Abstract
Background
Synthetic cathinones are popularly referred to in the media as “bath salts.” Through the direct and indirect activation of the sympathetic nervous system, smoking, snorting, or injecting synthetic cathinones can result in tachycardia, hypertension, hyperthermia, myocardial infarction and death.
Objectives
The chemical structures and names of bath salts identified by the State of Ohio Bureau of Criminal Identification and Investigation Laboratory are presented. Based on their common pharmacophores the authors review the history, pharmacology, toxicology, detection methods and clinical implications of synthetic cathinones. Through the integration of this information, the pharmacological basis for the management of patients using synthetic cathinones is presented.
Discussion
Synthetic cathinones activate central serotonergic and dopaminergic systems contributing to acute psychosis and the peripheral activation of the sympathetic nervous system. The over stimulation of the sympathetic nervous system contributes to the many toxicities reported with bath salt use. The pharmacological basis for managing these patients is targeted at attenuating the activation of these systems.
Conclusions
Treatment of patients presenting after using bath salts should be focused on reducing agitation and psychosis, and supporting renal perfusion. The majority of successfully treated synthetic cathinones cases have used benzodiazepines and antipsychotics along with general supportive care.
Keywords: sympathomimetic, synthetic cathinones, amphetamine, phenethylamine, bath salts
Introduction
Phenylisopropylamine or α-methylphenethylamine are chemical names for the clinically used, but also commonly abused medication amphetamine. Amphetamine was initially synthesized in 1887 by the German chemist Edeleano (1) as part of a series of compounds to improve upon ephedrine. Thus, amphetamine is a synthetic compound that is not based on a natural product, like ephedrine. Physicians noted the potential for amphetamine abuse and addiction, even in the context of medical use, as early as the 1940’s (2). Amphetamine and its N-methyl analog methamphetamine were extensively used by both Japanese and German armies to stimulate soldier efforts during World War II (3). After the war, Japan made amphetamines readily available without a prescription. Subsequently, the rates of amphetamine abuse and addiction escalated (4). In the United States, the initial epidemic of amphetamine abuse emerged in the 1960’s, and regulatory efforts that included classifying amphetamine as a schedule II controlled substance under the Controlled Substance Act and increasing regulatory control over the manufacturing and distribution of amphetamine were marginally effective (5). However, as regulatory and law enforcement officials focused efforts to reduce amphetamine abuse, an amphetamine analog 3,4-methylenedioxymethamphetamine (MDMA) began to emerge in the illicit drug scene (6).
MDMA was first synthesized in 1912 by Merck Pharmaceutical, but the compound remained largely ignored by both the scientific community and illicit drug users until the late 1970’s (7), probably because of the availability of amphetamine and methamphetamine. In one of the first scientific reports, Shulgin and Nichols (8) noted that MDMA induced an “easily controlled altered state of consciousness with emotional and sensual overtones.” These pharmacological effects of MDMA were in stark contrast to the hyperarousal, compulsive and sometimes paranoid behaviors from amphetamine use (9). Reasons for these differential pharmacological effects between MDMA and methamphetamine are ostensibly linked to the chemical structure differences (discussed in the next section). MDMA was placed on the Drug Enforcement Agency’s Schedule I list of controlled substances in 1985 (10).
Presently, the latest versions of sympathomimetic compounds to emerge as abused drugs are the synthetic cathinones derivatives. Cathinone is a naturally occurring β-ketone analog of amphetamine found in the leaves of the Catha edulis plant indigenous to northeast Africa and the Arabian Peninsula. Methcathinone, the N-methyl analog of cathinone was first synthesized in 1928 (11). These compounds are commonly classified in the popular media as “bath salts” because of the packaging and distribution techniques used by the illicit manufacturers to circumvent the federal analog act. These synthetic cathinone compounds are not chemically or pharmacologically similar to Epsom “bath” salts, but are central nervous system active drugs that are chemically and pharmacologically similar to amphetamine and MDMA.
Chemistry and how it predicts pharmacology
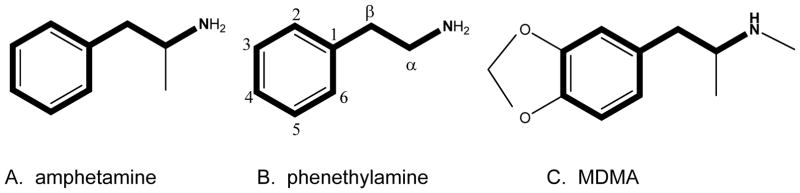
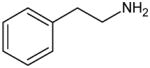
The chemical structure of amphetamine and MDMA are below (see Figure 1) and are presented in order to identify the region of the substance known as the pharmacophore. The pharmacophore of a chemical structure is the portion of the structure that confers the substance’s activity. In the case of amphetamine and MDMA, these drugs have the exact same pharmacophore (phenethylamine; see Figure 1). Because of this, MDMA would be considered a chemical analog of amphetamine. Comparing the three structures further, the phenethylamine pharmacophore can be identified in all agents. Amphetamine has the addition of a methyl group off the α carbon; hence the chemical name for amphetamine is α-methylphenethylamine. MDMA has the addition of the methyl group off the terminal amine generating the methamphetamine portion of the molecule. Increasing carbon substitutions has the ability to increase lipophilicity and in some cases protect against enzyme degradation. MDMA further has the methylenedioxy substitution off the three and four carbons. All these substitutions are responsible for MDMA’s chemical name of 3,4-methylenedioxy-methamphetamine. Thus, amphetamine and MDMA would have a predictably similar pharmacological activity (12,13).
