Abstract
Differentiated cells had long been thought to be non‐dividing, though we now know many can proliferate after injury. A new study by Leushacke et al (2017) shows how injury recruits mature, Lgr5‐expressing gastric chief cells to become stem cells that can either regenerate damaged tissue or fuel precancerous lesions.
Subject Categories: Cancer, Development & Differentiation, Stem Cells
During homeostasis, all epithelial cells lining the main portion (corpus) of the stomach are thought to be maintained by stem cells. These stem cells are in the isthmus region, located below the mucous pit cells covering the stomach surface and above the long gastric gland that dives down deeper toward the musculature. The gland comprises two main differentiated cell types: acid‐producing parietal cells and digestive‐enzyme‐secreting chief cells. Upon certain types of injury, for example, in some patients infected with the bacterium Helicobacter pylori, mature parietal and chief cells are replaced by proliferating cells secreting mucins and wound repair proteins in a lesion known as spasmolytic polypeptide‐expressing metaplasia (SPEM). A recent study by Leushacke et al (2017) uses mouse models to reveal that SPEM is derived from a subpopulation of chief cells expressing the Wnt target gene Lgr5 (Fig 1). These LGR5+ chief cells are recruited back into the cell cycle to serve as stem cells that in the short term repair glandular damage and in longer term can also fuel SPEM that persists as a precancerous lesion.
Figure 1. Lgr5‐expressing chief cells in the gastric corpus can repair the stomach and are sources of metaplasia and precancerous lesions after injury.
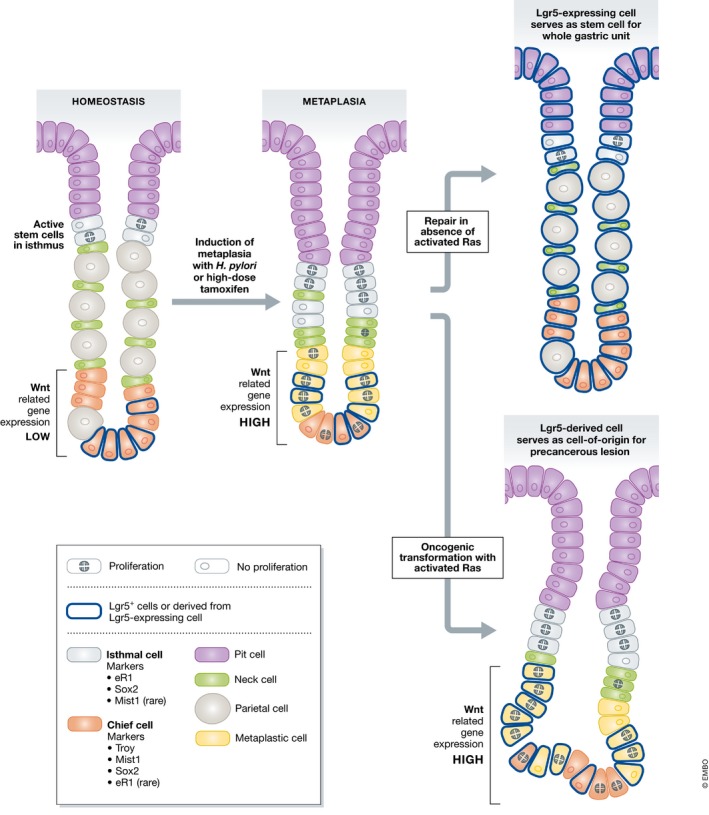
At homeostasis, Lgr5‐expressing chief cells remain in the base of the corpus unit and are not proliferative, while active stem cells in the isthmus give rise to all other epithelial cell types. However, after metaplasia‐inducing injury (as can be induced in the laboratory setting with high doses of tamoxifen; Huh et al, 2012), Lgr5‐expressing, “reserve” stem cells in the bottom part of the gland activate Wnt signaling, become metaplastic, and re‐enter the cell cycle in a reprogramming event. Without incurring additional damage, Lgr5‐expressing cells have potential to generate all epithelial cells in the gastric gland. If chief cells acquire oncogenic mutations (e.g., activate Ras; Choi et al, 2016), they can form dysplastic lesions, thus being a precursor cell to gastric cancer formation.
