Abstract
Evidence from observational studies shows that hypertension may be a risk factor for knee osteoarthritis (OA). However, the relationship between hypertension and knee OA risk remains controversial. This study aimed to quantitatively assess the relationship between hypertension and risk of knee OA.
Three electronic databases (PubMed, Embase, and Cochrane Library) were searched up to July 25, 2016. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were extracted from the included observational studies. Publication bias, heterogeneity test, and subgroup analyses were performed.
Eight studies including 2 cohort studies and 6 cross-sectional studies with 9762 participants were finally included in this meta-analysis. The results showed that hypertension was significantly associated with higher radiographic knee OA and symptomatic knee OA risks of 2.01 (95% CI, 1.28–3.15, I2 = 90.2%, P for heterogeneity <.001) and 1.49 (95% CI, 1.26–1.77, I2 = 0%, P for heterogeneity <.412), respectively. No publication bias was detected. The subgroup analysis showed that the study design did not influence the results (radiographic knee OA: OR = 1.42, 95% CI, 1.19–1.71 for cross-sectional studies and OR = 2.17, 95% CI, 1.30–3.63 for cohort studies; and symptomatic knee OA: OR = 1.85, 95% CI, 1.10–3.13) for cross-sectional studies and OR = 2.74, 95% CI, 1.81–4.16 for cohort studies).
This meta-analysis showed that there was a significant relationship between hypertension and knee OA (both radiographic and symptomatic). However, further original studies are needed that use a better design.
Keywords: hypertension, knee osteoarthritis, meta-analysis, risk
1. Introduction
Osteoarthritis (OA), a severe joint disease, is the 6th leading cause of disability worldwide and a major cause of restricted activity, disability, and low quality of life.[1] OA is predicted to advance to the 4th leading cause of disability in 2020.[1–3] The prevalence of knee OA is 3.6% of the total population (nearly 250 million people), while the prevalence of painful disabling knee OA is nearly 35% in adults >60 years of age.[4–7] Moreover, knee OA may substantially contribute to higher therapeutic costs, the mean of which exceeds $12,000 annually.[4–7] However, therapies for knee OA are complicated, and joint replacement is the final choice.[4–7] Therefore, finding the independent risk factors of knee OA may offer potential preventive insight.
Hypertension, a component of metabolic syndrome and an independent risk factor for cardiovascular and cerebrovascular disease, has been acknowledged as the 3rd leading cause of disability worldwide.[8,9] In 2005, the National Health and Nutrition Examination Survey revealed that 65 million people in the United States suffered from hypertension.[8,10] Accordingly, the total number of individuals with hypertension worldwide is predicted to reach 1.56 billion in 2025.[11]
A new classification for phenotyping OA was recently suggested that includes metabolic syndrome, aging, and posttraumatic-related OA.[12–14] In metabolic syndrome-related OA, obesity may be the major pathomechanism. However, observational studies have suggested that other components of metabolic syndrome, such as hypertension, may be independent risk factors for knee OA.[15–22] Because of the conflicting results, the relationship between hypertension and knee OA remains controversial.[15–22]
To our knowledge, the relationship between hypertension and the risk of knee OA has not been systematically evaluated. Therefore, here we conducted a meta-analysis of observational studies to quantitatively assess the relationship between hypertension and knee OA (radiographic and symptomatic).
2. Methods
We performed a systematic review and meta-analysis of the available studies according to MOOSE guidelines and the PRISMA statement.[23,24] The ethical approval and written consent are not necessary for the meta-analysis, because the data of meta-analysis are collected from published literature.
2.1. Literature search
We searched PubMed, EMBASE, and the Cochrane Library up to July 25, 2016 for observational studies, with no restrictions on language or publication year. All of the relevant observational studies were performed using medical subject headings or free text words. We followed combined medical subject headings and free text words search strategies by using follow search terms: (hypertension OR high blood pressure) AND (degenerative knee OR osteoarthritis OR knee osteoarthritis OR knee joint osteoarthritis). To find additional references, we manually searched the reference lists of all retrieved studies and published reviews and included all identified relevant articles in the analysis.
