Abstract
Background:
The aim of the study was to observe the effect of polysaccharide of dendrobium candidum (PDC) and high glucose on proliferation, apoptosis of human corneal epithelial cells (HCEC).
Methods:
The MTT method was used to screen and take the optimal high-glucose concentration, treatment time, and PDC concentration using HCEC and divide it into 4 groups: control group (C), high glucose group (HG), PDC group, and HG + PDC group. We observed and compared the effect of the 4 groups on HCEC proliferation by MTT, apoptosis by Annexin V-FITC/PI double fluorescent staining and flow cytometry (FCM), and expression of bax mRNA and bcl-2 mRNA by RT-qPCR.
Results:
Compared with the control group, proliferative activity of HCEC cells was reduced; the cells apoptosis ratio was increased; the expression of bax mRNA was increased, and the expression of bcl-2 mRNA was reduced in the HG group. Proliferative activity of HCEC cells in the PDC group was increased, and the expression of bcl-2 mRNA was increased but that of bax mRNA was decreased. Proliferative activity of HCEC cells in the HG + PDC group was increased, but it could not restore to the normal level; the expression of bax mRNA was significantly decreased but the expression of bcl-2 mRNA was significantly increased.
Conclusions:
Our results demonstrate that high glucose can inhibit proliferative activity and induce apoptosis of HCEC. PDC can improve the proliferative activity of HCEC cells under the high glucose environment and reduce the apoptosis of cells by regulating the expression of bax and bcl-2. PDC play a very important role on protecting and repairing of corneal epithelial cells damage in high glucose.
Keywords: apoptosis, HCEC, high glucose, PDC, proliferation
1. Introduction
Diabetes mellitus (DM) is a metabolic disorder caused by the influence of environment and genetic factors and characterized by chronic hyperglycemia.[1] The diabetic eye complications such as diabetic retinopathy,[2] diabetic lens opacity, and so on, have become the important causes of growing the blindness. Ro et al[3] first proposed and named the diabetes keratopathy (DK) 35 years ago. Some studies reported[4–6] that about 47% to 70% patients with the DM would suffer from corneal abnormalities and the primary symptoms were characterized by the decrease of corneal sensitivity, edema and thickening of cornea, and so on. After receiving the eye surgery or corneal injuries, it represented the repair delay of corneal epithelium, corneal epithelial erosions, corneal ulcers, and so on, and eventually would lead to corneal perforation or blindness. At present, the specific pathogenesis of the DK is still not clarified fully and the effect of related drug treatment is not good enough.
Dendrobium candidum (DC) is the dendrobium orchid herb. DC has the pharmacological effects of enhancing the immunity, resisting again the oxidation, regulating the glucose and lipid metabolism, controlling blood pressure, and inhibiting the bacteria. It is clinically used for the adjuvant therapy of hypertension, xerostomia, diabetes, and so on.[7] Polysaccharide of dendrobium candidum (PDC) is the major active substance of the dendrobe. The action mechanism of PDC includes the following 3 aspects[7]—(1) hypoglycemic effect: inhibit the gluconeogenesis and regulate the secretion of islet cells and regeneration and healing of pancreatic β cells; (2) antioxidant effect: inhibit glucose and lipid peroxidation, and protect islet cells and vascular endothelial cells from the damage; (3) improve the insulin resistance: improve the sensitivity of target organs such as liver, skeletal muscle on the insulin, and effectively balance the glucose and lipid metabolism using the insulin. Our previous experiments also proved[8] that PDC had the protective effect on the umbilical vein endothelial cells under the high-glucose action.
Hence, this study observed the effect of PDC of proliferation, apoptosis, and the expression of apoptosis regulatory factor bax and bcl-2 of HCEC in high glucose. This study was designed to discuss the protective effect of PDC in the diabetic corneal epithelial cells and the action mechanism of promoting the healing of diabetic corneal epithelial cell injury so as to provide the theoretical basis and experimental foundation for PDC to treat and improve the diabetic corneal epithelial cells.
2. Materials and methods
2.1. Main reagents and instrument
Human corneal epithelial cells (HCEC) were from Abcam (Cambridge, UK http://abcam.bioleaf.com), and HCEC have been proved the identity of human corneal epithelial cell line by STR analysis. Annexin V-FITC/PI double-stain apoptosis detection kit was from KeyGen Biotech. ELISA kit was from Flarebio Biotech LLC. Reverse transcription kit was from Beiing Cowin Biotech Co. Ltd. Dendrobium candidum was from Hunan Loongshare Dendrobium Base Co. Ltd. Primer was from Nanjing GenScript. Preparation of main solution and buffer solution:
2.1.1. Preparation of glucose solution (1.0 mol/L)
1.8 g glucose powder was dissolved in 10 mL distilled water.
