Abstract
Background:
Due to increasing antimicrobial resistance, a bismuth-based quadruple regimen has been recommended as an alternative first-line therapy for Helicobacter pylori (H pylori) eradication. However, different results are varied greatly and the availability of bismuth was limited in some countries. We assessed the efficacy and safety of 14-day berberine-containing quadruple therapy as an alternative regimen for H pylori eradication.
Methods:
In a randomized, open-label, non-inferiority, phase IV trial between November 25, 2014, and October 15, 2015, 612 treatment-naive patients were randomly assigned to 14-day berberine-containing (n = 308) or 14-day bismuth-containing (n = 304) quadruple therapy. The primary outcomes were eradication rates determined by the 13C urea breath test (13C-UBT) 28 days after the end of treatment. The secondary outcomes were adverse events and compliance.
Results:
The baseline demographic data including age, gender, body mass index (BMI), general condition and severity score were not statistically different in both groups. The eradication rates in bismuth and berberine groups were 86.4% (266/308) and 90.1% (274/304) in intention-to-treat (ITT) analysis (P = .149), and 89.6% (266/297) and 91.3% (273/299) in per-protocol (PP) analysis (P = .470), respectively. No statistically significant difference was found in the overall incidence of adverse events between both groups (35.7% vs 28.6%, P = .060).
Conclusions:
Both regimens achieved the recommended efficacy for H pylori eradication. The berberine-containing quadruple regimen was not inferior to bismuth-containing quadruple regimen and can be recommended as an alternative regimen for H pylori eradication in the local region.
Keywords: berberine, bismuth, eradication, Helicobacter pylori, quadruple therapy
1. Introduction
Helicobacter pylori (H pylori) infection is a major public health problem that affects 20% to 80% population all over the world[1,2] and 40% to 90% in China.[3]H pylori play a crucial role in the pathogenesis of a variety of gastrointestinal diseases, including peptic ulcer, gastric cancer, and mucosa-associated lymphoid tissue (MALT) lymphoma.[1] Eradication of H pylori alleviates the symptoms of chronic gastritis,[4] promotes the healing of ulcer, reduces the recurrence of peptic ulcer,[5] and helps more than 80% of early low-grade gastric MALT lymphoma to achieve complete remission.[6] Eradication of H pylori in areas with high incidence of gastric cancer can prevent or reduce its occurrence[7] and decrease the risk of metachronous gastric tumor formation in gastric cancer patients underwent endoscopic resection or gastrectomy.[8]
Previous data in China indicated that the eradication rate of standard triple therapy was less than 80%.[9,10] Also, the eradication rate of sequential therapy was not better than bismuth quadruple therapy,[11] and the data of concomitant therapy was absent in China. Therefore, bismuth quadruple therapy was recommended as the first-line regimen in “Fourth Chinese National Consensus Report on the Management of H pylori Infection.”[12] The addition of bismuth to triple therapy can improve cure rates of H pylori resistant strains, but the overall cure rates depend on both the cure rates with resistant strains and the prevalence of resistance.[13] If the prevalence of resistance is high, triple therapy plus bismuth will not achieve the ideal eradication rate, which elicits pursuing of nonbismuth regimen for H pylori eradication.
Researchers in many countries and regions have been trying to use medicinal plants to inhibit or kill H pylori in vitro,[14–16] especially in China.[17–22] Accumulated evidences confirmed that besides the effects of regulating lipid, decreasing glycemia, anti-arrhythmic and anti-cancer, berberine also shows great inhibition and eradication on H pylori.[23–26] In addition, recent studies have shown that berberine can effectively suppress multiresistant strains of H pylori.[27,28] Therefore, the present study was designed to evaluate the efficiency and safety of berberine-containing quadruple therapy for H pylori eradication compared to standard bismuth quadruple therapy.
2. Materials and methods
2.1. Participants
This single-center, randomized, open-label, noninferiority phase IV clinical trial was conducted in Xijing Hospital of Fourth Military Medical University, Xi’an, Shaanxi Province in China from November 25, 2014, to October 15, 2015. The study protocol was approved by the Human Research Ethics Committee of Xijing Hospital. The study was carried out in accordance with the principles of the Declaration of Helsinki.