2.2. Selection criteria
We evaluated all studies independently that presented quantitative evaluations of the relationship between hypertension and the risk of knee OA; the studies that met selection criteria were included in this meta-analysis. Studies were included in the meta-analysis according to PICOs criteria:
-
(1)
P: adult patients with radiographic or symptomatic knee OA. Kellgren–Lawremce grade is scored on AP view radiograph, joint space narrowing and osteophytes were scored on lateral view radiographs. A knee is defined as having ROA in the tibiofemoral joint if it had Kellgren and Lowrence grade >2 on the AP view, and as having ROA in the patellofemoral joint if it had any osteophyte grade >2, or any osteophyte grade >1 plus joint space narrowing grade >2 in the patellofemoral joint on the lateral view.[25–27] Symptomatic knee OA defined as the presence of knee pain and at least 1 knee with a K/L grade 2. Adult patients definite age ≥18 years.[27,28]
-
(2)
I: any definition of hypertension, the detail definition is given in Table 1.
-
(3)
C: patients without hypertension.
-
(4)
O: relative risks, odds ratios (ORs), and their corresponding 95% confidence intervals (CIs) specifically provided or sufficient data present to compute them.
-
(5)
S: study design were observational studies, included the cross-sectional studies, cohort studies, or case–control studies.
Table 1.
Characteristics of each included study.
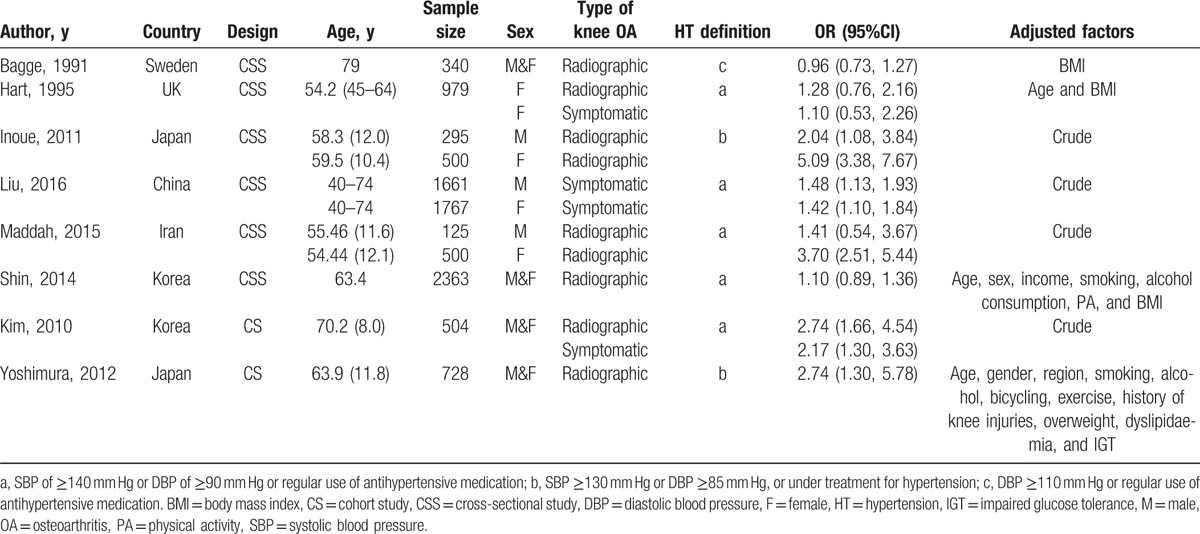
If several reports described the same study, we chose the most recent report as the main report, which typically was the latest full-text publication in a peer-reviewed journal.[29] We excluded letters, comments, reviews, and meta-analyses.
2.3. Data extraction and quality assessment
Two authors extracted the data from each study independently by using a standardized data collection form. The standardized data collection form included the 1st author's name, year of publication, country, study design, age and sex breakdown of the study population, sample size, knee OA type, and hypertension definition. Any disagreements were resolved through consensus.