Figure 1.
General chemistry of phenethylamine, amphetamine and 3,4-methylenedioxy-methamphetamine (MDMA). The phenethylamine pharmacophore is bolded in each of the structures. The α and β-carbons are the sites of many substitutions to the “bath salts.”
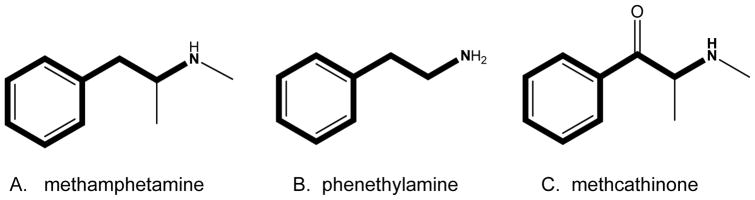
The synthetic cathinones are chemical analogs of methcathinone and are classified chemically as β-ketone due to the carbonyl group (=O) at the β-carbon (see Figure 2). The synthetic cathinones also differ between each other in the length of carbon substitutions off the α-carbon and nitrogen (N) terminus. Through the addition of electron withdrawing groups such as fluorine (F) or increasing carbon length, the lipophilic nature of the synthetic analog can be increased. The addition of carbons to the N-terminus is referred to N-alkylation. N-alkylation maintains the stimulant activity of phenethylamine analogs (14,15,16).
Figure 2.
Comparison of the substituted cathinones (methcathinone) to substituted amphetamines (methamphetamine) and the phenethylamine pharmacophore. The phenethylamine pharmacophore is bolded in each of the structures. Methamphetamine has a methyl (single carbon) off the α-carbon and nitrogen (N) terminus of phenethylamine. Methcathinone has carbonyl group (=O) off the β-carbon of methamphetamine.
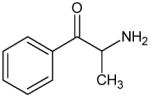
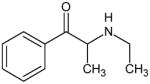
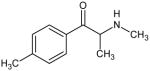
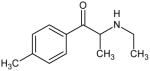
Based on the structure-activity relationships of these phenethylamine analogs, synthetic cathinones and MDMA analogs would be predicted to have substantially similar pharmacological effects. Table 1 presents the chemical structures and names of novel bath salts identified by the State of Ohio Bureau of Criminal Identification and Investigation Laboratory. Not all the agents listed in Table 1 have been pharmacologically tested in controlled human or animal studies. Most of the agents presented have also not been scheduled by the Drug Enforcement Administration. However, these synthetic cathinones, in general, have been shown to increase monoamine concentrations in the synaptic cleft (12,17,19). The increase in synaptic monoamines results in the stimulant and hallucinogenic effects of these phenethylamine analogs (12,17,19). The endogenously produced catecholamine monoamines (dopamine, norepinephrine and epinephrine) are also phenethylamines.
Table 1.