Eligibility criteria: Patients with documented H pylori infections (either urea breath test [UBT] positive or rapid urease test positive) and upper gastrointestinal symptoms were screened. The eligible participants were between 18 and 70 (male or female, medical contraception is required during the test and within 30 days after the end of the trial for women of childbearing age) and were willing to receive eradication therapy for H pylori. Patients were excluded if any one of the following criteria was present: (1) Previously H pylori eradication treatment; (2) contraindications to study drugs; (3) severe organ dysfunction, severe or unstable cardiopulmonary or endocrine disease; (4) continuously consumption antiulcer drugs including PPI in the past 2 weeks, antibiotics or bismuth complexes within 1 month of screening; (5) pregnant and lactating women; (6) history of gastrectomy; (7) patients with Barrett's esophagus or high-grade dysplasia, having dysphagia; (8) bleeding or evidence of iron deficiency anemia; (9) history of malignancy; (10) history of drug or alcohol abuse; (11) concurrent use of systemic corticosteroids, nonsteroidal anti-inflammatory drugs, anticoagulants, platelet aggregation inhibitors (except aspirin ≤ 100 mg/d); (12) participants of other clinical trial in the past 3 months. Written informed consent was obtained from all the patients prior to enrollment.
Clinical symptoms included acid reflux, heartburn, abdominal pain, flatulence, nausea, vomiting, dyspepsia, diarrhea, hiccup, belching, anorexia, and fetid breath. Severity of symptoms was divided into 4 degree from 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). Frequency of symptoms was divided into 4 degree from 0 to 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). Severity and frequency score was accumulated by the degree and frequency of all the symptoms. Total score = frequency score + severity score.
2.2. Procedure
Patients were randomly assigned (1:1) to berberine-containing or bismuth-containing quadruple therapy according to a predetermined list. Investigators generated the randomization schedule through a third part without accessing or influencing the randomization list. Study drugs include 20 mg esomeprazole magnesium enteric-coated tablets (Nexium, AstraZeneca Pharmaceutical Co., Ltd); 500 mg clarithromycin sustained-release tablets (Jiangbo Pharmaceutical Co., Ltd, Laiyang, China); 500 mg amoxicillin capsules (Zhongshan Branch, Zhuhai United Pharmaceutical Co., Ltd, China); 55 mg capsules colloidal bismuth tartrate (Bitenuoer, Yinxin Pharmaceutical Co., Ltd, Shantou, China); 100 mg berberine hydrochloride tablets (Jinhua Pharmaceutical Co., Ltd, Chengdu, China). In the 14-day berberine-containing quadruple regimen, 1 esomeprazole tablet, 2 amoxicillin capsules, 1 clarithromycin tablets, and 5 berberine tablets were taken twice a day (Esomeprazole before breakfast and dinner, other drugs after breakfast and dinner) and swallowed whole with 250 mL of water. In the 14-day bismuth-containing quadruple regimen, 1 esomeprazole tablet, 2 amoxicillin capsules, 1 clarithromycin tablet, and 4 tartrate bismuth potassium citrate were taken twice a day (Esomeprazole and bismuth potassium tartrate before breakfast and dinner, amoxicillin, and clarithromycin after breakfast and dinner) and swallowed whole with 250 mL of water. Patients were asked to refrain from consuming alcohol during the entire treatment period and for 48 hours after the last dose, and avoid taking the study drugs with milk, other daily products or antacids. Helicobacter pylori eradication was assessed on the 28th day after the end of treatment with 13C-UBT (Beijing Richen-Force Science & Technology, Beijing, China).
2.3. Outcomes
The primary outcomes of the study were H pylori eradication rates, based on negative 13C-UBT 28th day after the end of eradication treatment (follow-up at the 6th week). The secondary outcomes were incidence of adverse events during the treatment. Drug compliance was assessed through counting the number of used pills. Compliance was defined low when <80% of study medicines were taken. The patients were notified of the common side effects of study medicines, including nausea, vomiting, diarrhea, constipation, skin rash, headache, and dizziness at the time of enrollment. Written instructions were provided to all patients on how to take the drugs to reinforce the compliance. Enrolled patients were asked to record all adverse events. Standardized interviews were arranged at the 14th day of treatment and 28th day after the end of treatment. 13C-UBT could not be performed earlier than 28th day after the end of treatment.
