Supplemental Digital Content is available in the text
Keywords: aphasia, DTI, fMRI, rehabilitation, rTMS
Abstract
Rationale:
To date, little is known regarding the neural mechanisms of the functional recovery of language after repetitive transcranial magnetic stimulation (rTMS) in aphasia. Our aim was to investigate the mechanism that underlies rTMS and speech training in a case report.
Patient concerns and diagnoses:
We report the case of a 39-year-old woman who was initially diagnosed with conduction aphasia following a left hemisphere stroke.
Interventions:
The rTMS location comprised the left Broca area, and a frequency of 5 Hz for 20 min/d for 10 days during a 2-week period was used. She had received speech rehabilitation training 1 month after stroke. Functional magnetic resonance imaging (fMRI) and diffusion tensor imaging were used to investigate the functional and microstructural changes before and after rTMS treatment.
Outcomes:
The results demonstrated that the Western Aphasia Battery scores significantly improved for language ability at 2 weeks post-treatment, and the gains were steadily increased at 2.5 months post-treatment. The fMRI results indicated a more focused activation pattern and showed significant activation in the left dominant hemisphere relative to the right hemisphere, especially in the perilesional areas, post-treatment during 2 language tasks compared with pretreatment. Moreover, the fractional anisotropy increased in the left superior temporal gyrus, which comprises an important area that is involved in language processing.
Lessons:
Our findings suggest that rTMS combined with speech training improved the speech-language ability of this chronic conduction aphasia patient and enhanced the cerebral functional and microstructural reorganization.
1. Introduction
Aphasia comprises a combination of a speech and language disorders caused by damage to the brain. Approximately 21% to 38% of acute stroke patients suffer from aphasia, which is typically associated with high mortality, significant motor impairment, and severe limitations in social participation.[1,2] The traditional speech and language therapy (SLT) for aphasia is predominately based on compensatory strategies or training for lost functions.[3] Repetitive transcranial magnetic stimulation (rTMS) represents a relatively safe, effective, and noninvasive brain stimulation technique, and it has been applied to promote poststroke aphasia recovery in many research studies.[2,4] These studies have typically suppressed right hemisphere (RH) activity with low-frequency rTMS or excited the perilesional areas of the left hemisphere (LH) with high-frequency rTMS, and they showed that rTMS had a strong effect.[2–7] TMS can modulate synaptic plasticity that can last for days or even weeks and months.[8] Researchers have shown that rTMS has a long-term efficacy,[9,10] and rTMS together with SLT, they can consolidate the efficacy.[11] In this study, we hypothesized that rTMS combined with rehabilitation training would be more beneficial for the functional recovery of language in aphasic patients.[12,13] However, the neural mechanisms of the functional recovery of language post-TMS combined with rehabilitation training in aphasia remain elusive.
Aphasic patients exhibited atypical activation patterns and altered activity in the intact homotopic language areas in the nondominant RH.[14–16] Previous studies suggest that the RH activity may be transient and partially compensatory in subacute aphasic patients, and the recruitment of RH language homologues may be beneficial for early but not late language recovery.[15,17] Thus, studies have suppressed RH activity with low-frequency rTMS, whereas other studies have excited the perilesional areas of the LH with high-frequency rTMS to enhance language performance in chronic aphasic patients; previous reports indicate that rTMS may improve the speech-language ability of aphasic patients.[2–7] Moreover, the importance of right hemispheric homologues in the recovery process remains unclear. Thus, the patient in our study underwent LH activity excitement via high-frequency rTMS. In most cases, behavior scales, such as the Western Aphasia Battery (WAB), have been used to evaluate the curative effect of rTMS.[2] However, the neural mechanisms of language recovery following rTMS treatment are not clear. To date, functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) have been used to increase our understanding of poststroke brain reorganization of language recovery.[14–18] In this case report, a 39-year-old woman with poststroke conduction aphasia received rTMS and speech rehabilitation training. The patient had received SLT 1 month after stroke, and her speech-language ability improved substantially in the first 2 months after SLT. However, she had no obvious change in speech-language ability during the 3rd and 4th months after SLT. At the end of the 4th month after SLT, the patient received rTMS treatment while continuing the SLT, and her language skills were further enhanced. In this study, fMRI and DTI were used to investigate the functional and microstructural changes before and after the rTMS treatment.
