Abstract
Objectives:
To assess the ability of a 12-week primary care-based intensive lifestyle intervention (ILI), to facilitate a 5% reduction in baseline weight compared with an education-only active comparator (AC).
Methods:
A randomized clinical trial was conducted in a primary health care setting in Jeddah, Saudi Arabia between December 2014 and June 2015. Arab participants with obesity, but who were otherwise healthy (n=140), were randomized to the ILI (n=70) or AC (n=70) group. The ILI group received 8 clinical visits throughout the study. The AC group received only an initial health education session. The primary outcome was the proportion of participants who achieved clinically significant weight loss (≥5% of their baseline weight).
Results:
Participants in the ILI group were significantly more likely than those in the AC group to achieve the primary outcome (p=0.008, relative risk: 1.8 [95% confidence interval [CI]: 1.15 to 2.93). At week 12, the ILI group exhibited a mean weight decrease of 5.58 ± 5.60 kg (-5.37 ± 5.31%), significantly greater than that observed in the AC group (-2.8 ± 4.96 kg, -2.62 ± 4.34%, p=0.002), and corresponding to a weight loss advantage of 2.77 kg (95% CI: 1.01 to 4.54 kg) or 2.75% (95% CI: 1.13% to 4.37%).
Conclusion:
The 12-week primary care-based ILI program was effective in achieving a clinically meaningful weight reduction (≥5%) among Saudi and Arab patients with obesity.
The exponential increase in the incidence of obesity in recent decades has led the World Health Organization (WHO) to declare this condition a global epidemic and public health crisis.1 In Saudi Arabia, obesity is one of the most common health conditions, affecting people of all ages and both sexes.2,3 For example, the prevalence of overweight is 30.7% among adult Saudi men and 28.4% among Saudi women, while the prevalence of obesity is 14% and 23.6%, respectively.4 The main components of an effective high-intensity comprehensive lifestyle intervention for obesity management are: 1) a diet with a moderately-reduced calorie content, 2) increased physical activity, and 3) behavioral strategies that facilitate adherence to the prescribed diet and activities.5 These interventions are considered successful when they achieve a 5-10% reduction in the individual’s weight (according to the National Institute of Health).6,7 Among these components, behavioral treatments should be considered the first-line treatment for individuals who are overweight or obese.6 The most common psychological therapies for weight loss are behavioral and cognitive behavioral therapies.8 A variety of dietary approaches are associated with weight loss in overweight and obese individuals; all these approaches target a reduction in dietary energy intake. Among the various dietary approaches, the low-carbohydrate diet (<20 g/day of carbohydrates), low-fat diet (<20% of total calories from fat), vegetarian diet, and Mediterranean diet have been associated with significant weight loss.5
In a 2014 meta-analysis of 48 clinical trials, low-carbohydrate diets were found to provide the greatest weight loss after 6 months of follow-up (versus low-fat diets).9 Based on that finding, the authors concluded that adherence to the diet was the key factor in achieving successful weight loss, regardless of the diet’s food composition. A 2008 meta-analysis of 13 clinical trials reported that the attrition rates were higher for low-fat diets than for low-carbohydrate diets, which suggests that patients prefer a low-carbohydrate approach.10 This preference may be explained by the spontaneous reduction in calorie intake that is caused by carbohydrate restriction, compared with the calorie counting technique.11 In addition, the effects of low-carbohydrate diets on the hunger sensation may play an important role in patient adherence, as a low-carbohydrate diet can lead to hunger levels similar to those of a low-fat diet (even with 1,000 fewer kcal of daily caloric intake).12
In Saudi Arabia, healthcare-related efforts to reduce the prevalence of obesity have not been successful. Therefore, to successfully control obesity on a national scale, it is critical to identify the most appropriate and cost-effective obesity management techniques for Saudi and Arab patients, which can enable policymakers to make evidence-based decisions. The aim of this study was to evaluate the effectiveness of an intensive lifestyle intervention (ILI) to achieve weight loss in Saudi Arabian adults with obesity.
