Abstract
Our goal was to investigate the surgical procedures, postoperative complications, and survival with regard to different onset timing of necrotizing enterocolitis (NEC).
We performed a retrospective review of medical records with a diagnosis of NEC between 2005 and 2016. The cutoff was set at 10 days for early onset ≤10 days and late onset over 10 days. Propensity score matching was performed to adjust for any baseline differences. In 53 paired patients, clinical outcomes, including, mortality, postoperative complications, and length of neonatal intensive care unit (NICU) stay, were evaluated on the basis of early or late-onset NEC.
Successful 1:1 matching propensity score matching was performed with 208 infants. Mortality for early-onset NEC infants was lower than that of early late NEC infants (P = .026). A lower overall postoperative complication rate, including infectious complications [19 (35.8) vs 29 (54.7); odds ratio, 0.462, confidence interval (CI) 0.212–1.008, P = .039], was noted in patients with early-onset NEC compared with infants with late-onset NEC. NICU stay and major complication were marginal different between the 2 groups. Comparison of feeding outcomes revealed that the time to achieve full enteral feeds was significantly longer for those with late-onset NEC (18.1 ± 11.5 vs 26.3 ± 15.6, P = .008).
The infants who develop NEC after 10 days of life do influence postoperative outcome survival or other clinically important outcomes after laparotomy.
Keywords: mortality, necrotizing enterocolitis, postnatal ages, postoperative complications
1. Introduction
Necrotizing enterocolitis (NEC) is one of the major inflammatory gastrointestinal disease and a leading cause of prolonged hospitalizations, with higher morbidity and mortality in preterm infants.[1,2] In recent years, the survival percentage for more preterm infants have improved, but this advance also brings about the risk of acquiring NEC[3] and surgical treatment is necessary in almost 30% to 40% of this population. The mortality rate of approximately 50% with intestinal perforation (IP) following NEC remains essentially unchanged, although tremendous advances have been made in neonatal care.[4] We now believe that it is heterogeneous with the population of infants with NEC, and some infants might have a different and distinct pathology other than true and/or classic NEC.[5,6] If the distinction among this population could be made, we may be better to develop different therapies strategy and to predict prognosis for the different conditions.
It has been well documented that there is an inverse relationship between gestational age and the onset of NEC, with more mature infants presenting NEC in the early life.[7] Among infants who have suffered from NEC, little is known about the prognosis in different stage of NEC onset and outcomes following salvage laparotomy in patients with IP, which might be caused by NEC. On the basis of our clinical experience, we raise the question that whether there is a distinction pathogenesis between earlier onset of NEC, which appears to be presenting with NEC in the first week of life and later onset of NEC, usually emerging in the preterm infant population. Currently, the care of infants requiring surgical intervention depends mostly on the experiences of individual surgeon in the local biases of the treating institution. In the absence of rigorous evidence supporting the superiority of one approach over the other, there is considerable controversy regarding preferable surgical management of these infants with the different onset stage of NEC.
The aim of this study was to distinguish heterogeneous population preoperatively and the relevance of this distinction on surgical outcomes. We also evaluated the surgical outcomes (mortality, the dependence on total parenteral nutrition, or the NICU length of stay) in infants with different onset NEC stages who underwent initial laparotomy. The study utilized propensity score (PS) matching analysis to eliminate the heterogeneity of the study population and to minimize the effects of confounding variables.
2. Methods
This study is a retrospective review of the hospital-based cohort admitted to 3 newborn intensive care units (NICUs) at Yongchuan Hospital, Children's Hospital of Chongqing Medical University and Jinan maternity and child care hospital between January 1, 2003, and December 31, 2015. The study protocol was approved by the institutional review board. This retrospective research protocol was approved by the Institutional Review Board in the 3 institutions (IRB, No.: CHMU2015-046) and performed in accordance with the ethical standards prescribed by the Helsinki Declaration of the World Medical Association. The written informed consent was waived.
NEC was defined according to the criteria originally proposed by Bell et al and subsequently modified by Walsh and Kliegman.[8] On the basis of previous reported cutoff,[6,9] we adjusted and categorized early-onset NEC as occurring at <10 days of age and late-onset NEC occurring at ≥10 days. We limited eligibility to infants with a gestational age of more than 31 weeks and stage III NEC requiring laparotomy. The surgical approach to patients included surgical resection of the perforated intestine, necrotic intestine, the creation of intestinal stomas, and/or peritoneal drainage.