2.4. Sample size estimation and statistical analysis
Sample size estimation was based on antibiotic resistance rates of H pylori previously reported in China.[29] It is assumed that the eradication rate of bismuth quadruple therapy to be 75 to 90%[30] and 85% were used in this study. Δ (defined as noninferiority margin) was –10%, power of test 90% and α 0.05 (1-sided). This study was designed as a noninferiority trial. Given the primary efficacy variables selected, 218 patients were required to complete the trial in each group in order to show noninferiority of experimental drug as compared to active control. Since it is estimated that 25% of patients would be excluded from the per-protocol analysis for not meeting 1 or more major test program, 40% of patients were increased. As a result, 306 patients were randomly assigned to each group in order to ensure the power of test.
Statistical calculations were performed using SPSS 19.0 (SPSS Inc., Chicago, IL). Continuous variables were expressed as mean and evaluated using Student's t test. Categorical variables were evaluated using Chi-square or Fisher's exact test. Noninferiority test was carried out in patients meeting the program. H pylori eradication was confirmed by negative 13C-UBT 4 weeks after the end of therapy. Positive breath test indicated treatment failure and these patients were classified as eradication failed cases. Missing test results were not needed to be estimated in patients meeting the program, while breath test data missed or test performed not at specified time were considered eradication failure when intend-to-treat (ITT) patients were assessed. All the other results were evaluated based on observed-case. The primary outcome assessment was carried out in patients meeting the program.
The main analysis (comparing noninferiority of 2 regimens) was evaluated through hypothesis testing and derivation of a 2-sided 95% confidence interval (CI) using Student's t test. Berberine-containing quadruple therapy was considered noninferior to the bismuth-containing quadruple therapy if the lower limit of the CI was >−10%, whereas noninferiority was not supported if the lower limit was ≤−10%. If noninferiority of berberine-containing quadruple therapy compared to bismuth-containing quadruple therapy was confirmed in per-protocol (PP) population, then the corresponding CI was obtained in ITT population. The study was registered at ClinicalTrials.gov, number NCT 02296021.
3. Results
3.1. Participants flow
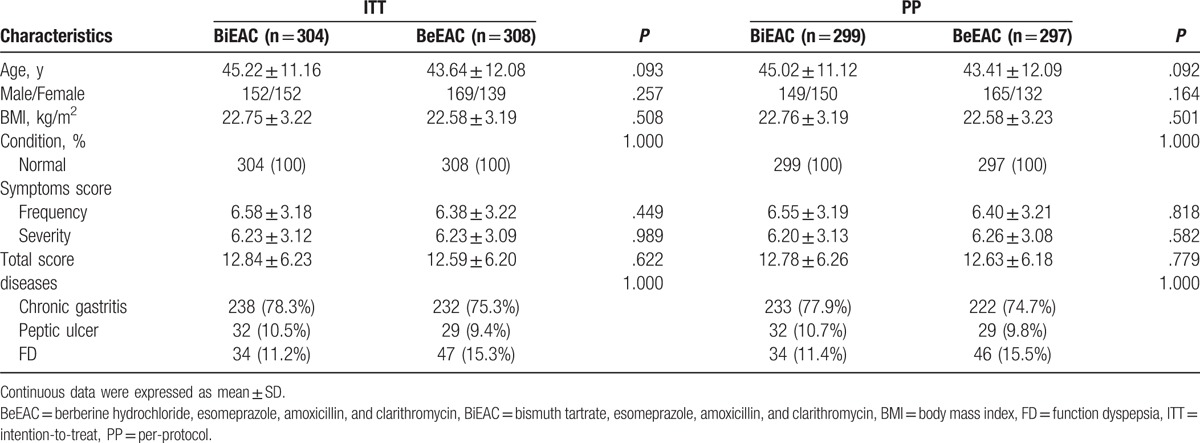
Participants flow for this study is shown in Fig. 1. A total of 865 patients positive for H pylori were screened, among which 253 were excluded, including 196 not meeting the inclusion criteria, 47 declining to participate and 10 for other reasons. As a result, 612 patients positive for H pylori were enrolled. Among them, 308 assigned to the bismuth group and 304 to the berberine group. The baseline demographic data during screening and enrolling time are shown in Table 1. There were no significant differences between both groups.