Methods
This study was conducted at Al-Safa 1, a primary health care center in Jeddah, Saudi Arabia, between December 2014 and June 2015. It was a 12-week interventional randomized controlled trial with open-label parallel assignment. The aim was to evaluate the effect of an ILI compared with an education-only active comparator (AC) to achieve a 5% reduction in weight for Arab men and women with obesity. The intervention was mainly administered with minimal help from the center’s staff. Ethical approval was obtained from the Scientific Committee and the Scientific Research Ethics Committee of Ministry of Health, Jeddah, before starting the data collection. The protocol has been registered at the ClinicalTrials.gov with registration identifier: NCT02464566. This study follows the principles of the Helsinki Declaration.
Participants were recruited through the study’s obesity clinic using a convenience sampling technique. Once a prospective participant was confirmed to be eligible for this study through a verbal medical interview, they received a detailed description of the study objectives, design, randomization process, and timetable. If the participant agreed to be included in the study, they were asked to provide informed consent and to sign a consent form. A copy of the consent form was given to them. The participants’ confidentiality was protected throughout the study, and participants were free to exit from the study at any time. Once the participant’s informed consent was received, he/she was randomized to either the ILI or the AC group, as described below. Detailed inclusion and exclusion criteria are described in Table 1.
Table 1.
Inclusion and exclusion criteria.

Sample size calculations were made for the success cut-point (weight loss ≥5%) based on the results of the Arab women study (success cut-point: weight loss ≥7%). The success rates was 24% after 6 months for the ILI and 6% after 6 months for moderate lifestyle intervention groups.13 Based on that binary outcome, it was found that 116 patients (58 in each group) are sufficient with an 80% power and significant level of 0.05 to detect an increase in the primary outcome measure from 6% in the AC group to 24% in the ILI group. However, the sample size (n=126 individuals [63 in each group]) was needed to be able to detect a 5% inter-group difference in weight loss at 0.05 significant level, a power of 80% and 10% standard deviation. This sample size was then adjusted to account for an anticipated drop-out rate of approximately 10%, increasing the requirement to 140 individuals, with 70 individuals per group. Calculations of sample sizes were performed using sealed envelope™ online service.14,15
Randomization and blinding
To control for possible bias in the response to the intervention due to gender differences, the consented participants were first classified into 2 equal strata: 70 men and 70 women. Within each stratum, the block randomization method ensured that equal numbers of participants were enrolled in the ILI and AC groups. For this randomization, 10 identical non-transparent envelops, with 5 envelopes containing a slip of paper indicating the ILI group and 5 envelopes containing a slip of paper indicating the AC group, were utilized. These envelopes were shuffled to ensure that the investigator was blinded to the contents of each envelope. After receiving the first participant’s informed consent, all 10 envelopes were presented to the participant, who was instructed to select a single envelope. This envelope was then removed from the block, and the next participant was presented with the remaining 9 envelopes. This process was repeated until a block of 10 participants had received their assignments, with 5 participants randomized to each study arm. Each subsequent block was randomized by re-sealing the slips in the envelopes, re-shuffling the envelopes to ensure random and blinded distribution, and presenting the envelopes to the next 10 participants. This process was continued until the required sample size was reached. No blinding was attempted, because it was impossible to blind the participants and investigator to the intervention.
Intensive lifestyle intervention program
Each participant in the ILI group received a modified form (namely, shorter duration and different dietary approach) of the recommended lifestyle intervention (diet, exercise, and behavioral techniques to facilitate adherence) based on the US Preventive Services Task Force (USPSTF) guidelines16 and the USPSTF’s 5-A framework,17 outlined as follows:
Assess: the behavioral factors and risks that contribute to progress in behavioral change.
Advise: the participant regarding the behavioral change process, with clear, specific, and personalized information regarding the benefits of weight control and the harmful effects of obesity.