Bath Salts Identified by the State of Ohio Bureau of Criminal Identification and Investigation Laboratory using Gas Chromatograph and Mass Spectrometer
| Structure | Name |
|---|---|
|
Phenethylamine Pharmacophore |
|
Cathinone DEA Schedule I |
|
Ethcathinone Not Scheduled by the DEA |
|
Mephedrone DEA Schedule I |
|
4-methylethcathinone (4-MEC) Not Scheduled by the DEA |
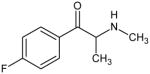
|
4-fluoromethcathinone DEA Schedule I |
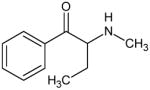
|
Buphedrone Not Scheduled by the DEA |
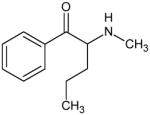
|
Pentedrone Not Scheduled by the DEA |
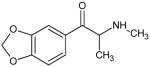
|
Methylone DEA Schedule I |
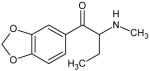
|
Butylone Not Scheduled by the DEA |
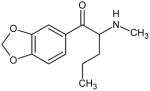
|
Pentylone Not Scheduled by the DEA |
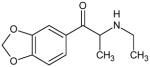
|
Ethylone Not Scheduled by the DEA |
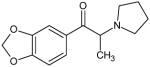
|
3,4-Methylenedioxy-α-pyrrolidinopropiophenone (MDPPP) Not Scheduled by the DEA |
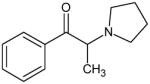
|
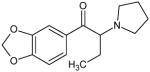
3,4-Methylenedioxy-α-pyrrolidinobutiophenone (MDPBP) Not Scheduled by the DEA |
|
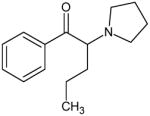
α-Pyrrolidinovalerophenone (Alpha-PVP) Not Scheduled by the DEA |
|
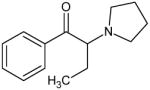
α-Pyrrolidinobutiophenone (Alpha-PBP) Not Scheduled by the DEA |
|
α-Pyrrolidinopropiophenone (Alpha-PPP) Not Scheduled by the DEA |
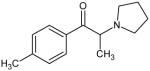
|
1-(4-methylphenyl)-2-(pyrrolidinyl)-1-propanone (MPPP) Not Scheduled by the DEA |
Pharmacology of phenethylamines
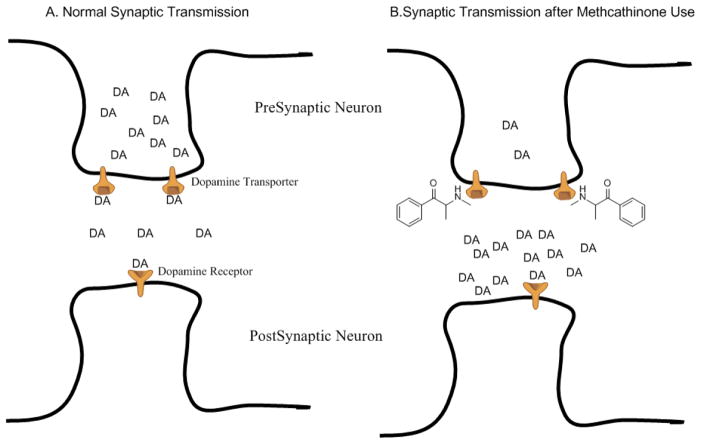
The shared phenethylamine pharmacophore between the endogenous monoamine neurotransmitters (dopamine and norepinephrine) and analogues of amphetamine and synthetic cathinones allows us to make predictions about the pharmacology of these abused compounds. For example, we would predict that these compounds would serve as substrates for the presynaptic monoamine transporters (dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT)); which are responsible for the re-uptake of released monoamines from the synaptic cleft into the pre-synaptic neuron to terminate the effects of that monoamine on the post-synaptic receptor and to recycle the monoamine for re-release (see Figure 3).
Figure 3.
Simplified schematic of synaptic neurotransmission for the endogenous monoamine dopamine (DA). Panel A shows that under normal (non-drug) conditions, DA is released from the presynaptic neuron into the synaptic cleft where DA can bind to post-synaptic dopamine receptors on the postsynaptic neuron to promulgate neurotransmission. DA can also bind to the dopamine transporter located on the presynaptic neuron and be translocated back into the presynaptic neuron for repackaging and subsequent release. Dopamine uptake by the dopamine transporter is the primary mechanism of terminating the DA-mediated neurotransmission. Panel B shows that under conditions of methcathinone use, there is an increased concentration of DA in the synaptic cleft that results in increased activation of post-synaptic dopamine receptors. Furthermore, methcathinone is a substrate for the dopamine transporter, blocking the ability of DA to bind to the transporter, and thus reducing one of the main mechanisms of dopaminergic neurotransmission termination.
Biochemical studies examining the effects of amphetamine and methamphetamine on these monoamine transporters confirm this prediction (19). Amphetamine is about 3-fold selective for the NET vs. DAT and about 70-fold selective for DAT vs. SERT. In contrast, methamphetamine is about 2-fold selective for the NET vs. DAT and about 30-fold selective for the DAT vs. SERT. Thus, the addition of the N-methyl group to amphetamine to produce methamphetamine resulted in a slight decrease in NET vs. DAT selective and a significant decrease in DAT vs. SERT selectivity. Next, adding a methylenedioxy bridge to the phenyl ring of methamphetamine changes the compound to MDMA. The addition of this group increases the selectivity for NET vs. DAT (~ 5-fold), but now MDMA is 7-fold more selective for SERT vs. DAT. Thus, the addition of the methylenedioxy bridge “flipped” the DAT vs. SERT selectivity in favor of more SERT selective. Finally, if we add the β-ketone group to the methamphetamine pharmacophore to make methcathinone, we lose selectivity for NET vs. DAT, such that the ratio is now 1:1, but significantly increase the selectivity for DAT vs. SERT (120-fold) compared to methamphetamine (20). Cyclization of the aliphatic chain off the terminal amine (as in MDPPP; table 1), has been demonstrated to decrease the neurotransmitter release activity. This cyclization of the aliphatic chain however maintains the reuptake inhibiting effects (21). Overall, these biochemical studies highlight that relatively simple changes in the chemical structure can have profound effects on the selectivity of these compounds to act as substrates for and induce release of the different monoamine neurotransmitters and ultimately alter the abuse potential of these compounds.
