Abstract
Obstructive sleep apnea (OSA) is prevalent in obese subjects. Plasma adiponectin level in obese subjects is decreased. Whether reduced adiponectin level is associated with OSA is unknown. Participants without a previous diagnosis of OSA or who have not been treated with continuous positive airway pressure were enrolled and parameters of interest were collected. Polysomnography was performed to evaluate the presence of OSA and the severity of OSA as indexed by the apnea-hypopnea index (AHI). Between-group differences were analyzed. Pearson correlation analysis was used to evaluate the association between body mass index (BMI) with plasma levels of adiponectin and C-reactive protein (CRP) and AHI; and the association between plasma adiponectin level with CRP and AHI was also evaluated. Logistic regression analysis was conducted to evaluate the association between per 1-SD standardized decrease of plasma adiponectin level and the prevalence of OSA using stepwise adjustment models. A total of 486 participants were enrolled and the mean BMI was 26.9 ± 6.2 kg/m2 with obesity prevalence of 28%; and the mean AHI was 12.6 ± 8.9 per sleep hour with OSA prevalence of 42%. The mean adiponectin level was 18.4 ± 10.6 μg/mL. Compared with the nonobese group, participants in the obese group had higher BMI, neck girth, waist circumference, and AHI (P < .05 for all comparisons). The prevalence of OSA (51% vs 37%) and the proportion of moderate OSA (49% vs 42%) were also significantly higher, while adiponectin level (14.6 ± 8.7 μg/mL vs 20.7 ± 10.5 μg/mL) was significantly lower. In the obese group, plasma adiponectin level was decreased gradually with the increasing severity of OSA, which was not observed in the nonobese group. BMI was negatively correlated with adiponectin while positively correlated with CRP and AHI; and adiponectin was negatively correlated with both CRP and AHI. After adjusted for covariates including BMI and waist circumference, adiponectin remained significantly associated with OSA prevalence with odds ratio of 1.20 (95% confidence interval 1.12–1.65). In summary, our preliminary study suggests that in obese subjects, plasma adiponectin level is associated with the prevalence of OSA.
Keywords: adiponectin, obesity, obstructive sleep apnea
1. Introduction
Obstructive sleep apnea (OSA) is a major risk factor for a variety of cardiovascular diseases such as coronary heart disease, ischemic stroke, and cardiovascular mortality.[1–4] Mechanistically,[5,6] through intermittent hypoxemia and reoxygenation, OSA promotes systemic inflammation and oxidative stress which in turn causes endothelial dysfunction and atherogenesis. In addition, OSA enhances sympathetic nerve activity which contributes to renin–angiotensin–aldosterone axis activation and targeted organ damage such as left ventricular hypertrophy and albuminuria.[7]
It is well known that obesity is an independent risk factor for OSA[8] and with the current epidemic of obesity, the prevalence and incidence of OSA is projected to increase dramatically in the coming decades.[9] Therefore, it is clinically relevant to investigate the potential biomarkers that are associated with OSA development in obese populations. Adiponectin is excreted by adipocytes and exerts numerous cardio-protective effects by means of anti-inflammation, endothelial-protection, and anti-oxidation.[10] Epidemiological studies have shown that compared with the lean control subjects, plasma adiponectin level in obese subjects was significantly lower;[11] and furthermore, patients with coronary heart disease also had lower plasma adiponectin level as compared with their counterparts without coronary heart disease.[12,13] Regarding the overlapped risk factor in terms of obesity but the opposed pathophysiological effects between OSA and adiponectin, we hypothesized that plasma adiponectin level might be associated with OSA in obese populations.
2. Methods
2.1. Enrollment of participants
Our present study was approved by the clinical research ethical committee of Shenzhen Sun Yat-sen Cardiovascular Hospital, and all the performances were processed in accordance with the Helsinki Declaration. Informed consent was provided before participants’ enrollment. Participants were enrolled from January of 2015 to December of 2016. The inclusion criteria were as follows: 30 to 60 years old; without documented coronary heart disease, ischemic stroke, congestive heart failure, or peripheral artery disease; and willing to participant in the present study; and the exclusion criteria were those with a previous diagnosis of OSA or have been treated with continuous positive airway pressure or device.
