Abstract
The aim of the study was to explore the relationship between the concentration of C-telopeptide fragments of type II collagen (CTX-II), Zn2+, and Ca2+ in urine and knee osteoarthritis (KOA).
Eighty-two patients with KOA and 20 healthy volunteers were enrolled. Anteroposterior and lateral position x-rays of knee joints were collected. The images were classified according to Kellgren-Lawrence radiographic grading criterion. The patients were divided into group grade I, group grade II, group grade III, and grade IV. The concentration of CTX-II in the urine was detected by enzyme-linked immunosorbent assay. The concentration of Zn2+ and Ca2+ in urine was detected by inductively coupled plasma atomic emission spectrometry.
Compared with the healthy individuals, the concentration of CTX-II was significantly higher in KOA patients. The concentration of CTX-II in KOA patients from high to low was as follows: group IV, group III, group II, and group I. There was no significant difference between group I and healthy individuals. The concentration of Zn2+ and Ca2+ in urine of KOA patients was higher than that in healthy individuals. There was no difference in each KOA group.
The concentration of CTX-II is instrumental to diagnose the progress of KOA. The concentration of Zn2+ and Ca2+ in urine is helpful for early diagnosis of KOA.
Keywords: Ca2+, collagen type II, knee osteoarthritis, urine, Zn2+
1. Introduction
Knee osteoarthritis (KOA) is a common disease for middle-aged and old people. The chronic and painful symptoms are seriously harmful to human health. Epidemiological investigation showed that over one-third of people aged over 60 years have KOA in United States.[1] In China, over 50% of people aged over 50 have KOA. Also, 80% female and 70% male aged over 65 suffered from the disease.[2] With the aging society, the population of KOA is still increasing. It is estimated that 25 million in United States and 8 million in Japan suffered from it.[3,4]
Traditionally, clinical symptoms and radiography were employed to diagnose KOA. Magnetic resonance imaging (MRI) was also used to diagnose the structural change of bone.[5] However, lack of early diagnostic markers and makers for progression lead to serious results. Some researchers identified several makers, such as C-telopeptide fragments of type II collagen (CTX-II) in urine, uric acid in joint fluid, serum cartilage oligometric matrix protein (sCOMP), and serum adipokines.[6–10] Proteomics also revealed some novel markers, such as hemopexin, custerin, alpha-1acid glycoprotein-2, macrophage stimulating protein, Fib3 (Fibulin 3 peptides)-1, and Fib3-2.[11,12] However, the predictive value should be further explored.
Some elements, such as Ca2+, play important role in keeping osmotic pressure of joint fluid and supplying nutrients. The unbalance of these elements occurs in different diseases. The test of these elements in joint fluid is helpful to diagnose the related diseases. Periodical examination of these elements is helpful to monitor the progress of the disease and effect of treatment.[13,14] Reagan et al[15] cultured chondrocyte with different elements, observed the effect, and found that elements have a certain role in repairing the cartilage cell regeneration. Krachler and Domej,[13] Krachler et al,[14] and Yazar et al[16] found that microelements have a certain role in diagnosing osteoarthritis. However, the potential of Zn2+ and Ca2+ in KOA is still unclear.
In this study, we will explore the predictive value of CTX-II, Zn2+, and Ca2+ in KOA, especially different progression.
2. Study population and methods
2.1. Study population
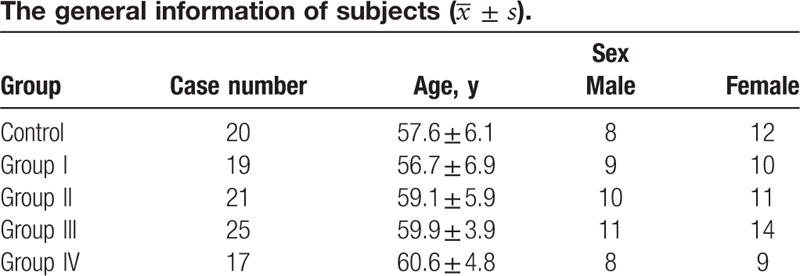
In all, 82 KOA patients and 20 healthy individuals were enrolled in this study. The study was approved by Review Board of Guilin Medical College. The basic information of subjects is shown in Table 1. The following patients were excluded: patients with liver and kidney dysfunction; patients with bone and joint disease in hip and ankle, cartilage metabolic diseases, such as ankylosing spondylitis, and rheumatoid arthritis; nonmenopause[17]; patients taking medicine of hormone and cartilage metabolism.
