Abstract
The aim of this study was to explore the effect of clomiphene citrate (CC) on the cycle characteristics and outcomes of obese women with polycystic ovarian syndrome (PCOS) undergoing ovarian stimulation for in vitro fertilization (IVF).
This is a retrospective cohort study, and it was conducted at the tertiary-care academic medical center.
This study included 174 obese PCOS patients undergoing IVF.
In the study group (n = 90), CC and human menopausal gonadotropin (HMG) were administered simultaneously beginning on cycle day 3, while in control group (n = 84) HMG was used only. Both of the 2 groups used medroxyprogesterone acetate (MPA) for preventing premature luteinizing hormone (LH) surges. Ovulation was cotriggered by a GnRH agonist and hCG when dominant follicles matured.
The primary outcome measure was the number of oocytes retrieved. Secondary outcomes included the number of top-quality embryos, maturation rate, fertilization rate, cleavage rate, incidence of premature LH surge, and OHSS.
The study group received obviously lower total HMG dose [1650 (975–4800) vs 2025 (1350–3300) IU, P = 2.038E–4] but similar HMG duration. While the antral follicle count (AFC) is higher in study group, the number of oocytes retrieved and top-quality embryos are remarkably less [5 (0–30) vs 13 (0–42), P = 6.333E–5; 2 (0–14) vs 3.5 (0–15), P = .003; respectively]. The mature oocyte rate is higher in study group (P = .036). No significant differences were detected in fertilization rate and cleavage rate between 2 groups.
CC has a positive influence on cycle characteristics, but might be correlated with the impaired IVF outcomes (less oocytes retrieved and top quality embryos, lower oocyte retrieval rate) in obese PCOS patients undergoing IVF, when HMG and MPA are used simultaneously.
Keywords: clomiphene citrate, IVF, obesity, ovarian stimulation, PCOS
1. Introduction
Polycystic ovary syndrome (PCOS) is a common female endocrine-metabolic disorder of reproductive age, characterized by a collection of manifestations, including hyperandrogenism, hypersecretion of LH, hyperinsulinemia, menstrual cycle irregularity, and infertility.[1,2] Among all of the features, obesity stands out because of its epidemic proportions in women with PCOS, with a prevalence of 74% in 2000 to 2002 as revealed by a US study.[3] Compared with lean-type patients, obese PCOS patients have greater risks of anovulation and infertility, as well as lower pregnancy rates even after treatment.[4]
Treatment of infertility in women with PCOS consists of lifestyle modification and use of clomiphene citrate (CC), exogenous gonadotropins, laparoscopic ovarian surgery, and in vitro fertilization (IVF).[5] Among these approaches, CC, the first-line option for ovulation induction (OI), has an advantage of low cost and easy administration. It is supposed to displace endogenous estrogen from hypothalamic estrogen receptor sites and block the negative feedback exerted by endogenous estrogen, resulting in a favorable increase in gonadotropin-releasing hormone (GnRH) secretion.[6,7] Adverse effects of CC over the endometrium and cervical mucus may decrease the pregnancy rates in OI and fresh embryo transfers, which has little to do with the pregnancy rates in frozen-thawed embryo transplantation (FET).
IVF is considered the third-line treatment, usually chosen when tubal factors and male factors are taken into account. Although infertile women with PCOS may typically present with increasing oocytes retrieved in IVF cycles, most oocytes are of poor quality, leading to lower maturation, fertilization, cleavage rate, and less top-quality embryos.[8] Up to now, a series of studies focused on controlled ovarian hyperstimulation (COH) protocols for PCOS patients have been carried out. Some are about the comparison of GnRH agonist long protocol and GnRH antagonist protocol, all of which lead to the conclusion that there is no difference in the pregnancy rate between the 2 protocols, while GnRH antagonist protocol shows a lower OHSS incidence.[9–11] A modified ultralong agonist protocol is recommended by another scholar in 2014, taking it as a novel but beneficial protocol for PCOS patients with a high body mass index (BMI) status.[12] However, it remains a controversial problem that which one is optimal, especially for obese PCOS patients. To our knowledge, few studies reported focus on the effect of CC on PCOS patients in IVF, as CC would not take effect in traditional protocols using GnRH analogue for downregulation, let alone obese PCOS, one of the most difficult type to treat.