2.2. Data collection
Demographic data including age, gender, smoking status, previous medical history, and current medication usage were collected using self-administered questionnaire with the help of investigators as necessary. Anthropometric data including neck girth, waist circumference, blood pressure, and heart rate at rest were measured as previously described.[14] Body mass index (BMI) was calculated by body weight in kilograms divided by height in meter squared, and those with BMI ≥ 30 kg/m2 was defined as obesity.[15] Biochemical data including fasting plasma glucose (FPG), fasting lipid profile, renal function, and C-reactive protein (CRP) level were measured using Automatic Biochemistry Analyzer (Hitachi 7150, Tokyo, Japan). In brief, plasma adiponectin level was measured using enzyme-linked immuno-sorbent assay (Otsuka Pharmaceutical Co Ltd, Japan) and all the performances were conducted in accordance with the manual instruction.
2.3. Polysomnography evaluation
Attended polysomnography was performed to evaluate the presence of OSA as well as the severity of OSA as indexed by the apnea-hypopnea index (AHI). In brief, according to the current AASM guideline,[16] complete blockage of airflow for more than 10 seconds, or >50% decrease in respiratory airflow accompanying >3% reduction in oxygen saturation for more than 10 seconds was defined as apnea or hypopnea, respectively. AHI was calculated by the total number of apnea and hypopnea per sleep hour, with AHI of 5 to 14 was defined as mild, 15 to 29 moderate, and ≥30 severe OSA.
2.4. Statistical analysis
Continuous variables were presented as mean ± SD and categorical variables were presented as number and percentages of cases. Between-group differences were analyzed by using Student t test for continuous variables and the χ2 or Fisher exact test for categorical variables. Pearson correlation analysis was used to evaluate the association between BMI with plasma levels of adiponectin and CRP, and AHI; and the association between plasma adiponectin level with CRP and AHI was also evaluated. Logistic regression analysis was conducted to evaluate the association between per 1-SD standardized decrease of plasma adiponectin level and OSA prevalence using stepwise adjustment models. Statistical analysis was computed using SPSS 17.0 (SPSS Inc, Chicago, IL). All statistical tests were 2-sided and considered statistically significant when P < .05.
3. Results
3.1. General characteristics
A total of 486 participants were enrolled and males accounted for nearly 65%. The mean age was 42.6 ± 5.9 years old and the percentages of participants with current smoking, hypertension, and diabetes mellitus were approximately 36%, 32%, and 23%, respectively. The mean BMI was 26.9 ± 6.2 kg/m2 with obesity prevalence of 28%; and the mean AHI was 12.6 ± 8.9 per sleep hour with OSA prevalence of 42%. The mean plasma adiponectin level was 18.4 ± 10.6 μg/mL and the other variables are presented in Table 1.
Table 1.
General characteristics.

3.2. Comparisons between obese and nonobese subjects
Based on diagnostic criterion,[15] participants were separated into the obese and nonobese groups and between-group differences were evaluated. As presented in Table 2, compared with the nonobese group, the participants in the obese group were more likely to be male, more elderly, and had higher BMI, neck girth, waist circumference, and AHI (P < .05 for all comparisons). The prevalence of OSA (51% vs 37%) and proportion of moderate OSA (49% vs 42%) were significantly higher in the obese groups (P < .05 for all comparisons). However, the percentage of mild OSA was lower in the obese group (30% vs 40%), and no statistically significant differences in the percentages of severe OSA were observed between the obese and nonobese groups (21% vs 18%). Regarding the biochemical parameters, levels of PFG, total cholesterol and low-density lipoprotein cholesterol and CRP were significantly higher in the obese group while plasma adiponectin level (14.6 ± 8.7 μg/mL vs 20.7 ± 10.5 μg/mL) was significantly lower when compared with the nonobese group (P < .05 for all comparisons).
Table 2.
Comparisons between obese and nonobese groups.