Agree: on treatments to achieve behavior change that is tailored to the patient’s personal interests and treatment goals.
Assist: with behavior change techniques that help the patient to achieve the previously planned goals.
This behavioral approach is based on the 3 main components of lifestyle interventions, for example, the Diabetes Prevention Program (DPP) methods; these components are goal setting, self-monitoring, and stimulus control.18
Goal setting
The weight loss goals were 0.5-1.0 kg/week, with an ultimate goal of a ≥5% reduction in their body weight at the end of the 12-week period.6 Self-monitoring: Daily food and exercise records were given to the ILI participants to self-monitor their behavior change progress. In addition, the participants were encouraged to describe their personal feelings, mood, or any other thoughts, for discussion during their next clinical visit.18
Stimulus control
Internal and external cues that were associated with the targeted eating and activity behaviors were addressed using stimulus control principles. The participants were taught to change their immediate environment (namely, in the home and workplace) to facilitate, rather than hinder, their behavior change.18
Arrange: in-person follow-up contact to ensure ongoing support and to adjust the treatment plan as needed. Each participant in the intervention group attended a total of 8 visits (15-20 min each) during the 12-week study period, including the initial recruitment visit and the final data collection session. Text messages were sent to the mobile phones of each participant to remind them of their clinic appointments. If necessary, phone calls were made.
The AC group received only a single clinical education session, during which the participants were given printed health education materials regarding the prescribed diet and physical activity only. This group did not receive any behavioral support.
Each participant was provided with a target for the average daily carbohydrate intake (20-25 g/day), selected to achieve a spontaneous reduction in calorie intake (namely, an energy deficit of 500-750 kcal/day). Participants in both study groups received booklets that explained the study’s diet, which was based on first stage of the latest edition of the Atkins diet (“New Atkins for new you”).19 This diet restricts the daily carbohydrate intake to <20-25 g/day. The content of the booklet was explained to each participant at the first meeting. The physical activity goal was ≥150 min/week of aerobic exercise (namely, 30 min of brisk walking for 5 days per week).
The main study outcome was the proportion of participants in the ILI group who achieved clinically significant weight loss (≥5% of baseline weight) after 12 weeks, compared to the proportion of participants in the AC group who achieved the same outcome. To measure this weight loss, we used a validated weigh scale (Cardinal Detecto, model No. 6129, Aloyayna Medical, KSA, Jeddah). Each participant’s height was measured by the physician at the initial session to calculate his/her body mass index (BMI). The secondary study outcomes were changes in weight, waist circumference, hip circumference, and systolic and diastolic blood pressure at 12 weeks. These parameters were measured at the initial session and at week 12 for participants in both groups. The measurement techniques (weight, height, waist circumference, and hip circumference) were performed according to the instructions contained in the WHO STEPS surveillance manual.20 A validated automatic oscillometric blood pressure monitoring device (Omron digital automatic blood pressure monitor, Zimmo Trading Company, KSA, Jeddah) was used to measure blood pressure, according to the Saudi Hypertension Management Society guidelines21 and the manufacturer’s instructions. All measurements were performed by the first author and qualified nurses. The Short Form Stages of Change Questionnaire was used because the intervention(s) can be customized to the patient’s level of readiness to change.22,23 We obtained permission from the original developers to use this form. The form was translated from English into Arabic and was then translated back into English to ensure lexical equivalence before the data collection began.
