) the brachial systolic BP during arterial stiffness measurement was < 130 mmHg; (3) there were no orthopedic injuries in the trunk or lower limbs; (4) sleep duration was > 5 h; and (5) there was no missing data, or the participants completed the fitness tests. The eligible data of 102 students (males, n = 56; females, n = 46) were analyzed in this study. All physical and physiological measurements were conducted in the morning.
Sleep Duration
On a typical weekday school day, the students' sleep duration was assessed using a survey that questioned the students “what time do you usually go to bed on weekdays” and “what time do you usually get out of bed in the morning on weekdays.” These questions were used to compute the average hours of weekday sleep.
Arterial Stiffness Index
Each student's CAVI, PWV, brachial systolic BP (SBP), brachial diastolic BP, and heart rate (HR) were simultaneously measured by an automatic waveform analyzer (VaSera VS-1000; Fukuda Denshi, Tokyo). The methods used to obtain the values of these parameters were as previously described19, 24). Briefly, the measurements were taken with the participant lying in a supine position after resting for at least 5 min. Occlusion and monitoring cuffs were placed around both sites of the participant's upper and lower extremities. The extremity BP was measured by oscillometry. Electrocardiography electrodes were attached to the upper arm. A microphone was placed on the sternal angle for phonocardiography.
PWV was calculated by dividing the distance from the aortic valve to the ankle artery by the sum of the difference between the time the pulse waves were transmitted to the brachium and the time the same wave was transmitted to the ankle, plus the time difference between the second heart sound on the phonocardiogram and the notch of the brachial pulse waves19). haPWV obtained from CAVI analysis indicates the velocity of the pulse wave from the heart to the ankle artery and thus differs from the brachial–ankle PWV. CAVI was obtained from the measurement of the participant's BPs and PWV19). The means of the right-side and left-side values of haPWV, CAVI, and SBP were used.
Anthropometric and Fitness Measures
Each participant's height and weight were measured using a standard stadiometer and a digital scale, respectively. The students reported their birth date, and we calculated their ages (in months) at the measurement day. The body mass index (BMI) was calculated from these measures and is expressed as a standard score (z-score) using a spreadsheet program of BMI norms for the students' age (in months) and sex in accordance with the guidelines of the Japanese Society for Pediatric Endocrinology (JSPE program)25). The participants' waist circumference was measured at the narrowest torso using a measuring tape. Physical fitness was assessed by a 20-m shuttle run test (20 mSRT) in which the participants ran between two lines 20 m apart until they twice failed to reach the front line within the required time. The initial running speed was 8.5 km/h. The pace of the test was increased by 0.5 km/h every minute, and an audio signal determined whether each lap was completed on time. Total laps were classified by 10 stages, as described by the Ministry of Education, Culture, Sports, Science and Technology of Japan26), and we used the stage reached by the participants as a parameter of systemic physical fitness.
Statistical Analyses
We report the data as means ± standard deviations unless otherwise noted. The normality of data distributions was tested using the Kolmogorov–Smirnov test. The correlations between two variables were assessed using Pearson's correlation analyses. We performed partial correlation analyses after controlling for confounding factors to examine the relationships between arterial stiffness indices and sleep duration. Model 1 was adjusted for age, BMI z-score, HR, and SBP for each gender. In addition, in the total of male and female students (overall), sex (male = 1, female = 2) was included into the model as control variables. Model 2 was adjusted for the control variables used for model 1 and 20 mSRT. We observed multicollinearity among study variables and therefore performed partial correlation analyses. All statistical analyses were calculated with PASW statistics ver. 18.0 software (SPSS, Chicago, IL), and P-values < 0.05 were considered significant.
