Abstract
Rationale:
The purpose of this study was to identify the chemical responsible for cholestatic hepatitis in a 55-year-old woman who ingested 1,1′-iminodi (octamethylene) diguanidinium triacetate (iminoctadine triacetate), a fungicide. The fungicide formulation was also composed of polyoxyethylene nonylphenol (NP-40) and methanol.
Patient concerns:
Severe cholestatic hepatitis developed, which led to the patient's death on day 88 of hospitalization. Post-mortem necropsy of the liver showed focal hepatocyte necrosis involving mostly the mid-zone, along with intracytoplasmic and intracanalicular cholestasis.
Diagnoses:
To identify the chemical responsible for hepatic injury, the cellular toxicity of all chemicals in the fungicide formulation was assessed in HepG2 cells using the 3-(4,5-dimethylthiaxol-2yl)-2, 5-diphenyl tetrazolium bromide test.
Outcomes:
Viability of cells treated with the surfactant NP-40 was significantly lower (P < .001), but that of cells treated with other components of the fungicide, including the active ingredient, iminoctadine triacetate, was unaffected. Fluorescence-activated cell sorting analysis confirmed that necrosis was induced in HepG2 cells treated with 25–80 μM of NP-40, while significant numbers of apoptotic cells were not detected.
Lessons:
NP-40 appears to be the chemical responsible for the patient's irreversible hepatic injury, accompanied by intracytoplasmic and intracanalicular cholestasis.
Keywords: cholestatic hepatitis, iminoctadine triacetate, pesticide poisoning, polyoxyethylene nonylphenol, surfactants
1. Introduction
Pesticides pose inherent hazards to human health. The World Health Organization has estimated that 3 million workers in developing countries experience severe poisoning from pesticides each year, and approximately 18,000 of them eventually die.[1,2] The degree of human exposure to pesticides varies widely, from a mild exposure of farm workers spraying crops to accidental or deliberate ingestion of undiluted pesticides. In some Asian countries, pesticide self-poisoning is a common method of suicide.[3,4] Under this circumstance, the majority of patients are hospitalized, and physicians undertake a lifesaving-treatment modality.
Common symptoms of pesticide toxicity from a lethal dose include shock, unconsciousness, and failure of organs such as the liver, kidney, and lungs.[5,6] However, data from the mammalian toxicological database are not informative enough to evaluate the acute toxicity induced by a lethal dose. One reason is that pesticide health hazard evaluations are carried out based on the lowest observed adverse effect level,[7] and the results do not consider the pathophysiology of acute lethal dose intoxication.
Another problem is the lack of toxicological data on additives in pesticide formulations. In some cases, the toxicity of the additives may be greater than that of the active ingredient.[8,9]
Solvents, emulsifiers, and anti-freeze agents, if added, are highly regulated and are evaluated regarding their safety. Hence, physicians should strive to collect data from the literature or the manufacturer. However, in the present case for this particular fungicide, there were no usage instructions or warnings in the form of labeling details or package insert. Accordingly, there were no available details concerning the chemical class of this fungicide or its potential toxicity.
In the following report, we present a case of acute cholestatic hepatitis after ingestion of the fungicide iminoctadine. The patient died of hepatic failure on day 88 of hospitalization. Pathologic findings from a post-mortem necropsy of the liver showed necrotic hepatitis with cholestasis. It is extremely rare for a patient to develop severe hyperbilirubinemia (peak level 47.3 mg/dL) and die of cholestatic hepatitis after ingesting a pesticide; this was the first such case at our pesticide intoxication institute in the past 3 decades. We could not elucidate the pathophysiology of hepatic injury in this patient. Our major aim was to determine the specific chemical responsible for the hepatic injury.
From the clinical findings, we surmised that the ingredient responsible was not the active pesticide chemical but the added surfactant. In this article, we present the results of in vitro experiments to test our hypothesis.
2. Materials and methods
2.1. Case report
2.1.1. Case presentation
A 55-year-old woman was transferred to our pesticide intoxication institute approximately 5 hours after ingesting 200 mL of a fungicide containing iminoctadine triacetate. The estimated amount ingested was 50 mL (total ingested volume 200 mL × inserted rate 25%, Table 1). As the clinical course of the patient was unusual with severe cholestatic hepatopathy, causing death, we attempted to identify the responsible chemicals in the fungicide formulation. The fungicide formulation is summarized in Table 1. This study was approved by the Institutional Review Board of Soonchunhyang Cheonan Hospital (IRB no. 2013–12–010). Written informed consent for necropsy and academic use (publication) of the pathologic findings was obtained from the patient's husband.