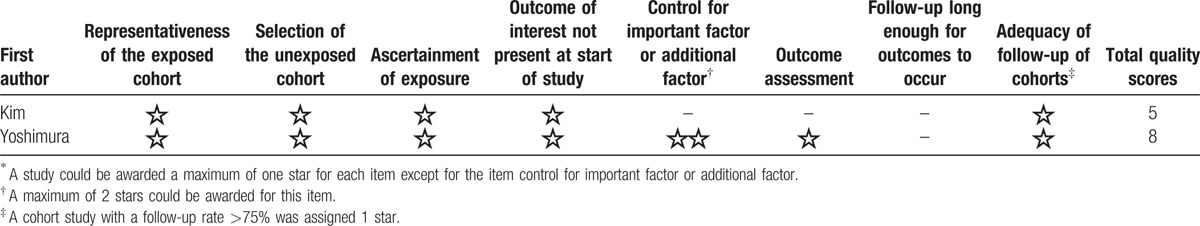
We utilized the Cross-Sectional/Prevalence Study Quality Scale recommended by the Agency for Healthcare Research and Quality (AHRQ)[30] to assess cross-sectional study quality. The AHRQ scale is a qualitative 11-item tool that is used to evaluate quality using questions with possible “Yes,” “No,” and “Unclear” answers. It means moderate risk of bias, if a study with more than 3 “YES.”[31] We also utilized the 9-star Newcastle-Ottawa Scale to assess case–control and cohort study quality; any study with ≥7 stars was considered of high quality.[32]
2.4. Statistical analysis
ORs were calculated as effect sizes to measure the association between hypertension and the risk of knee OA in the general population. As in prior studies, the multivariable-adjusted relative risks were transformed into ORs.[33,34] The potential interstudy heterogeneity was examined via Q[28] and I2 statistics.[35] A P value for heterogeneity of <0.1 or I2 of >50% indicated that the heterogeneity was statistically significant. Thus, the random-effects model was used to perform the analysis. Otherwise, we computed the summary effect using the fixed-effect model. Subgroup analyses were performed by variables including area, study design, hypertension definition, sex, and whether the data were adjusted (Yes or No). Publication bias was assessed using Egger test.[36] The meta-analysis was conducted using Stata version 11.0 (Stata Corporation, College Station, TX). Values of P < .05 were considered statistically significant.
3. Results
Figure 1 shows the study selection procedure. A total of 3550 studies were included from the primary electronic database search: 961 from PubMed, 393 from EMBASE, and 196 from the Cochrane library. After the assessment for duplicates, 637 studies were excluded. After title and abstract screening, 2848 studies were excluded for obvious irrelevance, while 29 articles remained. After full-text screening, 21 studies were excluded because for not examining the disease of interest (n = 10), not appropriate knee OA definition (n = 4), being reviews or meta-analyses (n = 4), or not examining hypertension and knee OA (n = 3). Finally, 8 studies[15–22] with a total of 9762 participants were included in the current meta-analysis.
Figure 1.
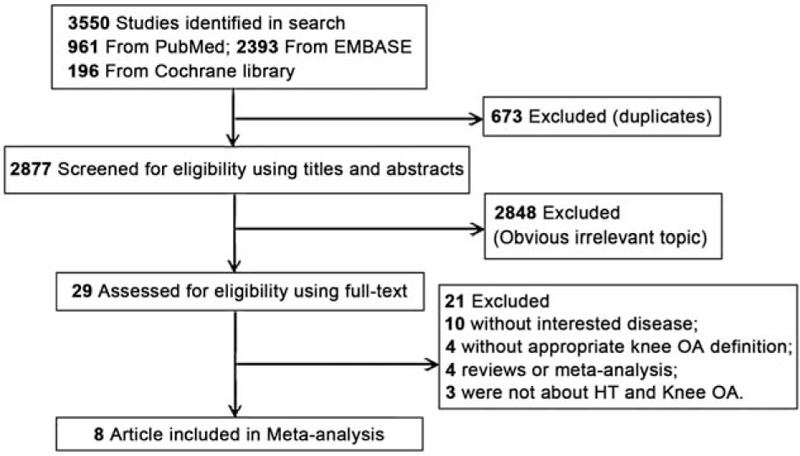
Literature search and study selection.
3.1. Study characteristics
Table 1 shows the characteristics of the included 8 studies: 2 were prospective cohort studies,[18,22] while the other 6 were cross-sectional studies.[15–17,19–21] Two of the 8 studies reported data for both radiographic and symptomatic knee OA[16,18]; 5 reported data for radiographic knee OA only[15,17,20–22] and only 1 reported data for symptomatic knee OA only.[19] The studies were performed in 6 different countries (2 each in Japan[17,22] and Korea[18,21] and 1 each in China,[19] Sweden,[15] the UK,[16] and Iran[20]). Seven studies included men and women,[15,17–22] while the other included only women.[16] Five studies[16,18–21] definite hypertension as a systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or regular use of antihypertensive medication; 2 studies[17,22] definite hypertension as a systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or under treatment for hypertension; and only 1 study[15] definite hypertension as a diastolic blood pressure ≥110 mm Hg or regular use of antihypertensive medication. All of the included studies were of moderate to high quality (Tables 2 and 3).