Exclusion criteria included infants with acute pulmonary bacterial infection, gastrointestinal anomalies (aproctia, intestinal atresia, or Hirschsprung disease) and severe cardiac dysfunction, or severe intraventricular hemorrhage. In addition, to minimize severity differences in the study population, patients managed in the respiratory support for more than 30 days were excluded. Two hundred nineteen patients underwent at least 1 operation for NEC and met the inclusion criteria for enrollment. In this second analysis, univariate analyses were performed to explore the characteristics of infants with early- and late-onset NEC.
2.1. Clinical assessment
Electronic clinical records were thoroughly reviewed with demographic information, clinical, nutritional, laboratory, radiographic, surgical, and histopathology results of each NEC cases. The operating surgeon determined the surgical intervention for all enrolled patients. Operative notes of surgical procedure for all patients were reviewed and all findings recorded. Duration of surgery, the type of fluid, operating time, intraoperative blood loss, and necessity for reoperation were also recorded. Pathological evaluation, when available, was also reviewed. Infectious complications were confirmed with microbiological analyses and positive cultures, including pneumonia (radiographic confirmation) and abdominal, or systemic [fever (oral temperature >38.5°C)] infection. Wound complications consisted of wound dehiscence, erythema, swelling, and pus. We also collected 90 days postoperative data after intervention to evaluate hospital outcomes, including days spent on mechanical ventilation and total parental nutrition, time to achieve full enteral feeds (defined as 140 mL/kg per day), and NICU length-of-stay and survival to 90 days after laparotomy. The primary outcome measure of this research was mortality 90 days after the intervention. Secondary outcome measures were the need for parenteral nutritional support on the 90th postoperative day (POD), the postoperative complications, and the NICU length-of-stay for patients surviving 90 days postoperatively.
2.2. Propensity scores and matching
Due to the presence of variables potentially associated with surgical outcome of early and late-onset NEC, PSM was used to increase the precision of the estimated discrepancy without increasing bias. The PS analyses were done using SPSS 20.0 (IBM, Armonk, NY) or R 3.1.2 (The R Foundation for Statistical Computing). PS were estimated using a multivariable logistic regression model. A 1:1 PS matching analysis was performed using nearest-neighbor analysis with demographic and clinical variables with potential biases related to the early- and late-onset NEC. The selected variables entered into the propensity model included gestational age, birth weight, probiotics, enteral feeding, respiratory support, etc (Table 1). Matching was performed based on the estimated PS of each patient with no replacement, and a 0.2 caliper width. The generalized additive model was used to check linear assumption in PS model. We also evaluated the interaction among all pre-test covariates. Our PS model discriminated well between early- and late-onset NEC. The characteristics of both early- and late-onset NEC patients were compared before and after PSM.
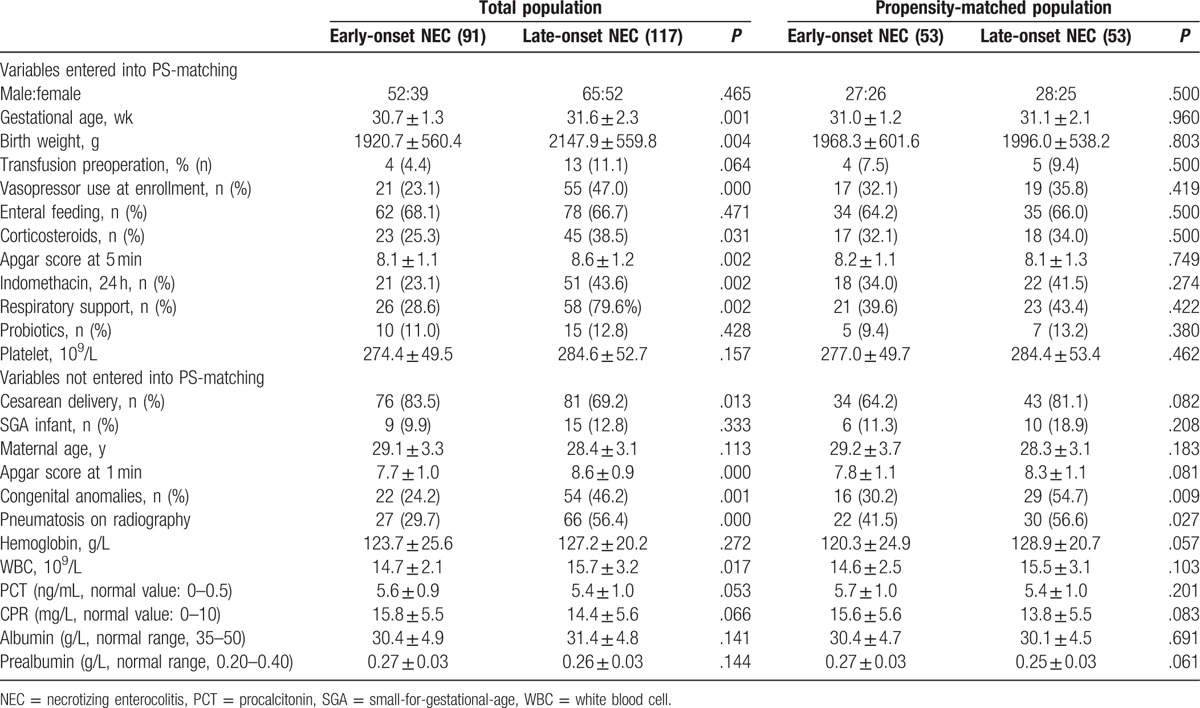
Table 1.