Table 1.
Formulation of the iminoctadine triacetate fungicide.

On arrival, the patient was mentally alert, and her blood pressure was 100/60 mm Hg, which she stated was normal for her; the respiratory rate was 20/min, and body temperature was 36.7°C. On physical examination, no specific abnormality was observed, except mild tachycardia (pulse rate 95/min). Conservative treatment was applied, including intravenous fluid administration. The patient lost her appetite, and total parenteral nutrition was provided from day 7 of hospitalization until death. As acute renal failure occurred, regular hemodialysis was conducted every other day until renal function was restored on day 50 of hospitalization. However, the jaundice continued to worsen, conjugated bilirubin levels increased >40 mg/dL, and the patient died of hepatic failure on day 88.
Basal laboratory findings, including complete blood count (CBC), blood chemistry, and routine urinalysis parameters, were within normal ranges, with the exception of mild leukocytosis (Table 2). From day 2 of hospitalization, both liver and renal function deteriorated. Sequential measurement of liver function is shown in Fig. 1. Clinically, the distinction between hepatocellular and intrahepatic cholestasis is indicated by the R value, the ratio of alanine aminotransferase (ALT) to alkaline phosphatase (ALP), both expressed as multiples of the upper limit of normal values. An R value >5.0 is associated with hepatocellular injury, R < 2.0 with cholestasis injury, and R values between 2.0 and 5.0 with mixed hepatocellular-cholestatic hepatopathy.
Table 2.
Change in the results of urinalysis, CBC, and blood chemistry during the admission.
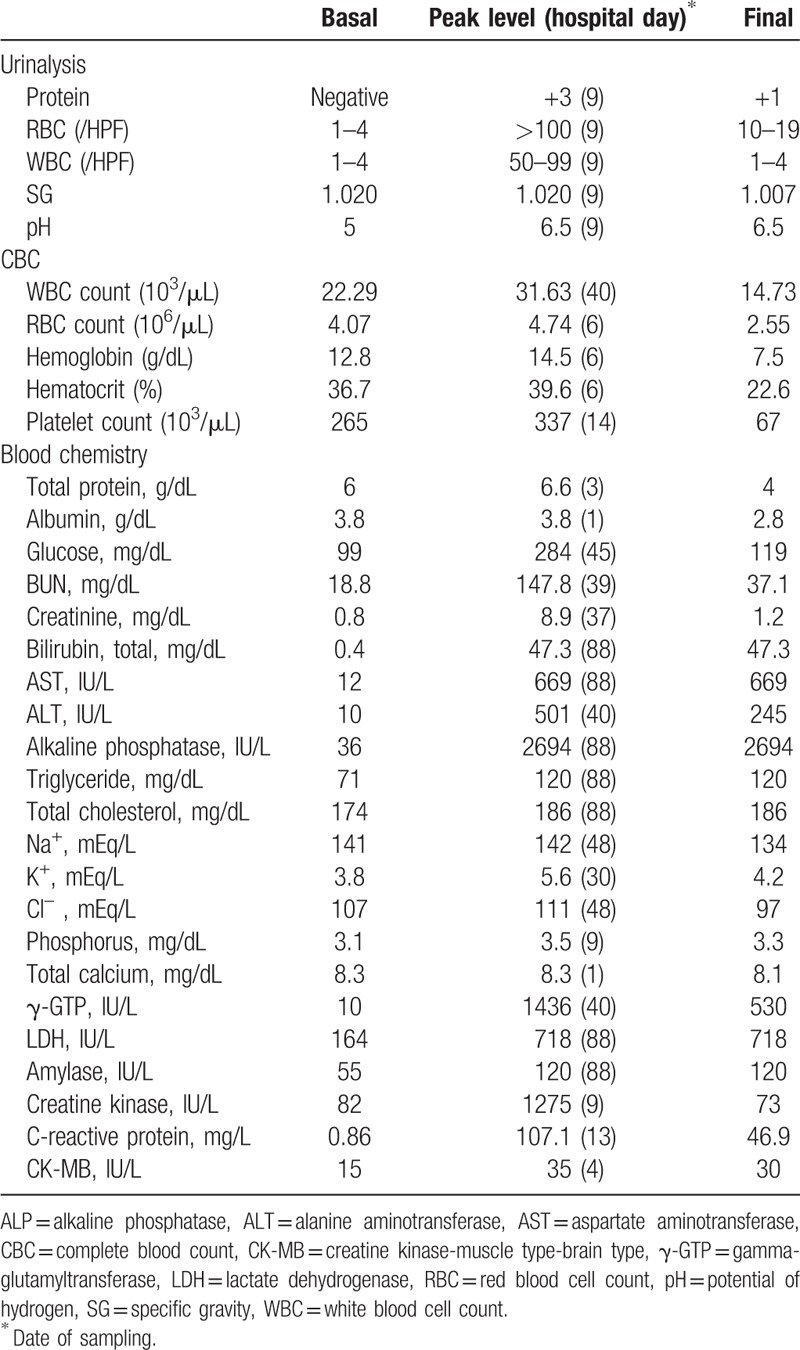
Figure 1.
