Abstract
Objectives:
To evaluate the diagnostic validity of GeneXpert for the detection of Mycobacterium tuberculosis (MTB) in pericardial and pleural effusions samples.
Methods:
A cross sectional study was conducted at the Mycobacteriology Laboratory, Allama Iqbal Medical College, Lahore, Pakistan. A total of 286 (158 pleural and 128 pericardial fluids) samples were received from tuberculosis (TB) suspects between January 2014 and August 2016. Every sample was processed for Ziehl-Neelsen (Zn) smear, Lowenstein Jensen (LJ) culture, GeneXpert MTB/RIF assay according to standard protocols. Validity of GeneXpert assay for the detection of MTB was evaluated using LJ culture as gold standard.
Results:
Out of 286 effusions samples, MTB was isolated by LJ culture in 51 (17.8%) samples followed by GeneXpert in 43 (15%), and acid- fast bacilli (AFB) was detected by Zn smear microscopy in 11 (3.8%) samples. GeneXpert showed high sensitivity (84.3%), specificity (100%), with positive predictive value (100%), and negative predictive value (96.7%), while Zn smear showed sensitivity 18.3%, specificity 99.1%, positive predictive value 81.8%, and negative predictive value 85.4%. A strikingly high sensitivity of 72.2% was observed for pericardial fluid by GeneXpert.
Conclusion:
GeneXpert assay is an innovative tool, for prompt detection of MTB and drug resistance. It is definitely an attractive point of care test, with high sensitivity and specificity along with turn around time of 2 hours, which facilitates timely diagnosis and appropriate management of TB pleuritis and pericarditis.
Despite molecular technological advances in diagnostic methodologies, reduction, and eradication of tuberculosis (TB) still remain a distant goal for clinicians. Tuberculosis is one of the most significant causes of morbidity and mortality worldwide. According to the World Health Organization (WHO), rapid diagnostic strategies and effective treatment of TB has extremely reduced mortality by 47% since 1990, yet TB remains a major source of death, especially in developing countries.1 Tuberculosis caused by Mycobacterium tuberculosis (MTB) manifests itself in 2 forms, pulmonary tuberculosis (PTB) or extra-pulmonary tuberculosis (EPTB). Pulmonary tuberculosis is the most common form, involving lungs while EPTB involves lymph nodes, bone, and joints, kidneys, intestine, abdominal and serous membranes such as pleural, pericardial, and meninges.2
Among 9.6 million reported TB patients in 2012 by WHO, approximately 15% were of EPTB while European Center for Disease Prevention and Control reported that 22% of notified TB patients in Europe had EPTB.3 Tuberculous pleural effusion is the second most common site of EPTB.4 Tuberculosis pericardial effusion is also one of the common manifestation of EPTB occurring in 1-8% patients.5 Tuberculosis has been the cause of acute pericarditis in 60-80% of patients in the developing world.6 Multiple diagnostic tools are in use to differentiate tuberculous pericarditis from non-tuberculous pericarditis. The Tygerberg score is also one of the famous clinical decision tool that allows the clinician to decide whether pericarditis is due to TB or not.1 For the diagnosis of TB pleural and pericardial effusion, conventional methods including Ziehl-Neelsen (ZN) smear microscopy and Löwenstein-Jensen (LJ) culture are available in low to middle-income countries. Although ZN staining microscopy is rapid and cheap; however, it is less sensitive for diagnosis of EPTB because of paucibacillary nature of samples and non-uniform circulation of MTB. Löwenstein-Jensen Culture is “Gold standard” method, but its long turnaround time of 2-6 weeks, as well as the complexity of procedure demanding highly skilled staff along with biosafety level III lab, limits its applicability for the routine use of a diagnostic test. For EPTB diagnosis, last few decades have witnessed the advancement of molecular techniques, with good positive predictive value (PPV): 98-99%.7,8 Moreover, the field of TB diagnosis is revolutionized by targeting specific genes or gene segments and has shortened the turnaround time of detection from weeks to days and days to hours.8 Precise and accurate TB diagnosis by new molecular tests necessitates specialized infrastructure of the laboratory with highly skilled and efficient staff, demanding high cost, limits their use in resource-constrained settings.7
In December 2010, WHO endorsed and implemented the use of a new technology GeneXpert MTB/RIF assay as a substitution over conventional techniques.9 Later on in October 2013, WHO dispensed policy updates and recommended the use of this novel technique for the rapid detection of TB infection among pediatric and extra-pulmonary cases.10 Cepheid GeneXpert system is innovative semi-automated real-time polymerase chain reaction (PCR) nucleic acid amplification technology, which can simultaneously detect MTB and Rifampicin (RIF) resistance in less than 2 hours. Molecular beacon technology and ultrasensitive Hemi-nested PCR are the basis of GeneXpert system.11 It is fully closed system; therefore, there is minimal risk of contamination and biohazard. It also does not require high expertise because of a very simple software-based reporting system.
