Abstract
Some statins, such as atorvastatin, have proven renoprotective effects. The comparative renoprotective potential of simvastatin is less clear. This study aimed to compare the renoprotective effects of simvastatin with atorvastatin in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI). This observational study examined the medical records of 271 patients who were treated at the Guangdong Cardiovascular Institute from April 2004 to February 2008. Patients had received either 40 mg simvastatin (n = 128) or 20 mg atorvastatin (n = 143), daily, for a period of at least 6 months following PCI. Declined renal function (DRF) was defined at the occurrence of chronic kidney disease (CKD) or elevated CKD stages at 6-months post-PCI. Results showed that the incidence of DRF was similar among patients taking simvastatin or atorvastatin (25.00% vs 26.57%, respectively). Kaplan–Meier survival analysis showed that patients who developed DRF had a higher incidence of mortality and major adverse cardiovascular events (MACEs) than those without DRF (17.41% vs 28.57%, P = .0308). Multivariate logistic regression analysis identified diabetes and baseline estimated glomerular filtration rate as independent risk factors for DRF. Collectively, our results indicate that simvastatin has comparable renoprotective effects to atorvastatin in ACS patients undergoing PCI. Further studies are warranted to confirm the comparative renoprotective effects of statins.
Keywords: acute coronary syndrome, atorvastatin, percutaneous coronary intervention, renal function, simvastatin
1. Introduction
Statins are a family of cholesterol-lowering drugs that inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, an enzyme with a central role in the production of cholesterol. Statins inhibit several pathological pathways involved in acute coronary syndrome (ACS), such as endothelial dysfunction, thrombus formation, and the activation of inflammatory and coagulation cascades.[1–3] Patients with ACS often require percutaneous coronary intervention (PCI) that can lead to complications such as contrast-induced nephropathy (CIN), which is associated with long-term morbidity, mortality, and increased health care costs. These inflict substantial burdens on the individual and society.[4,5]
Recently, meta-analyses[6,7] and large randomized controlled trials[8] have shown statins to have clinically relevant renoprotective effects that could justify their use during the early stages of chronic kidney disease (CKD).[9] Renoprotrotective effects have been established for atorvastatin,[10–18] simvastatin,[19–21] and rosuvastatin.[22,23] Moreover, several studies have compared these renoprotective effects in different clinical settings (atorvastatin vs rosuvastatin,[22–24] pravastatin vs simvastatin,[25] and rosuvastatin vs atorvastatin[26]). Collectively, these studies suggest that atorvastatin might have the strongest renoprotective effects.[6,10,22]
The renoprotective effects of simvastatin have been demonstrated in several pre-clinical studies.[19,27] Simvastatin ameliorated low-dose streptozotocin-induced nephropathy in an experimental rat model of type 2 diabetes. This was thought to be via its ability to lower cholesterol, reduce oxidative stress, and inflammation.[20] When applied to angiotensin II stimulated human mast cells, simvastatin also suppressed inflammation and alleviated oxidative stress. Furthermore, it had a protective effect in a pre-clinical model of CKD.[28] In another study, simvastatin dose-dependently increased the expression of ADAMTS13 in podocytes, which possibly explains its reported antithrombotic properties.[29] Simvastatin has also been shown to exhibit beneficial effect on vascular endothelium,[30] and to prevent the progression of renal fibrosis.[31] In a clinical setting, studies have shown beneficial effects of simvastatin on renal microvascular dysfunction, ischemia-reperfusion injury,[32] and the incidence of CIN.[21] Despite these promising findings, the renoprotective effects of simvastatin are yet to be compared with other statins.
This study aimed to determine the renoprotective effect of simvastatin versus atorvastatin in patients with ACS undergoing PCI.
2. Methods
This study was approved by the Guangdong General Hospital ethics committee and was conducting according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients involved.
2.1. Patients
We enrolled consecutive CHD patients undergoing PCI who agreed to attend “CHD club” at Guangdong Cardiovascular Institute, Guangdong General Hospital between April 2004 and February 2008. Patients in the “CHD club” receive CHD secondary prevention education, which include guiding lifestyle changes, taking medication, and biochemical investigation also conducted if necessary during hospitalization and follow-up period. The follow-up period includes 1, 3, 6, 12, 24, and 36 months after cardiac catherization. Adults with ACS and undergoing PCI were included in the study if they had received either simvastatin (40 mg daily) or atorvastatin (20 mg daily) from admission for PCI to the sixth month after PCI. Patients who had taken other statins, who changed statins during the 6 month period, who did not have recorded serum creatinine (SCr) concentrations at baseline or at 6-months follow-up, or who discontinued their use of simvastatin or atorvastatin due to adverse events, were excluded from the study (Fig. 1). Baseline demographics and clinical characteristics were identified from the time of PCI.
Figure 1.
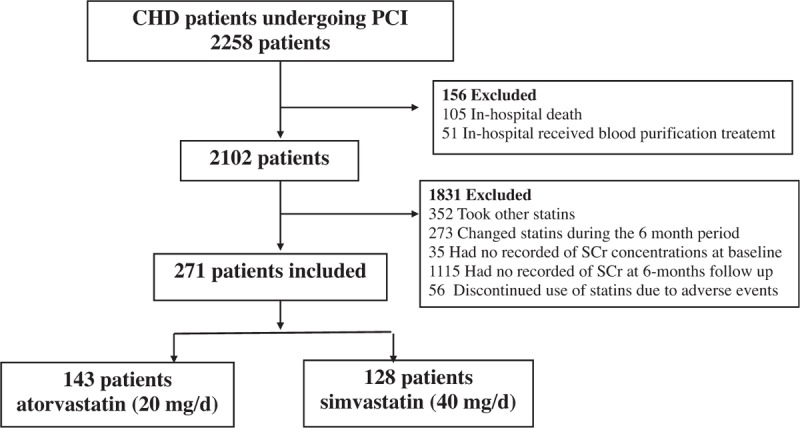
Patient study flow.