Table 2.
Methodological quality of cross-sectional studies included in the meta-analysis.
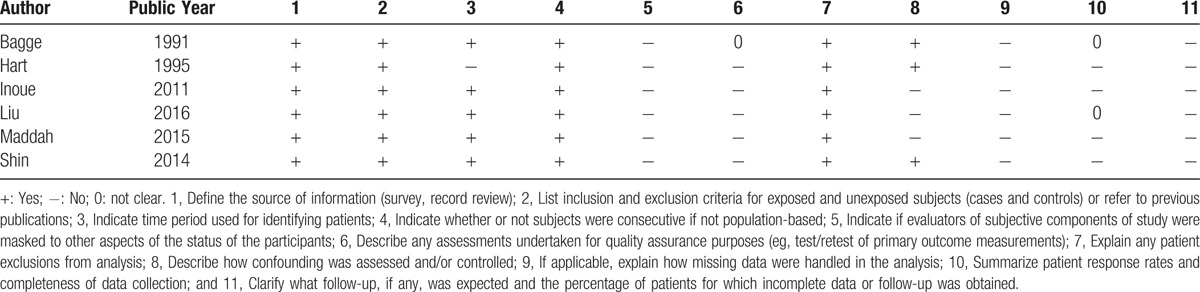
Table 3.
Methodological quality of case–control studies included in the meta-analysis∗.
3.2. Main analyses
Figure 2 shows the results of the relationship between hypertension and the risk of symptomatic knee OA. Hypertension was significantly associated with a higher symptomatic knee OA risk of 1.49 (95% CI, 1.26–1.77) with no interstudy heterogeneity (I2 = 0%, P for heterogeneity <.412).
Figure 2.
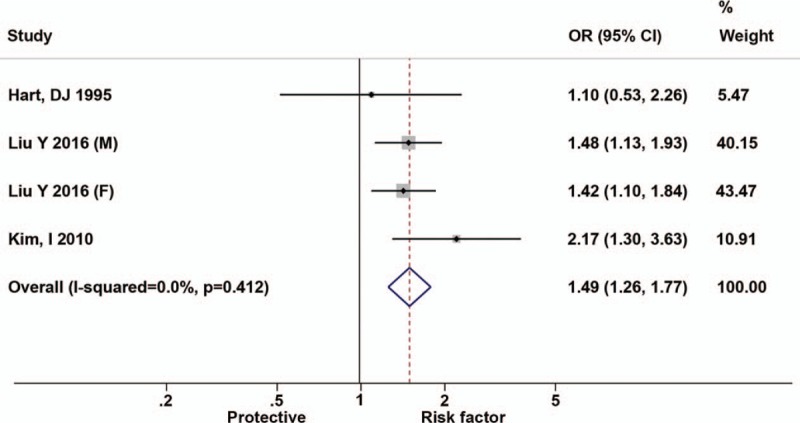
Forest plots for odds ratios of the association between hypertension and risk of symptomatic knee osteoarthritis.
Figure 3 shows the results of the relationship between hypertension and the risk of radiographic knee OA. The results showed that hypertension was significantly associated with a higher radiographic knee OA risk of 2.01 (95% CI, 1.28–3.15), with high interstudy heterogeneity (I2 = 90.2%, P for heterogeneity <.001).
Figure 3.
