Abstract
How endothelial cells adapt their metabolism to the rising energy and biomass demands of sprouting vessels is an exciting field of research in vascular biology with numerous open questions. Two new studies published in this issue of The EMBO Journal now show the importance of glutamine in endothelial metabolism, required to sustain endothelial cell proliferation and vascular expansion. These results provide insight into how endothelial cells selectively use nutrients for energy and biomass production and illuminate new levels of regulation of the angiogenic process.
Subject Categories: Metabolism, Vascular Biology & Angiogenesis
Endothelial cells (ECs) have special functions—functions that are critical for development and homeostasis, but also cause disease if not properly controlled. As inner lining of the blood vasculature, ECs provide nutrients and oxygen for tissues and remove waste products that result from the breakdown of these metabolic fuels. They also orchestrate the growth of the vasculature, a process referred to as angiogenesis. Activated by growth factors that are secreted from nutrient‐ and oxygen‐deprived tissues (e.g. VEGF), ECs sprout, migrate and proliferate to form new vessel branches. Only recently it has been recognized that such endothelial behaviour is not only dependent on growth factor‐induced signal transduction but also on endothelial metabolic state (Potente & Carmeliet, 2017). This is because the angiogenic process is metabolically demanding. ECs need nutrients and energy for migration as well as for the synthesis of cellular building blocks (nucleotides, amino acids, lipids) that are required for growth and proliferation. As a result, ECs must switch from a metabolic state that maintains basic cellular processes to a state of increased energy and biomass production. However, how ECs use their metabolic machinery to fulfil these requirements is poorly understood. Two studies in this issue shed light on this question by highlighting the importance of glutamine metabolism in growing vessels (Huang et al, 2017; Kim et al, 2017).
Figure 1. Glutamine metabolism in sprouting endothelial cells.
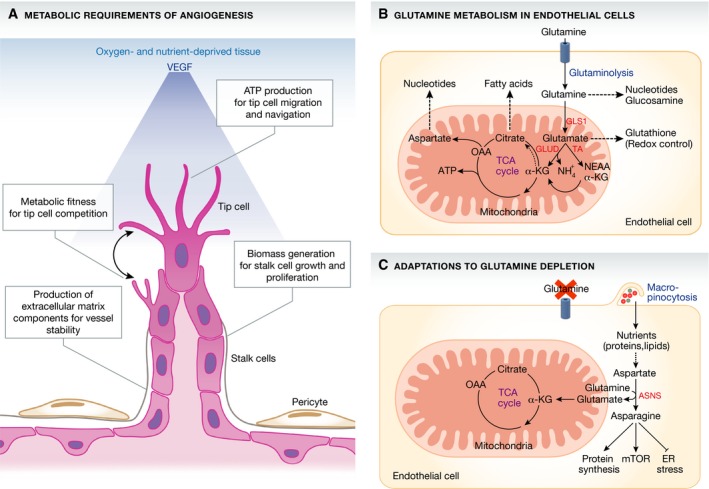
(A) ECs are confronted with several metabolic challenges when they invade avascular tissues. A gradient (blue) of VEGF, secreted by the nutrient‐ and oxygen‐deprived tissue, activates ECs to sprout, migrate and proliferate. Sprouting ECs need to increase their biosynthetic activity to produce sufficient energy (ATP) and biomass (nucleotides, protein and lipids) required for vascular expansion. (B) Huang et al (2017) and Kim et al (2017) show that ECs consume large amounts of glutamine to produce nucleotides, amino acids and lipids required for cell growth and duplication. Endothelial GLS1 activity was found to be essential to replenish the TCA cycle, to produce biomass and to maintain redox balance. Dashed arrow within the TCA cycle indicates reductive carboxylation, which denotes the carboxylation of α‐KG to citrate. (C) Under conditions of glutamine limitation, ECs can in part compensate for the lack of glutamine catabolism by using alternative nutrient acquisition pathways. When glutamine levels drop, ECs use macropinocytosis to take up extracellular nutrients such as asparagine. ECs use asparagine for protein synthesis, reactivation of mTOR signalling and the suppression of endoplasmic reticulum (ER) stress. EC, endothelial cell; VEGF, vascular endothelial growth factor; ATP, adenosine triphosphate; GLS1, glutaminase 1; GLUD, glutamate dehydrogenase; TA, transaminases; NEAA, non‐essential amino acids; α‐KG, α‐ketoglutarate; NH4 +, ammonium; OAA, oxaloacetate; ASNS, asparagine synthethase; mTOR, mechanistic target of rapamycin; ER, endoplasmic reticulum.