Figure 1.
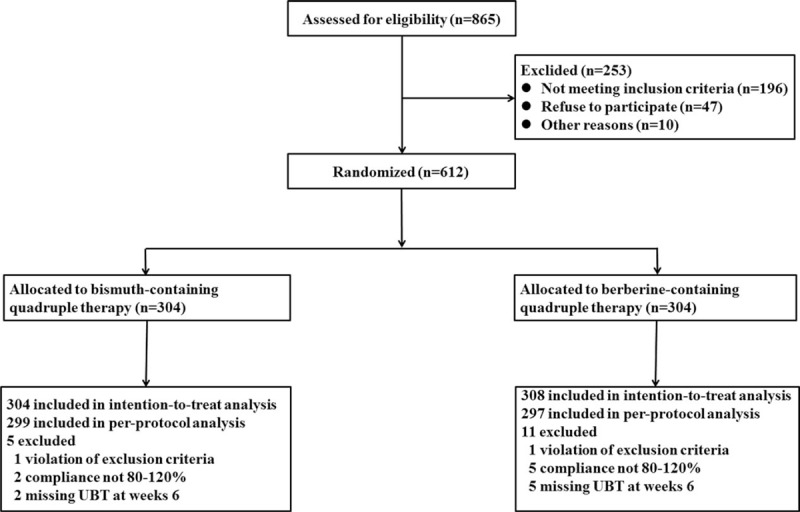
Participant flow.
Table 1.
Demographic data and baseline characteristics in the treated population.
3.2. Helicobacter pylori eradication rates
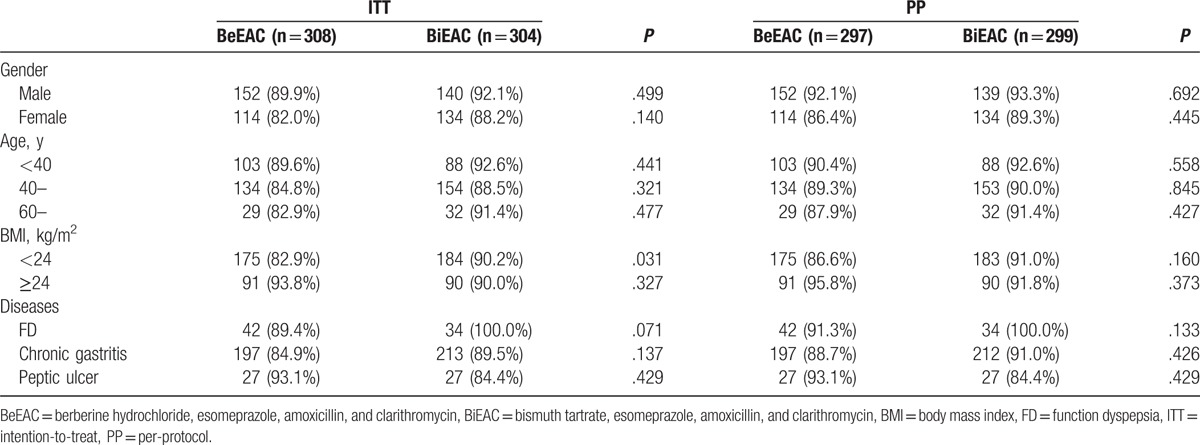
As shown in Fig. 1, 297 patients assigned to the berberine group and 299 assigned to the bismuth group completed the study. As shown in Table 2, in PP analysis, eradication rates between bismuth and berberine groups showed no significant differences (RD: –0.017, 95%CI: [−0.065, 0.030], P = .470). It is consistent with the noninferiority criteria since the lower limit of 95% CI of rate difference was higher than the noninferiority margin value Δ (−0.065 > −0.1). In the ITT analysis, the eradication rates were 90.1% and 86.4% when the missing data of 13C-UBT were estimated as positive (Table 2), which showed no significant difference (P = .148). The age, gender, BMI, and diseases had no obvious influence on H pylori eradication rates in both groups (Table 3).
Table 2.
Eradication rates in the ITT and PP populations.