Statistical analysis
Means and standard deviations were calculated for all continuous variables and frequency was used to describe qualitative variables. An intention-to-treat analysis was used for body weight and related outcome variables, using the last observation carried forward method (if necessary). The chi-square test was used to evaluate the proportion of participants in the AC and ILI groups who achieved clinically significant weight loss (≥5% of baseline weight). A test for normal distribution was performed for all data, and inter-group differences in normally distributed data were evaluated using the independent sample t-test. The paired t-test was used to evaluate intra-group differences over the study period. Even for some measures that exhibited minor violations of the assumption of normal data distribution, the t-test was used, because it is a robust test that can accommodate non-normally distributed data provided the sample size is large enough.24,25 IBM SPSS Statistics for Windows, Version 20.0. (Armonk, NY: IBM Corp.) was used to perform all statistical analyses, and differences were considered statistically significant at a p-value of <0.05.26
Results
The recruitment process started at the end of December 2014 and ended at the beginning of March 2015. During the 75-day recruiting period, 249 participants approached us, wanting to be part of the trial. However, after receiving the initial description of the required tasks/responsibilities expected from trial participants, 53 chose not to take part and 56 candidates were not eligible. After randomization, there were 70 participants in the AC group and 70 participants in the ILI group, with an equal number of men and women in each group. To execute the planned interventions for the ILI and AC groups, 700 clinic appointments were made (560 for the ILI group and 140 for the AC group). At the end of the intervention (June 2015), 462 clinical visits had been conducted for all participants, 66% of the planned visits: 349 for ILI group and 113 for AC group (Figure 1).
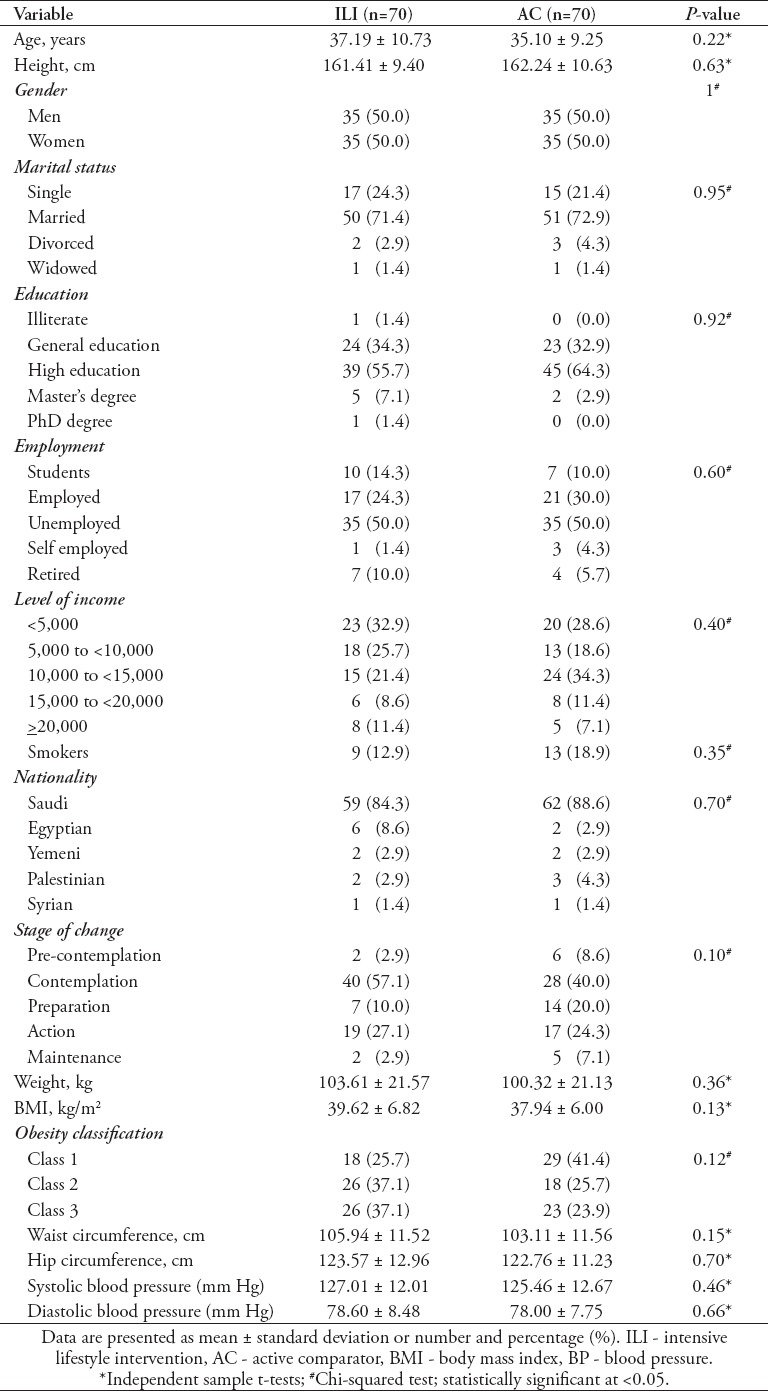
Table 2 provides the baseline characteristics of the participants in each group as well as the mean baseline scores (weight, BMI, waist circumference, hip circumference, and systolic and diastolic blood pressures), which were similar in the 2 groups.
