Abstract
In early-onset bacteremia among preterm neonates, Escherichia coli (E. coli) is the main pathogen and can cause a high mortality rate. Thus, the predictive factors of mortality and extended-spectrum β-lactamase (ESBL)-producing E. coli in preterm babies with E. coli early-onset bacteremia were reported.
We retrospectively reviewed preterm neonates who had E. coli bacteremia occurring within 3 days after birth between 2004 and 2015. Maternal and perinatal information were collected from their medical records and analyzed by comparing the survival and nonsurvival groups, and also the ESBL-producing and non-ESBL-producing E. coli bacteremia groups. Mann–Whitney U test, Fisher exact test, and multivariate Cox proportional-hazard model were used for statistical analysis.
A total of 27 preterm babies had E. coli bacteremia. The overall mortality rate was 55.56% (15 deaths). Five babies had ESBL-producing E. coli. The low systolic blood pressure of <48 mm Hg and low absolute neutrophil count of <2318 cells/mm3 were the most significant factors in predicting mortality. Moreover, the level of serum alanine aminotransferase was significantly lower in the ESBL-producing E. coli group than that in the non-ESBL-producing E. coli group.
Therefore, the lower systolic blood pressure and absolute neutrophil count were the risk factors of mortality in preterm babies with early-onset E. coli bacteremia, and alanine aminotransferase could be a significant factor in predicting ESBL-producing E. coli.
Keywords: Escherichia coli, neonate, premature, sepsis
1. Introduction
According to the data from the World Health Organization (WHO), approximately 2.8 million deaths occurred during the neonatal period in 2013, with more than one-third (36%) resulting due to infections.[1] Neonatal sepsis occurred more frequently in premature babies due to their immature immune system. In a cohort study, the incidence of early-onset neonatal sepsis (occurring within the first 72 hours of life) was 15 to 19 per 1000 live births, with Gram-negative organisms being the causative factor in more than half of these cases.[2] Unfortunately, the clinical signs and symptoms of neonatal bacteremia are unspecific, and the presence of feeding intolerance, self-resolving apnea or bradycardia, mild tachypnea or tachycardia, or decreased activity may be the only warning signs before fulminant clinical deterioration. Thus, a septic work-up that includes complete cell count and differential count, C-reactive protein, urinalysis, chest x-ray, and blood, urine, or cerebrospinal fluid were arranged at the time of admission for all neonates suspected of having bacterial infection.
Neonatal bacteremia is determined on positive blood cultures,[3] which may take at least 3 days. Thus, empiric antibiotics such as ampicillin and gentamicin or cefotaxime are routinely prescribed before the culture results are available for covering the common pathogens. Recently, the incidence of group B streptococci (GBS) infection was significantly decreased owing to the introduction of GBS intrapartum antibiotic prophylaxis, even if GBS remains a common pathogen in the early-onset bloodstream infection (BSI) in neonates.[4] Moreover, Escherichia coli (E. coli) is currently the most common pathogen and has a higher mortality rate than GBS in preterm infants.[5]
The frequency of antimicrobial resistance such as extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae is around 6% in the early-onset BSI.[6] Thus, BSI with ESBL-producing E. coli infection in preterm babies is a big challenge to clinicians, because the routinely prescribed antibiotics do not cover ESBL-producing E. coli, and can result in deaths.
This study retrospectively reviewed the data from the medical records to compare the clinical characteristics and laboratory data of preterm babies with E. coli BSI between the survival and nonsurvival groups, and also the ESBL-producing and non-ESBL-producing groups to determine the predictive factors of E. coli BSI in preterm babies.