Table 1.
2.2. Grouping
Kellgren-Lawrence radiographic grading criterion was employed to assess KOA patients. Three professional doctors analyzed the radiography. Group I: suspicious narrow in joint space and suspicious osteophyte; group II: osteophyte and normal or suspicious narrow in joint space; group III: medium osteophyte, obvious narrow in joint space, subchondral bone sclerosis, and possible deformity; group IV: large osteophyte, obvious narrow in joint space, serious subchondral bone sclerosis, and obvious deformity.
2.3. ELISA
Enzyme-linked immunosorbent assay (ELISA) was employed to detect the CTX-II level in urine. Morning urine (5–10 mL) was collected and centrifuged at 2500 rpm/min for 20 minutes. The supernate was transferred to centrifuge tube and kept at −80°C. After dissolution, the samples were diluted 5 times. The diluted samples and standard sample (100 μL) were added into coated wells and incubated at 37°C for 1 hour. The plate was washed 5 times and 50 μL enzyme was added into each well and incubated for at 37°C for 1 hour. Except for blank well, 50 μL color-developing agents A and B were added into each well for 15 minutes in dark place. Stop solution was used to stop the reaction. The optical density (OD) value at 450 nm was recorded.
2.4. Measurement of Zn2+ and Ca2+
HNO3 (8 mL) was added to 2 mL urine and mixed well. Full spectrum direct reading plasma spectrometer was used to examine the Zn2+ and Ca2+ in urine. The working parameters of instrument were as follows: incident power, 1.1 kW; argon flow, 0.5 L/min.
2.5. Statistical analysis
SPSS 18.0 was used to analyze the data. Independent-samples t test was used for statistical comparisons between 2 groups. One-way analysis of variance (ANOVA) followed by a least-significant difference test was used for statistical comparisons among multiple groups. The significance was assured when the P value was less than .05.
3. Results
3.1. The CTX-II concentration in different groups
Compared with the control group (218.341 ± 22.270) pg/mL, the CTX-II concentration in KOA (261.235 ± 39.944) pg/mL was higher (F = 43.722, P < .001). The concentration of CTX-II in KOA patients from high to low was as follows: group IV, group III, group II, and group I (F = 334.402, P < .001). There was no significant difference between group I and healthy individuals (P > .05) (Fig. 1).
Figure 1.
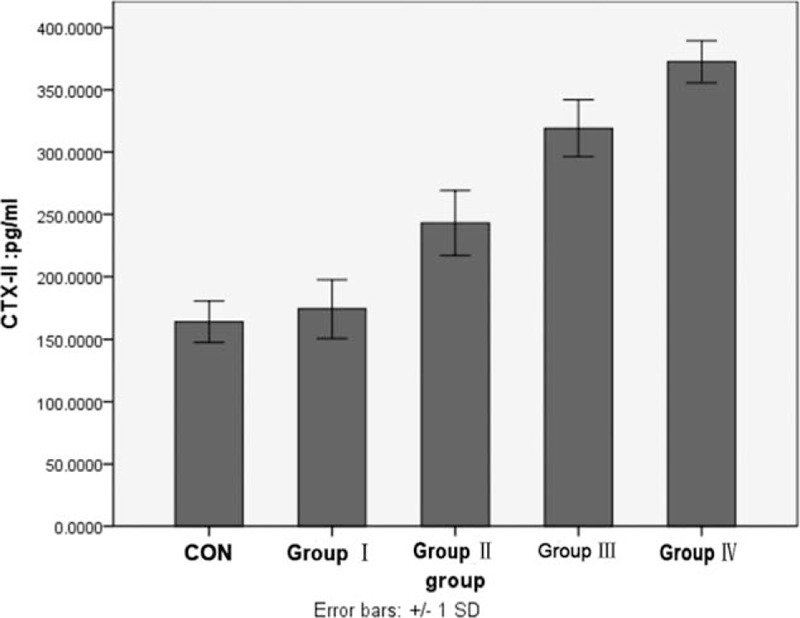
Comparison of the concentration of CTX-II in urine between control group and each KOA group (∗ means P < .05 vs control group). CTX-II = C-telopeptide fragments of type II collagen, KOA = knee osteoarthritis.