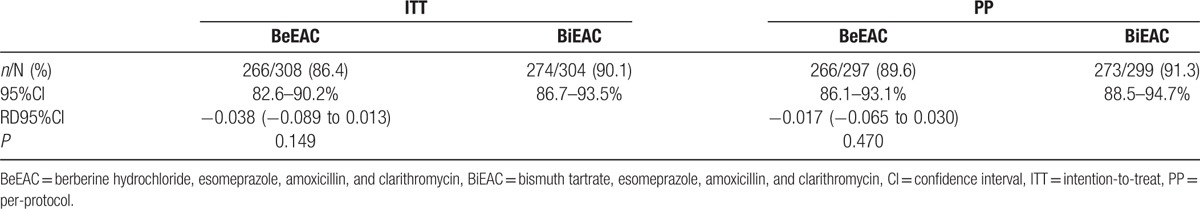
Table 3.
Influences of gender, age, BMI, and disease on the eradication rate in the ITT and PP populations.
3.3. Adverse effects and compliance
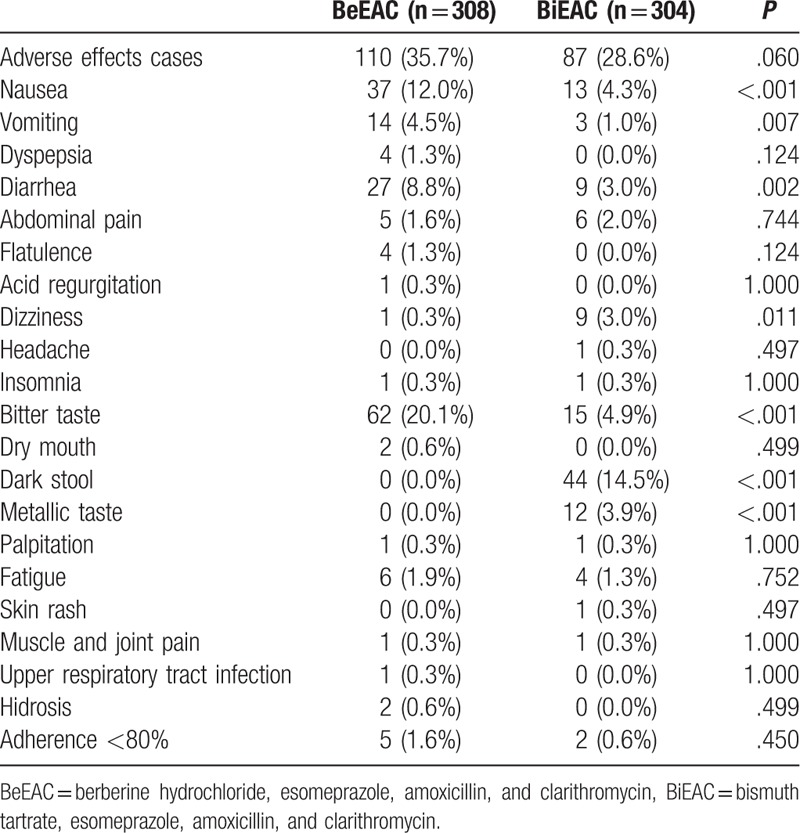
As shown in Table 4, no statistically significant differences were found in the overall incidence of adverse events between berberine and bismuth groups (35.7% vs 28.6%, P = .060). The most common adverse events were gastrointestinal symptoms (including indigestion, nausea, vomiting, and diarrhea), bitter taste, and dark stool. Gastrointestinal symptoms and bitter taste were more common in the berberine group than that in the bismuth group, and melena was only found in the bismuth group. The incidence of severe adverse events and discontinuation of medicines due to adverse events were similar between the 2 groups (< 1.0%). Two patients in the berberine group reported a total of 4 cases of severe adverse events (muscle and joint pain, palpitation, fatigue, and sweating) and thus terminated the trial. Two patients in the bismuth group reported 2 cases of severe adverse events (drug allergy and surgery because of acute exacerbation of chronic cholecystitis) and terminated the trail. No significant differences of medication compliance were found between the 2 groups (P = .450).
Table 4.
Adverse effects of treated population.