2.2. PCI and medications
PCI was performed by experienced interventional cardiologists according to standard national practice. Nonionic, low-osmolar contrast medium was employed for all patients (iopamiron or ultravist, both at 370 mg I/mL). Statins, antiplatelet agents (aspirin/clopidogrel), β-adrenergic blocking agents, angiotensin-converting enzyme inhibitors, diuretics, and inotropic drugs were used at the attending cardiologist's discretion, according to clinical protocol derived from national interventional guidelines.
2.3. Biochemical parameters measurement
Biochemical test results were isolated from medical records of the first, third, sixth, 24th, and 36th months following PCI. The concentrations of SCr, serum triglyceride concentration (TG), and low-density lipoprotein cholesterol (LDL-C) were identified at admission and at 6 months post-PCI. The levels of blood urea nitrogen (BUN), electrolytes, fasting glucose, fasting lipid, and other standard clinical parameters were obtained from the morning of the PCI. The estimated glomerular filtration rate (eGFR) was calculated using the 4-variable Modification of Diet in Renal Disease equation based on Chinese patients.
2.4. Clinical outcomes
The primary outcome was the occurrence of declined renal function (DRF), defined as the incidence of CKD or the elevated CKD stage at 6 months post-PCI. CKD was defined as an eGFR decline from ≥90 mL/min/1.73 m2 at baseline to < 90 mL/min/1.73 m2 at 6 months follow-up. Additional outcomes included major in-hospital or long-term adverse clinical events (major adverse clinical events; MACEs), such as mortality, nonfatal myocardial infarction, target vessel revascularization, or stroke.
2.5. Statistical analysis
Data were analyzed using IBM SPSS version 19 software. Comparisons between the groups of patients (simvastatin vs atorvastatin) were performed using Student t tests or the Wilcoxon rank sum test (if not normally distributed) for continuous variables. Alternatively, Chi-square or Fisher exact tests were used for categorical variables. All statistical significance was inferred if P < .05.
SCr and eGFR were compared between patients taking simvastatin and atorvastatin at baseline and at 6 months post-PCI.
A multivariate analysis was conducted to identify the clinical factors affecting the incidence of DRF (dependent variable). Age (≥75 years), serum TG, LDL-C, having diabetes, gender, and eGFR at baseline were included as potential variables. Those that were statistically significant according to a univariate analysis were included in the multivariate model.
Kaplan–Meier curves were calculated for patients who experienced DRF versus those who did not. Cumulative mortality or MACE event curves were compared using a log-rank test.
3. Results
3.1. Baseline characteristics of patients receiving simvastatin or atorvastatin
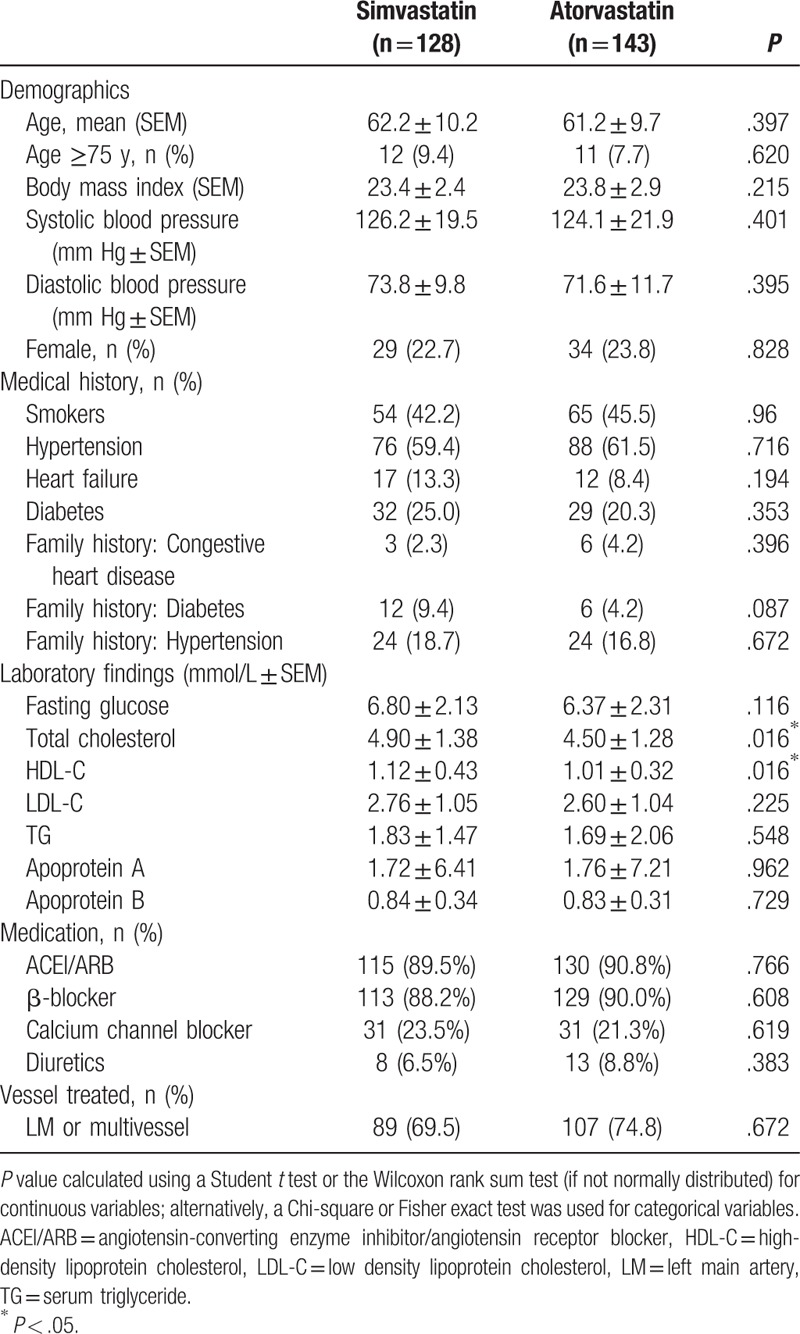
Of the 271 patients with ACS undergoing primary PCI whose medical records were included in the study, 128 had received daily simvastatin (40 mg) and 143 had received daily atorvastatin (20 mg) for at least 6 months post-PCI. As summarized in Table 1, there were no demographical or procedural differences between the groups. The concentrations of total serum cholesterol and high-density lipoprotein cholesterol (HDL-C) were higher in patients who had received simvastatin than in those who had received atorvastatin. These differences were not significant at 6 months post-PCI.