Sequential measurements of blood chemistry for liver and kidney function. Note: over 90% of the total bilirubin in plasma was in the conjugated form in all the sequential measurements.
2.1.2. Histopathology
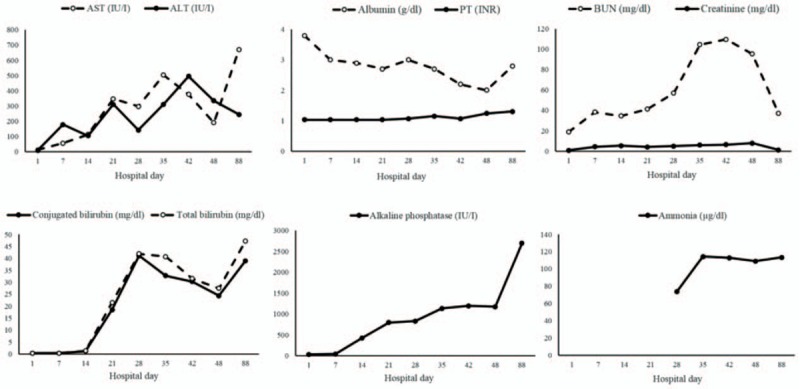
The general condition of the patient was too poor to allow renal or liver biopsy during her hospitalization. Instead, a post-mortem liver biopsy was conducted immediately after death (Fig. 2). Liver samples were stained with hematoxylin and eosin, as well as with the immunohistochemical stain cytokeratin 7, and examined microscopically.
Figure 2.
Pathologic findings from postmortem liver necropsy. (A) Focal hepatocyte necrosis involving the mid-zone (hematoxylin and eosin stain, original magnification ×200). (B) Bile accumulation in the cytoplasm of hepatocytes and canaliculi (hematoxylin and eosin stain, original magnification ×400). (C) Bile duct distortion and nuclear atypia in ductal epithelial cells (hematoxylin and eosin stain, original magnification ×400). (D) Bile duct distortion in the portal area (immunohistochemical cytokeratin 7 stain, original magnification ×400).
2.2. In vitro experiments
2.2.1. Cell line and compounds
A human liver cancer cell line (HepG2) was purchased from the Korean Cell Line Bank (KCLB; Seoul, South Korea) and maintained in DMEM media supplemented with 10% FBS and 1% penicillin-streptomycin antibiotic. Polyoxyethylene nonylphenol (NP-40, CAS number 9016-45-9), Triton X-114, iminoctadine triacetate (CAS number 39202-4-9), and methanol were purchased from Sigma-Aldrich (St. Louis, MO). Formaldehyde was obtained from Wako (Osaka, Japan), and formic acid from Daejung (Seoul, South Korea). 3-(4, 5-Dimethylthiaxol-2yl)-2, 5-diphenyl tetrazolium bromide (MTT) was obtained from Sigma-Aldrich.
2.2.2. Cell viability assay
To identify the chemical responsible for hepatic injury, cellular toxicity of all chemicals in the pesticide formulation was assessed in HepG2 cells. HepG2 cells were seeded and grown in a 96-well plate at 3 × 105/well and 37°C in a 5% CO2 humidified incubator. Cells were then allowed to reach 70% to 80% confluence overnight. Next, cells were treated with different concentrations (0.1, 1, 10, and 100 μM) of NP-40, Triton X-114, iminoctadine triacetate, methanol, formic acid, and formaldehyde for 6 hours. Formaldehyde and formic acid were not ingredients in the formulation, but were included because they represent toxic metabolites of methanol in vivo.