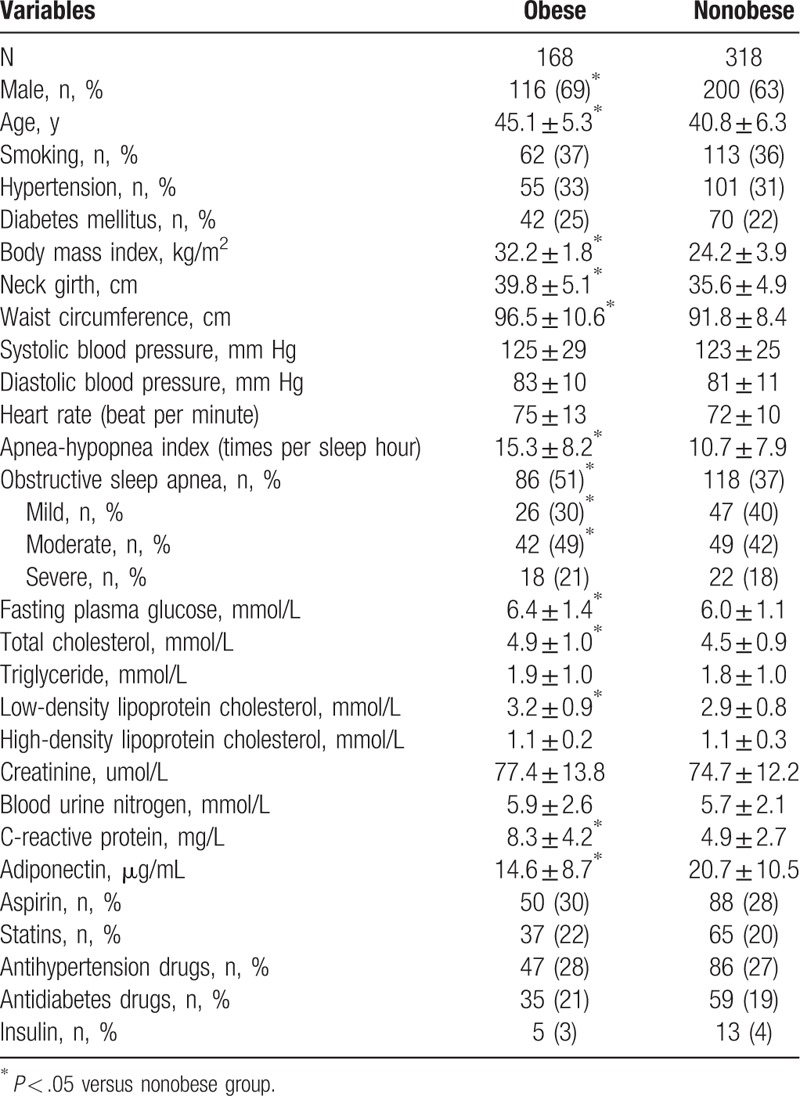
3.3. Adiponectin levels in participants with different degrees of OSA
As presented in Fig. 1, in the nonobese group, there was no significant difference in plasma adiponectin level between different degrees of OSA; nevertheless, in the obese group, compared with the without OSA group, adiponectin levels in the severe and moderate OSA groups were significantly lower (P = .002 and P = .017, respectively).
Figure 1.