Table 1.
Baseline clinical characteristics of study participants.
3.2. Effect of atorvastatin and simvastatin on renal function after PCI
In the 6 months following PCI, there was a significant deterioration in renal function in patients of both groups (Figs. 2 and 3 ), as shown by significant increases in SCr (Fig. 2 and Table 2) and significant decreases in eGFR (Fig. 3 and Table 2). There were no differences in the percentage increases in SCr, or the percentage decrease in eGFR, between the 2 groups (Fig. 4). DRF was defined in 70 of 271 patients (25.83%; Fig. 5). Among the 70 patients with DRF, there was a significantly higher incidence of mortality and MACEs than that in patients without DRF (17.41% vs 28.57%, P = .046; Fig. 5). The incidence of DRF was similar among patients treated with simvastatin or atorvastatin (25.00% vs 26.57%, P = .768).
Figure 2.
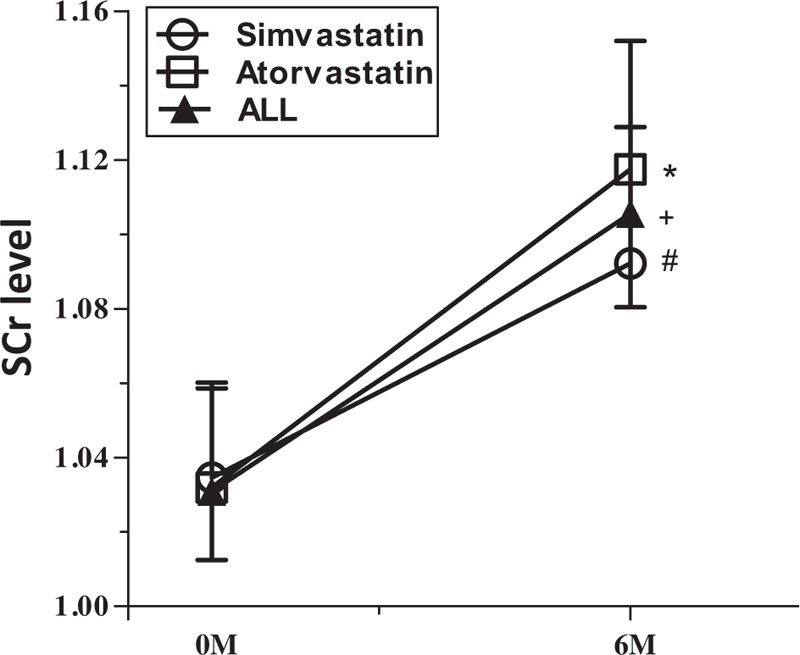
Time-course of serum creatinine (SCr) from baseline to 6 months post-percutaneous coronary intervention (PCI).
Figure 3.
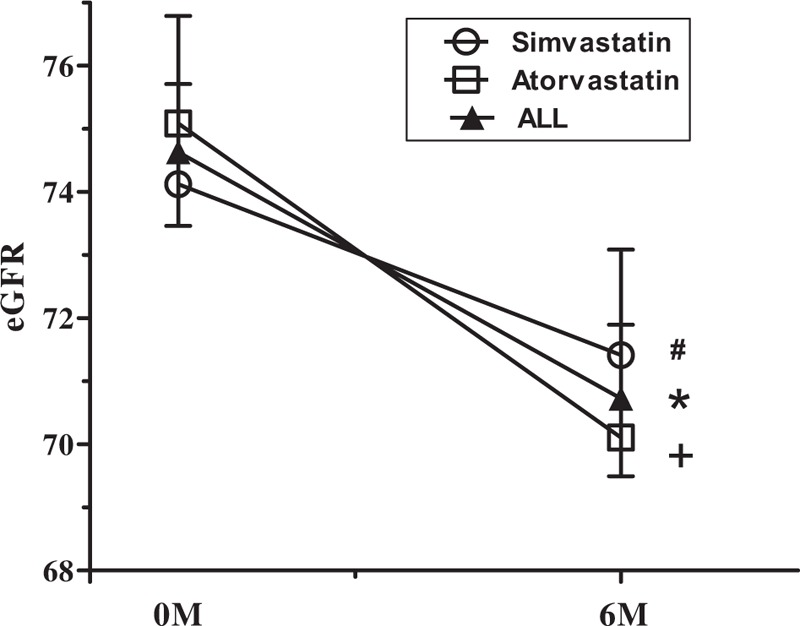
Time-course of estimated glomerular filtration rate (eGFR) from baseline to 6 months post-percutaneous coronary intervention (PCI).
Table 2.
Serum creatinine and estimated glomerular filtration rate levels at baseline and 6 months after percutaneous coronary intervention.