Increases in the neurotransmitter dopamine appear to be primarily responsible for producing the euphoric “abuse” effects of these compounds (22,23). Although Rothman and colleagues (19) have argued that the potency to release norepinephrine, compared to dopamine, is a better predictor of the subjective effects of amphetamine and amphetamine analogues in humans. As discussed earlier, norepinephrine also has a significant role in the activation of both central and peripheral mechanisms of the sympathetic nervous system. Increases in the neurotransmitter serotonin produced by these compounds appear to have two main effects. First, significant increases in serotonin may produce the serotonin “syndrome” clinically manifested as tachycardia, hypertension, diaphoresis, and hyperthermia (24). Secondly, the selectivity of these compounds to increase dopamine vs. serotonin levels appears to have an impact on the abuse of these compounds, such that dopamine-selective compounds, like amphetamine and 3,4-methylenedioxypyrovalerone (MDPV), have a higher abuse liability than serotonin-selective compounds, like fenfluramine and 4-methylmethcathinone (mephedrone) (25). How these compounds differentially alter levels of dopamine, norepinephrine, and serotonin in the brain and how this impacts both the abuse potential and the physiological consequences of these compounds that lead to emergency department visits are active areas of research.
Discussion
Implications for the Clinician
As their pharmacophores would predict, smoking, snorting, or injecting synthetic cathinones can cause clinical symptoms and medical complications that combine the worst features of methamphetamine and MDMA.
Like methamphetamine and MDMA, bath salts enhance sympathetic nervous system activity. This can result in tachycardia, hypertension, and occasionally self-reported discoloration of the hands (presumably from peripheral vasoconstriction) (34). The severity of cardiovascular effects is highly variable, with some case reports describing only mild tachycardia (e.g., HR<120 bpm) (35) and others more serious (e.g., HR>150, myocardial infarctions) (36). These differential effects are likely related to which synthetic cathinones are used, the amounts taken, and the time from drug use to presentation at a hospital. What is evident of these case reports is that tachycardia is more severe and more prevalent than hypertension (37); heart rates are commonly reported greater than 150 beats per minute while concomitant blood pressures may be normal or only mildly (Systolic BP<150 mmHg) elevated (38,39). This may reflect greater increases in circulating epinephrine and dopamine compared to norepinephrine. If true, this would make selective β-blockers a poor choice for controlling heart rate, as has been reported with cocaine, as they may cause unopposed α stimulation with worsening hypertension and coronary artery vasoconstriction (40). While their mechanisms of action likely increase the risk of developing myocardial ischemia to date ST-elevation myocardial infraction has not been reported. There have been cases of agitated delirium from bath salts where elevated cardiac enzymes are reported (41).
Consistent with previous case reports involving methamphetamine and MDMA, large ingestions or repetitive use of bath salts can cause a severe agitated delirium, seizures, and life-threatening hyperthermia with concomitant cardiovascular collapse, renal failure, hepatic dysfunction, disseminated intravascular coagulation and ultimately death (39,42,43). It is estimated that the mortality rate of patients using stimulants who present with body temps >40.5°C is upwards of 50% (26). Sympathomimetic agents such as MDMA and methamphetamine increase body temperature by preventing heat dissipation via α1-mediated peripheral vasoconstriction (27) and by generating heat through the activation of mitochondrial uncoupling proteins (UCP3; 28). Adrenergic activation by these agents results in peripheral norepinephrine release and subsequent activation of α1- and β3-adrengergic receptors (AR) (29,30,31). β3-AR activation leads to cAMP-mediated stimulation of hormone sensitive lipase (HSL) and subsequent release of free fatty acids (FFA). FFA can initiate facultative thermogenesis (28), a process by which ATP synthesis is “uncoupled” from substrate oxidation by FFA forming a proton-conductive pore in mitochondrial UCP3 located in skeletal muscle (32,33). Additionally, the elevations in plasma norepinephrine and epinephrine increase blood pressure and heart rate.
In addition to releasing norepinephrine, dopamine, and epinephrine many of the bath salts, by virtue of their ring substitutions, cause increases in extracellular concentrations of serotonin. Therefore, in addition to traditional sympathomimetic findings, patients can also develop serotonin syndrome with clonus and muscle rigidity (44). As it can be difficult to clinically differentiate between the sympathomimetic and serotonin syndrome the clinical management of these patients should focus on the use of benzodiazepines. While cyproheptadine, an anti-serotonergic agent, (45) has been employed in managing symptoms from bath salts it can only being given orally and should be considered an adjunct therapy(44).