3.2. The concentration of Zn2+ and Ca2+ in different groups
The concentration of Zn2+ in KOA (0.7000 ± 0.1736 mg/L) was higher than that in the control group (0.3764 ± 0.2163 mg/L) (F = 53.401, P < .001). The concentration of Ca2+ in KOA (204.3536 ± 71.6828 mg/L) was also higher than that in the control group (80.4635 ± 39.3493 mg/L) (F = 55.379, P < .0001). There was no significant difference among group I, group II, group III, and group IV (P > .05) (Figs. 2 and 3).
Figure 2.
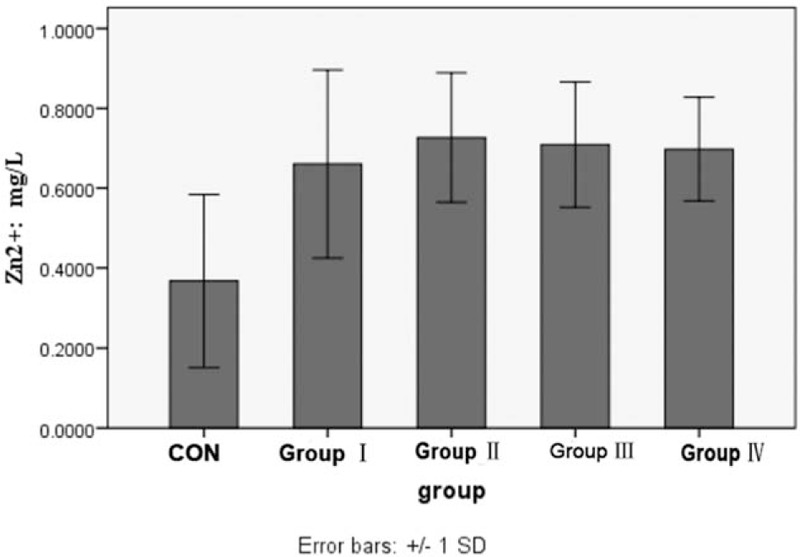
Comparison of the concentration of Zn2+ in urine between control group and each KOA group (∗ means P < .05 vs control group). KOA = knee osteoarthritis.
Figure 3.
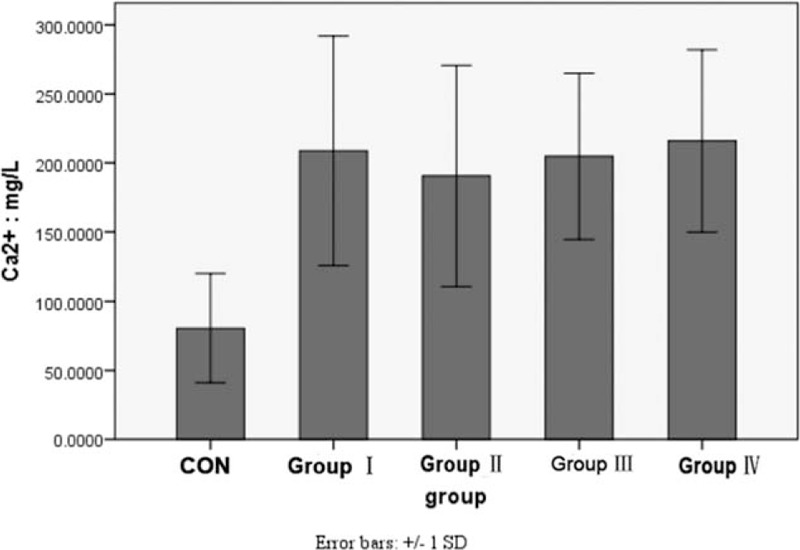
Comparison of the concentration of Zn2+ in urine between control group and each KOA group (∗ means P < .05 vs control group). KOA = knee osteoarthritis.