Baseline characteristics and surgical parameters of eligible patients.
2.3. Statistical analysis
After PS matching, the matched early and late-onset NEC infants were subjected to statistical comparisons. Data storage and analyses used SPSS Software (IBM Corp. Released 2013, IBM SPSS Statistics for Windows, Version 22.0; IBM Corp., Armonk, NY). Categorical and continuous variables for demographic, health care, and utilization characteristics were reported as frequencies (percentages) and means ± SDs, respectively. The primary outcome variable mortality 90 days postoperatively was measured with the Chi-square or the Fisher exact statistic test. The differences in other discrete variables were compared, first with the contingency tables and Chi-square tests, and then by estimation of the relative risk between treatment groups. Student t test was used to compare normally distributed continuous variables, and the Mann–Whitney U test, to compare abnormally distributed variables. We used the log-rank test to compare Kaplan–Meier survival curves for the 2 treatment groups. The statistical significance was evaluated using a 1-tailed 95% confidence interval (95% CI), and a P value less than .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
Among the initial cohort before PS matching, there are 208 preterm infants eligible for analysis, who had over NEC stage 3 and undergone laparotomy. As expected, preliminary analysis found that the smaller, sicker infants were significantly more likely to initial later onset NEC than healthier infants. The baseline features of the infants according to early or later onset NEC are presented in Table 1. After PS-matching, the selected variables, like birth weight, gestational age, vasopressor use at enrollment, respiratory support before or at the time of referral, and enteral feeding, etc, become comparable between the patients with early-onset NEC (n = 91) and late-onset NEC (n = 117) (Table 1), which suggested that the study population reasonably represented the spectrum of neonates with necrotizing enterocolitis in our institutions (Table 1). No differences were found between the 2 groups with regard to the other demographic features and clinical status of patients, which were entered the PS-matching. Under PS-matching, 53 patients with early-onset NEC were successfully matched to 53 patients with late-onset NEC. The other baseline characteristics and clinical status, which were not entered the PS-matching, were having similar (Table 1) significance before and after PS-matching, including congenital anomalies, pneumatosis on radiography, etc. Nutritional characteristics were also similar between the 2 groups, as assessed by mean serum prealbumin and albumin concentrations. All subsequent reported analyses are of these PS-matched cohorts.
3.2. Intraoperative feature and findings
In our institution, indications for laparotomy included pneumoperitoneum, condition aggravation, and stool or serous fluid (paracentesis). There were no significant differences in surgical indications between the 2 groups with PS-matched patients (Table 2).
Table 2.
Operative characteristics and findings in the matched population (Chi-square test).