A large body of literature is available regarding the role of GeneXpert for the diagnosis of EPTB including pleuritis, but a paucity of evidence exists concerning its use in TB pericarditis for the detection of MTB. Thus, the present study was planned to highlight the role of GeneXpert technology and to determine its validity to be used as a future diagnostic tool for TB pericardial and pleural effusions.
Methods
A cross sectional study was conducted at the Pathology Department, Mycobacteriology Laboratory, Allama Iqbal Medical College, which is one of the largest referral centers in Lahore, Punjab Pakistan. A total of 286 specimens including 158 pleural and 128 pericardial fluids samples were received from The Pulmonary and Cardiology Department of tertiary care hospital of Lahore, Pakistan, between January 2014 and August 2016.
Samples from the strong suspects of TB on the basis of i) clinical presentation, ii) relative lab investigation, iii) echocardiography, iv) radiological finding were included in the study. Contaminated samples, previously diagnosed TB cases and patients on anti-tuberculosis therapy (ATT) were excluded.
Every specimen was processed according to the following methods: 1) ZN smears microscopy according to WHO,2 2) GeneXpert MTB/RIF assay was performed directly according to the manufacturer’s instructions,3 and LJ cultures were processed and reported according to standard guidelines.4
Quality control and quality assurance
Ziehl-Neelsen smear: Positive and negative control slides were prepared and stained with every batch. Every slide was checked by 2 experienced medical laboratory technologist using a light microscope. Random positive and negative, doubtful cases were rechecked by highly experienced senior microbiologists for quality assurance purposes. Löwenstein-Jensen culture: culture media quality and mycobacterial growth were confirmed using American type culture collection (ATCC) strains of H37rv. A bottle of LJ media was inoculated with sterile water as negative control.5 GeneXpert assay: Bacillus globigiispores serve as an internal sample processing and PCR control and this assay are multiplexed with MTB assay.6
Statistical analysis
Statistical Package for Social Sciences version 21 (SPSS Inc., Chicago, IL, USA) was used for determination of the validity of GeneXpert & ZN smear microscopy, in terms of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Search criteria
Literature search was carried out using Pubmed with access to MEDLINE, the National Library of Medicine database of citations and abstracts in the fields of medicine, nursing, dentistry, veterinary medicine, health care systems, and preclinical sciences under Medical Subject Headings (MeSH) Key words sensitivity specificity, MTB, diagnostic accuracy and GeneXpert. Articles were downloaded and reviewed for eligibility criteria and outcome variables and results for diagnostic accuracy of GeneXpert.
Ethical approval
Study protocols were approved by the Institutional Board of Ethical Certification, Allama Iqbal Medical College and Jinnah Hospital, Lahore Pakistan.
Results
Out of the 286 effusions samples, MTB was detected in 51 (17.8%) samples by LJ culture, followed by GeneXpert in 43 (15%) samples and 11 (3.8%) by ZN smear microscopy (Figure 1).
Figure 1.