Results
The students' characteristics are summarized in Table 1. The mean CAVI values were 4.8 ± 0.9 and 4.7 ± 0.8 (arbitrary unit) for the 56 males and 46 females, respectively. haPWV was 5.5 ± 0.6 and 5.5 ± 0.6 m/s in the groups of males and females, respectively. Both males and females slept an average of 8.1 ± 1.1 h. The number (%) of students who slept less than 7.5 h per night (< 7.5 sleep duration) was two (6.9%) among the fourth graders, two (6.9%) among the sixth graders, and 25 (56.8%) among the junior high schoolers.
Table 1. Characteristics of subjects.
| Variable | Male, N= 56 | Female, N= 46 |
|---|---|---|
| Age, yr | 11.9 ± 1.7 | 11.9 ± 1.8 |
| Height, cm | 150.7 ± 12.8 | 148.9 ± 9.6 |
| Weight, kg | 45.8 ± 11.7 | 43.4 ± 9.7 |
| BMI, kg/m2 | 19.9 ± 3.2 | 19.4 ± 3.0 |
| BMI z-score | 0.42 ± 1.05 | 0.22 ± 1.03 |
| Waist, cm | 68.9 ± 8.9 | 65.3 ± 6.9 |
| HR, bpm | 75.1 ± 10.7 | 77.9 ± 9.2 |
| SBP, mmHg | 114.9 ± 8.7 | 113.8 ± 6.0 |
| CAVI | 4.82 ± 0.90 | 4.71 ± 0.82 |
| haPWV, m/s | 5.51 ± 0.62 | 5.47 ± 0.64 |
| 20mSRT, stage | 6.6 ± 2.7 | 4.8 ± 1.6 |
| Sleep duration, h | 8.1 ± 1.1 | 8.1 ± 1.1 |
BMI denotes body mass index; SBP, brachial systolic pressure; DBP, brachial diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; HR, heart rate; CAVI, cardio-ankle vascular index; PWV, pulse wave velocity; 20mSRT, 20-m shuttle run test.
Table 2 provides the Pearson's correlation coefficients between arterial stiffness measures and other study variables. In the total sample of males and females, CAVI and haPWV were positively correlated with age and 20 mSRT and negatively correlated with the BMI z-score, HR, and sleep duration. SBP was significantly correlated with haPWV but not with CAVI. Table 3 shows the correlation coefficients between sleep duration and other variables and between CAVI and other variables for students of each school grade. The correlations between age and sleep duration and between age and CAVI were not significant in any grade. The relationships between CAVI and sleep duration and between the haPWV values and sleep duration are illustrated in Fig. 1.
Table 2. Pearson's correlation coefficients between arterial stiffness indices and study variables.
| CAVI |
haPWV |
|||||
|---|---|---|---|---|---|---|
| Variable | Male | Female | Overall | Male | Female | Overall |
| Age, yr | 0.391** | 0.085 | 0.252* | 0.385** | 0.325* | 0.355** |
| BMI z-score | −0.529** | −0.342* | −0.441** | −0.429** | −0.278 | −0.356** |
| Waist, cm | −0.077 | −0.277 | −0.132 | 0.066 | −0.037 | 0.032 |
| SBP, mmHg | −0.114 | 0.085 | −0.041 | 0.239 | 0.439** | 0.308** |
| HR, bpm | −0.297* | −0.221 | −0.273** | −0.190 | −0.201 | −0.197* |
| Sleep duration, h | −0.395* | −0.199 | −0.309** | −0.406** | −0.220 | −0.319** |
| 20 mSRT, stage | 0.226 | 0.301* | 0.249* | 0.225 | 0.338* | 0.249* |
p < 0.05
p < 0.01
BMI, body mass index; SBP, systolic blood pressure; HR, heart rate; 20 mSRT, 20-m shuttle run test; CAVI, cardio-ankle vascular index; haPWV, heart-ankle pulse wave velocity.