Table 2.
Baseline characteristics of the participants in each group as well as the mean baseline scores (weight, body mass index, waist circumference, hip circumference, and systolic and diastolic blood pressures)
The dropout ratios were compared for the 2 groups using the Chi-square test: The dropout ratios were similar for the ILI and AC groups (p=0.48). Reasons for dropout were loss to follow-up (n=32), participants travelled or moved out of the area (n=15), pregnancy (n=2), and undergoing bariatric surgery (n=1).
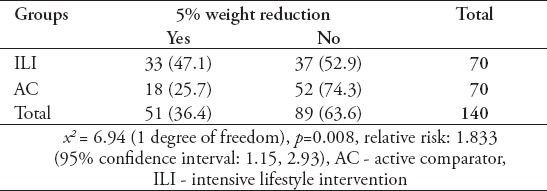
There was a significant difference in the proportion of participants who achieved a ≥5% weight reduction between the AC and ILI groups (x2 = 6.94 [1 degree of freedom], p=0.008). Relative risk analysis revealed that participants in the ILI group were 1.8-fold (95% CI: 1.15 to 2.93) more likely to achieve the ≥5% weight reduction goal, compared to the AC group (Table 3).
Table 3.
The ratios of a ≥5% weight reduction according to group.
At week 12, the ILI group exhibited a mean weight loss of 5.58 ± 5.60 kg (5.37 ± 5.31%), which was significantly greater than that achieved in the AC group (2.8 ± 4.96 kg, 2.62 ± 4.34%, p=0.002). This corresponded to a weight loss advantage of 2.77 kg (95% CI: 1.01 to 4.54 kg) or 2.75% (95% CI: 1.13% to 4.37%) in the ILI group. All the differences in study variables between the 2 groups are summarized in Table 4. Based on the results for both groups, the differences in all secondary outcomes (before and after) within the AC and ILI groups were significantly lower than the baseline values, except for systolic blood pressure in the AC group and diastolic blood pressure in the ILI group.
Table 4.
Inter- and intra- group differences in 3-months from baseline.