2.1.2. Preparation of complete culture medium
DMEM 450 mL + 50 mL FCS + 5 mL 100 × penicillin – streptomycin solution were stored in refrigerator at a low temperature.
2.1.3. Preparation of dendrobium polysaccharide solution (10 mg/mL)
2 g dry dendrobium was grinded into powder, and then put it into distilled water with 1:20 volume ratio for water bath for 4 hours (repeat it for 3 times) at 70 °C, and then collect the extract. After the concentration under reduced pressure, the extract was added 95% ethanol of 4 times the volume and precipitated overnight, and then centrifuged for 15 minutes at 12,000 rpm and the precipitate was collected. The precipitate was dissolved with distilled water and deproteinized using the TCA method (repeat it twice). PDC was obtained after the supernatant was centrifuged. Dilute supernatant was been a constant volume of 50 mL. The polysaccharide content of the supernatant was 13.7% measured by the phenol-sulfuric acid method. The mother solution prepared was diluted to 10 mg/mL by distilled water and ready for using after the filtration and sterilization.[9]
2.2. Experiment methods
2.2.1. In vitro culture of HCEC
HCEC was taken from the refrigerator of −80 °C and preheated in water bath, added the complete medium, and centrifuged for 5 minutes at 1000 rpm after a uniform percussion. The supernatant was removed. HCEC was re-added to the complete medium and then vaccinated in the culture flash, and then put into the CO2 culture incubator with saturated humidity. The solution was changed once every 2 to 3 days. Cells have been taken passage culture or harvested and digested after the cell density has reached 80%, and then centrifuged for 5 minutes at 1000 rpm. The supernatant was sucked and removed, and then HCEC was re-added to the complete medium. The admixture was divided into 2 parts for culture.
2.2.2. Screen the optimal high glucose, PDC concentration, and treatment time
2.2.2.1. Determine the optimal HG concentration and treatment time using the MTT method
HCEC in the logarithmic growth period were seeded or vaccinated in a 96-hole plate with a density of 104/hole, with 200 μL in each hole. The cells were settled in the serum-free medium for 24 hours after they were adhered. The glucose of 5.5 mmol/L was the normal control group. The treatment groups were added the high glucose of different concentrations (10, 15, 20, 25, 30, 35, 40, 45, and 50 mmol/L glucose). The complete medium was changed after the cells were cultured of 12 hours, 24 hours, 48 hours, and 72 hours, respectively. The cells were added with 5 mg/mL MTT and 20 μL/hole for a continuous incubation of 4 hours with 5% CO2 at 37 °C. The supernatant was sucked and remove. HCEC was added with DMSO and 150 μL/hole. The inhibition ratio of HECE cell proliferation was analyzed by the optical density (OD) value of medium at 490 nm using the Bio-Tek micro-plate reader. The optimal HG concentration A and time T1 had been taken. The inhibition ratio of cell proliferation (%) = (1 – treatment group OD value/control group OD value) × 100%.
2.2.2.2. Determine the optimal PDC concentration and treatment time using the MTT method
HCEC preparation was the same as the method above. The group of 5.5 mmol/L glucose was the normal control group. Control group and HG groups were added with the drugs (0,100, 200, and 400 μg/mL PDC), respectively. The method of HCEC culture and proliferation analysis was the same as stated above. The optimal PDC concentration B and time T2 had been taken.
2.2.3. Experimental grouping of cells
HG of A concentration treating the cells for T1 and PDC of B concentration treating the cells for T2 have been taken. Four groups were set up.
Control group: DMEM culture medium containing 5.5 mmol/L glucose.
HG group: DMEM culture medium containing A mmol/L glucose.
PDC group: DMEM culture medium containing B μg/mL PDC;
HG + PDC group: DMEM culture medium containing A mmol/l glucose + B μg/mL PDC.
It was noted that it was required to treat the cells in the HG + PDC group in the high glucose for T1 and add B μg/mL PDC to culture for T2.