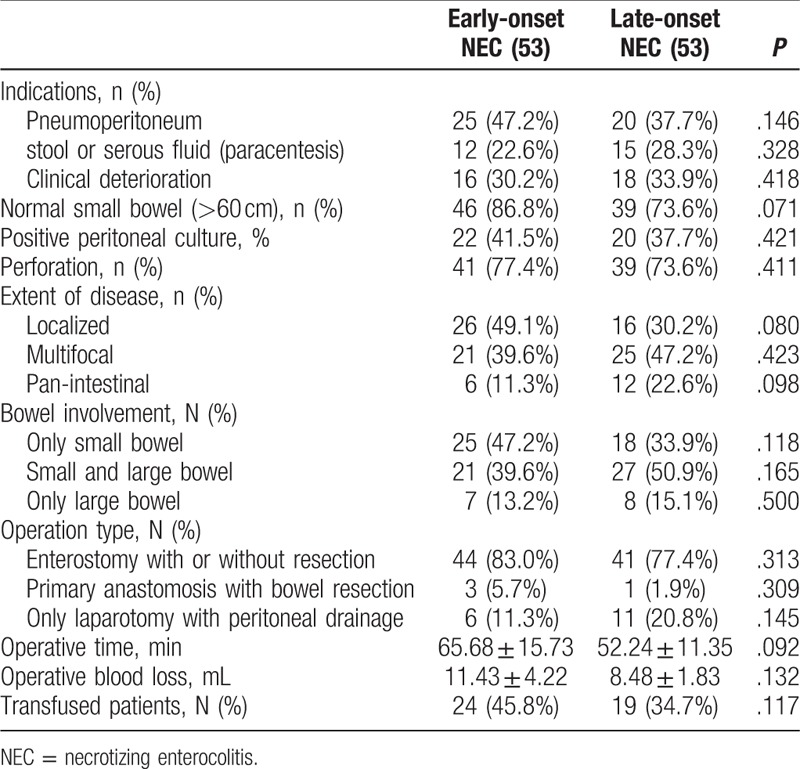
The disease involvement was assessed by the length of normal intestine, and diseased bowel at operation for patients undergoing initial laparotomy. The percentage of normal intestine over 60 cm was greater in early-onset NEC than in the infants with late-onset NEC, although the difference was just marginal significant (P = .071, Fisher exact test) (Table 2). The positive peritoneal culture [22 (41.5%) vs 20 (37.7%)] and incidence of perforation found [41 (77.4%) vs 39 (73.6%)] upon entering the abdomen did not have difference in the percentage of patients between late-onset NEC group and early-onset NEC group.
For late-onset NEC, located small bowel involvement (Ileum) was the most common affected location [16 (30.2%)], second with panintestinal disease, whereas multifocal disease was found almost in half patients [26 (49.1%)] with early-onset NEC, followed by 21 (39.6%) localized and 6 (11.3%) panintestinal. As for the surgical approaches, the choice of procedure has often depended upon the extent of disease as summarized in Table 2. Enterostomy creation, including jejunostomy, ileostomy, transverse colostomy, with or without necrotic bowel resection, was the initial procedure in the 2 groups. Three infants in early-onset NEC group and 1 infant in later-onset NEC required resection and primary anastomosis. The remaining infants underwent simple drainage during laparotomy because of panintestinal involvement with near total intestinal compromise. Only 2 early-onset NEC patients underwent ileocecal valve (ICV) resection during initial operation. The operative magnitude was evaluated by measurement of operative time, estimated blood loss, and total units of blood transfused within the 24-hour perioperative period. There were no statistically different among these measurements.
3.3. Gastrointestinal function
According to established criteria, a comparison of the postoperative complications between the 2 groups is summarized in Table 3. After PS-matching, the incidences of abdominal distention within 5 PODs in patients with early-onset NEC [35 (66.0%)] were similar with patients with late-onset NEC [39 (73.6%)]. First bowel movement was delayed in patients with early-onset NEC (5.67 ± 3.84 days) compared with the patients with late-onset NEC (6.82 ± 4.76 days), but this difference was not statistically significant [P = .089, analysis of variance (ANOVA)]. There were no differences in the incidence of serum electrolyte abnormalities between the 2 groups. Infants with late-onset NEC had significantly longer times to beginning enteral feeds (26.3 ± 15.6), than infants with early-onset NEC (18.1 ± 11.5) (P = .008, ANOVA) (Table 3). No significant differences were found in nutritional variables between the 2 groups within 5 PODs (albumin and prealbumin). In all groups, postoperative nutrition was continued for at least 30 days, and the mean total days spent on parenteral nutrition were 48.6 ± 29.8 and 66.8 ± 38.5 days for patients with early-onset NEC and late-onset NEC, respectively (P = .103, ANOVA). Furthermore, durations of mechanical ventilation were also significantly different between the 2 groups.
Table 3.
Outcome characteristics in the matched population (multivariate logistic regression).