Recently, medroxyprogesterone acetate (MPA) is reported to be an effective alternative for preventing premature LH surges in women undergoing COH in IVF.[13] It has been proved that using MPA cotreatment with gonadotropin during COH has similar IVF outcomes compared with short protocol. That means MPA can be used as a powerful medicine instead of GnRH analogue for suppressing premature LH surge. Nevertheless, little research has been published yet for applying this novel protocol to obese PCOS patients so far.
Different from the data reported by the US mentioned above, Asian women are usually much thinner with lower BMI, which presents with a small proportion of obesity, even in PCOS patients. However, given that the female obesity impairs IVF cycle characteristics and outcomes, including the need for higher doses of gonadotropins, fewer oocytes collected, as well as higher cancellation rate, obese PCOS patients, who are prone to be infertile and getting OHSS, are selected as the objectives of this study. And this research, focused on COH protocol for these obese PCOS, is surely of great importance. CC, a preferred OI drug and generally used in OI, is first used in obese PCOS women undergoing IVF in this study and hypothesized to have an effect on the IVF outcomes by adjuvant treatments. Hence, the aim of this retrospective study is to compare the cycle characteristics and IVF outcomes between 2 ovarian stimulation protocols in obese PCOS patients undergoing COH in IVF cycles: using CC and HMG together or using HMG alone for ovarian stimulation, both in conjunction with MPA to prevent premature LH surge.
2. Methods
2.1. Study settings and patients
This is a retrospective observational cohort study carried out at the Department of Assisted Reproduction of the Ninth People's Hospital of Shanghai JiaoTong University School of Medicine (Shanghai, People's Republic of China). In the study, which was approved by the hospital's ethic committee, PCOS patients who received IVF or intracytoplasmic sperm injection (ICSI) treatment between November 2014 and May 2016 were recruited. To avoid repeated inclusion, if a patient underwent more than 1 COH cycle during this period, then only the first cycle can be included. The diagnosis of PCOS was made according to the 2003 Rotterdam consensus, in which at least 2 out of 3 following criteria should be met after exclusion of the other causes for hyperandrogenism: oligo-ovulation and/or anovulation, clinical and/or biochemical signs of hyperandrogenism, and polycystic ovarian morphology by ultrasonography. As PCOS is considered a diagnosis of exclusion, it should be differential diagnosed with following diseases: nonclassic congenital adrenal hyperplasia, primary hypothyroidism, hyperprolactinemia, Cushing syndrome, virilizing adrenal or ovarian tumors, and so on.[14–16]
The inclusion criteria were as follows: PCOS women, undergoing mild ovarian stimulation protocol (HMG+MPA or HMG+CC+MPA), age between 24 and 38 years old, BMI between 25 and 33 kg/m2, infertility duration less than 10 years, normal ovarian reserve (basal FSH <10 IU/L), no IVF-ICSI history or no more than 3 unsuccessful cycles previously (cycles with no viable embryos), and IVF indications being tubal factors or male factors or repeated failure of OI (equal or more than 6 times) or repeated failure of intrauterine insemination (IUI, equal or more than 2 times).
Any other infertility factors other than the indications mentioned above were considered the exclusion criteria of the study, such as poor ovarian reserve or endometriosis grade 3 or higher. Contraindications to ovarian stimulation treatment such as severe systemic disease were also the criteria for exclusion.
The final data, in the foregoing time frame, involved a total of 174 patients meeting the criteria. These patients were divided into 2 groups according to whether CC was used besides HMG and MPA in the course of ovarian stimulation: 84 IVF cycles of HMG+MPA protocol and 90 IVF cycles of HMG+MPA+CC protocol.
2.2. Procedures
Patients were first evaluated clinically for BMI and evidence of hyperandrogenism. Then, on day 3 of their menstrual cycle, a transvaginal ultrasound (TVS) was performed for antral follicle count (AFC), and serum FSH, LH, E2, P, and T were assayed. From menstrual cycle 3 (MC3) onwards, patients in the control group were administrated HMG (Anhui Fengyuan Pharmaceutical Co., China) 225 IU/d and MPA 10 mg/d, while study group patients were given CC (Fertilan; Codal-Synto Ltd., France) 50 mg/d, HMG 225IU/d as well as MPA 10 mg/d.