Upon completion of incubation, MTT was added to the medium at a final concentration of 0.5 mg/mL, and cells were incubated for 3 hours at 37°C. MTT is a water-soluble dye that is readily incorporated into viable cells and reduced by mitochondrial dehydrogenase. The reduction product is water-insoluble blue formazan, which was solubilized in 100 μL of dimethylsulfoxide (DMSO; Sigma-Aldrich). In order to avoid any potential toxicity of the solvent, we included triton X-114 as a negative control. The color intensity was measured at 595 nm using a Victor X3 microplate reader (PerkinElmer, Waltham, MA).
2.2.3. Detection of apoptosis and necrosis by fluorescence-activated cell sorting (FACS)
Cells were grown overnight to 70% to 80% confluence to evaluate the effects of NP-40 on apoptosis and necrosis. Treated cells were washed with phosphate-buffered saline, trypsinized, and harvested by centrifugation, after which the apoptosis assay was carried out using an Alexa Fluor 488 Annexin V/Dead Cell Apoptosis Kit (Invitrogen, Carlsbad, CA). For the necrosis assay, cells were stained with 5 μL of fluorescein isothiocyanate (FITC)-conjugated annexin V and 1 μL of 100 μg/mL propidium iodide (PI) for each 100-μL cell suspension for 15 minutes at 25°C to 30°C. Apoptosis was measured by FITC and PI emissions at 515 to 545 nm and 564 to 606 nm, respectively; a minimum of 10,000 cells were examined per data point by flow cytometry (Becton Dickinson, San Jose, CA). Data were analyzed using FACSDiva v.6.1.3 software (Becton Dickinson). Cells that stained positive for annexin V and negative for PI were considered early apoptotic cells; those that were positive for both annexin V and PI were considered to be in the late stage of apoptosis, undergoing necrosis, or already dead. Cells that were not stained with either annexin V or PI were considered to be alive and not undergoing measurable apoptosis.
2.2.4. Data analysis
Statistical analyses were performed using SPSS software (version 14.0; IBM SPSS, Armonk, NY). All data were analyzed using Student's t-test and are expressed as the mean ± SD of at least 3 independent experiments, unless otherwise noted. Statistical significance was assessed at P < .05.
3. Results
3.1. Clinical outcome
The yellowish color of skin and sclera intensified over time. Results of sequential measurements of aspartate transaminase, ALT, albumin, prothrombin time, blood urea nitrogen, creatinine, bilirubin, ALP, and ammonium are presented in Fig. 1. The R value, calculated from the ratio of ALT to ALP, was >2 during the initial 2 weeks, peaked on day 7 (R = 11.8), and then decreased to < 2 and remained low until death on day 88.
Histopathology of the postmortem liver biopsy showed focal hepatocyte necrosis involving mostly the mid-zone and intracytoplasmic and intracanalicular cholestasis. Bile plug was observed in dilated ducts. In the portal area, bile duct distortion and atypical nuclei were noted on ductal epithelial cells without prominent inflammation (Fig. 2).
3.2. In vitro experiments
3.2.1. MTT assay
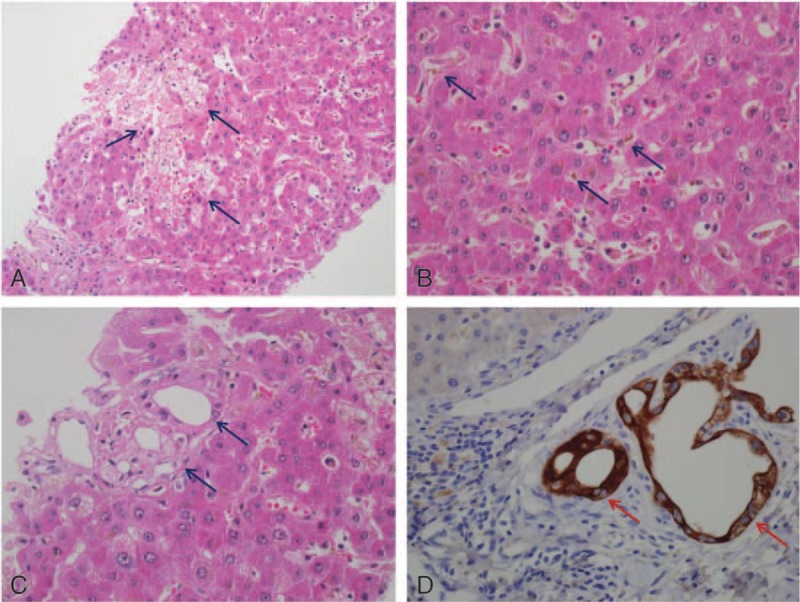
The viability of cells treated with different concentrations of NP-40, Triton X-114, iminoctadine triacetate, methanol, formic acid, and formaldehyde for 6 hours was compared using the MTT assay. Findings revealed that methanol and formaldehyde did not reduce cell viability at concentrations below 10 μM; treatment with 100 of μM methanol or formaldehyde reduced cell viability by 87% and 89%, respectively (P < .05). Formic acid displayed no cytotoxicity at any of the tested concentrations. Iminoctadine triacetate, the active ingredient in the pesticide formulation, displayed no toxic effects at 0.1 μM; however, 10%, 11%, and 22% reductions in cell viability were observed at 1, 10, and 100 μM, respectively (P < .05). Treatment with Triton X-114 reduced cell viability by 60% at a concentration of 100 μM (P < .001), but lower concentrations did not result in significant cytotoxicity. NP-40 reduced cell viability by 75% to 85% at concentrations of 0.1 to 10 μM (Fig. 3).