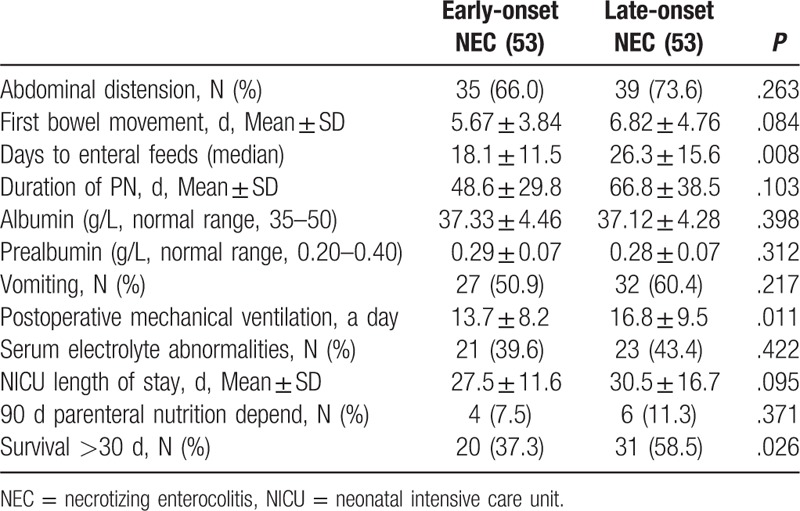
There were also no significant differences between the 2 groups in the rates of dependence on parenteral nutrition 90 days after surgery. All types of nutritional support were well tolerated in both groups. The mean NICU length of stay was 27.5 ± 11.6 days in patients with early-onset NEC, which was not significantly different from the mean length of stay 30.5 ± 16.7 days in patients with late-onset NEC (P = .095) (Table 3). Analysis of liver and kidney function did not demonstrate any alterations related to PGE1 treatment (data not shown).
3.4. Postoperative complications
Fewer postoperative complications were noted in patients with late-onset NEC than in patients with early-onset NECF or postoperative infectious complications (pneumonia, wound, peritonitis or abscess, and sepsis); there was a significant reduction in patients with early-onset NEC compared with infants with late-onset NEC (P = .039, odds ratio 0.462, CI 0.212–1.008) (Table 4). Wound infections developed in 21 infants and were managed by changing dressing. Furthermore, 16 patients had intra-abdominal or pelvic abscesses, of which some could be managed by percutaneous drainage. Minor stoma prolapse was noted in 2 infants, permitting manual reduction.
Table 4.
Postoperative complications in the matched population (Chi-square test).
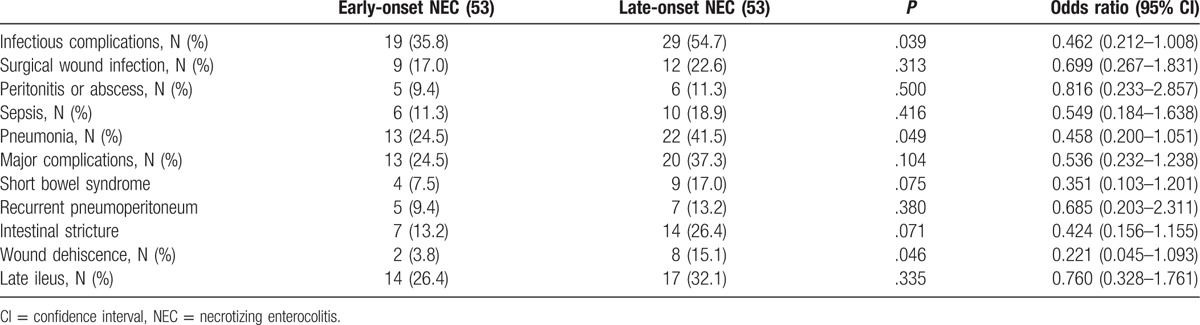
The most common and severe major complications for operation with NEC were short bowel syndrome (SBS) and intestinal stricture. Intestinal stricture was confirmed by a contrast study and operation in 16 children who underwent initial ileostomy. Thirteen of 53 patients (8.27%) with early-onset NEC developed major postoperative complications, which was not significantly less than the 20 of 53 patients (17.27%) with late-onset NEC who developed complications (P = .104; odds ratio 0.536, CI 0.232–1.238) (Table 4).
Although there was a trend toward an increased incidence of wound dehiscence (P = .046; odds ratio 0.221, CI 0.045–1.093) and intestinal stricture (P = .071; odds ratio 0.424, CI 0.156–1.155) in the early-onset NEC group compared with the patients with late-onset NEC, this did not achieve statistical significance. Although adhesion was frequently encountered during stoma takedown, the need of reoperation for adhesiolysis was required in none (Table 4).
3.5. Survival
The primary outcome variable, mortality 90 days after operation, was significantly different between the patients with late-onset NEC than in patients with early-onset NEC [20 (37.3%) vs 31 (58.5%); P = .026, Chi-square test] (Table 3). The median time to death after surgery was 15.8 days (range, 0–90 days) for early-onset NEC infants and 11.2 days (range, 0–90 days) for late-onset NEC infants. Most of the infants died within 7 days of surgery. No death occurred after stoma closure. The recorded cause of death was primarily attributed to sepsis, second by panintestinal NEC and severe short bowel. Furthermore, the remaining deaths resulted from respiratory distress syndrome or other pulmonary pathology.