For both the 2 groups, cycle monitoring started on MC7–8 and from then on performed every 2 to 4 days to adjust the HMG dose according to the development of the follicles. And for each monitoring, not only the number and the size of follicles were recorded by TVS, but also serum FSH, LH, E2, P, and T concentrations were measured on the same days. Once at least 1 dominant follicle reached 20 mm in diameter or 3 dominant follicles reached 18 mm in diameter, the final stage of oocyte maturation was induced by triptorelin (0.1 mg; Decapeptyl; Ferring GmbH, Germany) in conjunction with HCG (5000 IU; Lizhu Pharmaceutical Trading Co., China), in case that the LH response was not so optimal when using triptorelin only. TVS-guided oocyte retrieval was conducted 34 to 38 hours after trigger. All follicles with diameters of more than 10 mm were tried to be retrieved.
On the basis of semen parameters, either conventional insemination or ICSI was used for fertilization, which was carried out in vitro. Examinations for the number and regularity of blastomeres and the degree of embryonic fragmentation were then performed on the embryos on day 3, in which a score for the embryo was given according to Cummins criteria. On the same day of examination, all highest quality embryos (including grade 1 and grade 2 8-cell blastomere embryos) were frozen by vitrification. The rest of the embryos that were not so ideal of quality were placed in extended culture to day 5 or day 6 until the blastocyst stage, among which only good-morphology blastocysts were selected to freeze.
2.3. Statistical analysis
The primary outcome measure was the number of oocytes retrieved. The secondary measures included the number of top-quality oocytes, maturation rate, fertilization rate, cleavage rate, incidence of premature LH surge, and incidence of OHSS. Fertilization was defined as 2PN or 0PN embryos on day 1 observation after conventional insemination or ICSI. The oocyte retrieval rate was calculated by dividing the number of oocytes retrieved totally by the number of follicles with diameters larger than 10 mm on trigger day. The rate of fertilization/cleavage was calculated by dividing the number of fertilized/cleavaged embryos of the whole group by the total number of mature/fertilized oocyte. The criterion for cycle cancellation was no viable embryos for cryopreservation.
Statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL). Data were presented as means ± SD if they demonstrated normal distributions, or as medians (ranges) for non-normal distributions; qualitative data were presented as percentages. Different kinds of continuous parametric data were analyzed by different means: Student t test was used to compare the means and Mann–Whitney U tests used to compare the medians. Comparisons of rates between 2 groups were completed by χ2-test. P < .05 was considered statistically significant.