One of the hallmarks of ECs is their dependency on glucose (De Bock et al, 2013). Endothelial cells take up large amounts of glucose and metabolize it to lactate via aerobic glycolysis. Through this metabolic pathway, ECs satisfy most of their adenosine triphosphate (ATP) needs and also provide glycolytic intermediates that serve as precursors for biosynthetic pathways (De Bock et al, 2013). The non‐essential amino acid glutamine (abbreviated as Q) is another important growth‐supporting nutrient (Palm & Thompson, 2017). It is a major source of carbons and nitrogen, and its breakdown (glutaminolysis) is linked to multiple cellular functions. Once taken up into the cell, glutamine is sequentially converted to glutamate and α‐ketoglutarate, which replenishes the tricyclic acid (TCA) cycle with carbons (anaplerosis). Studies in cancer cells and other rapidly proliferating cells have shown that glutamine is used for the production of ATP, the biosynthesis of macromolecules including nucleotides, amino acids and lipids as well as the generation of antioxidants (DeBerardinis & Cheng, 2010; Zhang et al, 2017). Whether glutamine has similar roles in the endothelium was unclear.
To address this question, Huang et al (2017) and Kim et al (2017) performed metabolic labelling studies in cultured ECs. Both studies found that proliferating ECs consume high amounts of glutamine—more than any other amino acid. Consistent with a central role as an anaplerotic source of carbons, inhibition of glutamine consumption caused a collapse of the TCA cycle, which could not be compensated by glucose metabolism. As a consequence, glutamine‐depleted ECs were unable to synthesize cellular macromolecules and were cell cycle arrested. Surprisingly, Huang et al (2017) reported that glutamine metabolism is dispensable for ATP production, perhaps because ECs use aerobic glycolysis for this purpose. In contrast, Kim et al (2017) found that glutamine deprivation or knock‐down of glutaminase 1 (GLS1), the enzyme that catalyses the conversion of glutamine to glutamate, reduces the energy charge of ECs by half. Whether glutamine is used for endothelial ATP production might thus depend on environmental context and merits further investigation. Irrespective of the answer to this question, glutamine metabolism appears critical for endothelial redox balance. Huang et al (2017) showed that glutamine is used to produce the antioxidant glutathione (GSH) and that glutamine depletion increases reactive oxygen species (ROS) formation. These findings are of particular relevance for the endothelium, because ECs are long‐lived cells that must protect themselves against the harmful effects of ROS accumulation. Thus, by using the most abundant amino acid in the blood stream for redox regulation, ECs ensure that ROS levels can be kept in check.
The avidity of ECs for glutamine prompted both research groups to investigate its role in angiogenesis. To this end, they genetically inactivated GLS1 in ECs of newborn mice and analysed angiogenesis in the retina. Consistent with the in vitro studies, both groups found that loss of GLS1 impaired EC proliferation leading to a poorly branched and stunted vascular network. Notably, although both studies used the same conditional knockout allele, they also reported differences in some parameters. While Huang et al (2017) observed that GLS1‐deficient ECs are less migratory and have fewer filopodia, Kim et al (2017) did not find evidence for such changes. These disparities may be an indication that glutamine catabolism is not directly involved in the regulation of cellular rearrangements and migration during sprouting. However, it should be noted that both studies used different Cre lines to eliminate GLS1 expression in ECs, which could also explain some of the phenotypic differences.