2.2.4. Effect of PDC on HCEC proliferation activity in the high-glucose (HG) environment using the MTT method
There were Control group, HG group, PDC group, and HG + PDC group. The cells of the 4 groups have been taken in the logarithmic growth period and cell viability was detected. 5.5 mmol/L glucose was added into the C group and A mmol/L glucose was added into the HG group. Both groups were cultured for T1. B μg/mL PDC was added into the PDC group and cultured for T2. A mmol/L glucose was added into the HG + PDC group and cultured for T1, and then B μg/mL PDC was added and cultured for T2. Four complex holes were designed for each group. The MTT method was used to measure the proliferation activity of the cells.
2.2.5. Detect the effect of PDC on HCEC apoptosis in HG environment using annexin V-FITC/PI + flow cytometry
The cells of the 4 groups have been taken in the logarithmic growth period. After digested by pancreatin without EDTA, the cells of 4 groups were placed in the centrifugal tube and rinsed by PBS for twice, and centrifuged for 5 minutes at 2000 rpm each time. The cells about 1–5 × 105 were collected, and put into 1 mL EP tube, added 500 μL of 1 × Binding buffer to suspense cells. 5 μL Annexin V-FITC and 5 μL propidium iodide were added into the cells of 4 groups reacting for 5 to 15 minutes at the room temperature in a dark place. The cell apoptosis was detected using the flow cytometry.
2.2.6. Determine the effect of PDC on bax mRNA and bcl-2 mRNA expression of HCEC cells in HG environment using the RT-qPCR
The appropriate treatment were given to 4 groups; the cells were collected to determine the bax mRNA and bcl-2 mRNA Expression of HCEC cells using the RT-qPCR. Extraction of total RNA of HCEC cells and reverse transcription of RNA were performed according to Megha's method.[10] RT-qPCR was performed by the SYBR Method. The target gene sequence was searched in NCBI. Software primer 5 was used to design the primer. System composition and amplification program of quantitative PCR were performed according to Megha's method[10] (Table 1).
Table 1.
Sequence of specific primer of RT-qPCR.

2.3. Statistical treatment
All data were analyzed using software package of SPSS18.0. The metering data were presented according to the difference between average value ± standard value. The t test of individual samples was performed for the comparison of average values between 2 groups satisfying the normal distribution and homogeneity of variance. The single-factor ANOVA analysis of variance was used for the comparison of average values among many groups. The comparison of average values between 2 groups was tested using the LSD. P < .05 indicated that the difference was of the statistical significance.
3. Results
3.1. Effect of HG or PDC on the proliferation activity of HCEC cells
3.1.1. Determine the effect of HG on proliferation activity of HCEC cells using the MTT method
Compared with 5.5 mmol/L normal control group, the inhibition ratio of HG on cell proliferation was increased (P < .05) which was tended to be dependent on concentration and time after cells were treated by glucose of different concentrations and treatment time (Fig. 1). In combination with the inhibition ratio and subsequent experiments, glucose of 50 mmol/L with a proliferation inhibition ratio of 34.75% was selected (after action of 48 hours) for the subsequent experiment (Fig. 1).
Figure 1.

In combination with the inhibition ratio and subsequent experiments, glucose of 50 mmol/L with a proliferation inhibition ratio of 34.75% was selected (after action of 48 hours) for the subsequent experiment. Note: ∗P < .05 vs 5.5 mmol/L glucose group.
3.1.2. Determine the effect of PDC on HCEC proliferation activity using the MTT method
Compared with the control group, the inhibition ratio of cell proliferation in the HG group was increased at various time buckets (P < .05); compared with the HG group, inhibition ratios of cell proliferation for various groups added with PDC were all decreased (P < .05). The inhibition ratio of HG on cell proliferations was tended to be dependent on concentration and time. Hence, PDC of 200 μg/mL (after action of 72 hours) was chosen as the optimum treatment condition of PDC (Fig. 2).
Figure 2.