3. Results
As is summarized in Table 1, most of the basic characteristics of the 2 groups are comparable to each other. No statistical differences could be found regarding the values of age, BMI, duration of infertility, basal serum hormone (basal FSH, LH, E2, P, T), and basal LH/FSH ratio. Both groups had comparable proportions of primary or secondary infertility. And for the previous IVF history, 88.10% (74/84) of HMG+MPA group (control group) and 91.11% (82/90) of the HMG+MPA+CC group (study group) had no previous failed IVF/ICSI experience. The only existing difference of the baseline features was the AFC, for which study group was higher than control group (20 vs 17.5, P = .002).
Table 1.
The basic characteristics of women in the trial undergoing IVF/ICSI treatment.
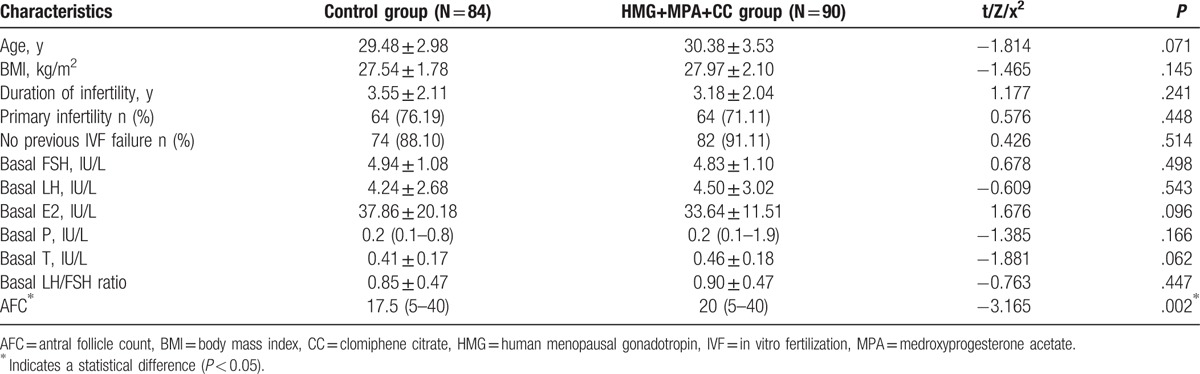
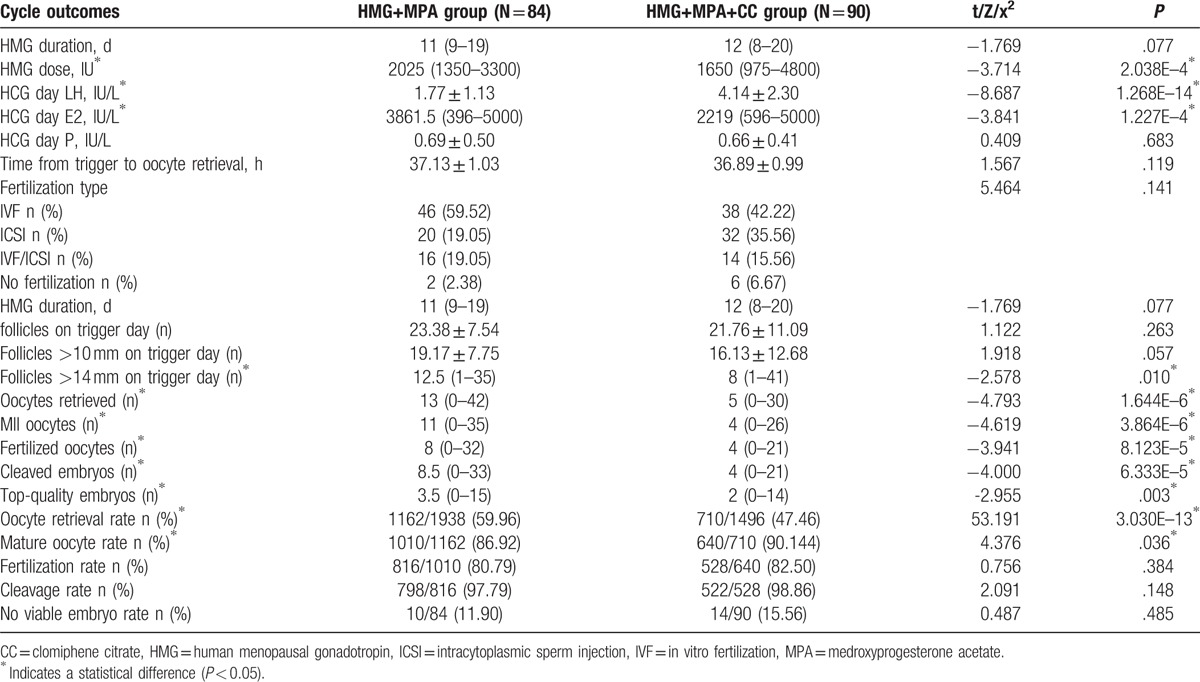
Table 2 describes the cycle characteristics and outcomes of COH treatment in both groups. Compared with HMG+MPA group, the HMG+MPA+CC group received obviously lower total HMG dose [1650 (975–4800) vs 2025 (1350–3300) IU, P = 2.038E–4] but comparable HMG stimulation duration [11 (9–19) vs 12 (8–20) days, P = .077]. The number of follicles with diameters larger than 14 mm on trigger day was higher in control group [12.5 (1–35) vs 8 (1–41), P = .01], while the follicles with diameters larger than 10 mm were similar between 2 groups. HCG day E2 was lower in HMG+MPA+CC group than in HMG+MPA group, but HCG day LH was higher and HCG day P was comparable (3861.5 vs 2219, P = 1.227E–4; 1.77 ± 1.13 vs 4.14 ± 2.30, P = 1.268E–14; 0.69 ± 0.50 vs 0.66 ± 0.41, P = .683; respectively). Comparing HMG+MPA group with HMG+MPA+CC group, the number of oocytes retrieved was distinctly higher [13 (0–42) vs 5 (0–30), P = 1.644E–6] with the time from trigger to oocyte retrieval similar. Although the study group showed a higher maturation rate, the number of mature oocytes, fertilized oocytes and cleaved embryos were all higher in HMG+MPA group [11 (0–35) vs 4 (0–26), P = 3.864E–6; 8 (0–32) vs 4 (0–21), P = 8.123E–5; 8.5 (0–33) vs 4 (0–21), P = 6.333E–5; respectively]. The number of top-quality embryos was statistically more in control group than in study group [3.5 (0–15) vs 2 (0–14), P = .003]. No significant differences were detected in fertilization type, fertilization rate, and cleavage rate between the 2 groups.
Table 2.