The median follow-up period for 74 discharged patients was 1 year (range, 3 months–4 years). In order to analyze whether the onset time is correlated with NEC patient survival, we used the log-rank test to compare Kaplan–Meier survival curves for the 2 groups postoperatively. The survival analysis comparing survival in patients is shown in Fig. 1. This survival analysis was used at the age of 10 days as a cutoff value. The 90-day overall survival for patients with late-onset NEC was 72% compared with 40% for those with early-onset NEC (P = .004, log-rank test) (Fig. 1).
Figure 1.
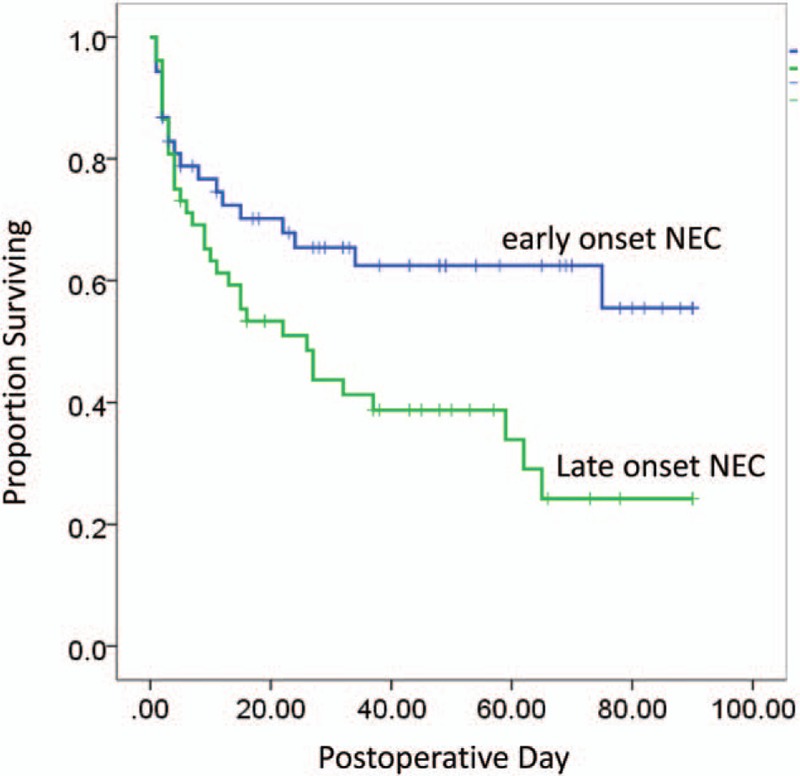
Kaplan–Meier survival curves for the infants with early and late-onset NEC. Survival curves were compared with the use of the log-rank test (P = .015).
4. Discussion
The present study was initiated to verify a different postoperative profile and presentation of NEC in the population of infants among the onset stage with different postnatal age. The distinction of NEC in the infants in respect to onset stage had a significant prognostic importance, with an increased likelihood for mortality for patients with late-onset NEC. Our data further supported that there is a clinically important distinction for postoperative complications between early and late-onset NEC. Because of the heterogeneity of the population, we preformed the PS matching and found significant differences in mortality among those with early and late-onset NEC who underwent laparotomy. We found significant differences in the reduced surgical complications, dependence on parenteral nutrition 90 days after operation, or in the duration of NICU stay in surviving infants between early and late-onset NEC groups.
It is possible that NEC exhibit different features, with differing prognostic result in different infant populations.[10,11] The long-term surgical outcomes of NEC may depend on the underlying variant pathogenesis in the population involved. Accurately distinguishing heterogeneous infant population is very important for the following reasons. First, as the infants suffering from NEC appear to have different risks for postoperative survival, it is thought to be potentially important to stratify the patient population based on the preoperative or postoperative pathogenesis features. Although the clinical picture of intestinal dysfunction and inflammation presented with NEC might be a common pathway, the etiology of NEC in the term and preterm infants may be existential discrepancy.[12] Results from a 2-center analysis recently reported that late onset of NEC in the full-term infant is associated with increased mortality.[9] However, neither study made a clear distinction between or analyzed separately patients who presented with a clinical postoperative course, in respect to earlier NEC as opposed to later NEC among all the infants population. Second, it is possible that the most effective surgical therapy will differ in the different infant subsets.[5,6,13–15] Identification of the appropriate patients suitable for bowel resection or peritoneal drainage will give the surgeon more objective information and might bring about the most appropriate treatments. Previous studies suggested that the surgical management might be differed greatly regarding time to onset of NEC in the term and preterm infant. As indicted, surgical intervention was more common in the early onset, older gestational age patients.[16,17] Here, we found that a significant large percentage of patients with bowel resection under the laparotomy had fallen into the categories of early-onset NEC and presentation within 10 days of life. It is important to apply different surgical options in the most efficacious manner. For this reason, we make up our cohort of the NEC infants undergoing laparotomy from the heterogeneous NEC population.