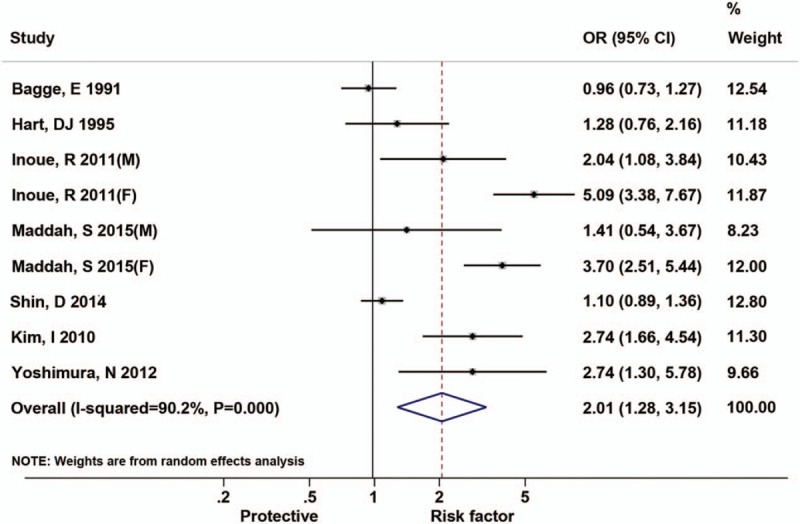
Forest plots for odds ratios of the association between hypertension and risk of radiographic knee osteoarthritis.
3.3. Subgroup analyses
Table 4 shows the results of the subgroup analyses of the relationship between hypertension and the risk of radiographic and symptomatic knee OA. For symptomatic knee OA, the subgroup analyses were assessed according to area, design, hypertension definition, sex, and adjusted factors. In the subgroup analysis by area, the OR was 1.52 (95% CI, 1.27–1.81) for Asian countries and 1.10 (95% CI, 0.53–2.27) for Western countries. In the subgroup analysis by study design, the OR was 2.17 (95% CI, 1.30–3.63) for cohort studies and 1.42 (95% CI, 1.19–1.71) for cross-sectional studies. In the subgroup analysis by sex, the OR was 2.17 (95% CI, 1.30–3.63) for both men and women, 1.48 (95% CI, 1.13–1.93) for men only, and 1.38 (95% CI, 1.08–1.76) for women only.
Table 4.
Subgroup analysis of studies included in the meta-analysis.
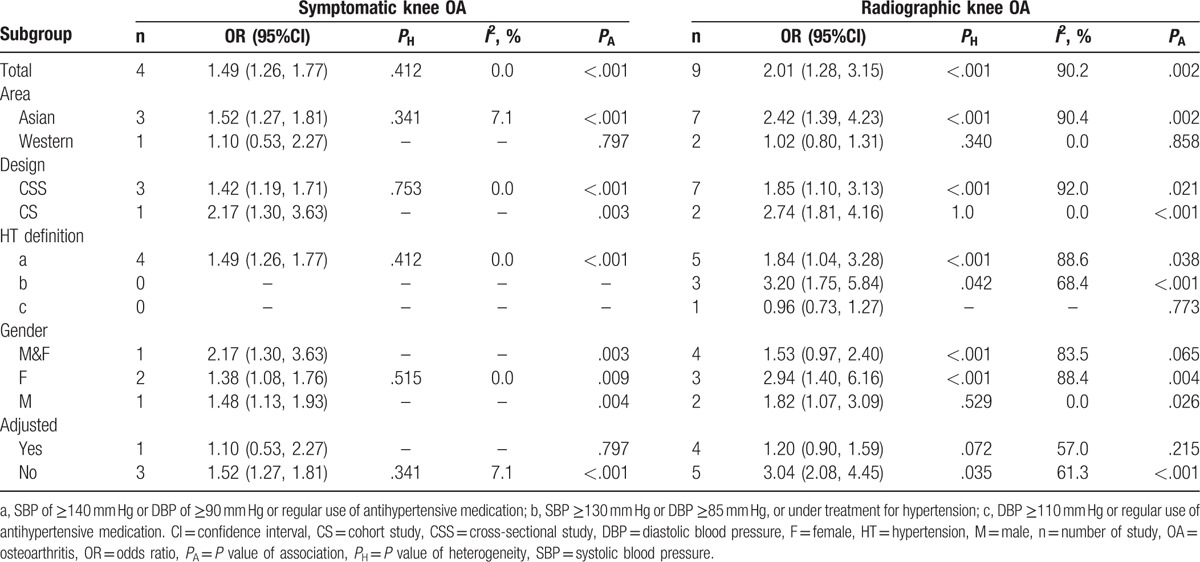
For radiographic knee OA, the subgroup analyses were assessed by area, design, hypertension definition, sex, and adjusted factors. In the subgroup analysis by area, the OR was 2.42 (95% CI, 1.39–4.23) for Asian countries and 1.02 (95% CI, 0.80–1.31) for Western countries. In the subgroup analysis by study design, the ORs were 2.74 (95% CI, 1.81–4.16) for cohort studies and 1.85 (95% CI, 1.10–3.13) for cross-sectional studies. In the subgroup analysis by sex, the OR was 1.53 (95% CI, 0.97–2.40) for both men and women, 1.82 (95% CI, 1.07–3.09) for men only, and 2.94 (95% CI, 1.40–6.16) for women only.