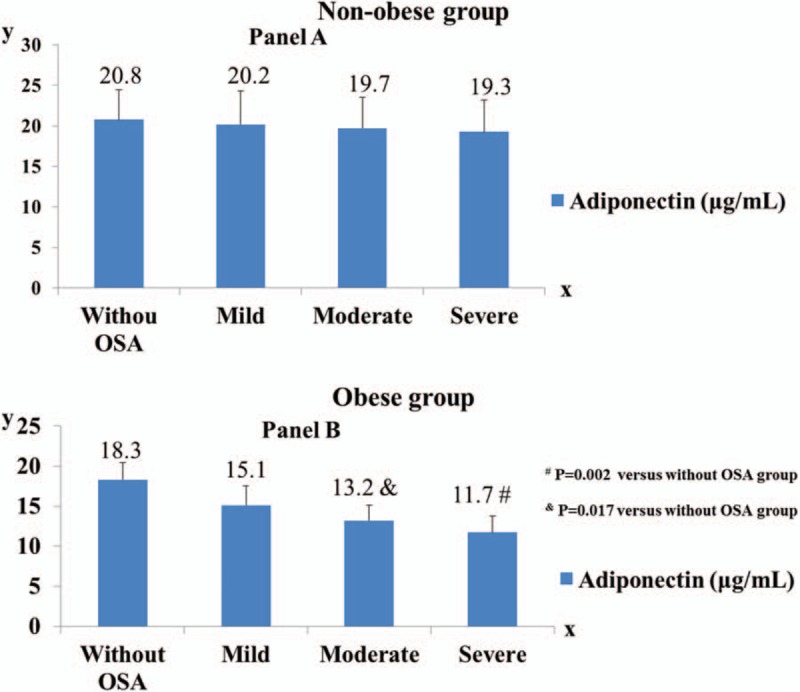
Comparison of adiponectin level in different OSA degrees by categories of obesity. A, In the nonobese group, no significant between-group difference in adiponectin level was observed (without OSA vs mild OSA, P = .265; without OSA vs moderate OSA, P = .173; without OSA vs severe OSA, P = .129; mild OSA vs moderate OSA, P = .304; mild OSA vs severe OSA, P = .154; moderate OSA vs severe OSA, P = .199). Without OSA group (n = 87, mean BMI = 23.8 ± 2.4 kg/m2), mild OSA group (n = 91, mean BMI = 23.5 ± 2.6 kg/m2), moderate OSA group (n = 72, mean BMI = 24.1 ± 3.0 kg/m2), severe OSA group (n = 68, mean BMI = 24.4 ± 3.7 kg/m2). B, In the obese group, compared with the without OSA group, adiponectin levels in the severe and moderate OSA groups were significantly lower (P = .002 and P = .017, respectively); no significant differences were observed between without OSA versus mild OSA (P = .098), mild OSA versus moderate OSA (P = .149), mild OSA versus severe OSA (P = .108), and moderate OSA versus severe OSA (P = .224). Without OSA group (n = 45, mean BMI = 31.6 ± 1.1 kg/m2), mild OSA group (n = 33, mean BMI = 32.0 ± 1.0 kg/m2), moderate OSA group (n = 46, mean BMI = 32.3 ± 1.7 kg/m2), severe OSA group (n = 44, mean BMI = 32.6 ± 1.9 kg/m2). BMI = body mass index, OSA = obstructive sleep apnea.
3.4. Pearson correlation analysis
Pearson correlation analysis was performed to evaluate the correlations between BMI with other variables as well as between adiponectin with other variables, respectively. As presented in Tables 3 and 4, BMI was negatively correlated with adiponectin while positively correlated with CRP and AHI; and adiponectin was negatively correlated with both CRP and AHI.
Table 3.
Correlations between BMI and other variables.
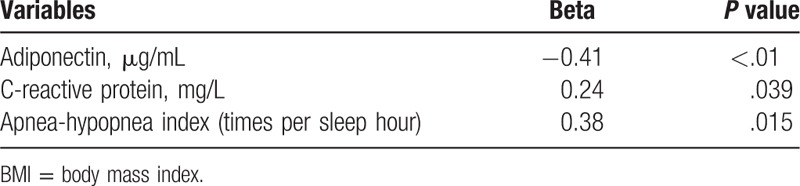
Table 4.
Correlations between adiponectin and other variables.

3.5. Logistic regression analysis
As shown in Table 5, in the unadjusted model, per 1-SD standardized decrease of plasma adiponectin level was associated with 2-folds higher prevalence of OSA. With adjusted for age and male gender, the odds ratio reduced to 1.90; additionally adjusted for smoking, hypertension, diabetes mellitus, CRP, and neck girth, the odds ratio was reduced slightly; while additionally adjusted for BMI and waist circumference, the odds ratio reduced prominently to 1.20 (95% confidence interval 1.12–1.65), suggesting that increased BMI and waist circumference might partially explain the association between reduced plasma adiponectin level and increased OSA prevalence.
Table 5.
Logistic regression analysis.