Lgr5 in other tissues, for example, in the intestine, tends to mark rapidly dividing stem cells (Barker et al, 2007); however, stem cell identity is proving to be dynamic. For instance, in intestine, differentiated cells can be recruited as stem cells if LGR5+ stem cells are lost (Mills & Sansom, 2015). Stem cell dynamics in the gastric corpus are still unclear. Previous studies have shown that chief cells expressing Mist1, Troy, or elements from the Runx1 promoter (designated eR1) can form SPEM and also, occasionally, serve as stem cells (Nam et al, 2010; Stange et al, 2013; Matsuo et al, 2017). It has also been shown that both isthmal and chief cells proliferate during the metaplastic response (Burclaff et al, 2017; Matsuo et al, 2017). In contrast, one report suggests that the isthmal stem cell is not only the source of normal homeostatic stem cell activity but also transdifferentiates to become the sole source for SPEM (Hayakawa et al, 2015).
Leushacke et al (2017) use an existing mouse pedigree expressing the human diphtheria toxin receptor and fluorescent GFP under control of the Lgr5 promoter. GFP‐expressing (i.e., LGR5+) cells in the corpus are a subpopulation of chief cells enriched for Wnt pathway‐related genes. Ablating the LGR5+ chief cells with diphtheria toxin caused some cystic changes in the mucus‐secreting surface cells, but parietal cells and the remaining non‐LGR5+ chief cells remained unaffected. Glands from mice depleted of LGR5+ cells also showed reduced potential for generating organoids (complex, multicellular three‐dimensional cultures). Organoids derive both from isthmal stem cells (Hayakawa et al, 2015; Matsuo et al, 2017) and from chief cells recruited back into the cell cycle (Stange et al, 2013; Matsuo et al, 2017). Next, the authors used a new mouse line engineered to express Cre recombinase in Lgr5‐expressing cells in a broader manner truer to endogenous Lgr5 expression than the famous Lgr5 Cre‐iRes‐EGFP allele used in many previous studies. The new allele allowed them to show that occasional whole gastric units could be derived from LGR5+ cells. Thus, Lgr5‐expressing chief cells can be recruited to serve as stem cells for organoids and, occasionally, in the gastric epithelium, but they do not seem to be an active, homeostatic stem cell.
Leushacke et al (2017) then explored LGR5+ cell behavior following injury by inducing SPEM using the high‐dose tamoxifen protocol (Huh et al, 2012). They found that proliferating, metaplastic cells were derived from LGR5+ chief cells that had re‐entered the cell cycle. Moreover, following recovery from tamoxifen, they noted that many gastric units were fully labeled, indicating that LGR5+ cells eventually served as stem cells to regenerate all epithelial cell lineages. In short, Lgr5‐expressing chief cells can dedifferentiate to a multipotent proliferative state and act as “reserve” stem cells upon gastric injury. The authors found that the two most differentially expressed genes in injured vs. uninjured LGR5+ chief cells are both Wnt‐related: Mmp7 and Sostdc1. Inducing expression of Mmp7 in organoids increased Lgr5 and Axin2 expression, and induced Sostdc1 decreased Lgr5 and Axin2 expression. Thus, Wnt signaling may be important for activating “reserve” chief cells to contribute to epithelial regeneration.
Previously published work has shown that expressing activated Kras in chief cells using Mist1 or eR1 causes formation of SPEM glands which eventually progress to more chronic intestinal‐type metaplasia (Choi et al, 2016; Matsuo et al, 2017). Leushacke et al (2017) show Lgr5 can also drive chief cells into such chronic metaplastic lesions and further that LGR5 expression correlates with tumors in humans. Thus, multiple reports now confirm chief cells in the gastric corpus may be a cell‐of‐origin for metaplasia and possibly human intestinal‐type gastric cancer.
The authors of the current study clarify and expand upon a rapidly expanding field. First, they confirm that chief cells are “reserve” stem cells that rarely proliferate at homeostasis but, upon injury, can act as a reservoir of recruitable stem cells for repair (Burclaff et al, 2017; Matsuo et al, 2017). Second, they show for the first time that targeted chief cell ablation is insufficient to induce SPEM, mirroring previous results showing that parietal cell ablation alone is insufficient to cause SPEM (Burclaff et al, 2017). Third, they show that the stomach corpus exhibits plasticity similar to other tissues. Fourth, they help clarify how LGR5+ cells in the corpus resemble those in the antrum. As in the corpus, antral LGR5+ cells are in the gland base, where differentiated antral cells reside—not in the isthmus where the fastest dividing cells reside (Barker et al, 2010). Antral LGR5+ cells, however, seem to have more stem cell activity without injury than those in the corpus. Perhaps either they are more stem‐like than their cousins in the corpus, or the antrum is simply more plastic in its stem cell dynamics overall.