Previous reports have cited higher postoperative complication rates after NEC surgery at times in excess of 50%.[18,19] Under the PS-matching, we also evaluated the postoperative outcomes with respect to early or late-onset NEC. Our data indicate that those patients with late-onset NEC are at risk for adverse hospital outcomes, including mortality and nutritional parameters, which included the dependence on total parenteral nutrition, or the length of NICU stay. On the basis of data stratification and screening, our data demonstrate that overall postoperative complications occurred less frequently in infants with early-onset NEC, who developed NEC within day of life 10. In our study cohort, postoperative intestinal stricture was the most common complication after laparotomy with initial drainage or section.
The infants in our study who developed late-onset NEC had been associated with congenital anomalies, and a significant increased risk of mortality. Motta et al[20] reported similar rates of congenital heart disease and that the infants in their population also had many associated illness and anomalies.[21] It is possible that more of hypoxic cardiac defects, associated with NEC patients, would place them at a higher risk for intestinal hypoperfusion, which might predispose infants to development NEC and the following complication. Although not proven, we speculated that late-onset NEC may lead to an increased cytokine response and, subsequently, adverse hospital outcomes. Previous results suggested that early-onset NEC might represent misclassified spontaneous IP (SIP).[22] And it has reported poorer hospital and neurodevelopmental outcomes in patients with surgical NEC than SIP.[23,24]
Furthermore, later, NEC patients tend to present pneumatosis intestinalis or necrosis of their intestines; although the sensitivity of the presence of pneumatosis intestinalis on plain films for NEC is only about 45%, the predictive value of radiologic findings is low.[25] We discovered radiographics consistent with pneumatosis intestinalis during plain films evaluation, which makes it clear that they are dissimilar, with earlier NEC lacking the inflammation and ischemia that is a hallmark of later NEC. Patients with pneumatosis might have more extensive intestinal involvement and might benefit from laparotomy and bowel resection.[25] Despite these differences, the difficulty in correctly identifying one disease state over the other at the time of presentation does need to be acknowledged, by which, we may be able to optimize our surgical and therapeutic approach to this group of patients. These difficulties reinforce the need to look for etiologies of NEC associated with preterm and term infants.
Although our study is the largest reported series of NEC that has undergone laparotomy, it has several limitations. Weaknesses of our study include the retrospective nature, in which we collected the data with inherent risk of selection bias. Furthermore, the results of this study were based on an intent-to-treat analysis. There was a number of infant withdrawal of support, making it difficult to interpret the survival data. The study also takes place over a long time period and outcomes from many patients may not reflect outcomes from current treatment algorithms; there have likely been many practice changes within both the surgery and the neonatology divisions, leading to different care practices between study patients. Misclassification bias is possible, as some diseases, such as SIP, Hirschsprung-associated enterocolitis, and milk protein allergy, share similar clinical features with NEC, although the pathological reports, operative notes, and radiographic images were reviewed in these infants for meeting the recruit criteria. To limit the influence of confounding variables on the actual effects of onset stage, we performed PS matching analysis to generate comparison groups of patients who had similar baseline factors regarding different onset age. However, we could not completely avoid variables that may affect this comparison.
In summary, clinical evidence from the present study suggest that late-onset infants were at an increased risk of mortality, the dependence on total parenteral nutrition, or the length of NICU stay. Early-onset NEC may have conditions not normally associated with the late-onset NEC for optimal care. We acknowledge that these results are based on a homogenous group of patients, although we performed a PS matching analysis. It will be necessary to conduct quality investigation in the future to best utilize the limited therapies available.
Acknowledgments
We thank Prof. XianqingJin for providing technical assistance and for insightful discussions during the preparation of the manuscript. We thank DrXiaoyong Zhang at the Wistar Institute, USA, for help with the linguistic revision of the manuscript.
Footnotes
Abbreviations: CRP = C-reactive protein, MMP = metalloproteinases, NEC = necrotizing enterocolitis, NICU = neonatal intensive care unit, PCT = procalcitonin, POD = postoperative days, PS = propensity score, SGA = small-for-gestational-age, WBC = white blood cell.