2. Materials and methods
This retrospective cross-sectional study reviewed all infants who were admitted to 2 medical centers located in the northern and southern Taiwan between January 2004 and July 2015. The Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital approved the study protocol. The patients were enrolled in this study if E. coli was isolated from their blood within 3 days after birth. All were preterm neonates, gestational age of <37 weeks, without any surgically indicated diseases, or congenital anomalies. These patients were further divided into the ESBL-producing E. coli and the non-ESBL-producing E. coli groups based on their antimicrobial sensitivity tests, and also divided into survival and nonsurvival groups based on their outcome. All data were obtained from the medical records. The perinatal and maternal characteristics, including the gestational age, mode of delivery, sex, birth body weight, Apgar score, ventilator settings, neonatal antibiotics use, maternal fever, maternal antibiotics use, hours of premature rupture of membrane, initial TPR (ie, body temperature, pulse rate, and respiratory rate), and blood pressure, were compared.
Neonatal laboratory data, which were collected at the same time of blood culture, such as arterial blood gas, aspartate aminotransferase (AST), alanine aminotransferase (ALT), complete blood count, C-reactive protein, electrolytes, blood sugar, blood urea nitrogen, creatinine, prothrombin time, and activated partial thromboplastin time, were analyzed. The maternal laboratory data upon delivery, such as complete blood count, C-reactive protein, and culture of the amniotic fluid, were also reviewed.