Discussion
The ILI program employed in the present study proved to be effective, with an average weight loss of 5.58 kg (5.37%) after the 12-week intervention, compared with an average weight loss of 2.8 kg (2.62%) in the AC group. Participants in the ILI program were also significantly more likely to achieve the desired 5% weight loss. This amount of weight loss (5%) is associated with an improved cardiovascular risk profile.5 It also had a significant effect on diabetes prevention in the DPP study, with individuals exhibiting a 16% reduction in the incidence of diabetes per kilogram of lost weight.27
The relative risk analysis revealed that participants in the ILI group were 1.8-times more likely than participants in the AC group to lose 5% of their basal weight. Although this program was only 12 weeks long and was administered in a primary care center, 33 (47.1%) participants from the ILI group achieved the 5% weight loss target, compared with only 18 (25.7%) participants from the AC group (p=0.008). The 47.1% success rate in the ILI group is comparable to results from other studies, such as the DPP study, in which 50% of the participants in the lifestyle intervention group achieved ≥7% weight loss within 24 weeks.28 In the 2011 ShapeUp Rhode Island program, which lasted for 12 weeks, 42% of the individuals who received an internet-based behavioral program achieved 5% weight loss, and optional group sessions increased the success rate to 54%.29 A Japanese study by Nakata et al30 found that 5% weight loss was achieved by 89% of participants who received 6 months of intensive intervention, compared with only 29% in a health education group. Thus, our findings indicate that a relatively short intervention (12 weeks versus 6 months) may not preclude achieving good success rates if the other supportive factors are addressed. The mean weight reduction in the present study was similar to those reported in previous studies. For example, the ILI program provided an additional weight loss of 2.77 kg, compared with the education-only group (ILI: 5.58 kg and 5.37% versus AC: 2.8 kg and 2.62%; p=0.002). In the ShapeUp Rhode Island study, a 3-month internet-based behavioral program and group session provided greater weight reduction (6.1%, standard error [SE] = 0.6) to participants than did an internet-only intervention (4.2%, SE = 0.6) or the standard intervention (1.1%; SE = 0.9).29 Furthermore, Nakata et al30 found that a 3-month intervention program provided 6.0 ± 3.0 kg of weight loss, compared with 2.4 ± 2.9 kg in the control group.30 It is well known that the group therapy in weight loss trials and the lifestyle intervention programs in academic or research centers have favorable effects on patient outcomes, which may partially explain the superior results of the Japanese study.18,31
The present study demonstrated that an effective ILI program can be administered in the primary care setting, despite previous studies frequently indicating that primary care programs are ineffective.5,18 Nevertheless, our findings may also emphasize the importance of establishing specialized obesity clinics or specialized obesity sessions, using a program similar to the one used in the present study, to overcome obstacles such as lack of physician time and training that impede these programs. The present study’s ILI program used several behavioral approaches, including goal setting, self-monitoring, and stimulus control, as these approaches are the main components of lifestyle interventions.18 In this program, the participants maintained daily records of their meals, sport activities, psychological status, and repeated weight measurements, which in turn allowed them to understand how eating and physical activity behaviors were related to weight loss or gain. It is likely that this knowledge helped the ILI group to achieve better outcomes, compared to the AC group. Moreover, the present study demonstrated that ILI programs can be effective among Arab patients, and that appropriate resource allocation can facilitate the successful large-scale execution of ILI programs in the primary care setting. The high prevalence of obesity among Saudis4 further emphasizes the need to implement similar programs, and to perform further research to evaluate their success.
Study limitations
First, despite significant inter-group differences in weight loss and waist and hip circumference measurements, the improvements in systolic and diastolic blood pressure were not statistically significant. This phenomenon is not exclusive to our study: the study by Nakata et al30 and the Arab women study13 found that the improvements in most secondary outcomes were not statistically significant. However, the baseline blood pressures of our participants were mostly within the normal range, which may have precluded observation of any significant intervention-related improvements. Furthermore, the present study may not have had sufficient statistical power to evaluate differences in blood pressure. Second, 36% of the participants dropped out of this study (similar proportions dropped out of the ILI and AC groups). High attrition rates are common in obesity studies, especially those that involve lifestyle interventions. For example, one systematic review found that attrition rates range from 10-80%, depending on the setting and the type of program,32 and an intervention trial of different diets found a mean attrition rate of >40% within the first 12 months.33 Because the present study was performed in a primary care setting, where the effectiveness of such interventions has often been shown to be poor duet to the lack of physician time and training5,18 and was mainly administered by the first author (with minimal help from the center’s staff), an attrition rate of 36% is understandable. In future studies or programs, better trial adherence may be achieved with the assistance of a specialized administrative team and electronic systems, which have been proven to enhance adherence and decrease the no-show of patients.34 Third, communication with the prospective participants was hampered by the absence of an official communication system with the primary care center’s patients. Thus, advertising the program and recruitment required an excessive amount of time and effort. Lastly, this trial lacked sufficient funding to evaluate more invasive secondary outcomes (namely, blood sugar levels and lipid profiles) and to follow-up the participants for a longer period (one year or more). Thus, future studies should incorporate these tests and plan for long-term follow-up to evaluate the immediate and long-term effects of ILI programs on these important cardiovascular risk factors.