4. Discussion
The present study demonstrated that berberine-containing quadruple therapy for H pylori eradication rates is not inferior to bismuth-containing quadruple therapy. Both bismuth-containing and berberine-containing quadruple therapy can be used as the first-line treatment for H pylori eradication in the region.
It was recommended in the Chinese guideline (updated in 2012) that bismuth-containing quadruple therapy is recommended as both first-line and second-line treatment for H pylori infection. Numerous studies have confirmed that the addition of bismuth to triple therapy can significantly improve the eradication rate, due to its direct antibacterial activity and the enhancement eradication of H pylori resistant strains.[13] The population eradication rate with bismuth-containing quadruple therapy is usually associated with the cure rate with resistant strains and the prevalence of resistance. Zhang et al[31] recently conducted a survey of H pylori resistance in the Xi’an area. They found the antibiotic resistant rates of isolated H pylori strains as following: metronidazole 61.3%, amoxicillin 6.5%, tetracycline 4.8%, clarithromycin 33.9% and levofloxacin 25.8%. According to the model suggested by Dore et al,[13] the eradication rate of bismuth quadruple therapy (containing amoxicillin and clarithromycin) should be approximately 87% in the Xi’an area. The present study was conducted in Xi’an and the eradication rate of bismuth-containing quadruple therapy in ITT and PP analysis were 90.1% and 91.3%, respectively. This is very close to the estimated eradication rate. However, a randomized, controlled, open-label clinical trial conducted in China from 2009 to 2011 showed that the eradication rate of 10-day bismuth quadruple therapy varied greatly in different cities, which was especially lower in Xi’an, as 50.0% and 53.6% in ITT and PP analysis.[32] The differences between the previous and the present studies may be explained with the following 2 facts. First, the bismuth dosage (220 mg, bid) used in the present study was standard dose recommend in the Chinese guideline (updated in 2012), whereas a lower dose of bismuth (110 mg, bid) was used in the previous study. Second, the treatment course was 14 days in this study but was 10 days in the previous study. Previous results showed that 14-day regimen is better than 7-day and 10-day regimen, and the eradication effect of full dosage bismuth-containing quadruple therapy is superior to low-dose regimens.[33]
Berberine, an isoquinoline alkaloid isolated from Coptis chinensis (Huanglian in Chinese), has been studied extensively and is known to have antiprotozoal, antibacterial, and anti-inflammatory properties. Also, berberine could be applied in clinical settings to treat diabetes and dyslipidemia.[34] Some pharmacological studies have demonstrated that berberine can inhibit H pylori activity in vivo and in vitro.[17] However, the mechanisms are still unclear. One study conducted by Chung JG showed that berberine could inhibit arylamine N-acetyltransferase activity of H pylori.[35] In addition, berberine could act as a urease inhibitors, which impairs the colonizing and virulent factor of H pylori.[36] The present study indicated berberine could substitute for bismuth in quadruple therapy with satisfactory eradication rates for treatment-naive patients. Accumulated evidence demonstrated that berberine can effectively inhibit and kill H pylori in vitro, even in multidrug resistant strains.[17,19,21,27] And, the present study is the first randomized, controlled trial that provided clinical evidence for berberine in quadruple therapy of H pylori.
In addition, the berberine-containing quadruple therapy is safe and tolerate to patients. Although adverse reactions in the berberine group are slightly higher than that in bismuth group in this study, most of them were mild and there were no significant differences between the 2 groups. The patients could tolerate and complete the 14-d eradication therapy. But when melena is excluded, the adverse events rates were 22.7% (69 / 304) and 35.7% (110 / 308) in the bismuth group and the berberine group, respectively, and the difference was statistically significant. But berberine quadruple therapy still has positive application prospect when considering the lower cost. The therapy cost of 14d berberine hydrochlorides tablets (¥ 11.9) is significantly lower than that of colloidal bismuth tartrate capsules (¥ 112.0) at least in China, which makes berberine quadruple therapy a cheaper regimen for H pylori eradication.
There are also some limitations in this study. First, this study is a single-center trial and patients are mainly from the northwest China (Shaanxi Province and its surrounding areas), whether the result of this study can be applied to other countries or regions is still questionable due to genetics and regions differences. So, further multi-center clinical studies of large samples in other areas are needed before its popularization and application. Second, the antibiotic susceptibility and resistance rate of H pylori are not detected in this study and drug selection is based on empirical medication. Therefore, it could not offer more useful information for antibiotic selection in H pylori eradication therapy in local areas.