Data were analyzed using the IBM SPSS Version 22.0 (IBM, Armonk, NY: IBM Corp) statistical software. The Mann–Whitney U test and Fisher exact test were used to compare the univariate analysis of continuous and binary variables of the ESBL-producing and non-ESBL-producing E. coli groups, and the survival and nonsurvival groups, respectively. Survival cases were defined as patients surviving during the first admission. The clinical parameters of mortality in premature infants with E. coli BSI were also analyzed using the multivariate Cox proportional-hazard model, which were adjusted for gestational age. Receiver-operating characteristic (ROC) statistics was applied and the areas under curve were compared to calculate their cut-off values. All data in tables were presented with the mean ± standard deviation. For all tests, statistical significance was set at P ≤ .05.
3. Results
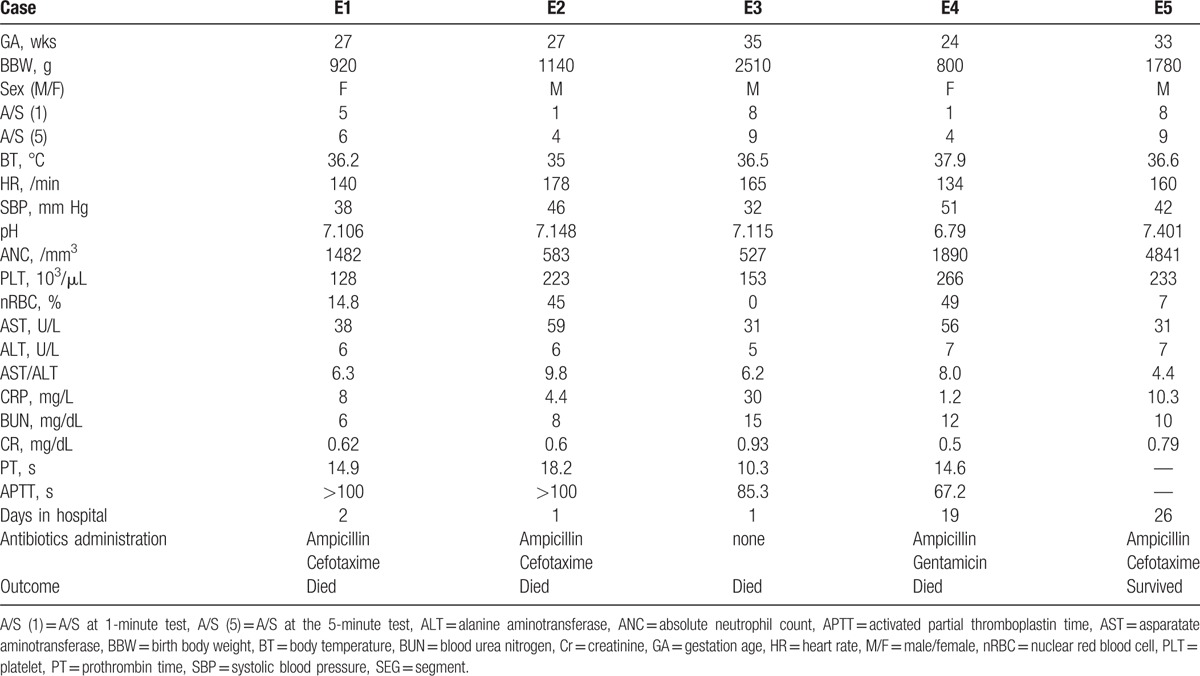
Between January 2004 and July 2015, 27 preterm babies were diagnosed as early-onset BSI, with E. coli based on the isolation of these bacteria from blood culture. Among these babies, 2 also had E. coli pneumonia, 3 had E. coli urinary tract infection, 1 had E. coli meningitis, and 1 had bowel perforation with E. coli ascites. Their mean gestational age was 31.2 weeks. The mean days of hospitalization was 9.19 ± 10.21 days. Immediately after birth, all patients were treated with empiric antibiotics of ampicillin and either gentamicin or cefotaxime. The overall mortality rate was 55.56% (15 deaths). Fourteen preterm babies died within 72 hours after birth due to septic shock and cardiopulmonary failure, and 1 baby died on 19th day after birth. According to the antibiotic susceptibility test, 5 neonates had BSI caused by ESBL-producing E. coli. Table 1 shows the characteristics of these 5 neonates.