4. Discussion
Healthy cartilage matrix mainly contains collagen type II. Type II collagen in cartilage was degraded continuously through matrix metalloproteinase (MMPs), synthesized by chondrocyte, osteoclast and synoviocytes.[18] Osteoarthritis is a disease characterized by continuous joint damage, including the wear of cartilage and change of synovial tissues. Studies showed that the increased expression of MMP-1, MMP-2, and MMP-9 proteins might be associated with the pathogenesis of osteoarthritis (OA).[19] MMPs are Zn2+ and Ca2+-dependent proteinases, and the activity of MMPs are regulated by Ca2+ concentration.[20] Type II collagen was degraded by MMP-1 into 3/4 or 1/4 fragments. These fragments can also be degraded by other MMPs into CTX-II.[21–26] The CTX-II is released into the serum and urine, and the CTX-II concentration in body fluids reflects OA progression.[27] Studies showed that serum CTX-II can be used to monitor the OA progression.[28] CTX-II in urine can be used to diagnose and assess the osteoarthritis.[29]
In this study, we also proved that CTX-II concentration in KOA was higher than healthy individuals. In consideration of different progress of KOA, we also analyzed the concentration of CTX-II in different grades according to Kellgren-Lawrence radiographic grading criterion. The concentration of CTX-II in KOA patients from high to low was as follows: group IV, group III, group II, and group I. There was no significant difference between group I and healthy individuals. These results suggested that CTX-II is not a biomarker for early KOA, but it can be used to indicate the progress of the disease.
Matrix metalloproteinases are Zn2+ and Ca2+-dependent proteinases. Wang et al[30] also found that concentration of Zn2+ and Ca2+ in synovial tissues was related to osteoarthritis. In this study, we found that the concentration of Zn2+ and Ca2+ from urine in KOA was higher than that in healthy individuals. There was no significant difference among groups I, II, III, and IV. These data suggested that Zn2+ and Ca2+ may be early markers of KOA, but not related to progress of KOA.
5. Conclusions
In summary, we examined the change of CTX-II, Zn2+, and Ca2+ in urine from KOA patients and found that CTX-II was positively related to the KOA radiographic grading. The change of Zn2+ and Ca2+ was higher in KOA patients than healthy individuals, but not related to KOA radiographic grading. The concentration of CTX-II is instrumental to diagnose the progress of KOA. The concentration of Zn2+ and Ca2+ in urine is also helpful for early diagnosis for KOA.
Footnotes
Abbreviations: ELISA = enzyme-linked immunosorbent assay, ICP-AES = inductively coupled plasma atomic emission spectrometry, KOA = knee osteoarthritis, MRI = magnetic resonance imaging, sCOMP = serum cartilage oligometric matrix protein.
Funding: This work was supported by the Scientific Research and Development Program of Guilin, China (20130120–5).
The authors report no conflicts of interest.
References
- [1].Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am 2013;95:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhai Y, Gao GD, Xu SY. [Basic research progress of knee osteoarthritis]. Zhongguo Gu Shang 2012;25:83–7. [PubMed] [Google Scholar]
- [3].Muraki S, Oka H, Akune T, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage 2009;17:1137–43. [DOI] [PubMed] [Google Scholar]
- [4].Attur M, Belitskaya-Levy I, Oh C, et al. Increased interleukin-1beta gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum 2011;63:1908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guermazi A, Hayashi D, Eckstein F, et al. Imaging of osteoarthritis. Rheum Dis Clin North Am 2013;39:67–105. [DOI] [PubMed] [Google Scholar]
- [6].van Spil WE, Drossaers-Bakker KW, Lafeber FP. Associations of CTX-II with biochemical markers of bone turnover raise questions on its tissue origin: data from CHECK, a cohort study of early osteoarthritis. Ann Rheum Dis 2013;72:29–36. [DOI] [PubMed] [Google Scholar]
- [7].Denoble AE, Huffman KM, Stabler TV, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci U S A 2011;108:2088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Erhart-Hledik JC, Favre J, Asay JL, et al. A relationship between mechanically-induced changes in serum cartilage oligomeric matrix protein (COMP) and changes in cartilage thickness after 5 years. Osteoarthritis Cartilage 2012;20:1309–15. [DOI] [PubMed] [Google Scholar]