In conclusion, our study is a useful exploration for H pylori eradication therapy in the local region. Based on the results of the study, both bismuth-containing and berberine-containing quadruple therapy can be used as first-line treatment for H pylori eradication in the region and berberine-containing quadruple therapy is an effective alternative in patients who would not take bismuth or in region where bismuth is not available. Whether the berberine-containing quadruple therapy can be applied to other countries or regions deserves further investigation.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, BMI = body mass index, H pylori = Helicobacter pylori, ITT = intention-to-treat, PP = per-protocol, UBT = urea breath test.
DZ and LK contributed equally to this study.
Funding: The study was supported by Promotion Project of Excellent Young Investigators in Xijing Hospital (XJZT12J06).
The authors have no conflicts of interest to disclose.
References
- [1].Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175–86. [DOI] [PubMed] [Google Scholar]
- [2].Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009;374:1449–61. [DOI] [PubMed] [Google Scholar]
- [3].Zhang WD, Hu FL, Xiao SD, et al. Prevalence of Helicobacter pylori infection in China. Xiandai Xiaohua Ji Jieru Zhenliao 2010;15:265–70. in Chinese. [Google Scholar]
- [4].Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597–604. [DOI] [PubMed] [Google Scholar]
- [6].Nakamura S, Matsumoto T. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: recent progress in pathogenesis and management. World J Gastroenterol 2013;19:8181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Venerito M, Malfertheiner P. Is mass eradication of Helicobacter pylori infection effective for preventing gastric cancer? Z Gastroenterol 2014;52:744–5. [DOI] [PubMed] [Google Scholar]
- [8].Asaka M, Kato M, Graham DY. Prevention of gastric cancer by Helicobacter pylori eradication. Intern Med 2010;49:633–6. [DOI] [PubMed] [Google Scholar]
- [9].Zheng Q, Chen WJ, Lu H, et al. Comparison of the efficacy of triple versus quadruple therapy on the eradication of Helicobacter pylori and antibiotic resistance. J Dig Dis 2010;11:313–8. [DOI] [PubMed] [Google Scholar]
- [10].Yan X, Zhou L, Song Z, et al. Sequential therapy for Helicobacter pylori eradication in adults compared with triple therapy in china: a multiplecenter, prospective, randomized, controlled trial. Helicobacter 2011;16:87. [Google Scholar]
- [11].Zhou L, Zhang J, Chen M, et al. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. Am J Gastroenterol 2014;109:535–41. [DOI] [PubMed] [Google Scholar]
- [12].Liu WZ, Xie Y, Cheng H, et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis 2013;14:211–21. [DOI] [PubMed] [Google Scholar]
- [13].Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870–8. [DOI] [PubMed] [Google Scholar]
- [14].Vitor JM, Vale FF. Alternative therapies for Helicobacter pylori: probiotics and phytomedicine. FEMS Immunol Med Microbiol 2011;63:153–64. [DOI] [PubMed] [Google Scholar]
- [15].Stamatis G, Kyriazopoulos P, Golegou S, et al. In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J Ethnopharmacol 2003;88:175–9. [DOI] [PubMed] [Google Scholar]
- [16].Bae EA, Han MJ, Kim NJ, et al. Anti-Helicobacter pylori activity of herbal medicines. Biol Pharm Bull 1998;21:990–2. [DOI] [PubMed] [Google Scholar]
- [17].Ma F, Chen Y, Li J, et al. Screening test for anti-Helicobacter pylori activity of traditional Chinese herbal medicines. World J Gastroenterol 2010;16:5629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shi DH, Liu YW, Liu WW, et al. Inhibition of urease by extracts derived from 15 Chinese medicinal herbs. Pharm Biol 2011;49:752–5. [DOI] [PubMed] [Google Scholar]