Table 1.
The characteristics of ESBL-producing Escherichia coli cases.
Comparing the perinatal conditions, neonatal outcomes, and maternal status between the ESBL-producing E. coli group (ESBL group) and the non-ESBL-producing E. coli group (non-ESBL group) (Tables 2 and 3), only the ALT was significantly lower in ESBL group than in the non-ESBL group. The mortality rates of ESBL and non-ESBL groups were 80% and 54.5%, respectively. Although the mortality rate was not significantly different from the 2 groups, that of the ESBL group was higher.
Table 2.
The perinatal conditions and neonatal outcome comparing ESBL-producing E. coli group (ESBL group) and non-ESBL-producing E. coli group (non-ESBL group).
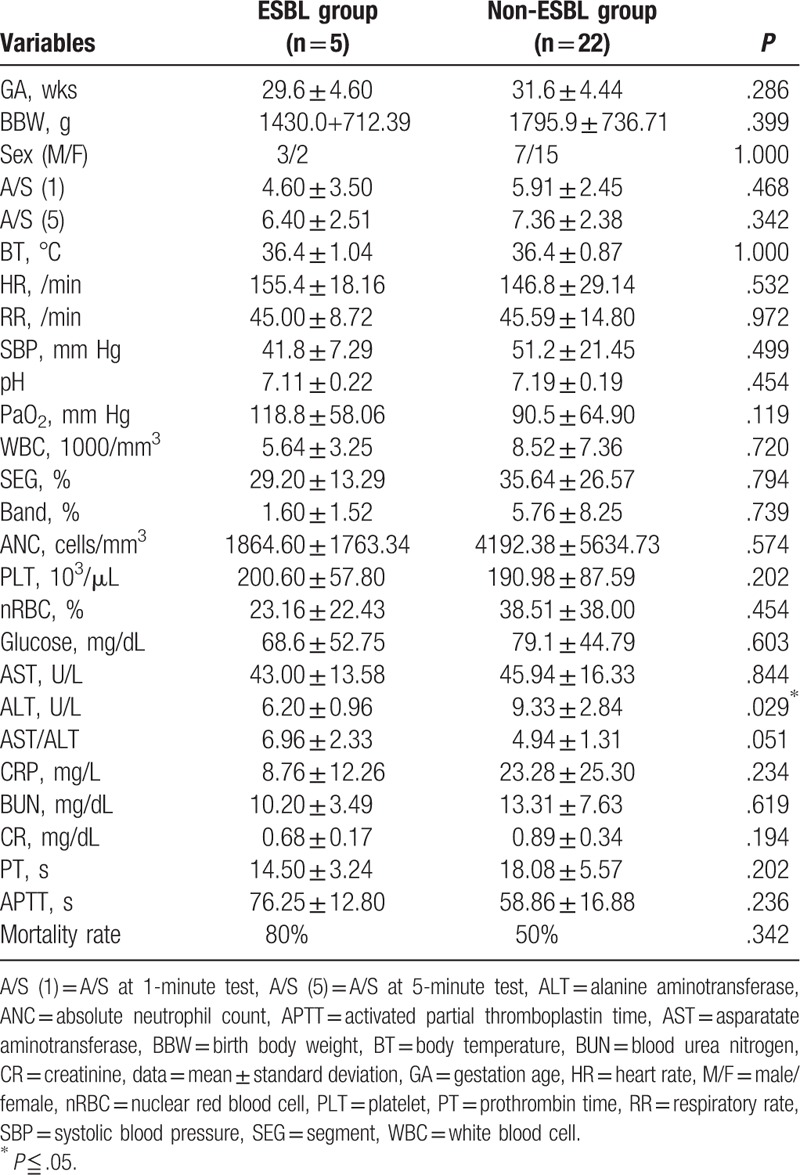
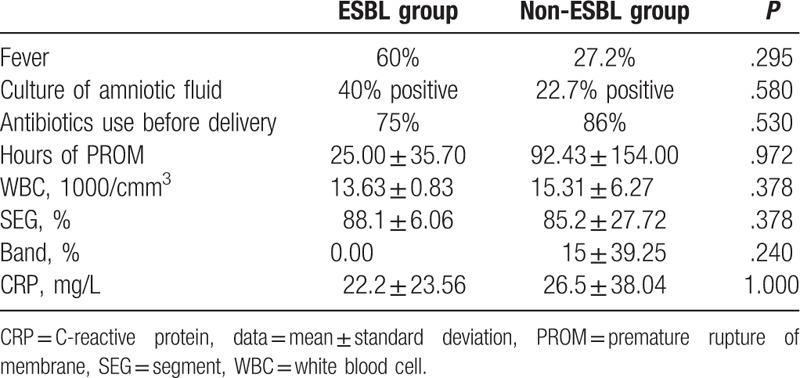
Table 3.
The maternal conditions comparing the ESBL-producing E. coli group (ESBL group) and non-ESBL-producing E. coli group (non-ESBL group).
The Mann–Whitney U test was used to determine the risk factors of mortality of the survivors and nonsurvivors. Blood acidosis, anemia, and low systolic blood pressure, gestational age, birth body weight, Apgar score, absolute neutrophil count (ANC), count of platelet count, and ALT were risk factors of mortality in preterm babies with E. coli BSI (Table 4). After adjusting the gestational age, low systolic blood pressure and ANC were the significant risk factors of mortality based on the multivariate Cox regression (Table 4).
Table 4.
The risk factors of mortality in premature infants with E. coli bloodstream infection.