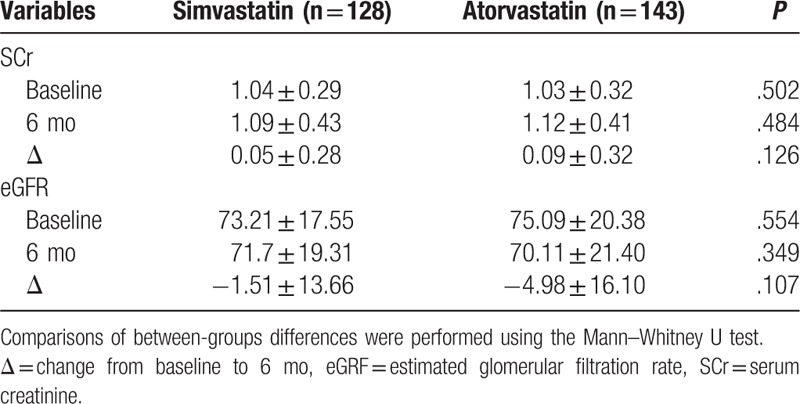
Figure 4.
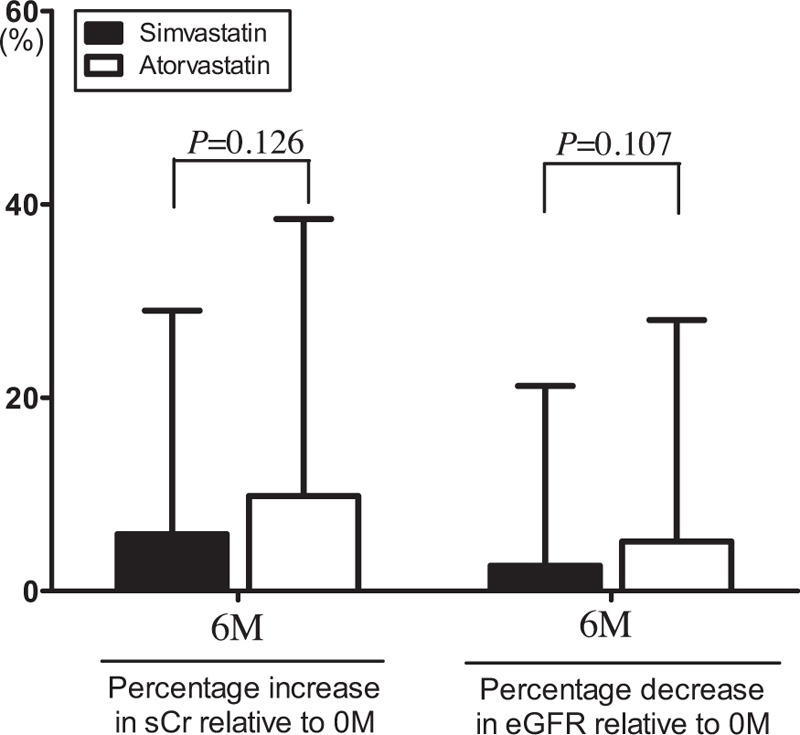
Comparison of the percentage increase in serum creatinine (SCr) and the percentage decrease in estimated glomerular filtration rate (eGFR) between simvastatin and atorvastatin group at 6 months post-percutaneous coronary intervention (PCI).
Figure 5.
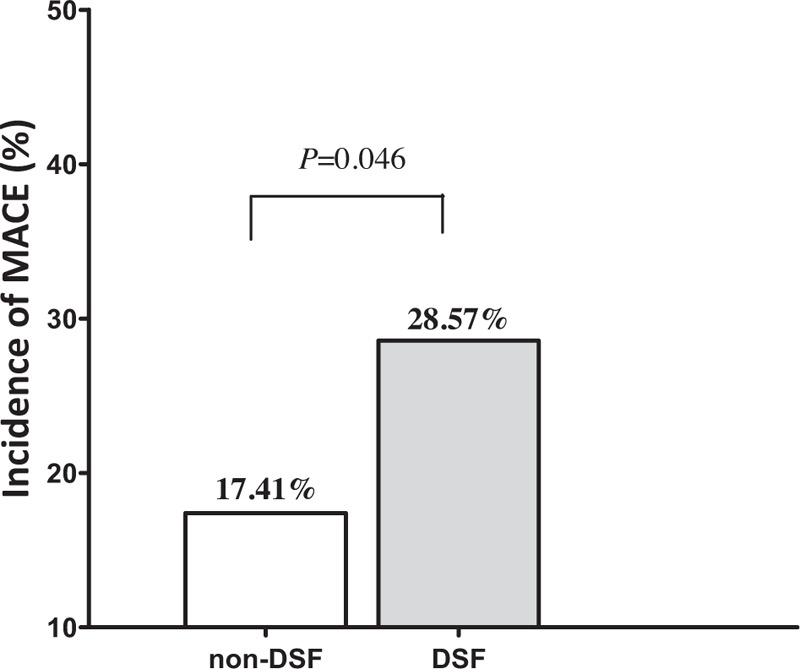
The prevalence of all-cause mortality and major adverse cardiovascular events (MACEs) in patients with or without declined renal function (DRF) during the 6 months post-percutaneous coronary intervention (PCI).
3.3. Analysis of clinical factors affecting the incidence of DRF in the 6 months post-PCI
Multivariate logistic regression analysis revealed that diabetes and the level of eGFR at baseline were independently associated with the incidence of DRF (Table 3).
Table 3.
Multivariate analysis of clinical factors affecting the incidence of declining renal function.