The cycle outcomes of controlled ovarian stimulation in 2 protocols.
In the study, 10 out of 84 women in control group and 14 out of 90 women in the study group had no viable embryos, as they had no oocytes retrieved, no oocytes fertilized, or poor-quality embryos. Therefore, these cycles were assigned to cancelled cycles. In the whole process of COH, no case of premature LH surge or moderate to severe OHSS happened.
4. Discussion
The present study evaluated comparative efficacy of 2 COH protocols (HMG+MPA protocol and HMG+MPA+CC protocol) in infertile obese women with PCOS. Considering the figures of Asian women, it is not easy to recruit such a number of obese patients, with the BMI of 25 to 33 kg/m2. In addition, the majority of PCOS patients achieved pregnancy through OI in our center, which leaves the obese PCOS women undergoing IVF much less. So, the sample size in this study seems to be limited, but the data of the 174 patients are still so precious. Another thing is that both of the 2 COH regimens used MPA to prevent premature LH surge in replacement of conventional GnRH analogue, which is a novel protocol not so widely used but suitable for PCOS for its low incidence of OHSS. The result of the study provides the first-time evidence that CC, as an adjuvant to stimulate, has a positive influence on cycle profiles and might be correlated with the impaired IVF outcomes in obese PCOS patients undergoing IVF treatment.
In our study, when the basic characteristics between groups are comparable, CC cotreatment with HMG on the basis of MPA priming has significantly reduced the total dose of HMG. This result is consistent with researches performed in recent years. Ghanem et al in 2013 made a comparison of CC cotreatment with low-dose urinary FSH (uFSH) and uFSH alone in the process of OI for CC resistant PCOS, and reached a conclusion that CC reduced both uFSH duration and uFSH dose.[17] Similar results have been reported in 2015 by a prospective study conducted between CC + HMG group and HMG-only group.[18] Even as early as a decade ago, it had been shown, by a nonrandomized study, that the average ampoules of HMG required was decreased by 65% when concomitant use of CC with HMG was employed.[19] All of these results give support to the preference of CC cotreatment with HMG protocol for its lightening patients’ financial burden and cost-effective benefit. Besides getting patients free from the inconvenience and pain resulting from daily injection is another advantage of it. It must be emphasized that patients in our study belonged to the obese-type PCOS, a particularly troublesome group to treat because of the already known large dose of gonadotropins they needed for ovarian stimulation.[20] So, this finding is of great importance and meets the urgency to find an approach to overcome such a problem.
However, it cannot be neglected that the decrease of HMG duration in our study has not reached a statistical significance. It can be explained partially by the discrepancy of treatment goals between our study and the above-mentioned investigations, namely COH in IVF and merely OI. In detail, it is known that the chronic low-dose step-up protocol has become the standard one in OI treatment, which is aimed at mono-follicle development and avoiding OHSS and multiple pregnancies.[21,22] Meanwhile, COH is devoted to inducing as many as follicles to develop within a certain range, so the initial and throughout daily HMG dose is larger than OI treatment, leading to the narrowed difference of stimulating duration between groups. In other words, the indistinctive effect of CC on HMG duration is compensated by relatively higher dose of HMG. The difference of HMG dose used in OI and COH makes it unreasonable to compare between our results and previous ones. Another possible reason is that our study is not large enough to attain statistical significance in HMG duration.
Moreover, it should be mentioned that in our study, in situation that the AFC is less in HMG+MPA group, the follicles with diameters larger than 14 mm on trigger day and the oocytes retrieved are significantly more than those in HMG+MPA+CC group, which is beyond expectation. This paradoxical result agrees well with the striking higher oocytes retrieval rate in control group. In fact, similar discoveries have been referred to in some literatures previously, but not highlighted due to the different primary points focused on. Ghanem et al in 2013 found that in PCOS patients undergoing OI, while there was no difference in large follicles (≥16 mm), the number of medium-sized follicles (12–15 mm) was 1.8 ± 2.03 in uFSH-only group compared with 1.1 ± 0.98 when using CC coadministered with uFSH.[17] This part of follicles may not be the targeted ones in OI treatment, but are potential to be retrieved after COH in IVF treatment. Hence, the fact that gonadotropins yield more medium-sized follicles gives a possible explanation for the contradiction between AFC and oocytes retrieved.