2. Case report
2.1. Participant
A 39-year-old, right-handed, Chinese woman with acute cerebral infarction in the left middle cerebral artery (see Supplemental Digital Content 1) presented to our rehabilitation clinic 1 month poststroke and was diagnosed with conduction aphasia. The patient had received speech training 1 month after stroke, her speech-language ability improved a lot in the first 2 months after SLT. However, she had no significant change in speech-language ability during 3rd and 4th months after SLT.
At the end of 4th month after SLT, the patient received rTMS treatment while continuing the SLT in our rehabilitation clinic. Before rTMS treatment, the Aphasia Quotient on the WAB-Revised was 83.2/100 (Table 1), and her performance was poor on speech-language tasks. Post-treatment, we used the Reliable Change Index (RCI) to assess whether there is a statistically meaningful change for the patient's score, the RCI defined as (X2 − X1)/SEdiff, where X2 is the post-treatment score, X1 is the pretreatment score, and SEdiff is the standard error of the difference between the 2 scores, and if RCI larger than 1.96 that would be a reliable change.[19,20]
Table 1.
Pre- and post-rTMS test scores of Western Aphasia Battery.

The experiments were conducted in accordance with the Declaration of Helsinki and the patient provided written informed consent on forms approved by the East China Normal University Committee on Human Research and the Independent Ethics Committee of Huashan Hospital.
2.2. rTMS stimulation
A 90-mm round coil stimulator (Yiruide CCY-II, Wuhan, China) was used to stimulate the left Broca area with 90% of the motor threshold, using a frequency of 5 Hz, 20 min/d, for 10 days during a 2-week period. The rTMS treatment program was similar to that described by most studies summarized in a review article.[2] The left Broca area was defined in the frontotemporal region as the crossing point between T3-Fz and F7-Cz according to the international 10 to 20 system.[21]
2.3. Speech and language therapy
Each rTMS session was immediately followed by SLT. In our rehabilitation clinic, the patient was administered SLT for 30 min/d and 5 times per week, and it was conducted one on one by a speech therapist. The SLT program was formulated based on her aphasic severity, which was evaluated after stroke and included free talk, corrections of mistakes in pronunciation, and the phonetic annotation of Chinese characters.
2.4. fMRI block design paradigm
Two speech-language tasks were performed before 1 week and after 2.5 months of rTMS treatment during the fMRI (Fig. 1).
Figure 1.

Schematic diagram of the speech-language tasks.
Phrase repetition task: The task consisted of 30 phrases, which were selected from the Western Aphasia Battery or Boston Diagnostic Aphasia Examination. In the task, the subject was asked to close her eyes naturally, listen carefully, and subsequently repeat the phrases. The paradigm consists of a 20-s rest epoch to direct attention to the task, followed by 5 blocks of each condition, which were presented in an alternating order. For the experimental condition, 6 phrases were presented pseudo-randomly in each block, and each phrase lasted 5 s. For the control condition, each block comprised a 20-s rest fixation.
Picture naming task:[22] The task consisted of 54 pictures, which were selected from the S&V database of black and white line drawings.[23] The paradigm consists of a 20-s rest epoch, following which 9 blocks of each condition were presented in an alternating order. For the experimental condition, 6 pictures were presented pseudo-randomly in each block, and each picture was presented for 4 s following by a 1-s black fixation crosshair. For the control condition, each block comprised a 20-s black fixation crosshair.
Prior to entering the scanner, the case was trained regarding the tasks (not shown during scanning). Verbal responses were recorded with a magnetic resonance imaging (MRI) compatible microphone to check the responses of the case during scanning. All stimuli were presented with the SAMRTEC SA-9900 (Shenzhen Sinorad Medical Electronics Inc., China), which determined the synchronization between the presentation and the scanner.
2.5. fMRI and DTI image acquisition
The functional and structural MRI data were acquired using a 3.0-Tesla Trio Tim system (Siemens, German). Functional MRIs were collected on 33 oblique slices (3.5 mm thick, 25% dist factor) using a  -weighted gradient echo pulse sequence, with the following acquisition parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 220 × 220 mm2, and acquisition matrix = 64 × 64. The DTI acquisition used a single-shot spin-echo echo planar imaging sequence in contiguous axial planes that covered the whole brain. The imaging parameters were set to the following values: TR = 8900 ms, TE = 86 ms, b-value = 0 and 1000 s/mm2, slice thickness = 2 mm, 70 slices, matrix = 128 × 128, FOV = 256 × 256 mm2, diff direction = 64, and the resolution = 2 × 2 × 2 mm3.