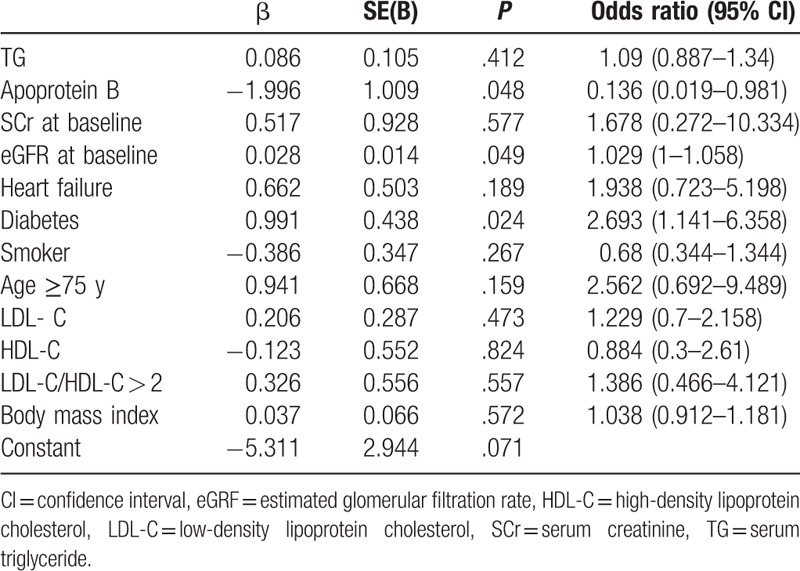
Other baseline characteristics [age (≥75 years), serum TG, LDL-C, and gender] did not affect the incidence of DRF.
3.4. Clinical outcomes during the entire follow-up
The median follow-up period for all patients was 25.23 ± 2.94 months. Kaplan–Meier survival analysis showed that patients who developed DRF had a higher rate of mortality and MACEs than those without DRF (17.41% vs 28.57%, P = .031; Fig. 6).
Figure 6.
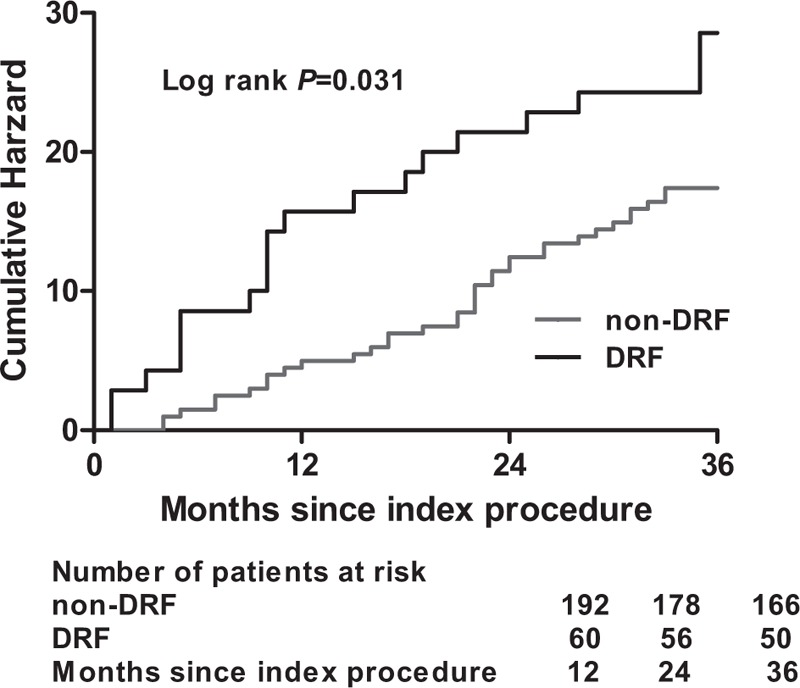
Cumulative rate of follow-up all-cause mortality and major adverse cardiovascular events (MACEs) in patients with or without declined renal function (DRF) during the 6 months post-percutaneous coronary intervention (PCI).
4. Discussion
Cardiovascular events and impaired renal function are associated with increased morbidity, decreased quality of life, and higher mortality rates.[4,5] Several risk factors have been identified for renal impairment following cardiovascular events, including dyslipidemia, hypertension, and diabetes.[4,5,8] Fortunately, there are some well characterized therapeutics available to manage these risk factors.[9]
Statins are a family of drugs widely used to lower serum cholesterol. Several statins have been shown to have renoprotective effects in patients with cardiovascular events (e.g., ACS).[1,8,9,33] Atorvastatin,[10–18] simvastatin,[19–21] rosuvastatin,[22,23,26] and pravastatin[25] have all been shown to have beneficial effects in different clinical settings; however, this is the first comparative study of simvastatin versus another statin in a clinical setting. In the present study, we reviewed the clinical records of patients with ACS who received simvastatin or atorvastatin for at least 6 months following PCI.
Previous studies[34,35] have suggested that simvastatin exerts a renoprotective effect. We found that the effects of simvastatin and atorvastatin were comparable in our study population at 6 months post-PCI; this was in terms of the concentration of SCr, eGFR, and the incidence of DRF.
As expected, patients experiencing DRF had poorer clinical outcomes. There is currently a lack of effective strategies to prevent DRF; therefore, the renoprotective effects of statins hold much promise. Statins are well characterized in the clinic and are widely available for use in at-risk patients.