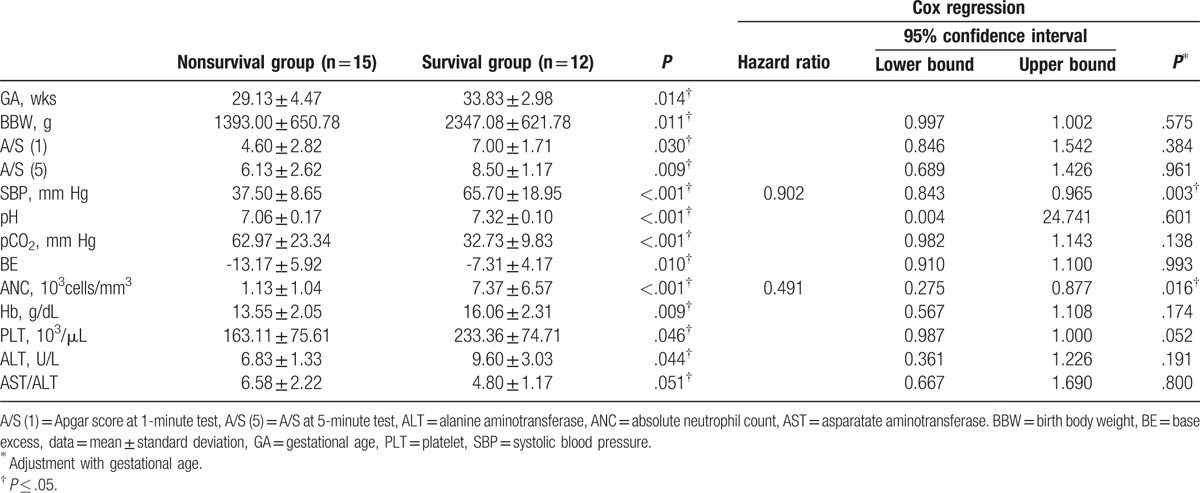
The predictive values of the systolic blood pressure and ANC associated with mortality were analyzed using the ROC statistics. Systolic blood pressure and ANC had a total area under the ROC curve (AUC) of 0.937 and 0.968, respectively. Their cut-off values were 48 mm Hg (77.8% sensitivity, 92.9% specificity) and 2318 cells/mm3 (100% sensitivity, 85.7% specificity), respectively.
4. Discussion
To date, this is the first study to compare the ESBL-producing E. coli and non-ESBL-producing E. coli BSI in preterm neonates. The results demonstrated that lower ALT level might be a predictive factor for ESBL-producing E. coli BSI. Moreover, systolic blood pressure of <48 mm Hg and neutropenia of <2318 cells/mm3 might be the most significant risk factors of mortality in these cases.
The liver is an important organ in response to sepsis as it produces proinflammatory cytokines and acute-phase proteins.[7] Like the spleen, the liver is also the first defense against the pathogens in the blood. The interaction of Kupffer and natural killer T cells in the liver can induce an important intravascular immune response.[8] A previous in vitro study revealed that cells infected with ESBL-producing or non-ESBL-producing E. coli could induce different host–response mechanisms and elicit different levels of interleukin (IL)-6 and IL-8.[9] The ability of ESBL-producing E. coli to stimulate or evoke the production of reactive oxygen species (ROS) from polymorphonuclear neutrophil cells is higher than that of non-ESBL-producing E. coli.[9] In an animal study, ROS and reactive nitrogen species were reported to cause hepatic microvascular dysfunction during sepsis.[10]
This study demonstrated that the ratio of serum AST and ALT in the ESBL group was almost significantly higher than that in the non-ESBL group (P = .051). The increased ratio of AST and ALT in infants with liver disease puts them at risk of poor clinical outcome.[11] This suggested that the ESBL group might have more severe liver damage and poorer prognosis than the non-ESBL group. Moreover, serum ALT in the ESBL group was lower than that in the non-ESBL group.
Lower ALT concentration can occur in the setting of vitamin B6 deficiency.[10] Vitamin B6 is an antioxidant and is a strong quencher of ROS. In the ESBL group, vitamin B6 may be consumed largely due to high ROS, resulting in decreased ALT levels.
The overall mortality rate of early-onset neonatal sepsis in premature babies was known to be as high as 34%.[12] Neonates with multidrug-resistant (MDR) Gram-negative bacteremia had higher rates of fatality and infectious complications than those with non-MDR strain.[13] However, a large cohort study revealed that the association of ampicillin-resistant E. coli BSI and mortality was not significant, and effective empiric agents could not decrease the mortality. [14] The population of this study included full-term babies, and early and late-onset BSI were analyzed together. Our study was focused on early-onset E. coli BSI on premature babies and revealed that ESBL group had higher mortality rate (80%) than non-ESBL group (54.5%). More than 90% of the babies in our study died within 72 hours after birth; clinicians usually knew the result of the blood culture after these babies died.