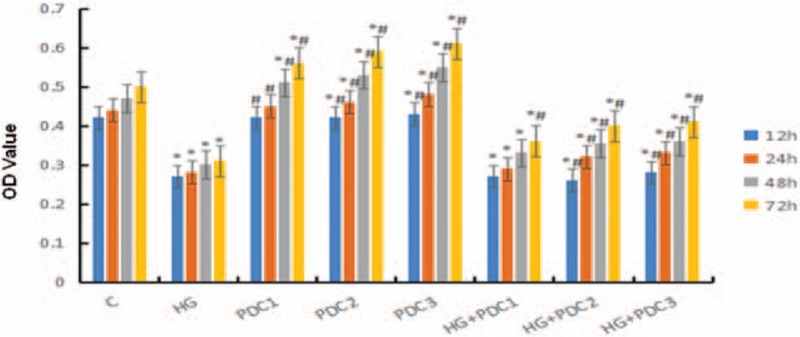
Inhibition ratio of HG on cell proliferations was tended to be dependent on concentration and time. Hence, PDC of 200 μg/mL (after action of 72 hours) was chosen as the optimum treatment condition of PDC. Notes: C: Control Group, containing 5.5 mmol/L glucose; HG Group, containing 50 mmol/L glucose. PDC1: containing 100 μg/mL PDC; PDC2: containing 200 μg/mL PDC; PDC3: containing 400 μg/mL PDC; HG + PDC1: containing 50 mmol/L glucose + 100 μg/mL PDC; HG + PDC2: containing 50 mmol/L glucose + 200 μg/mL PDC; HG + PDC3: containing 50 mmol/L glucose + 400 μg/mL PDC. ∗P < .05 vs control group. #P < .05 vs HG group. HG = high glucose group, PDC = polysaccharide of dendrobium candidum.
3.2. Determine the effect of PDC on proliferation activity of HCEC cells in the HG environment using the MTT method
Compared with the control group, proliferation of HCEC cells (P < .05) was inhibited in the HG group but promoted in the PDC group (P < .05); compared with the HG group, proliferation of HCEC cells was promoted in the HG + PDC group (P < .05), but there was still a difference from the normal control group (P < .05) (Fig. 3).
Figure 3.

Compared with the control group, proliferation of HCEC cells (P < .05) was inhibited in the HG group but promoted in the PDC group (P < .05). Compared with the HG group, the proliferation of HCEC cells was promoted in the HG + PDC group (P < .05). Notes: C: control group. Containing 5.5 mmol/L glucose; HG: high glucose group, containing 50 mmol/L glucose; PDC group: containing 200 μg/mL PDC; HG + PDC Group: containing 50 mmol/L glucose + 200 μg/mL PDC. ∗Compared with control group. P < .05. #Compared with HG Group. P < .05. HG = high glucose group, PDC = polysaccharide of dendrobium candidum.
3.3. Determine apoptosis rate of HCEC cells in HG environment using annexin V-FITC/PI + flow cytometry
Compared with the normal control group, the apoptosis rate of HCEC cells was increased in the HG group and the PDC + HG group (P < .05); compared with the HG group, the apoptosis rate of HCEC cells in the PDC + HG group was decreased (P < .05) (Figs. 4 and 5).
Figure 4 and Figure 5.

Apoptosis rate of HCEC cells was increased in the HG group and the PDC + HG group (P < .05). Compared with the HG group, the apoptosis rate of HCEC cells in the PDC + HG group was decreased (P < .05). Notes: Control: control group, containing 5.5 mmol/L glucose; HG: high glucose group, containing 50 mmol/L glucose; PDC: PDC group, containing 200 μg/mL PDC; HG + PDC: HG + PDC Group: containing 50 mmol/l glucose + 200 μg/ml PDC. Notes: Control: control group, containing 5.5 mmol/L glucose; HG: high glucose group, containing 50 mmol/L glucose; PDC group: containing 200 μg/mL PDC. HG + PDC Group containing 50 mmol/L glucose + 200 μg/mL PDC. ∗Compared with control group. P < .05. #compared with HG Group. P < .05. HG = high glucose group, PDC = polysaccharide of dendrobium candidum.
Figure 6.

bcl-2 mRNA expression was reduced (P < .05) and bax mRNA expression was increased (P < .05) in the HG group; the bcl-2 mRNA expression was increased (P < .05) and bax mRNA expression was reduced in the PDC group and the PDC + HG group. Compared with the HG group, bcl-2 mRNA expression was increased (P < .05) and bax mRNA expression was reduced in the PDC group and the PDC + HG group. Notes: Control: control group, containing 5.5 mmol/L glucose; HG: high glucose group, containing 50 mmol/L glucose; PDC group: containing 200 μg/mL PDC; HG + PDC Group containing 50 mmol/L glucose + 200 μg/mL PDC. ∗Compared with control group, P < .05; #Compared with HG Group, P < .05. HG = high glucose group, PDC = polysaccharide of dendrobium candidum.