The underlying mechanisms for the renoprotective effect of statins need to be further delineated. Statins are well known HMG-CoA reductase inhibitors, which is the mechanism by which they reduce of cholesterol biosynthesis, modulate hepatic lipid metabolism, and lower plasma lipid concentration.[36] Our analysis of the clinical factors affecting the incidence of DRF showed that plasma lipid concentration was not of key importance. Statins have pleotropic effects, and some of their actions may be mediated by other pathways. For example, HMG-CoA reductase is also the rate-limiting step in the conversion of HMG-CoA to mevalonic acid.[36,37]
It has been suggested that the renoprotective effects of statins could result from an effect on endothelial cell function. Nitric oxide (NO) mediates endothelial-derived vasodilation and also promotes both natriuresis and diuresis by increasing renal blood flow and GFR.[38] NO also increases renin secretion.[39] Statins have been shown to improve basal NO activity and NO-dependent endothelium-derived vasodilatation in healthy volunteers and in patients with heart disease.[40] They have also been shown to significantly ameliorate endothelial dysfunction in patients with carotid artery disease (CAD) via the regulation of endothelial NO synthase function and phosphatidylinositide 3-kinase/Akt signaling.[41–43] Atorvastatin increases NO availability, prevents the production of oxygen free radicals, and downregulates the expression of cyclooxygenase 2.[44] In keeping with these findings, short-term atorvastatin treatment in patients with CAD is associated with a reduction in endothelial apoptosis.[45] Other studies have also reported that simvastatin can improve endothelial function.[46,47]
Inflammation is highly prevalent in patients with CKD and CAD; it is associated with endothelial dysfunction, low endothelial NO production, microcirculation failure, and atherosclerosis.[48] The anti-inflammatory effects of statins are well known and may be of relevance to their renoprotective effects.[6,49]
The renoprotective effect of statins in patients with ACS undergoing PCI is likely to derive from the action of several synergistic pathways. This might include increased NO synthase activity, reduction in oxidative stress,[50–55] reduction in renal vascular permeability and tubular hypoxic injury,[51,56] or reduced incidence of CIN. Further interventional studies would be needed to delineate the actions of statins on these processes.
Our results suggest that the renoprotective effect of atorvastatin is similar to that of simvastatin for patients with ACS undergoing PCI. Interestingly, statins have been shown to have unique lipid-lowering, anti-inflammatory, and renoprotective profiles.[33,36,57] Atorvastatin is more expensive than other statins but has been reported to have stronger LDL-C lowering and renoprotective properties. Compared with 40 mg simvastatin, 10 mg atorvastatin further reduced hsCRP and anti-inflammatory effects in a crossover study of patients with type 2 diabetes.[58] As elevated C-reactive protein is a common feature of CKD, this finding would suggest that atorvastatin might have a stronger anti-inflammatory profile than simvastatin. However, the effects of simvastatin and atorvastatin were comparable in our study.
The beneficial renal and cardiovascular effects of atorvastatin and simvastatin might be situation dependent. In a rat model of cigarette smoke-induced acute lung inflammation, simvastatin attenuated inflammation and oxidative stress more than atorvastatin.[59] The ability of simvastatin to reduce oxidative stress and reverse the decline of NO was also observed in a study of CIN in rats.[21] Further studies are warranted to confirm the activity of statins in different cardiovascular and inflammatory disease scenarios.
5. Conclusion
We studied the medical records of patients with ACS undergoing PCI to compare the renoprotective effects of atorvastatin and simvastatin. Our study revealed that the decline of eGFR and increase in SCr was similar in patients who received atorvastatin or simvastatin for 6 months after PCI. Further studies are warranted to compare the clinical effectiveness of statins in situations where there is a risk of renal injury.
5.1. Limitations
There are several limitations of the current study. First, considering ethical requirement, we did not include a placebo control in the present study, and no definitive conclusions can be drawn in this regard. Second, MDRD study equation to estimate renal function was used in this study. However, certain study reported that this equation was less accurate in populations without CKD.[60] In their opinion, eGFR of less than 90 mL/min/1.73 m2 is lower than direct measurements. As such, more specific tests need to be used to test the potential effect of statins on renal function in future studies.
Footnotes
Abbreviations: ACEI/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, ACS = acute coronary syndrome, BUN = blood urea nitrogen, CAD = carotid artery disease, CIN = contrast-induced nephropathy, CKD = chronic kidney disease, DRF = declined renal function, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, HMG-CoA = the 3-hydroxy-3-methylglutaryl coenzyme A, LDL-C = low density lipoprotein cholesterol, LM = left main artery, MACEs = major adverse clinical events, NO = nitric oxide, PCI = percutaneous coronary intervention, SCr = serum creatinine, TG = triglyceride.
Funding/support: The study was supported by the grant (2014A020212734) from Guangdong Science and Technology Department and the grant (81602848) from The National Natural Science Fund, People's Republic of China.
The authors report no conflicts of interest.