Table 3. Pearson's correlation coefficients between sleep duration or CAVI and study variables in each grade.
| Sleep duration |
CAVI |
|||||
|---|---|---|---|---|---|---|
| 4th graders | 6th graders | Second year students of junior high school | 4th graders | 6th graders | Second year students of junior high school | |
| Age | −0.261 | 0.169 | 0.134 | −0.007 | −0.104 | 0.050 |
| BMI z-score | 0.207 | −0.204 | −0.091 | −0.296 | −0.427* | −0.409** |
| 20 mSRT | −0.060 | 0.132 | −0.129 | 0.021 | 0.089 | 0.206 |
| SBP | −0.041 | −0.060 | −0.259 | −0.333 | −0.344 | 0.063 |
| HR | −0.221 | 0.215 | −0.019 | 0.056 | −0.337 | −0.354* |
| CAVI | −0.378* | −0.172 | −0.155 | - | - | - |
| haPWV | −0.145 | −0.071 | −0.272 | 0.699** | 0.832** | 0.922** |
p < 0.05
p < 0.01
BMI, body mass index; SBP, systolic blood pressure; HR, heart rate; 20 mSRT, 20-m shuttle run test; CAVI, cardio-ankle vascular index; haPWV, heart-ankle pulse wave velocity.
Fig. 1.
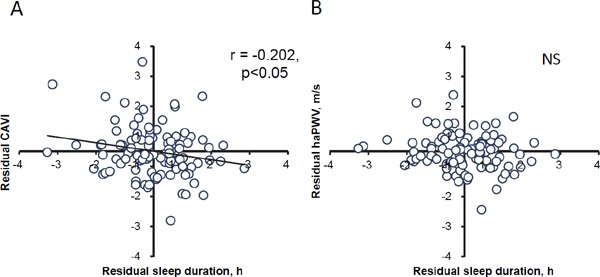
Correlations between the arterial stiffness parameters and sleep duration in the 102 Japanese children and adolescents.
A: The correlation between the residual CAVI and residual sleep duration after adjusting for age and sex. B: The correlation between residual PWV and residual sleep duration after adjusting for age and sex. NS, no significant.
To adjust for confounding factors, we performed a partial correlation analysis using CAVI, haPWV, and sleep duration (Table 4). After controlling for age, sex, BMI z-score, SBP, and HR, the partial correlation coefficient of CAVI and sleep duration and of haPWV and sleep duration in overall students was not significant (CAVI, r = −0.185; PWV, r = −0.101; both p > 0.05). As shown in Table 4, model 2 analyses after controlling for control variables of model 1 and 20 mSRT demonstrated significant correlations between CAVI and sleep duration in male and overall students. In model 2, haPWV was negatively associated with sleep duration in male students. After adjusting for age, sex, BMI z-score, HR, and SBP, the 20 mSRT was not significantly correlated with CAVI and haPWV (data not shown).
Table 4. Partial correlation analyses.
| Sleep duration |
|||||||
|---|---|---|---|---|---|---|---|
| Males |
Females |
Overall |
|||||
| partial r | p value | partial r | p value | partial r | p value | ||
| Model 1 | CAVI | −0.226 | 0.106 | 0.123 | 0.439 | −0.185 | 0.061 |
| haPWV | −0.232 | 0.098 | 0.085 | 0.594 | −0.101 | 0.310 | |
| Model 2 | CAVI | −0.414 | 0.003 | −0.135 | 0.401 | −0.250 | 0.014 |
| haPWV | −0.378 | 0.006 | 0.081 | 0.615 | −0.137 | 0.18 | |
CAVI, cardio-ankle vascular index; PWV, pulse wave velocity; model 1 was adjusted by BMI z-score, HR, age, and SBP; model 2 was adjusted by model 1 and 20mSRT. Italic letters are statistically significant.
Discussion
The main finding from the present study is that there was a significant inverse association between sleep duration and arterial stiffness in Japanese children and early adolescents, even after adjusting for age, HR, BP, adiposity, and physical fitness. Our results suggest that inadequate sleep duration could enhance arterial stiffening in healthy children and may be a behavioral target for primary prevention of early vascular aging.