3.4. Publication bias
Egger tests suggested nonsignificant publication bias for the association between hypertension and radiographic knee OA (P = .172) and symptomatic knee OA (P = .855).
4. Discussion
The new classification for phenotyping OA, including metabolic syndrome, aging, and posttraumatic-related OA, has been certified by epidemiological studies.[12–14] However, the relationship between each component, such as hypertension, of metabolic syndrome and OA requires further investigation. Therefore, this meta-analysis has systematically gathered the peer-reviewed evidence regarding the role of hypertension in the development of knee OA. Eight studies including 2 cohort studies and 6 cross-sectional studies with a total of 9762 participants were finally included in this meta-analysis.
Regarding the explanation for the associations between hypertension and knee OA risk, Hart et al[16] found that metabolic factors such as hypertension, blood glucose, and hypercholesterolemia were associated with knee OA independent of obesity. Puenpatom and Victor[37] reported that the component factors of metabolic syndrome (MetS), including hypertension, abdominal obesity, and hyperglycemia, were more prevalent in the population with OA than in that without OA.[38] OA and MetS share the same mechanisms of inflammation, obesity increased mass and joint load and altered proinflammatory factor adipokines secretion, leading to the chronic low grade inflammatory status in joint tissues. Additionally, cholesterol accumulation in the cartilage can impair the efflux function of cartilage, hence inducing OA.[39,40]
In order to found the confound factors, the subgroup analysis of studies included in the meta-analysis were performed. Subgroup analysis shown that the area (Asian vs Western), the definition of hypertension, and adjusted analysis might confounded the association between hypertension and knee OA.
However, the precise mechanism of this relationship is unclear. Hypertension and OA are both highly prevalent diseases. Some studies suggest that there may be several shared risk factors implicated in the plausible mechanisms of the relationship between hypertension and knee OA.[41,42] One possible explanation for the relationship between hypertension and knee OA is shared traditional risk factors, such as aging, obesity, and chronic inflammation.[2,9,43,44] Moreover, some studies have proven that multiple genes are involved in both hypertension and knee OA. The proinflammatory cytokine interleukin-6 plays an important role in hypertension and knee OA.[45–48] During bone resorption and formation, OPG and LDL Receptor Related Protein 6 play an important role in the OPG/receptor activator of nuclear factor kappa-B ligand (RANKL) and Wnt signaling pathways.[48,49] Both OPG and LDL Receptor Related Protein 6 were shown to be associated with hypertension as well.[49] In addition, some studies showed that polymorphisms in the vitamin D receptor may be associated with low bone mineral density, OA, and hypertension.[27,50–53]
Our study has several advantages. First, this is the 1st meta-analysis to evaluate the relationship between hypertension and the risk of knee OA. We did a comprehensive search and included a large number of participants (9762 total). Therefore, the statistical power of this study is sufficient. Second, we extracted the data, preformed the analysis, independently evaluated study quality, and resolved disagreements by consensus to minimize bias. Third, all types of observational studies were included in the meta-analysis, and the result of publication bias test was nonsignificant.
Our meta-analysis has some limitations. First, due to the inclusion of observational studies, confounding factors were inherent and the risk estimates may be exaggerated or underestimated. Second, the use of medicine (s) for hypertension were not described in the included studies, whether antihypertension or diuretic drugs, which might offer a further explanation for the relationship. Third, we did not conduct a subgroup analysis of knee OA stage, so a further study is required to investigate the relationship between hypertension and the risk of different knee OA stages. Finally, heterogeneity may have been introduced in this meta-analysis by the methodological differences among the included studies, which may reduce the strength of our conclusions.
In conclusion, this meta-analysis showed a significant relationship between hypertension and knee OA (radiographic and symptomatic). However, a study that is community-based, well-powered, and primarily designed for the target question should be considered in the future to identify true association.