Tissue regeneration from differentiated cells has been observed in many organs such as lung, pancreas, and intestines. The Leushacke et al (2017) work highlights how the stomach corpus might have at least one advantage for studying stem cell recruitment. Similar to the intestines, there are both constitutively active stem cells and cells with stem cell potential, equivalent to stem cells formerly known as “+4” in the intestines. Unlike the intestines, however, the base where chief cells reside is physically separate from the isthmus, whereas in the intestinal crypt, stem and recruitable stem cells are virtually intermixed.
Leushacke et al (2017) also add another example of how differentiated cells, called back into the cell cycle, can serve as cells of origin for cancer. In pancreas, similar experiments have shown that mutant Kras expressed in the enzyme‐secreting cousins of chief cells, the acinar cells, has little effect until acinar cells are recruited back into the cell cycle by injury‐induced metaplasia. Metaplastic acinar cells expressing mutant Kras can no longer redifferentiate and can progress to neoplasia (Mills & Sansom, 2015). Why might recruiting differentiated cells be so important in tumorigenesis? One possibility is that differentiated cells are long‐lived, so they can store mutations. Cycles of induced proliferation could cause gradual accrual of mutations that are “stored” long term as long as the cells can redifferentiate. We have proposed the importance of differentiation–dedifferentiation cycles as the “cyclical hit” model of how mutations accumulate until they result in cancer (Mills & Sansom, 2015).
Key questions persist. For example, do specific chief cell subsets serve stem cell functions? Recent work has shown that more mature chief cells may have less potential to convert to SPEM (Weis et al, 2017). Another big question is: What are the mechanisms dictating how differentiated cells “know” to re‐enter the cell cycle? Are those mechanisms conserved across tissues and species? One thing that is becoming clear is that the fields of cancer biology and regenerative medicine may be related as the plasticity of differentiated cells seems to be playing a leading role in fueling both repair and tumorigenesis.
See also: M Leushacke et al (July 2017)
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H (2010) Lgr5 + ve stem cells drive self‐renewal in the stomach and build long‐lived gastric units in vitro . Cell Stem Cell 6: 25–36 [DOI] [PubMed] [Google Scholar]
- Burclaff J, Osaki LH, Liu D, Goldenring JR, Mills JC (2017) Targeted apoptosis of parietal cells is insufficient to induce metaplasia in stomach. Gastroenterology 152: 762–766.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E, Hendley AM, Bailey JM, Leach SD, Goldenring JR (2016) Expression of activated Ras in gastric chief cells of mice leads to the full spectrum of metaplastic lineage transitions. Gastroenterology 150: 918–930.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Ariyama H, Stancikova J, Sakitani K, Asfaha S, Renz B, Dubeykovskaya Z, Shibata W, Wang H, Westphalen C, Chen X, Takemoto Y, Kim W, Khurana S, Tailor Y, Nagar K, Tomita H, Hara A, Sepulveda A, Setlik W et al (2015) Mist1 expressing gastric stem cells maintain the normal and neoplastic gastric epithelium and are supported by a perivascular stem cell niche. Cancer Cell 28: 800–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh WJ, Khurana SS, Geahlen JH, Kohli K, Waller RA , Mills JC (2012) Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142: 21–24.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leushacke M, Tan SH, Wong A, Swathi Y, Hajamohideen A, Tan LT, Goh J, Wong E, Denil SLIJ, Murakami K, Barker N (2017) Lgr5‐expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol 19: 774–786 [DOI] [PubMed] [Google Scholar]
- Matsuo J, Kimura S, Yamamura A, Koh CP, Hossain MZ, Heng DL, Kohu K, Voon DC, Hiai H, Unno M, So JBY, Zhu F, Srivastava S, Teh M, Yeoh KG, Osato M, Ito Y (2017) Identification of stem cells in the epithelium of the stomach corpus and antrum of mice. Gastroenterology 152: 218–231.e14 [DOI] [PubMed] [Google Scholar]
- Mills JC, Sansom OJ (2015) Reserve stem cells: differentiated cells reprogram to fuel repair, metaplasia, and neoplasia in the adult gastrointestinal tract. Sci Signal 8: re8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KT, Lee H, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero‐Gallo J, Shi G, Mills JC, Peek RM, Konieczny SF, Goldenring JR (2010) Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange DE, Koo B‐K, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, Peters PJ, van Es JH, van de Wetering M, Mills JC, Clevers H (2013) Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell 155: 357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis VG, Petersen CP, Weis JA, Meyer AR, Choi E, Mills JC, Goldenring JR (2017) Maturity and age influence chief cell ability to transdifferentiate into metaplasia. Am J Physiol Gastrointest Liver Physiol 312: G67–G76 [DOI] [PMC free article] [PubMed] [Google Scholar]