PWV measurement is a non-invasive and useful tool for assessing arterial stiffness, based on the increasing evidence of PWV as a significant predictor of CV events16) and CV mortality27). However, PWV is affected by BP levels at the time of measurement, and thus, when PWV of sensitive persons (e.g., some children and individuals with white coat hypertension) is evaluated, there is a possibility of obtaining an overestimated PWV value. CAVI showed no correlation with BP in previous studies19, 20) or in the present study, suggesting that CAVI measurement is a suitable method for assessing the arterial stiffness of such individuals. Although Philip et al. (2016)20) examined CAVI values in children, recent papers have shown relationships between PWV and intima-media thickness or flow-mediated dilation even in children28–30). Based on the results of these previous studies, it appears that a non-invasive evaluation of arterial stiffness in childhood would be a surrogate marker for vascular health.
On the other hand, poor sleep habits, including short sleep duration and low sleep quality, are associated with high BP and increased CV risk factors in children and adolescents10, 13, 15, 31–34). To our knowledge, the present study is the first report of an inverse association between sleep duration and arterial stiffness evaluated using CAVI after controlling for potential confounding factors in 9- to 13-year-old children. Our present findings support the previous studies' results regarding abnormalities in great artery elasticity early in life and also imply that unhealthy sleep habits in childhood can accelerate the process of arteriosclerosis.
We found no correlation between CAVI and physical fitness after adjusting for confounders. Another study also showed no significant association between physical fitness and PWV after adjusting for age, sex, SBP, HR, mean arterial pressure, and adiposity5). With the use of the second derivative of the finger photoplethysmogram, it was demonstrated that low physical fitness was related to higher arterial stiffness parameters in children35). A recent study has revealed that the correlations between arterial stiffness parameters assessed by PWV and the second derivative of the finger photoplethysmogram were only mild36), and the parameters reflected different physiological properties at central and peripheral sites in the arterial system37, 38). In addition, systemic physical fitness assessed using the 20 mSRT is influenced by adiposity because greater body fat would be a load on skeletal muscles of the lower limbs during the repeated stopping and running in this test. Although it is possible that there is an association between arterial stiffness and physical fitness in children, further studies are needed to clarify the influences of physical fitness on arterial stiffness using more precise evaluation methods and/or a longitudinal study design.
The underling mechanisms whereby short sleep duration may lead to arterial stiffening cannot be determined using our cross-sectional data. However, one possible explanation is that short sleep duration could induce a sustained activation of the neuroendocrine system. Earlier studies showed that low sleep quality was related to an unhealthy pattern of sympathetic and parasympathetic nervous system activity in 5- to 11-year-old children32) and that children with short sleep duration had higher salivary cortisol levels as a biomarker of sympathoadrenal system activity than children with average or long sleep duration39). It was also shown that CAVI was mediated by the α 1-adrenergic receptor pathway40, 41).
Given the results of these previous studies, it appears that short sleep duration could be related to higher sympathetic nervous activity, which may concomitantly mediate alterations in arterial function in children with habitual short sleep durations. On the other hand, CAVI increases with age, especially after middle age, which would be attributed to structural changes in the vascular wall as an inevitable consequence of aging40). Considering that our present participants were 9- to 13-year-old children, there is only a slight possibility of the structural alteration in the vascular wall in children with short sleep duration.
There are several limitations to this study. It had a cross-sectional study design, and thus, our results do not provide a cause-effect relationship between predictors and outcomes. In addition, sleep duration was assessed only by a self-reported questionnaire and not by objective sleep monitors. In previous studies, sleep quality and quantity were assessed using actigraphy monitors worn on the wrist33, 42, 43). Actigraphy methods for sleep assessment showed a tentative low to moderate correlation with sleep polysomnography44), which is a gold standard for evaluating sleep quantity and quality. Given that it is important for students to have self-awareness regarding their sleep habits to improve their daily lifestyle, our results based on self-reported sleep duration could have clinical and/or educational implications. Our results do not show the true effects of childhood obesity and low fitness on arterial stiffness in children and early adolescents because the study's design was cross-sectional. Further research should be performed to determine the relationship between sleep status and arterial stiffness in children and early adolescents in a longitudinal study design using larger sample sizes.