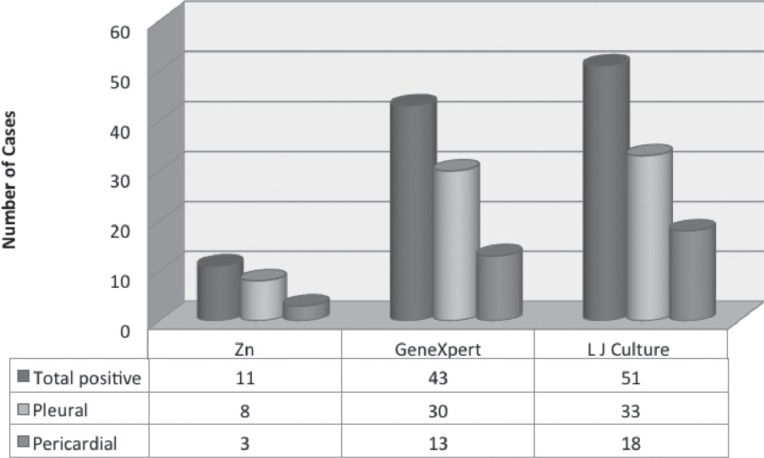
Frequency of different techniques for the detection of tuberculosis in 286 specimens including 158 pleural and 128 pericardial fluids samples received from the Pulmonary and Cardiology Department of tertiary care hospital in Lahore, Pakistan. Zn - Ziehl-Neelsen smear, LJ - Lowenstein Jensen
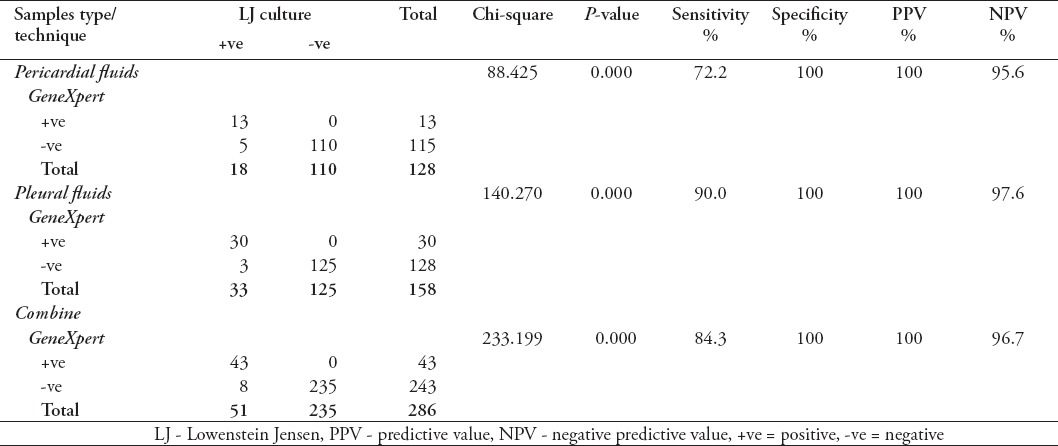
It was seen that the sensitivity of GeneXpert for pericardial fluid was 72.2% and specificity 100%, while for pleural fluids the sensitivity was 90% and specificity was 100%. Diagnostic validity of GeneXpert for detection of MTB in effusion overall was also determined in this study and a sensitivity of 84.3%, specificity 100%, PPV 100%, and NPV of 96.7% was observed for total samples (Table 1).
Table 1.
Diagnostic validity of Gene Xpert Assay among 286 specimens including 158 pleural and 128 pericardial fluids samples received from the Pulmonary and Cardiology Department of tertiary care hospital in Lahore, Pakistan.
It was noted that the sensitivity of Zn smear for pericardial fluid was 11.7%, specificity 99%, PPV 66.6%, and NPV 88% while for pleural fluids the sensitivity was 21.8%, specificity 99.2%, PPV 87.5% and NPV 83.3% (Table 2).
Table 2.
The sensitivity and specificity of Zn smear microscopy in different fluids.