4. Discussion
Our present research has 2 principal findings: first, as presented in the Pearson correlation analysis, plasma adiponectin level is inversely correlated with the AHI; and it seems that this relationship may exist only in the obese participants as revealed in Fig. 1; second, after extensively being adjusted for potential confounding factors including BMI and waist circumference, plasma adiponectin level remains independently associated with OSA. These findings indicate that decreased plasma adiponectin level is positively associated with the prevalence of OSA in obese populations. A prospective cohort study is warranted to evaluate whether the baseline and change of plasma adiponectin level over time is associated with the incidence of OSA in obese populations.
In the past few decades, numerous epidemiological studies have consistently revealed the independent association between obesity and OSA. For example, recently, a recent study showed that BMI was positively correlated with OSA with odds ratio of 1.064 after extensively adjusted for potential covariates.[17] In addition, the Wisconsin Sleep Cohort study also showed that a 10% increase in body weight contributed to 32% increase in AHI and 6-fold increase risk in developing moderate-severe OSA.[18] The Sleep Heart Health study further corroborated the independent predictor of obesity for OSA development.[19] Consistent with previous studies, our current research also showed that compared with the nonobese subjects, the overall prevalence of OSA was significantly higher in the obese subjects; and obese patients also had higher proportion of moderate OSA. Although the proportion of severe OSA was higher in the obese group, the difference did not achieve statistical significance. In addition, the proportion of mild OSA was significantly lower in the obese group. Pearson correlation analysis also showed a positive relationship between BMI and AHI, suggesting that losing weight may be favorable for OSA management which deserves further investigation in the future.
Despite compelling evidence supporting the independent relationship between obesity and OSA, the underlying mechanisms are still elusive. It has been speculated that compared with the lean control subjects, the obese populations commonly have more para-pharyngeal fat deposition which in turn causes upper airway caliber narrowing and collapsing. Moreover, through reduced lung volume, obesity results in decreased tracheal tug and increased airway resistance. Extending previous findings, our current research showed that decreased plasma adiponectin level might be associated with obesity-associated OSA development. Indeed, we observed that plasma adiponectin level was negatively correlated with both BMI and AHI. Furthermore, in the logistic regression models, after extensively being adjusted for potential confounding factors, the reduced plasma adiponectin level was still significantly associated with higher prevalence of OSA. Some previous observational studies also showed the negative association between adiponectin level and AHI. For example, Zhang et al[20] reported that compared with the control group, the adiponectin level was significantly lower in the OSA group, and adiponectin levels were also negatively correlated with AHI and BMI. In another study, Masserini et al[21] also reported that the adiponectin level was significantly reduced in obese patients compared with healthy normal weight subjects and adiponectin showed a trend to decrease in accordance with the severity of OSA. Although these 2 previous studies were limited by their small sample size, these aggregated data together strongly indicated that adiponectin might be an important mediator linking obesity and OSA. Interestingly, we observed that in the nonobese group, there was no significant association between adiponectin level and OSA degree as presented in Fig. 1, which further implicated that adiponectin might be only associated with OSA in obese populations. Nevertheless, our current study was unable to demonstrate a causal relationship between reduced plasma adiponectin level and OSA incidence.
There were another 2 significant findings of our current study. First, we showed that adiponectin was negatively correlated with CRP which was consistent with previous report,[13] suggesting that decreased plasma adiponectin level might be associated with systemic inflammation and adiponectin reduction might predispose OSA subjects to develop atherosclerosis. Second, as shown in the logistic regression model 3, after being adjusted for BMI and waist circumference, the odds ratio reduced from 1.78 to 1.20, which indicated that visceral obesity and adiponectin might have potential interactive action on OSA development, suggesting that in the future randomized trials, it is necessary to stratify subjects into different categories of BMI so as to avoid potential interaction between BMI and adiponectin.
There are several limitations of our current research. First, the cross-sectional design could not allow us to draw causal relationship between decreased adiponectin level and increased risk of OSA development. Nevertheless, these findings provide more insights into these potential significant associations. Moreover, to the best of our knowledge, this was the largest study to evaluate the association between adiponectin and OSA. Second, the participants in our study were relatively young and without overt cardiovascular diseases. Therefore, these findings could not be generalized to other populations. Nevertheless, our present study provides evidence to support the notion that OSA was epidemic even in the relatively healthy status. Third, despite being extensively adjusted for potential covariates, it was conceivable that some unmeasured and residual confounding factors might still exist. However, in both the Pearson correlation analysis and the logistic regression analysis, we observed that the adiponectin level remained independently correlated with both AHI and OSA.