In conclusion, we found that sleep duration was inversely associated with arterial stiffness after adjusting for confounders. Our data suggest that inadequate sleep duration could enhance the process of arterial stiffening in healthy children who are ≥ 10 years old. Our findings also suggest that sleep assessments, even by a self-reported questionnaire, may be a useful screening tool for identifying children with early vascular aging and future CV risks.
Acknowledgement
This study was supported by a Grant-in-Aid for Scientific Research (C, #22500619) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1). Juonala M, Järvisalo MJ, Mäki-Torkko N, Kähönen M, Viikari JS, Raitakari OT. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation, 2005; 112: 1486-1493 [DOI] [PubMed] [Google Scholar]
- 2). Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, Chen W, Srinivasan SR, Daniels SR, Kähönen M, Laitinen T, Taittonen L, Berenson GS, Viikari JS, Raitakari OT. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation, 2010; 122: 2514-2520 [DOI] [PubMed] [Google Scholar]
- 3). Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA, 2003; 290: 2271-2276 [DOI] [PubMed] [Google Scholar]
- 4). Li S, Chen W, Srinivasan SR, Berenson GS. Childhood Blood Pressure as a predictor of arterial stiffness in young adults: the Bogalusa Heart Study. Hypertension, 2004; 43: 541-546 [DOI] [PubMed] [Google Scholar]
- 5). Sakuragi S, Abhayaratna K, Gravenmaker KJ, O′Reilly C, Srikusalanukul W, Budge MM, Telford RD, Abhayaratna WP. Influence of adiposity and physical activity on arterial stiffness in healthy children the lifestyle of our kids study. Hypertension, 2009; 53: 611-616 [DOI] [PubMed] [Google Scholar]
- 6). Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol, 2009; 54: 2396-2406 [DOI] [PubMed] [Google Scholar]
- 7). Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med, 2007; 357: 2329-2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ, 2012; 345(November): e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Yoshinaga M, Ichiki T, Tanaka Y, Hazeki D, Horigome H, Takahashi H, Kashima K. Prevalence of childhood obesity from 1978 to 2007 in Japan. Pediatr Int, 2010; 52: 213-217 [DOI] [PubMed] [Google Scholar]
- 10). Guo X, Zheng L, Li Y, Yu S, Liu S, Zhou X, Zhang X, Sun Z, Wang R, Sun Y. Association between sleep duration and hypertension among Chinese children and adolescents. Clin Cardiol, 2011; 34: 774-781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Moraes W, Poyares D, Zalcman I, de Mello MT, Bittencourt LR, Santos-Silva R, Tufik S. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Med, 2013; 14: 312-318 [DOI] [PubMed] [Google Scholar]
- 12). Eguchi K, Pickering TG, Schwartz JE, Hoshide S, Ishikawa J, Ishikawa S, Shimada K, Kario K. Short sleep duration is an independent predictor of cardiovascular events in Japanese hypertensive patients. Arch Intern Med, 2008; 168: 2225-2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Sleep quality and elevated blood pressure in adolescents. Circulation, 2008; 118: 1034-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Lytle LA, Pasch KE, Farbakhsh K. The relationship between sleep and weight in a sample of adolescents. Obesity (Silver Spring), 2011; 19: 324-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Narang I, Manlhiot C, Davies-Shaw J, Gibson D, Chahal N, Stearne K, Fisher A, Dobbin S, McCrindle BW. Sleep disturbance and cardiovascular risk in adolescents. CMAJ, 2012; 184: 913-920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-Ankle elasticity index: A systematic review and meta-Analysis. Hypertension, 2012; 60: 556-562 [DOI] [PubMed] [Google Scholar]