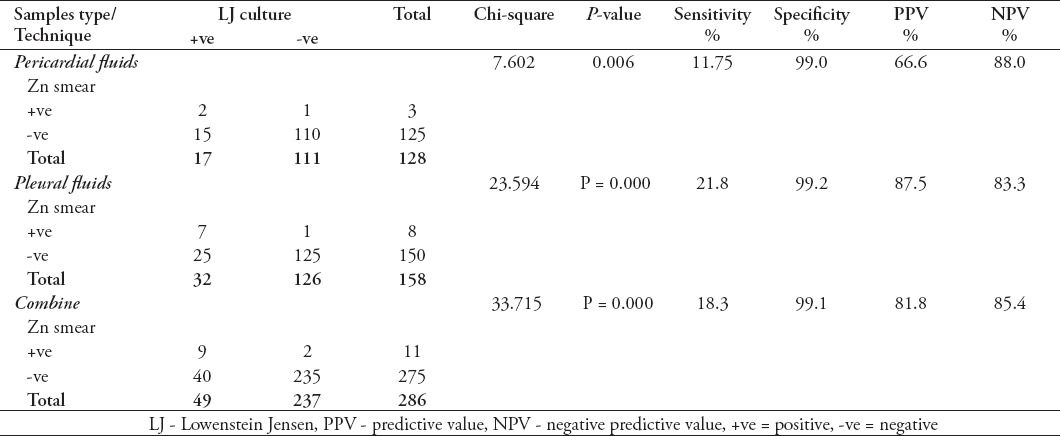
Table 3.
Previous studies reported sensitivity and specificity of GeneXpert in tuberculosis pleural fluids.
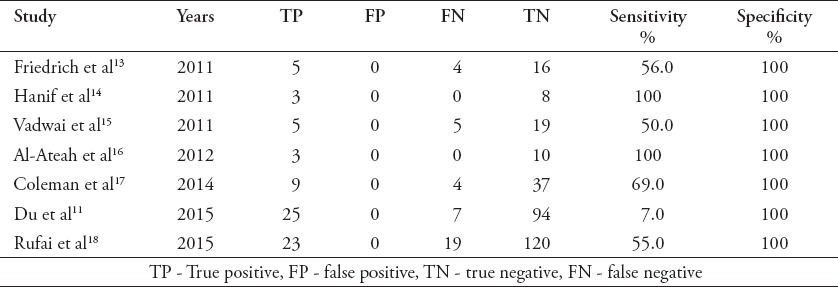
Figure 2 showed sample-wise frequency distribution of RIF resistance detected by GeneXpert. Among 13 MTB positive pericardial fluids detected by GeneXpert, 2 (15.3%) were drug resistance and out of 30 MTB positive pleural fluids 9 (30%) were drug resistant. Out of total 43 GeneXpert positive samples, 11 (25.5%) were multidrug resistance tuberculosis (MDR).
Figure 2.
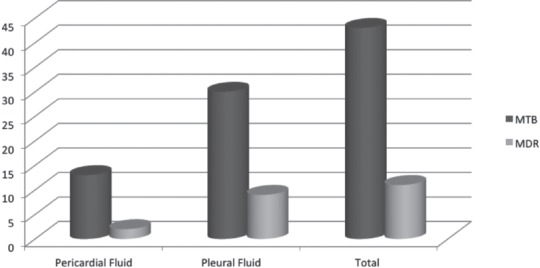
Distribution of multidrug resistance tuberculosis (MDR) cases detected pericardial and pleural fluids in 286 specimens including 158 pleural and 128 pericardial fluids samples received from the Pulmonary and Cardiology Department of tertiary care hospital in Lahore, Pakistan. Zn - Ziehl-Neelsen smear, LJ - Lowenstein Jensen
Discussion
Diagnosis of TB has always been a challenge for health services and clinicians. Despite the availability of anti-TB treatment for more than 60 years, it is still a cause of an unacceptably high mortality rate. Extra-pulmonary TB is also responsible for life-threatening consequences due to delayed diagnosis and inappropriate management. To overcome this problem, there is an urgent need for rapid diagnosis and proper treatment. The WHO endorsed GeneXpert has emerged as a breakthrough technique for the diagnosis of EPTB, but limited literature is available about its diagnostic validity in effusions. The present study highlighted the role of GeneXpert for rapid diagnosis of TB pericarditis and pleuritis in terms of sensitivity, specificity, PPV, NPV with LJ culture being the gold standard. In the current study, overall MTB was detected in 17.8% of samples by LJ culture, 14% detected in pericardial, and 20.9% in pleural fluids. It was observed that GeneXpert has a high sensitivity (84.3%) and specificity (100%) for detection of MTB effusions. Furthermore, the sensitivity was 72.2% and specificity 100% of GeneXpert for pericardial fluids and for pleural fluids sensitivity was 90% and specificity 100%. According to the report published by WHO, the pooled sensitivity of GeneXpert in pleural fluid was 43.7%, this low sensitivity can be due to the use of different gold standards in various studies included in this meta-analysis.7 A recent meta-analysis reported pooled sensitivity of GeneXpert for pleural fluid as 46.4% and specificity 99.1%, taking culture as the gold standard.8 While another systematic review conducted on 24 studies reported even a higher pooled sensitivity of GeneXpert as 51.4% and specificity as 98.6% in pleural effusion.9 The high frequency of detection in the current study can be due to the fact that Pakistan is a TB endemic country with higher disease burden as compared to the other regions in which previous studies have been conducted. In this study, a strict patient selection criteria in which strong suspects of TB patients were included on the basis of clinical and radiological evidence could have attributed to high sensitivity.