-weighted gradient echo pulse sequence, with the following acquisition parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 220 × 220 mm2, and acquisition matrix = 64 × 64. The DTI acquisition used a single-shot spin-echo echo planar imaging sequence in contiguous axial planes that covered the whole brain. The imaging parameters were set to the following values: TR = 8900 ms, TE = 86 ms, b-value = 0 and 1000 s/mm2, slice thickness = 2 mm, 70 slices, matrix = 128 × 128, FOV = 256 × 256 mm2, diff direction = 64, and the resolution = 2 × 2 × 2 mm3.
2.6. fMRI and DTI image analysis
Functional images were analyzed with statistical parametric mapping software (SPM8; http://www.fil.ion.ucl.ac.uk./spm/spm8.html) and MATLAB (The Math Works, Natick, MA) software on a personal computer. Images from the first 10 TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium. Images preprocessing included slice-timing correction, realignment of functional data to the subject's middle image, and coregistration of functional and structural images. The sessions were normalized to the Montreal Neurological Institute (MNI) stereotaxic space. Spatial smoothing was performed on the functional images using a Gaussian filter (6-mm full width half-maximum). The subject exhibited head movement <0.5 mm, regardless of rotation, and translation. In the first-level analysis, a block statistical model was constructed using a general linear model with SPM8, and 2 conditions were modeled for each task. We constructed a contrast between the experimental condition and control condition for the subject to evaluate the degree of brain activation specific for the speech-language tasks.
FSL v5.0 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and SPM8 were used to conduct the DTI data analysis. First, the DTI images were preprocessed for the correction of eddy current distortions and head motion artifacts. Fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD) maps were subsequently created in the individual space and were coregistered between the pre- and post-treatment sessions for voxel-based comparison. Finally, we calculated the mean values of these indices within 2 types of regions of interest (ROIs): the ROIs extracted from the post-treatment activation map in the tasks, which were warped to the individual space from the MNI space by applying the inverse spatial transformation in the fMRI data analysis; and the regions that exhibited apparent differences in the voxel-based FA map comparison. For location of ROIs, please see Supplemental Digital Content 2.
3. Results
3.1. Behavioral results
Table 1 provides the details regarding the Western Aphasia Battery test scores. There were almost no changes during long-term pre-rTMS, and the language function was relatively stable. However, when rTMS was applied and SLT was continued, the language ability significantly improved at 2 weeks post-TMS, and the gains were steadily increased at 2.5 months post-rTMS compared with pre-rTMS. The WAB administered at 2.5 months post-treatment indicated significant improvements in spontaneous speech, auditory comprehension, repetition, and naming compared with pre-rTMS. Specifically, there were 9.2-point significant improvements in the Aphasia Quotient (RCI = 6.84) and 3-point significant improvements in repetition (RCI = 2.60).
3.2. Functional MRI and DTI results
Table 2 and Fig. 2 provide details regarding the activity associated with the experimental condition versus the control condition for the phrase repetition task and the picture naming task before and after treatment.
Table 2.
Number of activated voxels associated with the experimental condition > control condition for the phrase repetition task and the picture naming task before and after treatment.