Figure 3.
Cytotoxic effect of NP-40, Triton X-114, iminoctadine triacetate, and methanol and its metabolites on HepG2 cells by the MTT assay. Concentrations of the chemicals were 0.1, 1, 10, and 100 μM. Untreated cells were used as controls. Data are presented as the mean ± SD of 3 independent experiments (∗P < .05, ∗∗P < .01, ∗∗∗P < .001). MTT = 3-(4,5-dimethylthiaxol-2yl)-2,5-diphenyl tetrazolium bromide, SD = standard deviation.
We also tested multiple concentrations of NP-40 to establish whether cytotoxicity was concentration-dependent in HepG2 cells. The cell viability at 12.5, 25, 50, 80, 100, and 200 μM of NP-40 was 86.6%, 83.3%, 74.3%, 69.3%, 22%, and 5.68%, respectively (Fig. 4).
Figure 4.
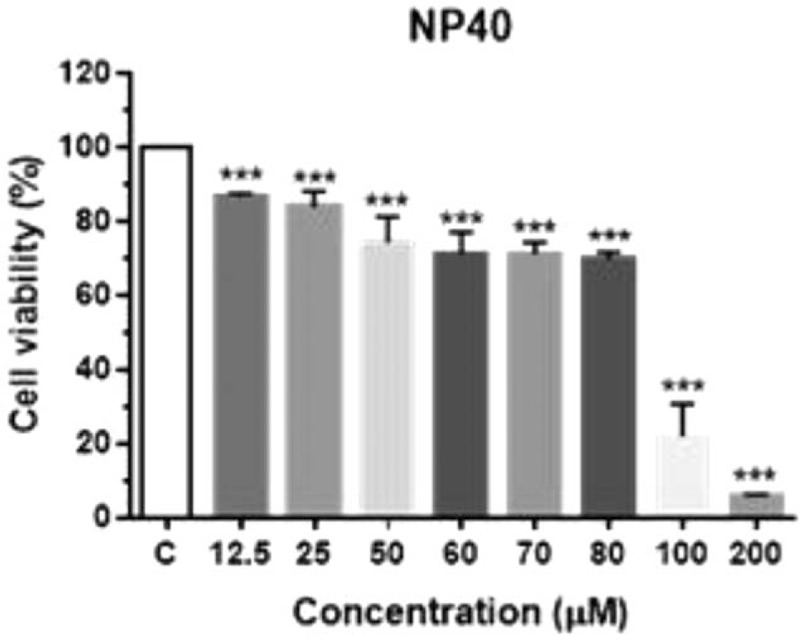
HepG2 viability following NP-40 treatment. Cells were exposed to NP-40 (12.5 μM to 200 μM) for 6 hours. Untreated cells were used as controls. Data are presented as the mean ± SD of 3 independent experiments (∗P < .05, ∗∗P < .01, ∗∗∗P < .001). HepG2 = a human liver cancer cell line, NP-40 = polyoxyethylene nonylphenol, SD = standard deviation.
3.2.2. NP-40-induced necrosis in HepG2 cells
FACS analysis confirmed that necrosis was induced in HepG2 cells treated with 25 to 80 μM of NP-40, whereas a significant number of apoptotic cells was not detected. Treatment with 25 μM of NP-40 resulted in 1.16% early and late apoptotic cells, and 11.1% necrotic cells (P < .001). When the concentration was increased incrementally, a similar trend of dominant necrosis and negligible apoptosis was observed. At an NP-40 concentration of 70 μM, the proportion of early and late apoptotic cells was only 2%, but that of necrotic cells was 61.74% (Fig. 5).
Figure 5.