Renal failure is a clinical manifestation of bath salt use that has also been reported with methamphetamine and MDMA. Renal failure after bath salts can be part of the multisystem organ failure seen in cases of severe sympathomimetic syndrome (39,41), or it can present as a complication of isolated rhabdomyolysis (45). Although bath salts are relatively new to the drug abuse scene, there have been numerous cases of rhabdomyolysis (36,38), and three reported cases of rhabdomyolysis from muscle compartment syndrome (46). The large percentage of cases reported with bath salts suggests they may have a great predilection for causing muscle damage. Clinicians should be aware of this potential in young adults presenting to the hospital with acute muscle pain. Renal failure can also be present without rhabdomyolysis or multisystem organ failure (36,47). While the mechanism behind these “isolated” cases of renal failure is not known, many of the patients abusing bath salts go on multiday-binges during which they exert themselves excessively but eat and drink little. As such, it is likely that many of these cases are pre-renal acute tubular necrosis from dehydration; a supposition that is supported by the finding that in many of these cases renal insufficiency rapidly resolves with fluid resuscitation (48).
While MDMA use has rarely been associated with isolated liver failure, there have not been similar cases reported to date with bath salts. Whether this is secondary to differences in the pharmacophore of MDMA and the synthetic cathinones, or differences in contaminants, or is simply of a matter of time is not yet known. Liver failure can be seen however in associated with multisystem organ failure from bath salts (39,43).
The most common clinical effect associated with bath salt use is the development of acute psychosis. While this has been well described with methamphetamine (49), and occasionally reported after MDMA (50), it appears to be particularly problematic for bath salt users. In published case series (35,37,51,52), and in most of case reports, the most common presenting clinical sign of patients taking bath salts is psychosis. In many of these cases, patients suffer from paranoia, visual and auditory hallucinations, and can be self-injurious or homicidal (37,53). Many of these patients also report amnesia surrounding their psychotic breaks (54,55). As with methamphetamine, the cause is likely related to altered dopaminergic neurotransmission (50). Antipsychotics attenuate dopaminergic activity and have been successfully utilized in the management of synthetic cathinones induced psychosis (37,51). Another contributing factor may be insomnia; in many of the cases, patients report being awake for days (54,55). It is important for the clinician to note that the psychosis induced by bath salts can present without the presence of acute sympathomimetic effects (53,56,57). Bath salts, therefore, should be considered in any young adult presenting with new onset psychosis. While the majority of patients with bath salt psychosis have had a history of prior drug abuse, most of them had not had prior episodes of psychosis (37). It is not clear if the increased incidence of psychosis with these cathinones is related to drug chemistry, potency, contaminants, or may be a consequence of a more frequent use. This is of particular interest as many of these drugs have been legally sold in corner convenient stores making them much easier to obtain than illegal methamphetamine or MDMA.
Similar to MDMA, use of bath salts has been associated with the development of hyponatremia and cerebral edema (58, 59). As with MDMA, bath salts likely cause hyponatremia by increasing the release of vasopressin (60), an effect which maybe mediated by serotonin.
A rare complication reported that appears to be somewhat unique to bath salts is hypoglycemia. To date there have been three cases reported in which patients have had low blood sugars (43,61). The etiology of this is not known, but bath salts users often binge for several days during which they may eat very little. As both methamphetamine and MDMA, and presumably bath salts, release insulin in animals (62,63), this combined with decreased local stores of glucose could cause hypoglycemia. It is of interest however that hypoglycemia is not commonly reported with MDMA or methamphetamine suggesting that there could metabolic effects unique to bath salts.
Detection of synthetic cathinones
Synthetic cathinones are not always detected in routine urine drug screens. Therefore if confirmation testing is required urine or blood samples need to be sent to forensic laboratories specializing in the detection of cathinones. As send-out testing can take a week or longer, the decision to test for bath salts should be based primarily on diagnostic, forensic, or public health needs. For instance, a young patient presenting with new onset psychosis or agitated delirium should have samples collected for testing to help clinicians differentiate between drug induced psychosis and mental illness. Similar testing should be done for new onset seizures, stroke, renal failure, or rhabdomyolysis in a young adult. Bath salts have a variety of structures and as soon as one compound is made illegal to sell and possess and new one arrives on the black market. This can result in clusters of clinical presentations and deaths. This makes it imperative for ED physicians to work with their local Poison Center, Toxicology Center, Health Department, and Law enforcement to help identify what is currently being sold and distributed in their area. This can help facilitate both educational and law enforcement efforts.