- 17). Lurbe E, Torro I, Garcia-Vicent C, Alvarez J, Fernández-Fornoso JA, Redon J. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension, 2012; 60: 550-555 [DOI] [PubMed] [Google Scholar]
- 18). Dangardt F, Osika W, Volkmann R, Gan L-M, Friberg P. Obese children show increased intimal wall thickness and decreased pulse wave velocity. Clin Physiol Funct Imaging, 2008; 28: 287-293 [DOI] [PubMed] [Google Scholar]
- 19). Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y, Saiki A, Takahashi M, Suzuki K, Takata M. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb, 2011; 18: 924-938 [DOI] [PubMed] [Google Scholar]
- 20). Philip R, Alpert BS, Schwingshackl A, Huang X, Blakely D, Rovnaghi CR, Tran QT, Velasquez A, Arevalo A, Anand KJ. Inverse relationship between cardio-ankle vascular index and body mass index in healthy children. J Pediatr, 2015; 167: 361-365.e1 [DOI] [PubMed] [Google Scholar]
- 21). Saiki A, Sato Y, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Nagayama D, Ohira M, Endo K, Tatsuno I. The role of a novel arterial stiffness parameter, cardio-ankle vascular index (CAVI), as a surrogate marker for cardiovascular diseases. J Atheroscler Thromb, 2016; 23: 155-168 [DOI] [PubMed] [Google Scholar]
- 22). Alberto EC, Tanigawa T, Maruyama K, Kawasaki Y, Eguchi E, Mori H, Yoshimura K, Tanno S, Sakurai S, Hitsumoto S, Saito I. Relationships between nocturnal intermittent hypoxia, arterial stiffness and cardiovascular risk factors in a community-based population: the Toon health study. J Atheroscler Thromb, 2014; 21: 1290-1297 [DOI] [PubMed] [Google Scholar]
- 23). Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Ohira M, Endo K, Kurosu T, Tomaru T, Shirai K, Tatsuno I. Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb, 2016; 23: 596-605 [DOI] [PubMed] [Google Scholar]
- 24). Kumagai T, Kasai T, Kato M, Naito R, Maeno K, Kasagi S, Kawana F, Ishiwata S, Narui K. Establishment of the cardio-ankle vascular index in patients with obstructive sleep apnea. Chest, 2009; 136: 779-786. 10.1378/chest.09-0178 [DOI] [PubMed] [Google Scholar]
- 25). Kato N, Takimoto H, Sudo N. The cubic functions for spline smoothed L, S and M values for BMI reference data of Japanese children. Clin Pediatr Endocrinol, 2011; 20: 47-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Ministry of Education, Culture, Sports S and T of J Standard operating procedure for physical fitness and exercise performance tests 12-19 yr. ed. http://www.mext.go.jp/a_menu/sports/stamina/05030101/002.pdf http://www.e-stat.go.jp/SG1/estat/List.do?bid=000001055014&cycode=0 Accessed September 8, 2015
- 27). Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension, 2001; 37: 1236-1241 [DOI] [PubMed] [Google Scholar]
- 28). Lim J, Pearman M, Park W, Alkatan M, Tanaka H. Interrelationships Among Various Measures of Central Artery Stiffness. Am J Hypertens, (in press). 10.1093/ajh/hpw045 [DOI] [PubMed] [Google Scholar]
- 29). Ridha M, Nourse SE, Selamet Tierney ES, Tierney ESS. Pediatric interventions using noninvasive vascular health indices. Hypertension, 2015; 65: 949-955 [DOI] [PubMed] [Google Scholar]
- 30). Litwin M, Feber J, Ruzicka M. Vascular aging: lessons from pediatric hypertension. Can J Cardiol, 2016; 32: 642-649 [DOI] [PubMed] [Google Scholar]
- 31). Au CT, Ho CKW, Wing YK, Lam HS, Li AM. Acute and chronic effects of sleep duration on blood pressure. Pediatrics, 2013; 133: e64-e72 [DOI] [PubMed] [Google Scholar]