3.4. Determine bax mRNA & bcl-2 mRNA expressions of HCEC Cells using RT-qPCR
Compared with the control group, bcl-2 mRNA expression was reduced (P < .05) and bax mRNA expression was increased (P < .05) in the HG group; bcl-2 mRNA expression was increased (P < .05) and bax mRNA expression was reduced in the PDC group and the PDC + HG group. Compared with the HG group, bcl-2 mRNA expression was increased (P < .05) and bax mRNA expression was reduced in the PDC group and the PDC + HG group (Figs. 6A and B and 7).
4. Discussion
The normal corneal epithelial cells showed a proliferating state under the physiologic conditions, and they ultimately formed the terminally differentiated cells via the migration and differentiation to thus maintain the physiological functions. When the corneal epithelial cells are injured or wounded, their proliferation and differentiation is significantly enhanced. The epithelial cells in the normal areas will be migrated to the injured areas to repair the wounds and substitute for the normal barrier function via the proliferation, differentiation, and deformation and in the form of amoeba. The whole healing process needs the participation and regulation of growth factors and related signal pathways. The growth factors can identify and bind the specific receptors on the cell surfaces to activate the transduction of related signal molecules and complete the said repair or the healing process.
The studies found that it was easier to lead to the delay of wound healing than the normal people in the case of any corneal damage in the patients with diabetes. It might be considered that it was associated with the cell dysfunction.[11,12] The reasons may be as follows—(1) dysfunction: a lot of proteins should be synthesized during the repair, and the glucose is the major functional substance in this process. The hyperglycemia is mainly metabolized in a form of glucolysis in the body or organism, resulting in the lack of energy in the body; (2) oxidative stress[12]: the high glucose can increase the reactive oxygen metabolites to damage the stability of cell DNA, down-regulate the relevant protein and mRNA expressions, which may lead to the abnormal cell factors, and affect the signal pathway and cell immune responses so as to inhibit the healing or repair of corneal epithelial cells and resulting in the declined cell function and increased cell apoptosis; (3) inhibition of PI3-K/AKT signal pathway:[13] the PI3-K/AKT signal pathway was inhibited in the HG environment, resulting into the declined cell activity and promoting the cell apoptosis. Corneal epithelia barrier was damaged, and the harmful factors continued to stimulate the wound area so as to thus delay the wound healing.
The possible protection mechanism of PDC on corneal epithelial cells in HG environment was discussed in this study by establishing the HG model of HCEC cells and observing the effect of PDC on wound healing of HCEC cells. In this experiment, we found that the low-concentration (<25 mmol/L) high glucose was considered to promote HCEC proliferation, but high-concentration (35 mmol/L–50 mmol/L) high glucose promote HCEC apoptosis. This was consistent with the situation that the DK degree clinically became progressively worsened with the extended course of disease and the hyperglycemic state. The wound healing condition of HCEC cells was researched in this experiment. We chose the best glucose concentration of 50 mmol/L and 48 hours to treat the HCEC cells and establish the HG model of HCEC, and concentration of 200 μg/mL and treatment time of 72 hours were eventually recommended for the PDC.
Cell proliferation is a key link for wound healing of corneal epithelia, and it will complete the repair process together with the cell migration. Whether PDC can induce the proliferation and differentiation of HCEC cells has not been reported. This study showed that the proliferation of HCEC cells was inhibited in HG environment. However, this inhibition of proliferation has been improved after adding PDC even though it cannot be restored to the normal level. The mechanism of PDC to induce the cell proliferation is still unclear and need more study.
Cell apoptosis is a kind of programmed death for the organism to protect itself which can remove the aging or abnormal cells. Excessive apoptosis after corneal epithelial injury can be observed in rats with diabetes,[14] and the excessive apoptosis is not conducive to the healing. The increased expression of bax and decreased expression of bcl-2 could induce cells excessive apoptosis.[15–17] This experiment found that high glucose can induce the excessive apoptosis, high expression of bax, and low expression of bcl-2 of HCEC. However, PDC can improve the apoptosis state under the induction of the high glucose and reduce the apoptosis rate by regulating apoptosis-related gene expression of bax and bcl-2. Therefore, we believe that PDC can improve the apoptosis of HCEC in the HG environment, so it is considered to be associated with the anti-oxidant mechanism of PDC.[18]
In summary, our studies showed that high glucose can inhibit proliferative activity and induce apoptosis of HCEC. PDC can improve the proliferative activity of HCEC cells under the high glucose environment and reduce the apoptosis of cells by regulating the expression of bax and bcl-2. PDC play a very important role on protecting and repairing of corneal epithelial cells damage in high glucose.