The most commonly used instrumentation for detection of controlled substances is a gas chromatograph (GC) coupled to a detection system to confirm structure, such as a mass spectrometer (MS) or an infrared detector (IRD). This combination is desirable because the GC separates compounds in a mixture based on size while the MS or IRD can deduce the different functional groups that can identify an individual compound. While no one system is perfect, using multiple systems on a single sample will often yield more conclusive results.
Separating a mixture using a GC is essential in identifying each individual compound. An illicit drug is often “cut” or diluted with an additional, inexpensive substances in order to increase profit for the supplier or dealer. This “cutting” can complicate detection of the illicit substance. The separation facilitated by the GC simplifies this problem. Although coupling the GC with a MS or IRD is preferable, valuable information can also come from size separation alone. A secondary test is to run the evidence sample and a known standard consecutively and comparing the migration time. While not confirmatory, this method is often utilized as a secondary assay once the identity of a substance is known.
The MS is likely the most often used detector in drug chemistry. A mass spectrum is easily created using now common instruments with highly reproducible results and mass spectra of nearly all known licit and illicit chemicals can be found in the scientific community.
The IRD has an advantage over the MS in the respect that the IRD can distinguish positional and optical isomers. While the MS may provide data suggesting an isomer, further testing would be required to prove the existence of that isomer. The method of structure analysis used by an IRD would immediately indicate the identity of an isomer as the vibration of the bonds would be different resulting in a different IR spectrum. Two common advantages of the IRD are differentiating cocaine base from cocaine hydrochloride and methamphetamine from phentermine, something that is not possible on normal GC/MS runs. The disadvantage of the IRD is that a pure or nearly pure sample is required. As discussed earlier, this is not the normal case as most drugs are “cut” or contaminated due to poor manufacturing practices.
Conclusions
Treatment of patients presenting after using bath salts should be focused on reducing agitation and psychosis, and supporting renal perfusion. The majority of successfully treated cases have used benzodiazepines and antipsychotics along with general supportive care (37,51,52). While many of these patients will have excited delirium, it is important that the clinician strives to achieve chemical rather than physical restraint. The use of physical restraints has been associated with sudden death in persons with stimulant-induced psychosis (64).
Combining the worst of both methamphetamine and MDMA, bath salts are dangerous drugs and clinicians need to be aware of their clinical effects as well as their addictive and psychiatric manifestations. There use should be suspected in any young adult presenting with new onset psychosis, renal failure, or manifesting sympathomimetic symptoms and agitated delirium.
Article Summary.
1. Why is this topic important?
Over the past several years, emergency physicians have seen a growing number of bath salt abuse cases. Currently, there is a lack of concise information on the pharmacology, toxicology and clinical management of these patients.
2. What does this review attempt to show?
This review attempt to show how differences in the chemistry between the different bath salts influences the pharmacology and toxicology. The pharmacology and toxicology is also used as the basis for the clinical management of patients exposed to bath salts.
3. What are the key findings?
This review discusses the pharmacology, toxicology and chemistry of previously and recently identified synthetic cathinones.
4. How is patient care impacted?
Patient care is impacted by increasing the knowledge of emergency physicians of bath salts. Further, the pharmacological basis for treating patients is discussed in order to assist emergency physicians in treating their patients.
References
- 1.Edeleano L. Ueber einige Derivate der Phenylmethacrylsäure und der Phenylisobuttersäure. Berichte der deutschen chemischen Gesellschaft. 1887;20:616–22. [Google Scholar]
- 2.Friedenberg S. Addiction to amphetamine sulfate. JAMA. 1940;114:956. [Google Scholar]
- 3.Morimoto K. [Accessed 6/2013];The problem of the abuse of amphetamines in Japan. 1957 :8–12. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1957-01-01_3_page003.html.