- 32). Michels N, Clays E, De Buyzere M, Vanaelst B, De Henauw S, Sioen I. Children's sleep and autonomic function: low sleep quality has an impact on heart rate variability. Sleep, 2013; 36: 1939-1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Iglayreger HB, Peterson MD, Liu D, Parker CA, Woolford SJ, Sallinen Gafka BJ, Hassan F, Gordon PM. Sleep duration predicts cardiometabolic risk in obese adolescents. J Pediatr, 2014; 164: 1085-1090.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Xi B, He D, Zhang M, Xue J, Zhou D. Short sleep duration predicts risk of metabolic syndrome: A systematic review and meta-analysis. Sleep Med Rev, 2014; 18: 293-297 [DOI] [PubMed] [Google Scholar]
- 35). Veijalainen A, Tompuri T, Haapala EA, Viitasalo A, Lintu N, Väistö J, Laitinen T, Lindi V, Lakka TA. Associations of cardiorespiratory fitness, physical activity, and adiposity with arterial stiffness in children. Scand J Med Sci Sports, 2016; 26: 943-950 [DOI] [PubMed] [Google Scholar]
- 36). von Wowern E, Östling G, Nilsson PM, Olofsson P. Digital photoplethysmography for assessment of arterial stiffness: repeatability and comparison with applanation tonometry. PLoS One, 2015; 10: e0135659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Bortolotto LA, Blacher J, Kondo T, Takazawa K, Safar ME. Assessment of vascular aging and atherosclerosis in hypertensive subjects: second derivative of photoplethysmogram versus pulse wave velocity. Am J Hypertens, 2000; 13: 165-171 [DOI] [PubMed] [Google Scholar]
- 38). Hashimoto J, Chonan K, Aoki Y, Nishimura T, Ohkubo T, Hozawa A, Suzuki M, Matsubara M, Michimata M, Araki T, Imai Y. Pulse wave velocity and the second derivative of the finger photoplethysmogram in treated hypertensive patients: their relationship and associating factors. J Hypertens, 2002; 20: 2415-2422 [DOI] [PubMed] [Google Scholar]
- 39). Räikkönen K, Matthews KA, Pesonen AK, Pyhälä R, Paavonen EJ, Feldt K, Jones A, Phillips DI, Seckl JR, Heinonen K, Lahti J, Komsi N, Järvenpää AL, Eriksson JG, Strandberg TE, Kajantie E. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenalmedullary system activity in children. J Clin Endocrinol Metab, 2010; 95: 2254-2261 [DOI] [PubMed] [Google Scholar]
- 40). Shirai K, Utino J, Saiki A, Endo K, Ohira M, Nagayama D, Tatsuno I, Shimizu K, Takahashi M, Takahara A. Evaluation of blood pressure control using a new arterial stiffness parameter, cardio-ankle vascular index (CAVI). Curr Hypertens Rev, 2013; 9: 66-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Takahashi M, Shiba T, Hirano K, Hitsumoto T, Shirai K. Acute decrease of cardio-ankle vascular index with the administration of beraprost sodium. J Atheroscler Thromb, 2012; 19: 479-484 [DOI] [PubMed] [Google Scholar]
- 42). Martikainen S, Pesonen AK, Feldt K, Jones A, Lahti J, Pyhälä R, Heinonen K, Kajantie E, Eriksson J, Räikkönen K. Poor sleep and cardiovascular function in children. Hypertension, 2011; 58: 16-21 [DOI] [PubMed] [Google Scholar]
- 43). Arora T, Broglia E, Pushpakumar D, Lodhi T, Taheri S. An investigation into the strength of the association and agreement levels between subjective and objective sleep duration in adolescents. PLoS One, 2013; 8: e72406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Johnson NL, Kirchner HL, Rosen CL, Storfer-Isser A, Cartar LN, Ancoli-Israel S, Emancipator JL, Kibler AM, Redline S. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. Sleep, 2007; 30: 899-905 [DOI] [PMC free article] [PubMed] [Google Scholar]