Infectious complications included any abscess formation, neurologic complications, acute renal failure, and intracardiac vegetation. BSI with septic shock could be an independent risk factor for infectious complications, which finally lead to mortality.[15] In our study, the systolic blood pressure of <48 mm Hg and ANC of <2318 cells/mm3 might be the most significant risk factors of mortality. Septic shock and subsequent disseminated intravascular coagulation could cause intraventricular hemorrhage leading to poor outcomes. Although platelet count, prothrombin time, and activated partial thromboplastin time were not associated with mortality in our study, a previous study revealed that lower level of plasma fibrinogen was associated to death in neonatal sepsis. [16] Correct neutrophil counts provide the first line of innate immune system defense to pathogens through phagocytosis. The ANC of <1500 cells/mm3 is considered neutropenia, which could be congenital or acquired. In premature infants, neutropenia is a common finding, particularly in extremely low-birth-weight babies, which might be associated with an increased incidence of early-onset sepsis.[17] Neutrophilia and neutropenia could happen during neonatal sepsis based on the bone marrow storage pool or the infectious pathogens. Neutropenia was usually found during sepsis in sick premature infants who had Gram-negative bacterial infection.[18]
Bloodstream infection is 1 of the most common causes of morbidity and mortality in preterm babies.[19] Preterm neonates are prone to infections due to their immature immune function, and thus, starting antibiotic treatment immediately after birth is recommended. Nevertheless, empiric antibiotic treatment, even intravenous immunoglobulin (IVIG) or IgM-enriched IVIG[20], may not prevent disease progression and mortality. A study had revealed that preterm infants who survived from severe infections were associated with white matter injury and neurodevelopmental sequelae.[21] Thus, early diagnosis and timely intervention are needed to reduce disease morbidity. Previous studies had identified indicators for the early detection of neonatal sepsis. These included the ratio of abnormal immature and mature neutrophils in the cord blood [19]and 16S rRNA gene sequencing in neonatal peripheral blood.[22] However, phlebotomy examination of premature babies were difficult to perform because of their small blood volume and vessels. Therefore, other bio-fluid, such as urine, was reported to detect neonatal sepsis through an elevated IL-6.[23]
This study has shown that lower ALT was correlated with ESBL E. coli bacteremia in preterm neonates. Early shifting of antibiotics from empirical to an appropriate one may be needed to control the infection. Furthermore, clinicians should be alert in cases with low systolic blood pressure and neutropenia as these may lead to mortality. Early and aggressive management for these babies are warranted.
One of the limitations of this retrospective study was the information bias because of incomplete profiles as some of the babies were transferred from local hospitals after birth, so some data with regard to maternal conditions were missing. However, these mothers’ data were recalled by looking up their medical charts, and only 6 babies were admitted to our hospital via transportation system. Furthermore, owing to the small number of the ESBL group cases, we were unable to recommend directly on the association with low ALT and ESBL group. Further prospective studies with larger cohorts are needed to clarify the difference between ESBL E. coli and non-ESBL E. coli in premature babies with BSI, and determine the method to early detect ESBL E. coli using other bio-fluids instead of blood.
Acknowledgment
We appreciated the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for statistical work.
Footnotes
Abbreviations: ALT = alanine aminotransferase, ANC = absolute neutrophil count, AST = aspartate aminotransferase, AUC = area under the ROC curve, BSI = bloodstream infection, E. coli = Escherichia coli, ESBL = extended-spectrum β-lactamase, GBS = group B streptococci, MDR = multidrug-resistant, ROC = receiver-operating characteristic, ROS = reactive oxygen species.
The authors report no conflicts of interest.