- 4.Goto A. Personal and social factors in connection with the etiology of amphetamine addiction. Folia Psychiat Neurol Jap Suppl. 1963;7:376–7. [Google Scholar]
- 5.Ellinwood E. Drug Use in America: Problem in Perspective. Washington DC: US Government Printing Office; 1973. Amphetamine and stimulant drugs; pp. 140–57. [Google Scholar]
- 6.Gaston T, Rasmussen G. Identification of 3,4-methylenedioxymethamphetamine. Microgram. 1972;5:60–3. [Google Scholar]
- 7.Freudenmann RW, Oxler F, Bernschneider-Reif S. The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents. Addiction. 2006;101:1241–5. doi: 10.1111/j.1360-0443.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 8.Shulgin AT, Nichols DE, Stillman RC, Willette RE. The Psychopharmacology of Hallucinogens. New York: Pergamon; 1978. Characterization of Three New Psychotomimetics; pp. 1–9. [Google Scholar]
- 9.Kramer J, Fischman VS, Littlefield DC. Amphetamine abuse: Pattern and effects of high doses taken intravenously. JAMA. 1967;201:305–9. doi: 10.1001/jama.201.5.305. [DOI] [PubMed] [Google Scholar]
- 10.Lawn J. Schedules of controlled substances: temporary placement of 3,4-methylenedioxymethamphetamine (MDMA) into Schedule I. Fed Regist. 1985;50:23118–20. [Google Scholar]
- 11.Hyde JF, Browning E, Adams R. Synthetic homologs of d,l-ephedrine. J Am Chem Soc. 1928;50:2287–92. [Google Scholar]
- 12.Cozzi NV, Sievert MK, Shulgin AT, Jacob P, III, Ruoho AE. Inhibition of plasma membrane monoamine transporters by -ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- 13.Glennon RA. Stimulus properties of hallucinogenic phenalkylamines and related designer drugs: Formulation of structure-activity relationships. NIDA Res Mono. 1989;94:43–67. [PubMed] [Google Scholar]
- 14.Braun U, Shulgin AT, Braun G. Centrally active N-substituted analogs of 3,4-methylenedioxyphenylisopropylamine (3,4-methylenedioxyamphetamine) J Pharm Sci. 1980;69:192–195. doi: 10.1002/jps.2600690220. [DOI] [PubMed] [Google Scholar]
- 15.Dal Cason T. Cathinone: An investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav. 1997;58:1109–16. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- 16.Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- 17.Nagai F, Nonaka R, Satoh K, Kamimura H. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Euro J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 18.Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared to MDMA (Ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in the nucleus accumbens of awake rats. Brit J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman R, Baumann M, Dersch C, Romero D, Rice K, Carroll F, Partilla J. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In Vitro Characterization of Ephedrine-Related Stereoisomers at Biogenic Amine Transporters and the Receptorome Reveals Selective Actions as Norepinephrine Transporter Substrates. J Pharmacol Exp Ther. 2003;307:138–45. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- 21.Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive “Bath Salts” Products. Neuropsychopharmacology. 2013;38:552–62. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonci A, Bernardi G, Grillner P, Mercuri NB. The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends Pharmacol Sci. 2003;24:172–7. doi: 10.1016/S0165-6147(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 23.Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology. 2012;37:1192–203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer EW, Shannon M. The Serotonin Syndrome. N Engl J Med. 2005;352:1112–20. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 25.Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the Serotonergic Activity and Reinforcing Effects of a Series of Amphetamine Analogs. J Pharmacol Exp Ther. 2005;313:848–54. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- 26.Gowing LR, Henry-Edwards SM, Irvine RJ, Ali RL. The health effects of ecstasy: a literature review. Drug Alcohol Rev. 2002;21:53–63. doi: 10.1080/09595230220119363. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills E, Rusyniak D, Sprague JE. The role of sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. J Mol Med. 2004;82:787–799. doi: 10.1007/s00109-004-0591-7. [DOI] [PubMed] [Google Scholar]
- 29.Kuusela P, Rehnmark S, Jacobsson A, Cannon B, Nedergaard J. Adrenergic stimulation of lipoprotein lipase gene expression in rat brown adipocytes differentiated in culture: mediation via beta3- and alpha1-adrenergic receptors. Biochem J. 1997;321:759–767. doi: 10.1042/bj3210759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J, Cannon B, Nedergaard J. alpha1-Adrenergic stimulation potentiates the thermogenic action of beta3-adrenoreceptor-generated cAMP in brown fat cells. J Biol Chem. 1997;272:32847–32856. doi: 10.1074/jbc.272.52.32847. [DOI] [PubMed] [Google Scholar]
- 31.Himms-Hagen J, Cerf J, Desautels M, Zaror-Behrens G. Thermogenic mechanisms and their control. Experientia Suppl. 1978;32:119–134. doi: 10.1007/978-3-0348-5559-4_14. [DOI] [PubMed] [Google Scholar]
- 32.Echtay KS, Winkler E, Frischmuth K, Klingenberg M. Uncoupling proteins 2 and 3 are highly active H+ transporters and highly nucleotide sensitive when activated by coenzyme Q (ubiquione) Proc Natl Acad Sci USA. 2001;98:1416–1421. doi: 10.1073/pnas.98.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone) Drug Test Anal. 2011;3:454–63. doi: 10.1002/dta.312. [DOI] [PubMed] [Google Scholar]