Footnotes
Abbreviations: DC = dendrobium candidum, DK = diabetes keratopathy, DM = diabetes mellitus, FCM = flow cytometry , HCEC = human corneal epithelial cells, HG = high glucose group, PDC = polysaccharide of dendrobium candidum.
Funding: This work was supported financially by the grants from National Natural Science Foundation of China (81170823), Science & Technology Program Project of Hunan Provincial Science & Technology Department (No. 2015JC3011, No. 2015JC3118), Scientific Research Program Key Projects of Hunan Traditional Chinese Medicine (No. 201533), Hunan High-Level Health Professionals “225” Project Training Found; Scientific Research Program Project of Hunan Provincial Health Department (No. B2015-70).
The authors have no conflicts of interest to disclose.
References
- [1].Chai W, Dong Z, Wang N, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 2012;61:888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang J, Xu X, Elliott MH, et al. Muller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes 2010;59:2297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schultz RO, Van Horn DL, Peters MA, et al. Diabetic keratopathy. Trans Am Ophthalmol Soc 1981;79:180–99. [PMC free article] [PubMed] [Google Scholar]
- [4].Yamada N. Promotion of corneal epithelial wound healing with a tetrapeptide (SSSR) derived from insulin-like growth factor-1. Nippon Ganka Gakkai Zasshi 2008;112:984–93. [PubMed] [Google Scholar]
- [5].Yu FS, Yin J, Xu K, et al. Growth factors and corneal epithelial wound healing. Brain Res Bull 2010;81:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology 2001;108:586–92. [DOI] [PubMed] [Google Scholar]
- [7].Fang H, Hu X, Wang M, et al. Anti-osmotic and antioxidant activities of gigantol from Dendrobium aurantiacum var. denneanum against cataractogenesis in galactosemic rats. J Ethnopharmacol 2015;172:238–46. [DOI] [PubMed] [Google Scholar]
- [8].Li XQ, Shi ML, Cai GX. Effects of polysaccharides of dendrobium candidum on high glucose-induced mitochondrial membrane potential in cultured human umbilical vein endothelial cells. J Hyg Res 2014;22:1022–4. [Google Scholar]
- [9].Chen J, Qi H, Li JB, et al. Experimental study on Dendrobium candidum polysaccharides on promotion of hair growth. China J Chin Materia Medica 2014;2:291–5. [PubMed] [Google Scholar]
- [10].Barot M, Gokulgandhi Mitan R, Haghnegahdar Megan, et al. Effect of emergence of fluoroquinolone resistance on intrinsic expression of P-glycoprotein phenotype in corneal epithelial cCells. J Ocul Pharmacol Ther 2011;27:553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lu W, Ebihara N, Miyazaki K, et al. Reduced expression of laminin-5 in corneal epithelial cells under high glucose condition. Cornea 2006;25:61–7. [DOI] [PubMed] [Google Scholar]
- [12].Saghizadeh M, Soleymani S, Harounian A, et al. Alterations of epithelial stem cell marker patterns in human diabetic corneas and effects of c-met gene therapy. Mol Vis 2011;17:2177–90. [PMC free article] [PubMed] [Google Scholar]
- [13].Chang JH, Huang YH, Cunningham CM, et al. Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea. Surv Ophthalmol 2016;4:478–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bhan S, Mitra R, Arya AK, et al. A study on evaluation of apoptosis and expression of bcl-2-related marker in wound healing of streptozotocin induced diabetic rats. ISRN Dermatol 2013;2013:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Drel VR, Pacher P, Ali TK, et al. Aldose reductase inhibitor fidarestat counteracts diabetes-associated cataract formation, retinal oxidative - nitrosative stress, glial activation, and apoptosis. Int J Mol Med 2008;21:667–76. [PMC free article] [PubMed] [Google Scholar]
- [16].Mo ZT, Li WN, Zhai YR, et al. Icariin attenuates OGD/R-induced autophagy via Bcl-2-dependent cross talk between apoptosis and autophagy in PC12 cells. Evid Based Complement Alternat Med 2016;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lauterwasser J, Todt F, Zerbes RM, et al. The porin VDAC2 is the mitochondrial platform for Bax retrotranslocation. Sci Rep 2016;13:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yao J, Cao X, Zhang R, et al. Protective effect of baicalin against experimental colitis via suppression of oxidant stress and apoptosis. Pharmacogn Mag 2016;12:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