Theoretically, attempts are made for explaining such a result from several aspects. First, as is known to all, approximately 25% of women with PCOS will not respond adequately to CC and are classified to the group of “CC resistance,”[23] which is termed as failure of ovulation with CC treatment at a maximum dosage.[6] Risk factors for CC resistance include obesity, insulin resistance, hyperandrogenism, and older age.[24,25] That is to say, patients in our study, all of whom are obese, have a greater tendency to be CC resistant. From this perspective, it provides a possible reason why patients cotreated with CC have not presented a superior result. Second, some studies indicate that CC has an inhibiting effect on ovaries in COH by making the ovary insensitive and unresponsive to gonadotropins.[26] The third point we should pay attention to is that the percentage of relatively small follicles (with diameters larger than 10 mm and less than 14 mm) on trigger day is higher in HMG+MPA+CC group. This kind of follicle has less of a chance to be retrieved in light of its unsatisfactory size. Given the mechanism of CC, the effect of it may not as direct as HMG, for it takes effect by inducing a discharge of endogenous gonadotropins from the anterior pituitary.[27] And the use of less gonadotropins leads to less middle-sized follicles. However, these are just hypotheses, needing to be verified by further experiments.
One distinctive difference of endocrine characteristics is the higher LH level on trigger day in CC group. According to a study, women with a regular menstrual cycle present an increased LH frequency rather than amplitude when treated with CC, probably by inducing an increase in the frequency of GnRH secretion.[28] It is also believed that there is a LH increase in absence of the hypothalamic participation, resulting from the direct effect of CC on pituitary.[26] Other researchers, on the contrary, hold the opinion that when administered to PCOS patients, CC mainly increased GnRH amplitude, but not frequency, which is already elevated in these patients.[7] Although disputed, something in common still exists among these views that there is an increase of LH in CC treatment, which is in consistence with our results.
There is an ongoing debate about the effect of high level of LH on the outcomes of IVF. A general belief is that high LH levels have been associated with significant decreases in oocyte maturation rate, fertilization rate, and impaired embryo quality.[29–31] Exposure of the ovaries to high concentrations of LH during phase of follicular growth seems to be deleterious to the developing oocytes. However, on the contrary, LH is essential in the development of follicles, especially playing an important role in the production of estrogen and maturation of oocytes.[32] Thus, a “threshold” and “ceiling” level for LH (therapeutic window) is proposed, below which E2 production is not adequate and above which LH may be detrimental to follicular development.[33]
Considering the hypersecretion of LH during the follicular phase in PCOS patients, along with the higher LH level presented in the group treated with CC, it seems that HMG+MPA+CC group will have a poor performance in oocyte maturation and fertilization. Nevertheless, this indication conforms not so well to our results, which shows no decrease in oocyte maturation rate and fertilization rate in CC cotreatment group.
Possible interpretations for this conflict can be searched from following aspects. First, several literatures have reported some gene mutations of LH and LH receptor, which are associated with the high LH level in PCOS patients. In other words, such patients may be LH-dependent in the development and maturation of oocyte.[34–36] Second, a retrospective study in 2014 conducted in our center in China gave evidence to this hypothesis. It makes a conclusion that for women with PCOS, the high LH levels in mild ovarian stimulation protocol do not mean impaired quality of oocytes and decreased fertilization rate.[37] For these reasons, high-quality embryos still can be obtained with the use of CC, even if resulting in an elevated LH.
What is worth mentioning is that there is not even one case of premature LH surge or OHSS in our study. Compared with conventional COH protocol using GnRH analogue, it is generally acknowledged that mild ovarian stimulation protocol has a remarkable advantage in decreasing the incidence of OHSS.[38,39] However, the premature LH surge is a long-lasting but urgent problem needing to be solved in this protocol. By using MPA, an oral alternative to progesterone with the function of blocking the premature LH surge, this disturbing problem is settled in our study.[13]
One weakness in this trail is the lack of another control group of traditional COH protocol, such as GnRH agonist protocol or GnRH antagonist protocol, through which we can compare the efficacy of mild ovarian stimulation and conventional COH protocol at the same time. The other limitation is that it is a retrospective study with a relatively small sample, though we strictly executed this study according to good clinical practice guidelines. Hence, a large sample randomized controlled trial is necessary to be performed in the future, not only for providing more evidence about the difference of the 2 protocols but also for finding out the optimal COH protocol for obese PCOS patients.