References
- [1].Vale N, Nordmann AJ, Schwartz GG, et al. Statins for acute coronary syndrome. Cochrane Database Syst Rev 2011;CD006870. [DOI] [PubMed] [Google Scholar]
- [2].Ostadal P. Statins as first-line therapy for acute coronary syndrome? Exp Clin Cardiol 2012;17:227–36. [PMC free article] [PubMed] [Google Scholar]
- [3].Vale N, Nordmann AJ, Schwartz GG, et al. Statins for acute coronary syndrome. Cochrane Database Syst Rev 2014;9:CD006870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol 2005;95:13–9. [DOI] [PubMed] [Google Scholar]
- [5].Caruso M, Balasus F, Incalcaterra E, et al. Contrast-induced nephropathy after percutaneous coronary intervention in simple lesions: risk factors and incidence are affected by the definition utilized. Intern Med 2011;50:983–9. [DOI] [PubMed] [Google Scholar]
- [6].Krane V, Wanner C. Statins, inflammation and kidney disease. Nat Rev Nephrol 2011;7:385–97. [DOI] [PubMed] [Google Scholar]
- [7].Geng Q, Ren J, Song J, et al. Meta-analysis of the effect of statins on renal function. Am J Cardiol 2014;114:562–70. [DOI] [PubMed] [Google Scholar]
- [8].Sharp Collaborative G. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J 2010;160:785–94. e10. [DOI] [PubMed] [Google Scholar]
- [9].Wanner C, Tonelli M. Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014;85:1303–9. [DOI] [PubMed] [Google Scholar]
- [10].Fassett RG, Robertson IK, Ball MJ, et al. Effect of atorvastatin on kidney function in chronic kidney disease: a randomised double-blind placebo-controlled trial. Atherosclerosis 2010;213:218–24. [DOI] [PubMed] [Google Scholar]
- [11].Li W, Fu X, Wang Y, et al. Beneficial effects of high-dose atorvastatin pretreatment on renal function in patients with acute ST-segment elevation myocardial infarction undergoing emergency percutaneous coronary intervention. Cardiology 2012;122:195–202. [DOI] [PubMed] [Google Scholar]
- [12].Patti G, Ricottini E, Nusca A, et al. Short-term, high-dose Atorvastatin pretreatment to prevent contrast-induced nephropathy in patients with acute coronary syndromes undergoing percutaneous coronary intervention (from the ARMYDA-CIN [atorvastatin for reduction of myocardial damage during angioplasty: contrast-induced nephropathy] trial. Am J Cardiol 2011;108:1–7. [DOI] [PubMed] [Google Scholar]
- [13].Toso A, Leoncini M, Maioli M, et al. Short-term high-dose atorvastatin for periprocedural myocardial infarction prevention in patients with renal dysfunction. J Cardiovasc Med (Hagerstown) 2011;12:318–21. [DOI] [PubMed] [Google Scholar]
- [14].Bybee KA, Lee JH, O’Keefe JH. Cumulative clinical trial data on atorvastatin for reducing cardiovascular events: the clinical impact of atorvastatin. Curr Med Res Opin 2008;24:1217–29. [DOI] [PubMed] [Google Scholar]
- [15].Takazakura A, Sakurai M, Bando Y, et al. Renoprotective effects of atorvastatin compared with pravastatin on progression of early diabetic nephropathy. J Diabetes Investig 2015;6:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fassett RG, Robertson IK, Ball MJ, et al. Effects of atorvastatin on biomarkers of inflammation in chronic kidney disease. Clin Nephrol 2014;81:75–85. [DOI] [PubMed] [Google Scholar]
- [17].Ueshima K, Kasahara M, Koya D, et al. Effects of atorvastatin on renal function in patients with dyslipidemia and chronic kidney disease: rationale and design of the ASsessment of clinical Usefulness in CKD patients with Atorvastatin (ASUCA) trial. Clin Exp Nephrol 2013;17:211–7. [DOI] [PubMed] [Google Scholar]
- [18].Prowle JR, Calzavacca P, Licari E, et al. Pilot double-blind, randomized controlled trial of short-term atorvastatin for prevention of acute kidney injury after cardiac surgery. Nephrology (Carlton) 2012;17:215–24. [DOI] [PubMed] [Google Scholar]
- [19].Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang S, Xu H, Yu X, et al. Simvastatin ameliorates low-dose streptozotocin-induced type 2 diabetic nephropathy in an experimental rat model. Int J Clin Exp Med 2015;8:6388–96. [PMC free article] [PubMed] [Google Scholar]
- [21].Al-Otaibi KE, Al Elaiwi AM, Tariq M, et al. Simvastatin attenuates contrast-induced nephropathy through modulation of oxidative stress, proinflammatory myeloperoxidase, and nitric oxide. Oxid Med Cell Longev 2012;2012:831748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shepherd J, Kastelein JJ, Bittner V, et al. Treating to new targets I. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary heart disease: the Treating to New Targets (TNT) study. Clin J Am Soc Nephrol 2007;2:1131–9. [DOI] [PubMed] [Google Scholar]
- [23].de Zeeuw D, Anzalone DA, Cain VA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol 2015;3:181–90. [DOI] [PubMed] [Google Scholar]
- [24].Kaya A, Kurt M, Tanboga IH, et al. Rosuvastatin versus atorvastatin to prevent contrast induced nephropathy in patients undergoing primary percutaneous coronary intervention (ROSA-cIN trial). Acta Cardiol 2013;68:489–94. [DOI] [PubMed] [Google Scholar]
- [25].Munoz MA, Maxwell PR, Green K, et al. Pravastatin versus simvastatin for prevention of contrast-induced nephropathy. J Cardiovasc Pharmacol Ther 2011;16:376–9. [DOI] [PubMed] [Google Scholar]
- [26].Liu Y, Liu YH, Tan N, et al. Comparison of the efficacy of rosuvastatin versus atorvastatin in preventing contrast induced nephropathy in patient with chronic kidney disease undergoing percutaneous coronary intervention. PLoS One 2014;9:e111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Haynes R, Lewis D, Emberson J, et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol 2014;25:1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shahbazian H, Atrian A, Yazdanpanah L, et al. Anti-inflammatory effect of simvastatin in hemodialysis patients. Jundishapur J Nat Pharm Prod 2015;10:e17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shen L, Lu G, Dong N, et al. Simvastatin increases ADAMTS13 expression in podocytes. Thromb Res 2013;132:94–9. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Z, Wang M, Xue SJ, et al. Simvastatin ameliorates angiotensin II-induced endothelial dysfunction through restoration of Rho-BH4-eNOS-NO pathway. Cardiovasc Drugs Ther 2012;26:31–40. [DOI] [PubMed] [Google Scholar]