Authorship: YW and CG designed and analyzed the data. LL helped design the experiments and evaluate the manuscript. CG performed the statistic measurement and analyzed the data. CG analyzed the data, and wrote the paper.
Funding/support: The research was supported by National Natural Science Foundation of China (No: 30973440, 30770950), and the key project of the Chongqing Natural Science Foundation (CSTC, 2008BA0021, cstc2012jjA0155).
No potential conflicts of interest relevant to this article are reported.
References
- [1].Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Patel RM, Kandefer S, Walsh MC, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015;372:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clin Perinatol 2013;40:135–48. [DOI] [PubMed] [Google Scholar]
- [5].Blakely ML, Lally KP, McDonald S, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg 2005;241:984–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stey A, Barnert ES, Tseng CH, et al. Outcomes and costs of surgical treatments of necrotizing enterocolitis. Pediatrics 2015;135:e1190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yee WH, Soraisham AS, Shah VS, et al. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129:e298–304. [DOI] [PubMed] [Google Scholar]
- [8].Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Short SS, Papillon S, Berel D, et al. Late onset of necrotizing enterocolitis in the full-term infant is associated with increased mortality: results from a two-center analysis. J Pediatr Surg 2014;49:950–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McElhinney DB, Hedrick HL, Bush DM, et al. Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics 2000;106:1080–7. [DOI] [PubMed] [Google Scholar]
- [11].Sankaran K, Puckett B, Lee DS, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr 2004;39:366–72. [DOI] [PubMed] [Google Scholar]
- [12].Gordon PV, Clark R, Swanson JR, et al. Can a national dataset generate a nomogram for necrotizing enterocolitis onset? J Perinatol 2014;34:732–5. [DOI] [PubMed] [Google Scholar]
- [13].Hull MA, Fisher JG, Gutierrez IM, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J Am Coll Surg 2014;218:1148–55. [DOI] [PubMed] [Google Scholar]
- [14].Kelleher J, Mallick H, Soltau TD, et al. Mortality and intestinal failure in surgical necrotizing enterocolitis. J Pediatr Surg 2013;48:568–72. [DOI] [PubMed] [Google Scholar]
- [15].Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med 2006;354:2225–34. [DOI] [PubMed] [Google Scholar]
- [16].Alexander F, Smith A. Mortality in micro-premature infants with necrotizing enterocolitis treated by primary laparotomy is independent of gestational age and birth weight. Pediatr Surg Int 2008;24:415–9. [DOI] [PubMed] [Google Scholar]
- [17].Henry MC, Moss RL. Neonatal necrotizing enterocolitis. Semin Pediatr Surg 2008;17:98–109.18395659 [Google Scholar]
- [18].Wright NJ, Thyoka M, Kiely EM, et al. The outcome of critically ill neonates undergoing laparotomy for necrotising enterocolitis in the neonatal intensive care unit: a 10-year review. J Pediatr Surg 2014;49:1210–4. [DOI] [PubMed] [Google Scholar]
- [19].Pierro A, Hall N. Surgical treatments of infants with necrotizing enterocolitis. Semin Neonatol 2003;8:223–32. [DOI] [PubMed] [Google Scholar]
- [20].Motta C, Scott W, Mahony L, et al. The association of congenital heart disease with necrotizing enterocolitis in preterm infants: a birth cohort study. J Perinatol 2015;35:949–53. [DOI] [PubMed] [Google Scholar]
- [21].Fisher JG, Bairdain S, Sparks EA, et al. Serious congenital heart disease and necrotizing enterocolitis in very low birth weight neonates. J Am Coll Surg 2015;220:1018–26.e14. [DOI] [PubMed] [Google Scholar]
- [22].Singh R, Shah B, Allred EN, et al. The antecedents and correlates of necrotizing enterocolitis and spontaneous intestinal perforation among infants born before the 28th week of gestation. J Neonatal Perinatal Med 2016;9:159–70. [DOI] [PubMed] [Google Scholar]
- [23].Wadhawan R, Oh W, Hintz SR, et al. Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J Perinatol 2014;34:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shah TA, Meinzen-Derr J, Gratton T, et al. Hospital and neurodevelopmental outcomes of extremely low-birth-weight infants with necrotizing enterocolitis and spontaneous intestinal perforation. J Perinatol 2012;32:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Munaco AJ, Veenstra MA, Brownie E, et al. Timing of optimal surgical intervention for neonates with necrotizing enterocolitis. Am Surg 2015;81:438–43. [PubMed] [Google Scholar]