There is limited information on the diagnostic utility of this test particulary for pericardial fluid, which is a striking feature of this study. The results of this study showed that GeneXpert exhibits a high diagnostic validity for detection of MTB in a pericardial fluid with a sensitivity of 72.2% and specificity of 100%. Pandie et al10 reported comparable results with similar specificity of 100% and a sensitivity of 63.8%. Previously, the test used for diagnosis of pericardial effusion included ZN smear microscopy and LJ culture, which have their own limitations. ZN smear has a low sensitivity for pericardial fluid similar results were obtained in our study as well for pericardial fluid with a sensitivity of 11.7% and specificity of 99.2%. A significantly higher sensitivity of GeneXpert achieved in the present study for diagnosis of TB pericarditis further strengthens the recommendation for its use as screening and diagnostic tool.
We also evaluated sensitivity and specificity of Zn smear microscopy in pleural and pericardial effusions for detecting AFB. Regarding ZN smear, the overall sensitivity of 18.3% for both the sample with a specificity of 99.1%, was observed. While sensitivity of 21.8% and specificity of 99.2% were seen for pleural fluids. The results of our study are significantly higher as compared to those in the study from Spain in which a sensitivity of 7.3% and specificity of 100% was achieved by Zn smear microscopy in tuberculous pleural effusion.11 The variation in the results might be attributed to the difference in the endemicity of the disease in the 2 regions with Pakistan being a high burden country as compared to Spain. In a study from South Africa in 2012, it is documented that GeneXpert assay has replaced the Zn smear microscopy for rapid detection of Mycobacterium tuberculosis, and has been implemented on a very large scale among each and every sector of the country.12 For the provisional diagnosis and initiation of empirical therapy of TB pericarditis, Tygerberg scoring system is usually applied in clinical settings. However, due to increase in MDR TB around the globe, the desirable treatment success rate is not achieved with first-line drug making efficacy of therapy questionable. The application of GeneXpert provides additional information on drug resistance in optimizing and increasing the effectiveness of therapy. The result of this study has pinpointed 25.5% MDR cases, 15% in pericardial and 30% in pleural fluids. All these drug resistance cases can be timely managed according to the protocol of drug resistance TB to achieve a higher treatment success rate in minimum time and to break the chain of transmission of MDR TB.
In conclusion, considering the diagnostic validity along with detection of drug resistance in 2 hours turnaround time, GeneXpert assay is a useful technique for resource-limited settings making it an attractive tool for accurate diagnosis of TB pleuritis and pericarditis with high sensitivity and specificity. It is recommended to scale up this brilliant technology in the future, which will help in reducing not only the disease burden, but also the cost of diagnosis. This will facilitate the timely management and appropriate treatment of patients to reduce the mortality and morbidity.