- [31].Hamasaki Y, Doi K, Okamoto K, et al. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor simvastatin ameliorates renal fibrosis through HOXA13-USAG-1 pathway. Lab Invest 2012;92:1161–70. [DOI] [PubMed] [Google Scholar]
- [32].Tuuminen R, Nykanen AI, Saharinen P, et al. Donor simvastatin treatment prevents ischemia-reperfusion and acute kidney injury by preserving microvascular barrier function. Am J Transplant 2013;13:2019–34. [DOI] [PubMed] [Google Scholar]
- [33].Elisaf M, Mikhailidis DP. Statins and renal function. Angiology 2002;53:493–502. [DOI] [PubMed] [Google Scholar]
- [34].Wang J, Gu C, Gao M, et al. Preoperative statin therapy and renal outcomes after cardiac surgery: a meta-analysis and meta-regression of 59,771 patients. Canad J Cardiol 2015;31:1051–60. [DOI] [PubMed] [Google Scholar]
- [35].Singh I, Rajagopalan S, Srinivasan A, et al. Preoperative statin therapy is associated with lower requirement of renal replacement therapy in patients undergoing cardiac surgery: a meta-analysis of observational studies. Interact Cardiovasc Thorac Surg 2013;17:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med 2001;5:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Buhaescu I, Izzedine H. Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem 2007;40:575–84. [DOI] [PubMed] [Google Scholar]
- [38].Ergin B, Kapucu A, Demirci-Tansel C, et al. The renal microcirculation in sepsis. Nephrol Dial Transplant 2015;30:169–77. [DOI] [PubMed] [Google Scholar]
- [39].Mose FH, Larsen T, Jensen JM, et al. Effects of atorvastatin on systemic and renal NO dependency in patients with non-diabetic stage II-III chronic kidney disease. Br J Clin Pharmacol 2014;78:789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Philips B, MacPhee I. Do statins prevent acute kidney injury? Expert Opin Drug Safety 2015;14:1547–61. [DOI] [PubMed] [Google Scholar]
- [41].Dai YL, Luk TH, Siu CW, et al. Mitochondrial dysfunction induced by statin contributes to endothelial dysfunction in patients with coronary artery disease. Cardiovasc Toxicol 2010;10:130–8. [DOI] [PubMed] [Google Scholar]
- [42].Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 1998;273:24266–71. [DOI] [PubMed] [Google Scholar]
- [43].Kureishi Y, Luo Z, Shiojima I, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med 2000;6:1004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Virdis A, Colucci R, Versari D, et al. Atorvastatin prevents endothelial dysfunction in mesenteric arteries from spontaneously hypertensive rats: role of cyclooxygenase 2-derived contracting prostanoids. Hypertension 2009;53:1008–16. [DOI] [PubMed] [Google Scholar]
- [45].Schmidt-Lucke C, Fichtlscherer S, Rossig L, et al. Improvement of endothelial damage and regeneration indexes in patients with coronary artery disease after 4 weeks of statin therapy. Atherosclerosis 2010;211:249–54. [DOI] [PubMed] [Google Scholar]
- [46].Zhu J, Gao B. Simvastatin combined with aspirin increases the survival time of heart allograft by activating CD4(+)CD25(+) Treg cells and enhancing vascular endothelial cell protection. Cardiovasc Pathol 2015;24:173–8. [DOI] [PubMed] [Google Scholar]
- [47].Tong XK, Hamel E. Simvastatin restored vascular reactivity, endothelial function and reduced string vessel pathology in a mouse model of cerebrovascular disease. J Cereb Blood Flow Metab 2015;35:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Heeba G, Hassan MK, Khalifa M, et al. Adverse balance of nitric oxide/peroxynitrite in the dysfunctional endothelium can be reversed by statins. J Cardiovasc Pharmacol 2007;50:391–8. [DOI] [PubMed] [Google Scholar]
- [49].Antonopoulos AS, Margaritis M, Lee R, et al. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Design 2012;18:1519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Luvai A, Mbagaya W, Hall AS, et al. Rosuvastatin: a review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin Med Insights Cardiol 2012;6:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sabbatini M, Pisani A, Uccello F, et al. Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol 2004;15:901–9. [DOI] [PubMed] [Google Scholar]
- [52].Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 2006;17:1503–20. [DOI] [PubMed] [Google Scholar]
- [53].John S, Schneider MP, Delles C, et al. Lipid-independent effects of statins on endothelial function and bioavailability of nitric oxide in hypercholesterolemic patients. Am Heart J 2005;149:473. [DOI] [PubMed] [Google Scholar]
- [54].Ridker PM, Rifai N, Clearfield M, et al. Air Force/Texas Coronary Atherosclerosis Prevention Study I. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 2001;344:1959–65. [DOI] [PubMed] [Google Scholar]
- [55].Mithani S, Kuskowski M, Slinin Y, et al. Dose-dependent effect of statins on the incidence of acute kidney injury after cardiac surgery. Ann Thorac Surg 2011;91:520–5. [DOI] [PubMed] [Google Scholar]
- [56].Yasuda H, Yuen PS, Hu X, et al. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 2006;69:1535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Agarwal R. Effects of statins on renal function. Am J Cardiol 2006;97:748–55. [DOI] [PubMed] [Google Scholar]
- [58].Sathyapalan T, Shepherd J, Atkin SL, et al. The effect of atorvastatin and simvastatin on vitamin D, oxidative stress and inflammatory marker concentrations in patients with type 2 diabetes: a crossover study. Diabetes Obes Metab 2013;15:767–9. [DOI] [PubMed] [Google Scholar]
- [59].Ferreira TS, Lanzetti M, Barroso MV, et al. Oxidative stress and inflammation are differentially affected by atorvastatin, pravastatin, rosuvastatin, and simvastatin on lungs from mice exposed to cigarette smoke. Inflammation 2014;37:1355–65. [DOI] [PubMed] [Google Scholar]
- [60].Pyorala K, Ballantyne CM, Gumbiner B, et al. Scandinavian Simvastatin Survival S. Reduction of cardiovascular events by simvastatin in nondiabetic coronary heart disease patients with and without the metabolic syndrome: subgroup analyses of the Scandinavian Simvastatin Survival Study (4S). Diabetes care 2004;27:1735–40. [DOI] [PubMed] [Google Scholar]