When Huang et al (2017) aimed to rescue the endothelial defects elicited by glutamine limitation, they found that only a mixture of α‐ketoglutarate and asparagine was able to restore the angiogenic defects, indicating that glutamine and asparagine metabolism are linked. In agreement with these data, inhibiting asparagine synthetase (ASNS), which generates asparagine from aspartate and glutamine, or manipulating asparagine levels, rendered ECs unable to proliferate. Interestingly, Kim et al (2017) also noted that glutamine‐depleted ECs started to ingest extracellular macromolecules via macropinocytosis to provide alternative sources of nutrients including asparagine. These compensatory mechanisms might explain the observation by Kim et al (2017) that GLS1 mutant mice developed to adulthood and are grossly normal. They also indicate that ECs possess different nutrient acquisition strategies allowing them to sprout in metabolically challenging environments. However, the in vivo relevance of these findings is still elusive, and future studies with mice lacking ASNS or essential components of the macropinocytotic pathway are needed to understand the connection between glutamine and asparagine metabolism in detail.
An intriguing concept from these studies is that ECs compartmentalize their metabolic machinery to maximize the use of nutrients during angiogenic growth. In sprouting ECs, glycolytic enzymes are found near lamellipodia and filopodia, where high ATP levels are needed to drive migration (De Bock et al, 2013). In contrast, glutamine‐consuming enzymes are mainly found in the mitochondria, located largely peri‐nuclear and away from the migratory edge. Notably, intermediates of glutamine metabolism are co‐substrates for proteins that control epigenetic gene expression including Jumonji C‐domain containing (JmjC) histone demethylases and ten‐eleven translocation (TET) DNA demethylases (Kaelin & McKnight, 2013). It is thus tempting to speculate that the peri‐nuclear localization of mitochondria helps to channel glutamine‐derived (signalling) metabolites to the nucleus, where active gene regulation is needed for cell growth and duplication. The compartmentalization of glucose and glutamine metabolism could provide a spatial configuration to effectively coordinate EC sprouting. However, how ECs couple all these processes remains an outstanding question that warrants further study.
The reports by Huang et al (2017) and Kim et al (2017) provide novel insights into the complexities of EC metabolism and have implications for a number of angiogenesis‐related diseases such as cancer. Given that cancer metabolism‐targeting drugs are already entering the clinic, the present reports provide a rationale to consider GLS1 and ASNS as potential therapeutic targets to limit aberrant angiogenic responses in tumours.
Acknowledgements
The work of M.P. is supported by the Max Planck Society, the European Research Council (ERC) Starting Grant ANGIOMET (311546), the Deutsche Forschungsgemeinschaft (SFB 834), the Excellence Cluster Cardiopulmonary System (EXC 147/1), the LOEWE grant Ub‐Net, the DZHK (German Center for Cardiovascular Research), the Stiftung Charité and the European Molecular Biology Organization (EMBO) Young Investigator Programme.
See also: H Huang et al (August 2017) and B Kim et al (August 2017)
References
- De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquiere B, Cauwenberghs S, Eelen G, Phng LK, Betz I, Tembuyser B, Brepoels K, Welti J, Geudens I, Segura I, Cruys B, Bifari F, Decimo I et al (2013) Role of PFKFB3‐driven glycolysis in vessel sprouting. Cell 154: 651–663 [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Cheng T (2010) Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Brüning U, Visnagri A, Yuldasheva N, Goveia J, Cruys B, Brepoels K, Wyns S, Rayport S, Ghesquière B, Vinckier S, Schoonjans L, Cubbon R, Dewerchin M, Eelen G, Carmeliet P (2017) Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J 36: 2334–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG Jr, McKnight SL (2013) Influence of metabolism on epigenetics and disease. Cell 153: 56–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Li J, Jang C, Arany Z (2017) Glutamine fuels proliferation but not migration of endothelial cells. EMBO J 36: 2321–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, Thompson CB (2017) Nutrient acquisition strategies of mammalian cells. Nature 546: 234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Carmeliet P (2017) The link between angiogenesis and endothelial metabolism. Annu Rev Physiol 79: 43–66 [DOI] [PubMed] [Google Scholar]
- Zhang J, Pavlova NN, Thompson CB (2017) Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J 36: 1302–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]