Footnotes
Abbreviations: CI = confidence interval, OA = osteoarthritis, OR = odds ratio.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Bedson J, Jordan K, Croft P. The prevalence and history of knee osteoarthritis in general practice: a case–control study. Fam Pract 2005;22:103–8. [DOI] [PubMed] [Google Scholar]
- [2].Silverwood V, Blagojevic-Bucknall M, Jinks C, et al. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:507–15. [DOI] [PubMed] [Google Scholar]
- [3].Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646–56. [PMC free article] [PubMed] [Google Scholar]
- [4].Dillon CF, Rasch EK, Gu Q, et al. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–1994. J Rheumatol 2006;33:2271–9. [PubMed] [Google Scholar]
- [5].Gupta S, Hawker GA, Laporte A, et al. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford) 2005;44:1531–7. [DOI] [PubMed] [Google Scholar]
- [6].Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhou ZY, Liu YK, Chen HL, et al. Body mass index and knee osteoarthritis risk: a dose-response meta-analysis. Obesity (Silver Spring) 2014;22:2180–5. [DOI] [PubMed] [Google Scholar]
- [8].Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347–60. [DOI] [PubMed] [Google Scholar]
- [9].Huai P, Xun H, Reilly KH, et al. Physical activity and risk of hypertension: a meta-analysis of prospective cohort studies. Hypertension 2013;62:1021–6. [DOI] [PubMed] [Google Scholar]
- [10].Taylor B, Irving HM, Baliunas D, et al. Alcohol and hypertension: gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction 2009;104:1981–90. [DOI] [PubMed] [Google Scholar]
- [11].Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217–23. [DOI] [PubMed] [Google Scholar]
- [12].Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26. [DOI] [PubMed] [Google Scholar]
- [13].Louati K, Vidal C, Berenbaum F, et al. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 2015;1:e000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yusuf E, Nelissen RG, Ioan-Facsinay A, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis 2010;69:761–5. [DOI] [PubMed] [Google Scholar]
- [15].Bagge E, Bjelle A, Eden S, et al. Factors associated with radiographic osteoarthritis: results from the population study 70-year-old people in Goteborg. J Rheumatol 1991;18:1218–22. [PubMed] [Google Scholar]
- [16].Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women: the Chingford Study. J Rheumatol 1995;22:1118–23. [PubMed] [Google Scholar]
- [17].Inoue R, Ishibashi Y, Tsuda E, et al. Medical problems and risk factors of metabolic syndrome among radiographic knee osteoarthritis patients in the Japanese general population. J Orthop Sci 2011;16:704–9. [DOI] [PubMed] [Google Scholar]
- [18].Kim I, Kim HA, Seo YI, et al. The prevalence of knee osteoarthritis in elderly community residents in Korea. J Korean Med Sci 2010;25:293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu Y, Zhang H, Liang N, et al. Prevalence and associated factors of knee osteoarthritis in a rural Chinese adult population: an epidemiological survey. BMC Public Health 2016;16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maddah S, Mahdizadeh J. Association of metabolic syndrome and its components with knee osteoarthritis. Acta Med Iran 2015;53:743–8. [PubMed] [Google Scholar]
- [21].Shin D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: results of a national survey. J Clin Endocrinol Metab 2014;99:3177–83. [DOI] [PubMed] [Google Scholar]
- [22].Yoshimura N, Muraki S, Oka H, et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis Cartilage 2012;20:1217–26. [DOI] [PubMed] [Google Scholar]
- [23].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [24].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Monira Hussain S, Wang Y, Cicuttini FM, et al. Incidence of total knee and hip replacement for osteoarthritis in relation to the metabolic syndrome and its components: a prospective cohort study. Semin Arthritis Rheum 2014;43:429–36. [DOI] [PubMed] [Google Scholar]
- [26].Oliveria SA, Felson DT, Reed JI, et al. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum 1995;38:1134–41. [DOI] [PubMed] [Google Scholar]
- [27].Franczyk A, Stolarz-Skrzypek K, Wesolowska A, et al. Vitamin D and vitamin D receptor activators in treatment of hypertension and cardiovascular disease. Cardiovasc Hematol Disord Drug Targets 2014;14:34–44. [DOI] [PubMed] [Google Scholar]