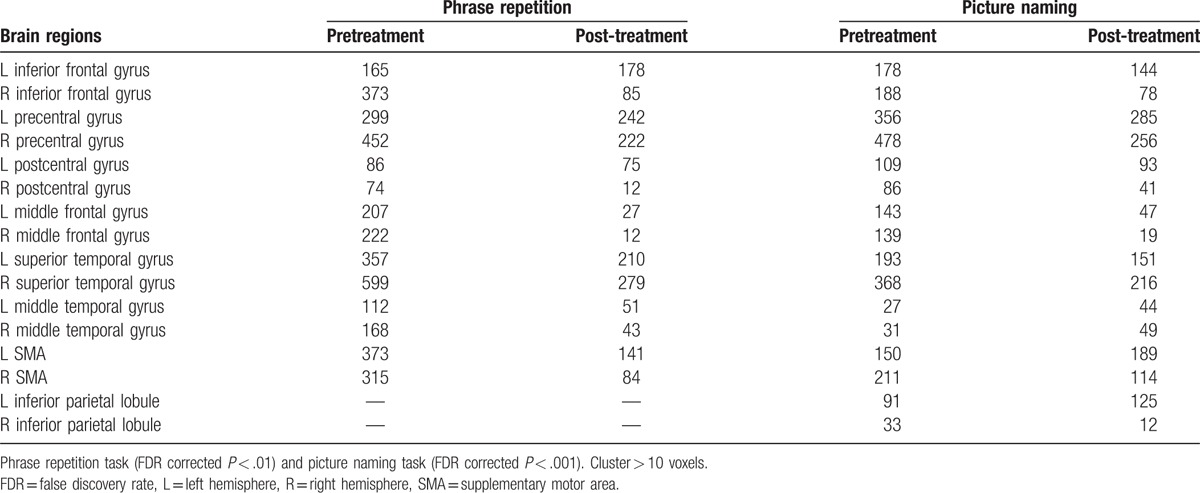
Figure 2.

Brain activation images associated with the experimental condition versus control condition for the Phrase repetition task (FDR corrected P < .01) and the Picture naming task (FDR corrected P < .001) 1 week pretreatment and 2.5 months post-treatment. At 2.5 months post-treatment, the fMRI results exhibited significant activation in the LH language areas relative to the RH, especially in the perilesional areas such as the inferior frontal gyrus (as in yellow circle 2), precentral gyrus (as in yellow circle 3), middle temporal gyrus extended to superior temporal gyrus (as in yellow circle 1), and inferior parietal lobule (as in yellow circle 4) and so on. fMRI = functional magnetic resonance imaging, LH = left hemisphere, RH = right hemisphere.
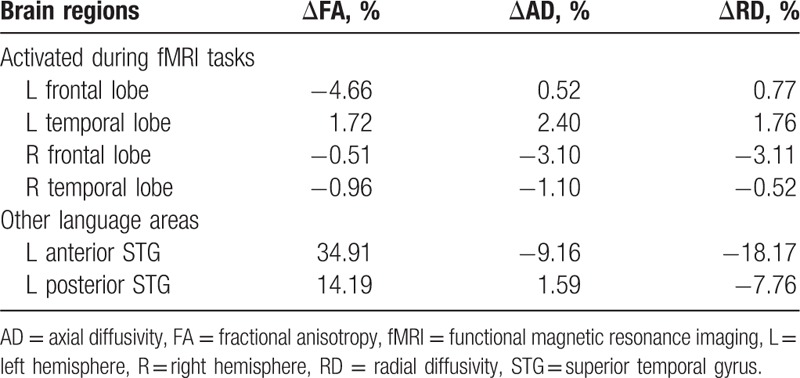
There was no obvious change (<5%) in the FA in the bilateral frontal and temporal areas, which were activated during both fMRI tasks before and after treatment. The FA increased by 34.9% in the left anterior superior temporal gyrus and by 14.2% in the left posterior superior temporal gyrus post-treatment compared with pretreatment (Table 3 and Supplemental Digital Content 2); these 2 regions comprise important areas involved in language processing.[24]
Table 3.
Location and changes post- > pretreatment in fractional anisotropy in language-related brain regions.
4. Discussion
Conduction aphasia is a relatively rare form of aphasia that is characterized by intact auditory comprehension, relatively fluent spontaneous speech, and poor speech repetition.[25,26] In this case study, the patient had no significant change in speech-language ability in the 2 months before rTMS treatment. When rTMS was applied and SLT was continued, the language ability significantly improved at 2 weeks post-TMS, and the gains were steadily increased at 2.5 months post-TMS compared with pre-TMS. The significant and reliable changes in language function, we thought that rTMS played a important role.[2–7] In addition, we could see the long-term efficacy at 2.5 months post-TMS.[9,10] Furthermore, SLT was a constant condition, which would consolidate the changes.[11] Moreover, the patient was more willing to communicate with other individuals.