In conclusion, this study found that participants in a primary care-based ILI program were significantly more likely to achieve a 5% weight reduction, and exhibited significantly greater weight loss at 3 months than the AC group. Thus, this ILI program was effective in promoting weight loss among Arab adults with obesity in the primary care setting and is a practical option for policy makers to implement in primary care centers. However, further studies are needed to evaluate the long-term effectiveness of ILI in this population, and to identify effective methods for long-term weight loss maintenance.
Acknowledgment
I would like to express my deepest appreciation to Dr. Rajaa Alraddadi for her supervision and continuous encouragement during this project. I also thank Dr. Adel Ibrahim (statistical advisor) for assistance and advice during the study’s design and statistical analysis. Furthermore, I thank the residency program supervisors, staff, and my colleagues for their kind assistance. Finally, I thank the Al Safa 1 center’s staff and all other individuals who assisted with the trial execution and data collection.
References
- 1.World Health Organization. Preventing chronic diseases : a vital investment : WHO global report. [Internet] Geneva (GE): World Health Organization; 2005. Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Preventing+Chronic+Diseases:+A+Vital+Investment#3 . [Google Scholar]
- 2.Al-Shehri F, Moqbel M, Abu-Melha W, Al-Khaldi Y, Al-Shahrani A. Management of obesity: Saudi Clinical Guideline. Saudi J Obes [Internet] 2013;1:18–30. [Google Scholar]
- 3.Aburizaiza OS, DeNicola E, Siddique A, Khwaja H, Carpenter DO. Obesity and public health in the Kingdom of Saudi Arabia. Rev Env Heal. 2015;30:191–205. doi: 10.1515/reveh-2015-0008. [DOI] [PubMed] [Google Scholar]
- 4.Al-Othaimeen AI, Al-Nozha M, Osman AK. Obesity: an emerging problem in Saudi Arabia. Analysis of data from the National Nutrition Survey. East Mediterr Health J. 2007;13:441–448. [PubMed] [Google Scholar]
- 5.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. [[cited 1998]]. Available from URL: https://www.nhlbi.nih.gov/files/docs/guidelines/obesity_guidelines_archive.pdf . [PubMed]
- 7.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Shaw K, O’Rourke P, Del Mar C, Kenardy J. Psychological interventions for overweight or obesity. Cochrane Database Syst Rev. 2005;2:CD003818. doi: 10.1002/14651858.CD003818.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, et al. Comparison of weight loss among named diet programs in overweight and obese adults. JAMA. 2014;312:923–933. doi: 10.1001/jama.2014.10397. [DOI] [PubMed] [Google Scholar]
- 10.Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009;10:36–50. doi: 10.1111/j.1467-789X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 11.Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86:276–284. doi: 10.1093/ajcn/86.2.276. [DOI] [PubMed] [Google Scholar]
- 12.Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403–411. doi: 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kalter-Leibovici O, Younis-Zeidan N, Atamna A, Lubin F, Alpert G, Chetrit A, et al. Lifestyle intervention in obese Arab women: a randomized controlled trial. Arch Intern Med. 2010;170:970–976. doi: 10.1001/archinternmed.2010.103. [DOI] [PubMed] [Google Scholar]
- 14.Sealed Envelope. Power calculator for binary outcome superiority trial [Internet] [[cited 2017 April 30]]. Available from: https://www.sealedenvelope.com/power/binary-superiority/
- 15.Sealed Envelope. Power calculator for continuous outcome superiority trial [Internet] [[cited 2017 April 30]]. Available from: https://www.sealedenvelope.com/power/continuous-superiority/
- 16.Moyer VA. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:373–378. doi: 10.7326/0003-4819-157-5-201209040-00475. [DOI] [PubMed] [Google Scholar]