5. Conclusion
With the advantage of large sample size, our present study first shows that the plasma adiponectin level is inversely correlated with AHI; and the adiponectin level is gradually decreased with increasing OSA severity in obese subjects. Furthermore, independent of BMI and waist circumference, decreased adiponectin level is significantly associated with increased prevalence of OSA. These preliminary findings together suggest that the reduced plasma adiponectin level is associated with OSA in obese subjects. Prospective cohort studies are warranted to investigate whether the baseline and change of plasma adiponectin level over time are associated with the incidence of OSA in obese populations.
Footnotes
Abbreviations: AHI = apnea-hypopnea index, BMI = body mass index, CRP = C-reactive protein, FPG = fasting plasma glucose, OSA = obstructive sleep apnea.
This study is supported by the Guangdong Shenzhen Scientific Technology Project (No. JCYJ20160427174117767) and Sanming Project of Medicine in Shenzhen.
The authors have no conflicts of interest to disclose.
References
- [1].Chami HA, Resnick HE, Quan SF, et al. Association of incident cardiovascular disease with progression of sleep-disordered breathing. Circulation 2011;123:1280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013;62:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang L, Cai A, Zhang J, et al. Association of obstructive sleep apnea plus hypertension and prevalent cardiovascular diseases: a cross-sectional study. Medicine (Baltimore) 2016;95:e4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cao Z, Zhang P, He Z, et al. Obstructive sleep apnea combined dyslipidemia render additive effect on increasing atherosclerotic cardiovascular diseases prevalence. Lipids Health Dis 2016;15:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation 2012;126:1495–510. [DOI] [PubMed] [Google Scholar]
- [6].Jean-Louis G, Zizi F, Brown D, et al. Obstructive sleep apnea and cardiovascular disease: evidence and underlying mechanisms. Minerva Pneumol 2009;48:277–93. [PMC free article] [PubMed] [Google Scholar]
- [7].Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010;7:677–85. [DOI] [PubMed] [Google Scholar]
- [8].Goodfriend TL. Obesity, sleep apnea, aldosterone, and hypertension. Curr Hypertens Rep 2008;10:222–6. [DOI] [PubMed] [Google Scholar]
- [9].Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US preventive services task force recommendation statement. JAMA 2017;317:407–14. [DOI] [PubMed] [Google Scholar]
- [10].Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 2005;26:439–51. [DOI] [PubMed] [Google Scholar]
- [11].Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83. [DOI] [PubMed] [Google Scholar]
- [12].Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 2003;23:85–9. [DOI] [PubMed] [Google Scholar]
- [13].Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation 2003;107:671–4. [DOI] [PubMed] [Google Scholar]
- [14].Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [15].Eckel RH, Krauss RM. American Heart Association call to action: obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation 1998;97:2099–100. [DOI] [PubMed] [Google Scholar]
- [16].Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667–89. [PubMed] [Google Scholar]
- [17].Cai A, Zhou Y, Zhang J, et al. Epidemiological characteristics and gender-specific differences of obstructive sleep apnea in a Chinese hypertensive population: a cross-sectional study. BMC Cardiovasc Disord 2017;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015–21. [DOI] [PubMed] [Google Scholar]
- [19].Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005;165:2408–13. [DOI] [PubMed] [Google Scholar]
- [20].Zhang XL, Yin KS, Mao H, et al. Serum adiponectin level in patients with obstructive sleep apnea hypopnea syndrome. Chin Med J (Engl) 2004;117:1603–6. [PubMed] [Google Scholar]
- [21].Masserini B, Morpurgo PS, Donadio F, et al. Reduced levels of adiponectin in sleep apnea syndrome. J Endocrinol Invest 2006;29:700–5. [DOI] [PubMed] [Google Scholar]