rTMS modulates neural activity, which promotes changes and potential reorganization of the language networks, to improve behavior in poststroke aphasic patients.[27] Low-frequency rTMS may be used to suppress the disinhibition in the RH, which promotes the recruitment of the LH, and to improve the modulation of regions in each hemisphere.[5–7] Similarly, high-frequency rTMS excites the LH, which suggests that recruitment of the LH and a reduction in the inefficient recruitment of the RH may promote improved language functions.[3,15,18]
Our fMRI results indicated a loose and extensive activation pattern before treatment and a more focused activation pattern following rTMS and speech rehabilitation training during 2 language tasks. Furthermore, the patient exhibited significant activation pretreatment in the RH language areas, such as the inferior frontal gyrus, precentral gyrus, middle frontal gyrus, and superior temporal gyrus, compared with the LH. At 2.5 months post-treatment, the patient exhibited significant activation in a network of the LH language areas, especially the perilesional areas, such as the inferior frontal gyrus, precentral gyrus, postcentral gyrus, middle frontal gyrus, middle temporal gyrus, and inferior parietal lobule, compared with the RH. The results of the 2 speech-language tasks are consistent following rTMS and speech rehabilitation training.
We also determined that the FA increases the left superior temporal gyrus, which comprises an important area involved in language processing.[24] The increase in the FA combined with the respective changes in RD and AD suggests potential increases in the degree of myelination,[28] and thicker myelin may improve neuronal signal transduction. In our case, the rTMS adjusted the cortical excitability to induce or enhance neuroplasticity changes in brain activity,[8] and speech rehabilitation training consolidated and strengthened these changes.
5. Conclusion
This study demonstrated that rTMS combined with speech training improved the speech-language ability in a chronic conduction aphasia patient. The treatment induced language activation pattern changes and increased white matter integrity, which may reflect the recruitment of the LH and a reduction in inefficient recruitment of other brain regions through the functional reorganization and synaptic plasticity changes that were mediated by excitatory rTMS and speech training.
Supplementary Material
Footnotes
Abbreviations: AD = axial diffusivity, AQ = Aphasia Quotient, DTI = diffusion tensor imaging, FA = fractional anisotropy, FDR= false discovery rate, fMRI = functional magnetic resonance imaging, FOV = field of view, LH = left hemisphere, MNI = Montreal Neurological Institute, MRI = magnetic resonance imaging, RCI = Reliable Change Index, RD = radial diffusivity, RH = right hemisphere, ROIs = regions of interest, rTMS = repetitive transcranial magnetic stimulation, SLT = speech and language therapy, TE = echo time, TR = repetition time, WAB = Western Aphasia Battery.
Data contain patient-identifying information and sharing is restricted by the ethics committee. Data request may be sent to the corresponding author.
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor of this journal.
HZ and YC have equally contributed to this work.
HZ, RH, YW, and XD conceived and designed the experiments; YC, HZ, and HL prepared the samples and analyzed the data; MW, JZ, YW, and LY participated in interpreting and analyzing the data; HZ and XD wrote the paper.
This research was supported by grants from the National Natural Science Foundation of China (Nos. 81571658 and 81201082), and the Youth Project of Shanghai Zhabei District Health Bureau (No. 2011 QN02).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Engelter ST, Gostynski M, Papa S, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke 2006;37:1379–84. [DOI] [PubMed] [Google Scholar]
- [2].Shah PP, Szaflarski JP, Allendorfer J, et al. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Front Hum Neurosci 2013;7:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Szaflarski JP, Vannest J, Wu SW, et al. Excitatory repetitive transcranial magnetic stimulation induces improvements in chronic post-stroke aphasia. Med Sci Monit 2011;17:CR132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weiduschat N, Thiel A, Rubi-Fessen I, et al. Effects of repetitive transcranial magnetic stimulation in aphasic stroke: a randomized controlled pilot study. Stroke 2011;42:409–15. [DOI] [PubMed] [Google Scholar]