- [9].Van Spil WE, Welsing PM, Kloppenburg M, et al. Cross-sectional and predictive associations between plasma adipokines and radiographic signs of early-stage knee osteoarthritis: data from CHECK. Osteoarthritis Cartilage 2012;20:1278–85. [DOI] [PubMed] [Google Scholar]
- [10].Ishijima M, Kaneko H, Kaneko K. The evolving role of biomarkers for osteoarthritis. Ther Adv Musculoskelet Dis 2014;6:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fukuda I, Ishihara T, Ohmachi S, et al. Potential plasma biomarkers for progression of knee osteoarthritis using glycoproteomic analysis coupled with a 2D-LC-MALDI system. Proteome Sci 2012;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Henrotin Y, Gharbi M, Mazzucchelli G, et al. Fibulin 3 peptides Fib3-1 and Fib3-2 are potential biomarkers of osteoarthritis. Arthritis Rheum 2012;64:2260–7. [DOI] [PubMed] [Google Scholar]
- [13].Krachler M, Domej W. Clinical laboratory parameters in osteoarthritic knee-joint effusions correlated to trace element concentrations. Biol Trace Elem Res 2001;79:139–48. [DOI] [PubMed] [Google Scholar]
- [14].Krachler M, Domej W, Irgolic KJ. Concentrations of trace elements in osteoarthritic knee-joint effusions. Biol Trace Elem Res 2000;75:253–63. [DOI] [PubMed] [Google Scholar]
- [15].Reagan BF, McInerny VK, Treadwell BV, et al. Irrigating solutions for arthroscopy. A metabolic study. J Bone Joint Surg Am 1983;65:629–31. [PubMed] [Google Scholar]
- [16].Yazar M, Sarban S, Kocyigit A, et al. Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol Trace Elem Res 2005;106:123–32. [DOI] [PubMed] [Google Scholar]
- [17].Nevitt MC, Cummings SR, Lane NE, et al. Association of estrogen replacement therapy with the risk of osteoarthritis of the hip in elderly white women. Study of Osteoporotic Fractures Research Group. Arch Intern Med 1996;156:2073–80. [PubMed] [Google Scholar]
- [18].Krasnokutsky S, Samuels J, Abramson SB. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis 2007;65:222–8. [PubMed] [Google Scholar]
- [19].Zeng GQ, Chen AB, Li W, et al. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet Mol Res 2015;14:14811–22. [DOI] [PubMed] [Google Scholar]
- [20].Sondergaard BC, Henriksen K, Wulf H, et al. Relative contribution of matrix metalloprotease and cysteine protease activities to cytokine-stimulated articular cartilage degradation. Osteoarthritis Cartilage 2006;14:738–48. [DOI] [PubMed] [Google Scholar]
- [21].Paladini RD, Wei G, Kundu A, et al. Mutations in the catalytic domain of human matrix metalloproteinase-1 (MMP-1) that allow for regulated activity through the use of Ca2+. J Biol Chem 2013;288:6629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A 1990;87:5578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Seltzer JL, Welgus HG, Jeffrey JJ, et al. The function of Ca+ in the action of mammalian collagenases. Arch Biochem Biophys 1976;173:355–61. [DOI] [PubMed] [Google Scholar]
- [24].Housley TJ, Baumann AP, Braun ID, et al. Recombinant Chinese hamster ovary cell matrix metalloprotease-3 (MMP-3, stromelysin-1). Role of calcium in promatrix metalloprotease-3 (pro-MMP-3, prostromelysin-1) activation and thermostability of the low mass catalytic domain of MMP-3. J Biol Chem 1993;268:4481–7. [PubMed] [Google Scholar]
- [25].Zhang Y, Dean WL, Gray RD. Cooperative binding of Ca2+ to human interstitial collagenase assessed by circular dichroism, fluorescence, and catalytic activity. J Biol Chem 1997;272:1444–7. [DOI] [PubMed] [Google Scholar]
- [26].Welgus HG, Kobayashi DK, Jeffrey JJ. The collagen substrate specificity of rat uterus collagenase. J Biol Chem 1983;258:14162–5. [PubMed] [Google Scholar]
- [27].Park YM, Kim SJ, Lee KJ, et al. Detection of CTX-II in serum and urine to diagnose osteoarthritis by using a fluoro-microbeads guiding chip. Biosens Bioelectron 2015;67:192–9. [DOI] [PubMed] [Google Scholar]
- [28].Duclos ME, Roualdes O, Cararo R, et al. Significance of the serum CTX-II level in an osteoarthritis animal model: a 5-month longitudinal study. Osteoarthritis Cartilage 2010;18:1467–76. [DOI] [PubMed] [Google Scholar]
- [29].Garnero P, Conrozier T, Christgau S, et al. Urinary type II collagen C-telopeptide levels are increased in patients with rapidly destructive hip osteoarthritis. Ann Rheum Dis 2003;62:939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang SY, Tao SQ, Rong JS. Distribution of 16 elements in synovial menbrane of patients with osteoarthritis. Chinese J Bone Joint 2013;7:375–8. [Google Scholar]