- [28].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [29].Rutjes AW, Juni P, da Costa BR, et al. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012;157:180–91. [DOI] [PubMed] [Google Scholar]
- [30].Rostom A, Dube C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. 2013. [Google Scholar]
- [31].Almeida FT, Pacheco-Pereira C, Porporatti AL, et al. Oral manifestations in patients with familial adenomatous polyposis: a systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:527–40. [DOI] [PubMed] [Google Scholar]
- [32].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [33].Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280:1690–1. [DOI] [PubMed] [Google Scholar]
- [34].McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157:940–3. [DOI] [PubMed] [Google Scholar]
- [35].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med 2009;121:9–20. [DOI] [PubMed] [Google Scholar]
- [38].Niu J, Clancy M, Aliabadi P, et al. Metabolic syndrome, its components, and knee osteoarthritis: the framingham osteoarthritis study. Arthritis & Rheumatology 2017;69:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Haj Hamad W, Maraoui M, Sghir M, et al. Knee osteoarthritis and metabolic syndrome. Ann Phys Rehabil Med 2016;59s:e158. [Google Scholar]
- [40].Veronese N, Cereda E, Maggi S, et al. Osteoarthritis and mortality: a prospective cohort study and systematic review with meta-analysis. Semin Arthritis Rheum 2016;46:160–7. [DOI] [PubMed] [Google Scholar]
- [41].Morovic-Vergles J, Salamon L, Marasovic-Krstulovic D, et al. Is the prevalence of arterial hypertension in rheumatoid arthritis and osteoarthritis associated with disease? Rheumatol Int 2013;33:1185–92. [DOI] [PubMed] [Google Scholar]
- [42].Verdecchia P, Angeli F, Mazzotta G, et al. Treatment strategies for osteoarthritis patients with pain and hypertension. Ther Adv Musculoskelet Dis 2010;2:229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Richmond SA, Fukuchi RK, Ezzat A, et al. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J Orthop Sports Phys Ther 2013;43:B515–9. [DOI] [PubMed] [Google Scholar]
- [44].Lytsy P, Ingelsson E, Lind L, et al. Interplay of overweight and insulin resistance on hypertension development. J Hypertens 2014;32:834–9. [DOI] [PubMed] [Google Scholar]
- [45].Ma H, Sun G, Wang W, et al. Association between interleukin-6-572 C>G and -174 G>C polymorphisms and hypertension: a meta-analysis of case-control studies. Medicine (Baltimore) 2016;95:e2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cheung BM, Ong KL, Tso AW, et al. Relationship of plasma interleukin-6 and its genetic variants with hypertension in Hong Kong Chinese. Am J Hypertens 2011;24:1331–7. [DOI] [PubMed] [Google Scholar]
- [47].Qu XQ, Wang WJ, Tang SS, et al. Correlation between interleukin-6 expression in articular cartilage bone and osteoarthritis. Genet Mol Res 2015;14:14189–95. [DOI] [PubMed] [Google Scholar]
- [48].Blumenfeld O, Williams FM, Valdes A, et al. Association of interleukin-6 gene polymorphisms with hand osteoarthritis and hand osteoporosis. Cytokine 2014;69:94–101. [DOI] [PubMed] [Google Scholar]
- [49].Mani A, Radhakrishnan J, Wang H, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 2007;315:1278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu H, He H, Li S, et al. Vitamin D receptor gene polymorphisms and risk of osteoarthritis: a meta-analysis. Exp Biol Med (Maywood) 2014;239:559–67. [DOI] [PubMed] [Google Scholar]
- [51].Zhu ZH, Jin XZ, Zhang W, et al. Associations between vitamin D receptor gene polymorphisms and osteoarthritis: an updated meta-analysis. Rheumatology (Oxford) 2014;53:998–1008. [DOI] [PubMed] [Google Scholar]
- [52].Lee YH, Woo JH, Choi SJ, et al. Vitamin D receptor TaqI, BsmI and ApaI polymorphisms and osteoarthritis susceptibility: a meta-analysis. Joint Bone Spine 2009;76:156–61. [DOI] [PubMed] [Google Scholar]
- [53].Jia J, Shen C, Mao L, et al. Vitamin D receptor genetic polymorphism is significantly associated with decreased risk of hypertension in a Chinese Han population. J Clin Hypertens (Greenwich) 2014;16:634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