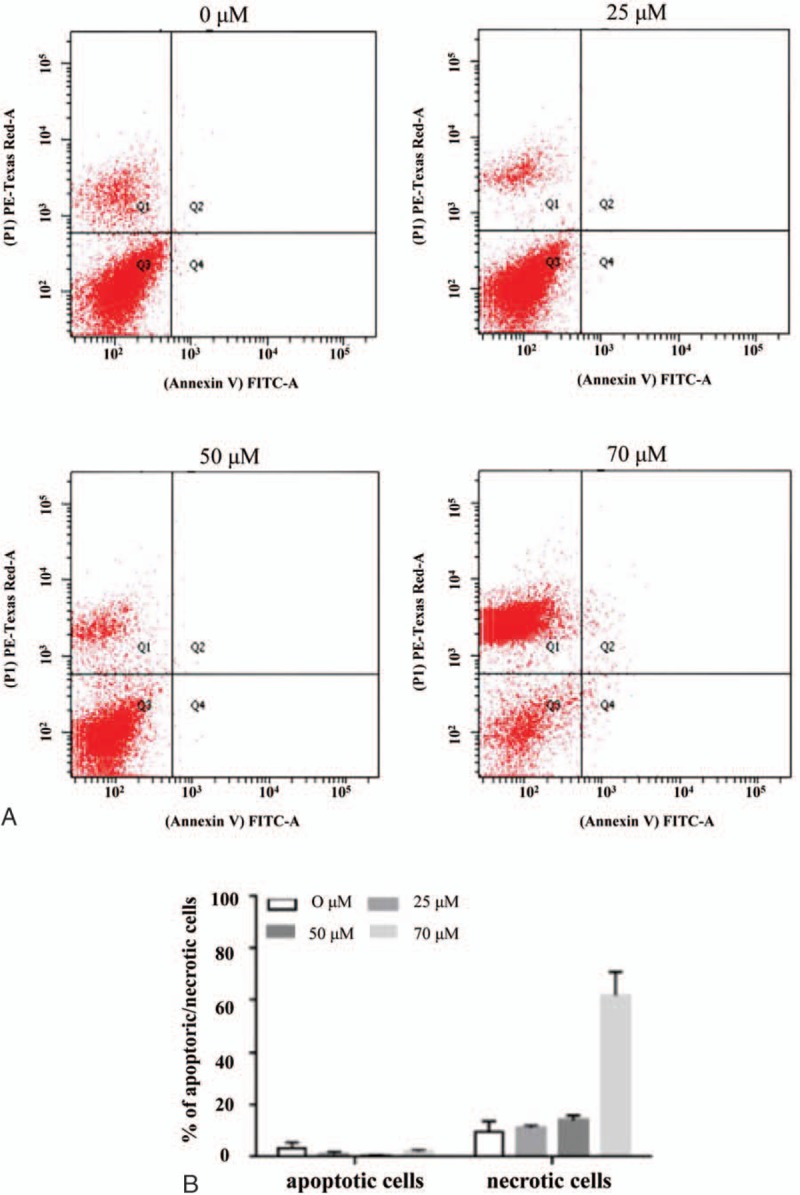
NP-40-induced necrosis in HepG2 cells. (A) Cells were treated with 25, 50, and 80 μM NP-40 for 6 hours. Apoptotic (annexin V +/PI − ) and necrotic (annexin V − /PI +) cells were detected by fluorescence-activated cell sorting. (B) Rate of apoptosis and necrosis was determined by counting apoptotic and necrotic cells as a percentage of the total number of cells. Data represent the mean ± SD of 3 independent experiments. HepG2 = a human liver cancer cell line, NP-40 = polyoxyethylene nonylphenol, SD = standard deviation.
4. Discussion
Iminoctadine [N,N′′′-(iminodi-8,1-octanediyl) bisguanidine] exerts a fungicidal effect by interrupting the biosynthesis of the fungal cell membrane.[10] The acute oral LD50 in the experimental animal has been reported as 187 mg/kg in rats and 308 mg/kg in mice (A world Compendium: The Pesticide Manual, Sixteenth Edition, Editor: MacBean, 2012, British Crop Production Council).
However, in humans, little is known about the pathophysiology of the toxicity of iminoctadine triacetate. It is not listed in WHO acute Hazard Toxicity information, and there is no consensus value in acute rating from U.S. EPA product labels (Pesticide Action Network North America: WWW.panna.org/).