- [19].Jiang C, Yan C, Liu W, et al. Experiment of 15 kinds of Chinese herbs in inhibiting Helicobacter pylori in vitro. Fujian Zhongyiyao Daxue Xuebao 2003;13:30–2. in Chinese. [Google Scholar]
- [20].Zhang W, Zhang X, Huang Z, et al. Screening test for the killing and inhibiting effect of Chinese herbs on Helicobacter pylori. Xiandai Xiaohua Ji Jieru Zhenliao 2001;6:55–6. in Chinese. [Google Scholar]
- [21].Wang X, Jiao W, Lu Z, et al. Preliminary screening of Chinese Herbal medicine in inhibiting Helicobacter pylori. Zhongguo Zhongxiyi Jiehe Zazhi 1994;14:534–6. in Chinese. [PubMed] [Google Scholar]
- [22].Xu Z, Zhou D, Zhou D, et al. Inhibition and killing effects of traditional Chinese medicine on Helicobacter pylori. Zhonghua Zhongyiyao Zazhi 1993;8:25–6. in Chinese. [Google Scholar]
- [23].Yao J, Kong W, Jiang J. Learning from berberine: treating chronic diseases through multiple targets. Sci China Life Sci 2015;58:854–9. [DOI] [PubMed] [Google Scholar]
- [24].Lan J, Zhao Y, Dong F, et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol 2015;161:69–81. [DOI] [PubMed] [Google Scholar]
- [25].Chen C, Yu Z, Li Y, et al. Effects of berberine in the gastrointestinal tract—a review of actions and therapeutic implications. Am J Chin Med 2014;42:1053–70. [DOI] [PubMed] [Google Scholar]
- [26].Derosa G, Maffioli P, Cicero AF. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther 2012;12:1113–24. [DOI] [PubMed] [Google Scholar]
- [27].Wu M, Huang Y, Huang Z, et al. In vitro bacteriostatic effect of berberine, emodin,schisandra, and baicalin on multidrug resistant strains of Helicobacter pylori. Shijie Huaren Xiaohua Zazhi 2013;21:3247–51. in Chinese. [Google Scholar]
- [28].Huang Y, Huang X, Zhao L, et al. Effects of emodin and other herbal extracts on resistance of Helicobacter pylori to clarithromycin. Shijie Huaren Xiaohua Zazhi 2014;22:825–30. in Chinese. [Google Scholar]
- [29].Cheng H, Hu F, Xie Y, et al. Prevalence of Helicobacter pylori resistance to antibiotics and its influence on treatment outcome in China: a Multicenter Clinical Study. Weichangbing Xue 2007;12:525–30. in Chinese. [Google Scholar]
- [30].Alahdab YO, Kalayci C. Helicobacter pylori: management in 2013. World J Gastroenterol 2014;20:5302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang LX, Song Y, Zhang L, et al. Antibiotics resistance status and change trend of Helicobacter pylori in Xi’an area. Xiandai Xiaohua Ji Jieru Zhenliao 2013;18:252–4. in Chinese. [Google Scholar]
- [32].Liang J, Li J, Han Y, et al. Helicobacter pylori eradication with ecabet sodium, omeprazole, amoxicillin, and clarithromycin versus bismuth, omeprazole, amoxicillin, and clarithromycin quadruple therapy: a randomized, open-label, phase IV trial. Helicobacter 2012;17:458–65. [DOI] [PubMed] [Google Scholar]
- [33].Lu H, Zhang W, Graham DY. Bismuth-containing quadruple therapy for Helicobacter pylori: lessons from China. Eur J Gastroenterol Hepatol 2013;25:1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wei S, Zhang M, Yu Y, et al. Berberine attenuates development of the hepatic gluconeogenesis and lipid metabolism disorder in type 2 diabetic mice and in palmitate incubated HepG2 cells through suppression of the HNF-4α miR122 pathway. PLoS One 2016;11:e0152097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chung JG, Wu LT, Chang SH, et al. Inhibitory actions of berberine on growth and arylamine Nacetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Int J Toxicol 1999;18:35–40. [DOI] [PubMed] [Google Scholar]
- [36].Tan LH, Li CL, Chen HB, et al. Epiberberine, a natural protoberberine alkaloid, inhibits urease of Helicobacter pylori and jack bean: susceptibility and mechanism. Eur J Pharm Sci 2017;(In press). [DOI] [PubMed] [Google Scholar]