5. Conclusion
This retrospective observational study made a comparison of 2 COH protocols for obese PCOS patients, both of which belong to the mild ovarian stimulation protocol. Results showed that CC reduced the total dose of HMG, when cotreatment with HMG on the basis of MPA priming. This protocol is more cost-effective and well tolerated than HMG+MPA protocol. However, patients received CC in COH presented with less oocytes retrieved, lower oocyte retrieval rate, and less top-quality embryos. Considering the comparable oocyte maturation rate and fertilized rate, the higher LH caused by CC should have no obviously detrimental effect on the oocyte quality. So, there is always a tradeoff between the convenient and affordable COH and the unsatisfactory IVF outcomes. Further blind randomized controlled trials remain to be conducted to confirm the feasibility of CC used in combination with HMG and MPA in obese PCOS patients undergoing IVF.
Footnotes
Abbreviations: AFC = antral follicle count, BMI = body mass index, CC = clomiphene citrate, COH = controlled ovarian hyperstimulation, FET = frozen-thawed embryo transplantation, GnRH = gonadotropin-releasing hormone, HMG = human menopausal gonadotropin, ICSI = intracytoplasmic sperm injection, IVF = in vitro fertilization, LH = luteinizing hormone, MC = menstrual cycle, MPA = medroxyprogesterone acetate, OI = ovulation induction, PCOS = polycystic ovarian syndrome, TVS = transvaginal ultrasound.
Shanghai Three-year Plan on Promoting TCM Development (ZY3-LCPT-2-2006 to Y.K.)
The authors disclose no conflicts of interest.
References
- [1].Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333:853–61. [DOI] [PubMed] [Google Scholar]
- [2].Rojas J, Chavez M, Olivar L, et al. Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med 2014;10:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction 2010;140:347–64. [DOI] [PubMed] [Google Scholar]
- [5].Tarlatzis B, Fauser B, Legro R. Thessaloniki ESHRE/ASRM-Sponsored PCOS consensus workshop group (2008), consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 2008;23:462–77. [DOI] [PubMed] [Google Scholar]
- [6].Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update 1997;3:359–65. [DOI] [PubMed] [Google Scholar]
- [7].Kettel LM, Roseff SJ, Berga SL, et al. Hypothalamic-pituitary-ovarian response to clomiphene citrate in women with polycystic ovary syndrome. Fertil Steril 1993;59:532–8. [PubMed] [Google Scholar]
- [8].Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update 2011;17:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Orvieto R, Meltcer S, Homburg R, et al. What is the preferred GnRH analogue for polycystic ovary syndrome patients undergoing controlled ovarian hyperstimulation for in vitro fertilization? Fertil Steril 2009;91:1466–8. [DOI] [PubMed] [Google Scholar]
- [10].Lainas TG, Sfontouris IA, Zorzovilis IZ, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: a prospective randomised controlled trial (RCT). Hum Reprod 2010;25:683–9. [DOI] [PubMed] [Google Scholar]
- [11].Xiao J, Chen S, Zhang C, et al. Effectiveness of GnRH antagonist in the treatment of patients with polycystic ovary syndrome undergoing IVF: a systematic review and meta analysis. Gynecol Endocrinol 2013;29:187–91. [DOI] [PubMed] [Google Scholar]
- [12].Tu J, Lin G, Lu C, et al. A novel modified ultra-long agonist protocol improves the outcome of high body mass index women with polycystic ovary syndrome undergoing IVF/ICSI. Gynecol Endocrinol 2014;30:209–12. [DOI] [PubMed] [Google Scholar]
- [13].Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril 2015;104:62–70. [DOI] [PubMed] [Google Scholar]
- [14].Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2013;98:4565–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alcalay M, Bider D, Lipitz S, et al. Polycystic ovarian syndrome. Med Clin N Am 2015;99:221–35.25456652 [Google Scholar]
- [16].Qiao J, Li R. The characteristics and antidiastole of PCOS hyperandrogenism. J Pract Obstet Gynecol 2005;21:524–6. [Google Scholar]