References
- [1].Lawn JE, Cousens S, Zupan J, et al. 4 million neonatal deaths: when? Where? Why? Lancet 2005;365:891–900. [DOI] [PubMed] [Google Scholar]
- [2].Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002-2003. Pediatr Infect Dis J 2005;24:635–9. [DOI] [PubMed] [Google Scholar]
- [3].Kurlat I, Stoll BJ, McGowan JE., Jr Time to positivity for detection of bacteremia in neonates. J Clin Microbiol 1989;27:1068–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bauserman MS, Laughon MM, Hornik CP, et al. Group B streptococcus and Escherichia coli infections in the intensive care nursery in the era of intrapartum antibiotic prophylaxis. Pediatr Infect Dis J 2013;32:208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stoll BJ, Hansen NI, Sanchez PJ, et al. Early onset neonatal sepsis: the burden of group B streptococcal and E. coli disease continues. Pediatrics 2011;127:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Han SB, Jung SW, Bae EY, et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae bacteremia in febrile neutropenic children. Microb Drug Resistance 2015;21:244–51. [DOI] [PubMed] [Google Scholar]
- [7].Dickson S. Sepsis and multiple organ failure. Anaesth Intensive Care Med 2009;10:4. [Google Scholar]
- [8].Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013;14:996–1006. [DOI] [PubMed] [Google Scholar]
- [9].El Gawhary S, El-Anany M, Hassan R, et al. The Role of 16S rRNA gene sequencing in confirmation of suspected neonatal sepsis. J Trop Pediatr 2016;62:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Singer G, Stokes KY, Neil Granger D. Reactive oxygen and nitrogen species in sepsis-induced hepatic microvascular dysfunction. Inflamm Res 2013;62:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosenthal P. Assessing liver function and hyperbilirubinemia in the newborn. National Academy of Clinical Biochemistry. Clin Chem 1997;43:228–34. [PubMed] [Google Scholar]
- [12].Lee SM, Chang M, Kim KS. Blood culture proven early onset sepsis and late onset sepsis in very-low-birth-weight infants in Korea. J Korean Med Sci 2015;30(Suppl 1):S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tsai MH, Chu SM, Hsu JF, et al. Risk factors and outcomes for multidrug-resistant Gram-negative bacteremia in the NICU. Pediatrics 2014;133:e322–9. [DOI] [PubMed] [Google Scholar]
- [14].Bergin SP, Thaden JT, Ericson JE, et al. Neonatal Escherichia coli bloodstream infections: clinical outcomes and impact of initial antibiotic therapy. Pediatr Infect Dis J 2015;34:933–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tsai MH, Lee CW, Chu SM, et al. Infectious complications and morbidities after neonatal bloodstream infections: an observational cohort study. Medicine 2016;95:e3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mitra P, Guha D, Nag SS3, et al. Role of plasma fibrinogen in diagnosis and prediction of short term outcome in neonatal sepsis. Indian J Hematol Blood Transfus 2017;33:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Del Vecchio A, Christensen RD. Neonatal neutropenia: what diagnostic evaluation is needed and when is treatment recommended? Early Human Develop 2012;88(Suppl 2):S19–24. [DOI] [PubMed] [Google Scholar]
- [18].Sarkar S, Bhagat I, Hieber S, et al. Can neutrophil responses in very low birth weight infants predict the organisms responsible for late-onset bacterial or fungal sepsis? J Perinatol 2006;26:501–5. [DOI] [PubMed] [Google Scholar]
- [19].Annam V, Medarametla V, Chakkirala N. Evaluation of cord blood: haematological scoring system as an early predictive screening method for the detection of early onset neonatal sepsis. J Clin Diagn Res 2015;9:SC04–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ohlsson A, Lacy JB. Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst Rev 2015;CD001239. [DOI] [PubMed] [Google Scholar]
- [21].Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics 2008;122:299–305. [DOI] [PubMed] [Google Scholar]
- [22].Liu CL, Ai HW, Wang WP, et al. Comparison of 16S rRNA gene PCR and blood culture for diagnosis of neonatal sepsis. Arch Pediatr 2014;21:162–9. [DOI] [PubMed] [Google Scholar]
- [23].Miklaszewska M, Korohodai P, Kwinta P, et al. Clinical validity of urinary interleukin 18 and interleukin 6 determinations in preterm newborns. Przeglad lekarski 2015;72:589–96. [PubMed] [Google Scholar]