Koyama et al[11] reported that iminoctadine caused circulatory failure in acute oral poisoning. Studies in rats concluded that severe hypotension induced by an iminoctadine triacetate-containing fungicide was attributable mainly to the vasodilatory effect of iminoctadine and partly to the cardiosuppressive effect of the surfactant polyoxyethylene alkylether.[12,13] However, were unable to find published reports of cholestatic hepatopathy in humans caused by ingestion of an iminoctadine triacetate fungicide using the PubMed-NCBI and Google Scholar search engines.
A remarkable clinical feature of our case was severe hyperbilirubinemia. Since over 90% of the total bilirubin in plasma was in the conjugated form in all the sequential measurements, and abdominal imaging showed no extrahepatic biliary tract occlusion, it seemed likely that our patient's cholestasis was caused by impaired bilirubin excretion, at the bile duct or bile canaliculi level. In that case, the chemicals in the pesticide formulation and the mechanism of toxicity responsible for the hepatitis and the accompanying severe cholestasis in the current patient should be identified.
Direct toxic hepatitis is known to occur with predictable regularity in individuals exposed to an injurious agent; the hepatitis is usually dose-dependent.[14] The latent period between exposure and liver injury is generally several hours, although clinical manifestation may be delayed for 24 to 48 hours.[15] In the current case, abnormal liver function was first detected 2 days after ingestion.
The MTT assay showed that cell viability was reduced not by the active ingredient, iminoctadine triacetate, but by the surfactant NP-40. We have previously performed a number of studies investigating the roles of surfactants in pesticide intoxication.[8,16] These studies revealed that surfactants stimulate cell damage in a variety of ways, including breaking down the cell membrane, altering metabolic and mitochondrial activity, disrupting protein synthesis, and facilitating mitochondrial damage-induced apoptosis and necrosis.[17] A study has shown that NP-40 induces gene expression changes leading to genotoxicity and activates genes involved in the cell death (necrosis signaling) pathway.[18] In accordance with the results from that study, the pathologic findings of the current case included an abnormal appearance of nuclei in bile duct epithelial cells without prominent inflammation in the portal area. These findings suggest that injury of bile duct epithelial cells by NP-40 was the cause of the severe cholestasis we observed in the patient.
The other characteristic of hepatopathy in the current case was its irreversibility. During hospitalization, the renal failure was completely reversed; in contrast, hepatic failure, in particular hyperbilirubinemia, was not reversed, leading to death.
What, then, was the cause of irreversible damage in the bile excretion system in this patient? Three mechanisms may explain the irreversible hepatopathy. First, NP-40 injures cells permanently, leading to hepatic cell death. In our in vitro experiment, NP-40 induced concentration-dependent cytotoxicity, supporting the hypothesis that direct toxicity from a high dose of NP-40 was responsible for the irreversible hepatic injury we observed. However, further study is required to elucidate the toxicokinetics of NP-40.
Second, the result of FACS analysis in the current study demonstrated that the majority of cells underwent necrotic change, bypassing apoptosis, even at low concentrations of NP-40. This finding suggests that the liver may be vulnerable to NP-40.
Third, genetic differences in the response to NP-40 may influence the severity of liver cell damage.[18,19] Further studies are required to assess whether NP-40 is hepatotoxic in the general population, or whether our patient was particularly vulnerable to its effects.
The current study had some limitations. First, owing to technical difficulties, we did not measure the plasma level of NP-40 in this patient. Furthermore, we were unable to find information on the absorption, metabolism, disposition, and excretion properties and toxicological effects of iminoctadine triacetate and NP-40 are not available in humans (search engines: PubMed-NCBI, Google Scholar).
Second, in the MTT assay system, reduction of MTT (tetrazolium dye) depends on cellular metabolic activity. However, assay conditions can alter metabolic activity and thus tetrazolium dye reduction without affecting cell viability. Therefore, the result of our MTT test may not represent the toxicity of iminoctadine triacetate.
In conclusion, the current in vitro experiments, in conjunction with previous reports regarding NP-40 toxicity,[8,16,17,18] suggest that NP-40 is a strong candidate for the chemical responsible for the irreversible hepatic injury, accompanied by intracytoplasmic and intracanalicular cholestasis, in a patient who ingested an iminoctadine fungicide.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CBC = complete blood count, CK-MB = creatine kinase-muscle type-brain type, FACS = fluorescence-activated cell sorting, γ-GTP = gamma-glutamyltransferase, HepG2 = a human liver cancer cell line, Iminoctadine triacetate = 1,1′-iminodi (octamethylene) diguanidinium triacetate, LDH = lactate dehydrogenase, MTT = 3-(4,5-dimethylthiaxol-2yl)-2,5-diphenyl tetrazolium bromide, NP-40 = polyoxyethylene nonylphenol, pH = potential of hydrogen, RBC = red blood cell count, SG = specific gravity, WBC = white blood cell count.