- [17].Me G, La E, Hassan M, et al. Clomiphene citrate co-treatment with low dose urinary FSH versus urinary FSH for clomiphene resistant PCOS: randomized controlled trial. J Assist Reprod Genet 2013;30:1477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xi W, Liu S, Mao H, et al. Use of letrozole and clomiphene citrate combined with gonadotropins in clomiphene-resistant infertile women with polycystic ovary syndrome: a prospective study. Drug Design Develop Ther 2015;9:6001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dickey RP, Olar TT, Taylor SN, et al. Sequential clomiphene citrate and human menopausal gonadotrophin for ovulation induction: comparison to clomiphene citrate alone and human menopausal gonadotrophin alone. Hum Reprod 1993;8:56–9. [DOI] [PubMed] [Google Scholar]
- [20].Hamilton-Fairley D, Kiddy D, Waston H, et al. Association of moderate obesity with a poor pregnancy outcome in women with polycystic ovary syndrome treated with low dose gonadotrophin. Br J Obstet Gynaecol 1992;99:128–31. [DOI] [PubMed] [Google Scholar]
- [21].Hamilton-Fairley D, Kiddy D, Waston H, et al. Low-dose gonadotrophin therapy for induction of ovulation in 100 women with polycystic ovary syndrome. Hum Reprod 1991;6:1095–9. [DOI] [PubMed] [Google Scholar]
- [22].Homburg R, Hendriks ML, Konig TE, et al. Clomifene citrate or low-dose FSH for the first-line treatment of infertile women with anovulation associated with polycystic ovary syndrome: a prospective randomized multinational study. Hum Reprod 2012;27:468–73. [DOI] [PubMed] [Google Scholar]
- [23].Mitwally MFm, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril 2001;75:305–9. [DOI] [PubMed] [Google Scholar]
- [24].Imani B, Eijkemans MJ, te Velde ER, et al. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab 1998;83:2361–5. [DOI] [PubMed] [Google Scholar]
- [25].Shokeir T, El-Kannishy G. Rosiglitazone as treatment for clomiphene citrate-resistant polycystic ovary syndrome: factors associated with clinical response. J Womens Health 2008;17:1445–52. [DOI] [PubMed] [Google Scholar]
- [26].Adashi EY. Clomiphene citrate: mechanism(s) and site(s) of action: a hypothesis revisited. Fertil Steril 1984;42:331–44. [DOI] [PubMed] [Google Scholar]
- [27].Homburg R. Clomiphene citrate: end of an era? A mini-review. Hum Reprod 2005;20:2043–451. [DOI] [PubMed] [Google Scholar]
- [28].Kerin JF, Liu JH, Phillipou G, et al. Evidence for a hypothalamic site of action of clomiphene citrate in women. J Clin Endocrinol Metab 1985;61:265–8. [DOI] [PubMed] [Google Scholar]
- [29].Adams J, Polson DW, Abdulwahid N, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet 1985;2:1375–9. [DOI] [PubMed] [Google Scholar]
- [30].Urman B, Tiras B, Yakin K. Assisted reproduction in the treatment of polycystic ovarian syndrome. Reprod Biomed Online 2004;8:419–30. [DOI] [PubMed] [Google Scholar]
- [31].Shoham Z, Borenstein R, Lunenfeld B, et al. Hormonal profiles following clomiphene citrate therapy in conception and nonconception cycles. Clin Endocrinol 1990;33:271–8. [DOI] [PubMed] [Google Scholar]
- [32].Chappel SC, Howles C. Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Hum Reprod 1991;6:1206–12. [DOI] [PubMed] [Google Scholar]
- [33].Shoham Z. The clinical therapeutic window for luteinizing hormone in controlled ovarian stimulation. Fertil Steril 2002;77:1170–7. [DOI] [PubMed] [Google Scholar]
- [34].Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev 2013;21:551–83. [DOI] [PubMed] [Google Scholar]
- [35].Liu N, Ma Y, Wang S, et al. Association of the genetic variants of luteinizing hormone, luteinizing hormone receptor and polycystic ovary syndrome. Reprod Biol Endocrinol 2012;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gielen SCJP, Hoon B, Ljpma A, et al. LH regulated gene expression in the ovary of PCOS women, a role for PEG3 and TCF21. Fertil Steril 2013;100:S361. [Google Scholar]
- [37].Wen HE, Kuang YP. Effects of high serum LH levels during mild ovarian stimulation protocol on IVF outcome of patients with polycystic ovary syndrome: a matched case-control study. J Reprod Med 2014;23:214–8. [Google Scholar]
- [38].Zhang JJ, Merhi Z, Yang M, et al. Minimal stimulation IVF vs conventional IVF: a randomized controlled trial. Am J Obstet Gynecol 2016;214:96e1–8. [DOI] [PubMed] [Google Scholar]
- [39].Shrestha D, La X, Feng HL. Comparison of different stimulation protocols used in in vitro fertilization: a review. Ann Transl Med 2015;3:137. [DOI] [PMC free article] [PubMed] [Google Scholar]