Footnotes
References
- 1.Liebenberg JJ, Dold CJ, Olivier LR. A prospective investigation into the effect of colchicine on tuberculous pericarditis:cardiovascular topics. Cardiovasc J Afr. 2016;27:350–355. doi: 10.5830/CVJA-2016-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iram S, Zeenat A, Hussain S, Wasim Yusuf N, Aslam M. Rapid diagnosis of tuberculosis using Xpert MTB/RIF assay - Report from a developing country. Pak J Med Sci. 2015;31:105–110. doi: 10.12669/pjms.311.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Xpert MTB/RIF implementation manual Technical and operational ‘how-to’:practical considerations. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 4.Chihota V, Grant A, Fielding K, Ndibongo B, Van Zyl A, Muirhead D, et al. Liquid vs. solid culture for tuberculosis:performance and cost in a resource-constrained setting. Int J Tuberc Lung Dis. 2010;14:1024–1031. [PubMed] [Google Scholar]
- 5.Tahseen S, Qadeer E, Khanzada F, Rizvi A, Dean A, Van Deun A, et al. Use of Xpert®MTB/RIF assay in the first national anti-tuberculosis drug resistance survey in Pakistan. Int J Tuberc Lung Dis. 2016;20:448–455. doi: 10.5588/ijtld.15.0645. [DOI] [PubMed] [Google Scholar]
- 6.Tyagi S, Kramer FR. Molecular beacons:probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance:Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 8.Denkinger CM, Schumacher SG, Boehme CC, Dendukuri N, Pai M, Steingart KR. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis:a systematic review and meta-analysis. European Respiratory Journal. 2014;44:435–446. doi: 10.1183/09031936.00007814. [DOI] [PubMed] [Google Scholar]
- 9.Sehgal IS, Dhooria S, Aggarwal AN, Behera D, Agarwal R. Diagnostic performance of Xpert MTB/RIF in tuberculous pleural effusion:systematic review and meta-analysis. J Clin Microbiol. 2016;54:1133–1136. doi: 10.1128/JCM.03205-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandie S, Peter JG, Kerbelker ZS, Meldau R, Theron G, Govender U, et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous pericarditis compared to adenosine deaminase and unstimulated interferon-ßin a high burden setting:a prospective study. BMC Med. 2014;12:101. doi: 10.1186/1741-7015-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du J, Huang Z, Luo Q, Xiong G, Xu X, Li W, et al. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay using pleural biopsy and pleural fluid specimens. J Res Med Sci. 2015;20:26–31. [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer-Rath G, Schnippel K, Long L, MacLeod W, Sanne I, Stevens W, et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PloS One. 2012;7:e36966. doi: 10.1371/journal.pone.0036966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich SO, von Groote-Bidlingmaier F, Diacon AH. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J Clin Microbiol. 2011;49:4341–4342. doi: 10.1128/JCM.05454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanif SN, Eldeen HS, Ahmad S, Mokaddas E. GeneXpert®MTB/RIF for rapid detection of Mycobacterium tuberculosis in pulmonary and extra-pulmonary samples [Correspondence] Int J Tuberc Lung Dis. 2011;15:1274–1275. doi: 10.5588/ijtld.11.0394. [DOI] [PubMed] [Google Scholar]
- 15.Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF:a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49:2540–2545. doi: 10.1128/JCM.02319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ateah SM, Al-Dowaidi MM, El-Khizzi NA. Evaluation of direct detection of Mycobacterium tuberculosis complex in respiratory and non-respiratory clinical specimens using the Cepheid Gene Xpert®system. Saudi Med J. 2012;33:1100–1105. [PubMed] [Google Scholar]
- 17.Coleman M, Finney L, Komrower D, Chitani A, Bates J, Chipungu G, et al. Markers to differentiate between Kaposi’s sarcoma and tuberculous pleural effusions in HIV-positive patients. Int J Tuberc Lung Dis. 2015;19:144–150. doi: 10.5588/ijtld.14.0289. [DOI] [PubMed] [Google Scholar]
- 18.Rufai SB, Singh A, Kumar P, Singh J, Singh S. Performance of Xpert MTB/RIF assay in diagnosis of pleural tuberculosis by use of pleural fluid samples. J Clin Microbiol. 2015;53:3636–3638. doi: 10.1128/JCM.02182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