JM and JH contributed equally to this study as first authors.
Funding: This work was conducted with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project no. PJ01083201), Rural Development Administration, Republic of Korea.
The authors have no conflicts of interest to disclose.
References
- [1].Litchfield MH. Estimates of acute pesticide poisoning in agricultural workers in less developed countries. Toxicolog Rev 2005;24:271–8. [DOI] [PubMed] [Google Scholar]
- [2].Qi H, Liu X. Review and further explain the new “guidelines for diagnosis and treatment of intrahepatic cholestasis of pregnancy (2015)’. Zhonghua Fu Chan Ke Za Zhi 2015;50:486–8. [PubMed] [Google Scholar]
- [3].Pitman A, Krysinska K, Osborn D, et al. Suicide in young men. Lancet 2012;379:2383–92. [DOI] [PubMed] [Google Scholar]
- [4].Wu KC, Chen YY, Yip PS. Suicide methods in Asia: implications in suicide prevention. Int J Environ Res Pub Health 2012;9:1135–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hulse EJ, Davies JO, Simpson AJ, et al. Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am J Resp Crit Care Med 2014;190:1342–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Woo JH, Lim YS. Severe human poisoning with a flufenoxuron-containing insecticide: report of a case with transient myocardial dysfunction and review of the literature. Clin Toxicol 2015;53:569–72. [DOI] [PubMed] [Google Scholar]
- [7].Zarn JA, Engeli BE, Schlatter JR. Study parameters influencing NOAEL and LOAEL in toxicity feeding studies for pesticides: exposure duration versus dose decrement, dose spacing, group size and chemical class. Regul Toxicol Pharmacol 2011;61:243–50. [DOI] [PubMed] [Google Scholar]
- [8].Hwang I, Lee JW, Kim JS, et al. Surfactant toxicity in a case of (4-chloro-2-methylphenoxy) acetic acid herbicide intoxication. Hum Exp Toxicol 2015;34:848–55. [DOI] [PubMed] [Google Scholar]
- [9].Song HY, Kim YH, Seok SJ, et al. In vitro cytotoxic effect of glyphosate mixture containing surfactants. J Korean Med Sci 2012;27:711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kawamoto T, Yano M, Makihata N. Analytical method for determining iminoctadine triacetate by LC/ESI/MS using hydrophilic interaction chromatography. Anal Sci 2006;22:489–90. [DOI] [PubMed] [Google Scholar]
- [11].Koyama K, Yamashita M, Miyauchi T, et al. A fungicide containing iminoctadine causes circulatory failure in acute oral poisoning. Vet Hum Toxicol 1993;35:512. [PubMed] [Google Scholar]
- [12].Koyama K, Goto K, Yamashita M. Circulatory failure caused by a fungicide containing iminoctadine and a surfactant: a pharmacological analysis in rats. Toxicol Appl Pharmacol 1994;126:197–201. [DOI] [PubMed] [Google Scholar]
- [13].Koyama K, Yamashita M, Miyauchi T, et al. Mechanisms of hypotension in iminoctadine poisoning: pharmacological analysis in rats. Eur J Pharmacol 1994;270:151–5. [DOI] [PubMed] [Google Scholar]
- [14].Chen M, Suzuki A, Borlak J, et al. Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol 2015;63:503–14. [DOI] [PubMed] [Google Scholar]
- [15].Fisher K, Vuppalanchi R, Saxena R. Drug-induced liver injury. Arch Pathol Lab Med 2015;139:876–87. [DOI] [PubMed] [Google Scholar]
- [16].Seok SJ, Park JS, Hong JR, et al. Surfactant volume is an essential element in human toxicity in acute glyphosate herbicide intoxication. Clin Toxicol 2011;49:892–9. [DOI] [PubMed] [Google Scholar]
- [17].Song HY, Kim YH, Seok SJ, et al. Cellular toxicity of surfactants used as herbicide additives. J Korean Med Sci 2012;27:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Park S, Hwang IW, Kim JS, et al. The effects of nonyl phenoxypolyethoxyl ethanol on cell damage pathway gene expression in SK-NSH cells. Korean J Int Med 2015;30:873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Urban TJ, Daly AK, Aithal GP. Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis 2014;34:123–33. [DOI] [PubMed] [Google Scholar]