- 17.Vallis M, Piccinini-Vallis H, Sharma AM, Freedhoff Y. Clinical review: modified 5 As: minimal intervention for obesity counseling in primary care. Can Fam physician Médecin Fam Can. 2013;59:27–31. [PMC free article] [PubMed] [Google Scholar]
- 18.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34:841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westman EC, Phinney SD, Volek JS. New Atkins For a New You: The Ultimate Diet for Shedding Weight and Feeling Great. UK: Kindle Store [Internet]; [[Update 2010; cited 2016 May 19]]. Available from: https://www.amazon.co.uk/New-Atkins-You-Ultimate-Shedding-ebook/dp/B005NHQ2NY?ie=UTF8&*Version*=1&*entries*=0 . [Google Scholar]
- 20.World Health Organization. STEPS Surveillance Manual. The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance. Geneva (SW): World Health Organization; 2008. [Google Scholar]
- 21.Saudi Hypertension Management Society. Saudi Hypertension Management Guidelines. Riyadh (SA): Saudi Hypertension Management Society; 2011. [Google Scholar]
- 22.Johnson SS, Paiva AL, Cummins CO, Johnson JL, Dyment SJ, Wright JA, et al. Transtheoretical model-based multiple behavior intervention for weight management: effectiveness on a population basis. Prev Med (Baltim) 2008;46:238–246. doi: 10.1016/j.ypmed.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wee CC, Davis RB, Phillips RS. Stage of readiness to control weight and adopt weight control behaviors in primary care. J Gen Intern Med. 2005;20:410–415. doi: 10.1111/j.1525-1497.2005.0074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fagerland MW. t-tests, non-parametric tests, and large studies--a paradox of statistical practice? BMC Med Res Methodol. 2012;12:78. doi: 10.1186/1471-2288-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skovlund E, Fenstad GU. Should we always choose a nonparametric test when comparing two apparently nonnormal distributions? J Clin Epidemiol. 2001;54:86–92. doi: 10.1016/s0895-4356(00)00264-x. [DOI] [PubMed] [Google Scholar]
- 26.Biau DJ, Jolles BM, Porcher R. P value and the theory of hypothesis testing: an explanation for new researchers. Clin Orthop Relat Res. 2010;468:885–892. doi: 10.1007/s11999-009-1164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leahey TM, Thomas G, Fava JL, Subak LL, Schembri M, Krupel K, et al. Adding evidence-based behavioral weight loss strategies to a statewide wellness campaign: a randomized clinical trial. Am J Public Health. 2014;104:1300–1306. doi: 10.2105/AJPH.2014.301870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakata Y, Okada M, Hashimoto K, Harada Y, Sone H, Tanaka K. Comparison of education-only versus group-based intervention in promoting weight loss: a randomised controlled trial. Obes Facts. 2011;4:222–228. doi: 10.1159/000329619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cresci B, Tesi F, La Ferlita T, Ricca V, Ravaldi C, Rotella CM, et al. Group versus individual cognitive-behavioral treatment for obesity: results after 36 months. Eat Weight Disord. 2007;12:147–153. doi: 10.1007/BF03327591. [DOI] [PubMed] [Google Scholar]
- 32.Moroshko I, Brennan L, O’Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obes Rev. 2011;12:912–934. doi: 10.1111/j.1467-789X.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 33.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 34.Kim H. A Study on the Effects of Online Appointment Systems on Patients and Hospitals. Int J Appl Eng Res. 2016;11:973–4562. [Google Scholar]
- 35.Gorin AA, Wing RR, Fava JL, Jakicic JM, Jeffery R, West DS, et al. Weight loss treatment influences untreated spouses and the home environment: evidence of a ripple effect. Int J Obes (Lond) 2008;32:1678–1684. doi: 10.1038/ijo.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