- [5].Abo M, Kakuda W, Watanabe M, et al. Effectiveness of low-frequency rTMS and intensive speech therapy in poststroke patients with aphasia: a pilot study based on evaluation by fMRI in relation to type of aphasia. Eur Neurol 2012;68:199–208. [DOI] [PubMed] [Google Scholar]
- [6].Hamilton RH, Sanders L, Benson J, et al. Stimulating conversation: enhancement of elicited propositional speech in a patient with chronic non-fluent aphasia following transcranial magnetic stimulation. Brain Lang 2010;113:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ren CL, Zhang GF, Xia N, et al. Effect of low-frequency rTMS on aphasia in stroke patients: a meta-analysis of randomized controlled trials. PLoS ONE 2014;9:e102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul 2010;3:95–118. [DOI] [PubMed] [Google Scholar]
- [9].Barwood CH, Murdoch BE, Riek S, et al. Long term language recovery subsequent to low frequency rTMS in chronic non-fluent aphasia. NeuroRehabilitation 2013;32:915–28. [DOI] [PubMed] [Google Scholar]
- [10].Barwood CH, Murdoch BE, Whelan BM, et al. Improved language performance subsequent to low-frequency rTMS in patients with chronic non-fluent aphasia post-stroke. Eur J Neurol 2011;18:935–43. [DOI] [PubMed] [Google Scholar]
- [11].Wang CP, Hsieh CY, Tsai PY, et al. Efficacy of synchronous verbal training during repetitive transcranial magnetic stimulation in patients with chronic aphasia. Stroke 2014;45:3656–62. [DOI] [PubMed] [Google Scholar]
- [12].Koganemaru S, Fukuyama H, Mima T. Two is more than one: how to combine brain stimulation rehabilitative training for functional recovery? Front Syst Neurosci 2015;9:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yoon TH, Han SJ, Yoon TS, et al. Therapeutic effect of repetitive magnetic stimulation combined with speech and language therapy in post-stroke non-fluent aphasia. NeuroRehabilitation 2015;36:107–14. [DOI] [PubMed] [Google Scholar]
- [14].Eaton KP, Szaflarski JP, Altaye M, et al. Reliability of fMRI for studies of language in post-stroke aphasia subjects. NeuroImage 2008;41:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Winhuisen L, Thiel A, Schumacher B, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke 2005;36:1759–63. [DOI] [PubMed] [Google Scholar]
- [16].Szaflarski JP, Eaton K, Ball AL, et al. Poststroke aphasia recovery assessed with functional magnetic resonance imaging and a picture identification task. J Stroke Cerebrovasc Dis 2011;20:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saur D, Lange R, Baumgaertner A, et al. Dynamics of language reorganization after stroke. Brain 2006;129:1371–84. [DOI] [PubMed] [Google Scholar]
- [18].Allendorfer JB, Storrs JM, Szaflarski JP. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci 2012;30:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–9. [DOI] [PubMed] [Google Scholar]
- [20].Leo A, De Luca R, Russo M, et al. Role of tDCS in potentiating poststroke computerized cognitive rehabilitation: lessons learned from a case study. Appl Neuropsychol Adult 2016;23:162–6. [DOI] [PubMed] [Google Scholar]
- [21].Friederici AD, Hahne A, von Cramon DY. First-pass versus second-pass parsing processes in a Wernicke's and a Broca's aphasic: electrophysiological evidence for a double dissociation. Brain Lang 1998;62:311–41. [DOI] [PubMed] [Google Scholar]
- [22].Martin PI, Naeser MA, Ho M, et al. Overt naming fMRI pre- and post-TMS: two nonfluent aphasia patients, with and without improved naming post-TMS. Brain Lang 2009;111:20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol 1980;6:174–215. [DOI] [PubMed] [Google Scholar]
- [24].Skeide MA, Friederici AD. The ontogeny of the cortical language network. Nat Rev 2016;17:323–32. [DOI] [PubMed] [Google Scholar]
- [25].Tomasino B, Marin D, Maieron M, et al. A multimodal mapping study of conduction aphasia with impaired repetition and spared reading aloud. Neuropsychologia 2015;70:214–26. [DOI] [PubMed] [Google Scholar]
- [26].Ardila A. A review of conduction aphasia. Curr Neurol Neurosci Rep 2010;10:499–503. [DOI] [PubMed] [Google Scholar]
- [27].Andoh J, Martinot JL. Interhemispheric compensation: a hypothesis of TMS-induced effects on language-related areas. Eur Psychiatry 2008;23:281–8. [DOI] [PubMed] [Google Scholar]
- [28].Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 2002;17:1429–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