- 35.Caffery TM, Musso M, Manausa R, Everett J, Perret J. Riding high on cloud 9. J La State Med Soc. 2012;16:186–9. [PubMed] [Google Scholar]
- 36.Penders TM. How to recognize a patient who’s high on “bath salts”. J Fam Pract. 2012;61:210–2. [PubMed] [Google Scholar]
- 37.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 38.Murray BL, Murphy CM, Buehler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young AC, Schwarz ES, Velez LI, Gardner M. Two cases of disseminated intravascular coagulation due to “bath salts” resulting in fatalities, with laboratory confirmation. Am J Emerg Med. 2013;31:e443–5. doi: 10.1016/j.ajem.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 40.Lange RA, Cigarroa RG, Flores ED, McBride W, Kim AS, Wells PJ, Bedotto JB, Danziger RS, Hillis LD. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann Intern Med. 1990;112:897–903. doi: 10.7326/0003-4819-112-12-897. [DOI] [PubMed] [Google Scholar]
- 41.Smith C, Cardile AP, Miller M. Bath salts as a “legal high. Am J Med. 2011;124:e7–8. doi: 10.1016/j.amjmed.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Warrick BK, Hill M, Hekman K, Christensen R, Goetz R, Casavant MJ, Wahl M, Mowry JB, Spiller H, Anderson D, Aleguas A, Gummin D, Thomas R, Nezlek C, Smolinske S. A 9-state analysis of designer stimulant, “bath salts,” hospital visits reported to poison control centers. Ann Emerg Med. 2013;62:244–251. doi: 10.1016/j.annemergmed.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 43.Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60:103–5. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 44.Rusyniak DE, Sprague JE. Toxin-induced hyperthermic syndromes. Med Clin N Am. 2005;89:1277–96. doi: 10.1016/j.mcna.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Mugele J, Nanagas KA, Tormoehlen LM. Serotonin syndrome associated with MDPV use: a case report. Ann Emer Med. 2012;60:100–2. doi: 10.1016/j.annemergmed.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 46.Levine M, Levitan R, Skolnik A. Compartment syndrome after “bath salts” use: a case series. Ann Emerg Med. 2013;61:480–3. doi: 10.1016/j.annemergmed.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 47.Regunath H, Ariyamuthu VK, Dalal P, Misra M. Bath salt intoxication causing acute kidney injury requiring hemodialysis. Hemodial Int. 2012;16:S47–9. doi: 10.1111/j.1542-4758.2012.00750.x. [DOI] [PubMed] [Google Scholar]
- 48.Adebamiro A, Perazella MA. Recurrent acute kidney injury following bath salts intoxication. Am J Kidney Dis. 2012;59:273–5. doi: 10.1053/j.ajkd.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Neurol Clin. 2011;29:641–55. doi: 10.1016/j.ncl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burgess CA, O’Donohoe, Gill M. Agony and ecstasy: a review of MDMA effects and toxicity. Eur Psychiatry. 2000;15:287–294. doi: 10.1016/s0924-9338(00)00396-5. [DOI] [PubMed] [Google Scholar]
- 51.Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, Dargan PI. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol (Phila) 2010;48:924–7. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- 52.Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. 2011;28:280–2. doi: 10.1136/emj.2010.092288. [DOI] [PubMed] [Google Scholar]
- 53.Sharma TR, Iskandar JW, Ali R, Shah UR. Bath salts-induced delirium and brief psychotic episode in an otherwise healthy young man. Prim Care Companion CNS Disord. 2012:14. doi: 10.4088/PCC.11l01224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antonowicz JL, Metzger AK, Ramanujam SL. Paranoid psychosis induced by consumption of methylenedioxypyrovalerone: two cases. Gen Hosp Psychiatry. 2011;33:640e645–6. doi: 10.1016/j.genhosppsych.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Penders TM, Gestring R. Hallucinatory delirium following use of MDPV: “Bath Salts”. Gen Hosp Psychiatry. 2011;33:525–6. doi: 10.1016/j.genhosppsych.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 56.Goshgarian AM, Benford DM, Caplan JP. Bath salt abuse: neuropsychiatric effects of cathinone derivatives. Psychosomatics. 2011;52:593–94. doi: 10.1016/j.psym.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Kyle PB, Iverson RB, Gajagowni RG, Spencer L. Illicit bath salts: not for bathing. J Miss State Med Assoc. 2011;52:375–7. [PubMed] [Google Scholar]
- 58.Boulanger-Gobeil C, St-Onge M, Laliberte M, Auger PL. Seizures and hyponatremia related to ethcathinone and methylone poisoning. J Med Toxicol. 2012;8:59–61. doi: 10.1007/s13181-011-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sammler EM, Foley PL, Lauder GD, Wilson SJ, Goudie AR, O’Riordan J. A harmless high? Lancet. 2010;376:742. doi: 10.1016/S0140-6736(10)60891-4. [DOI] [PubMed] [Google Scholar]
- 60.Fallon JK, Shah D, Kicman AT, Hutt AJ, Henry JA, Cowan DA, Forsling M. Action of MDMA (ecstasy) and its metabolites on arginine vasopressin release. Ann N Y Acad Sci. 2002;965:399–409. doi: 10.1111/j.1749-6632.2002.tb04181.x. [DOI] [PubMed] [Google Scholar]
- 61.Falgiani M, Desai B, Ryan M. “Bath salts” intoxication: a case report. Case Rep Emerg Med. 2012:976314. doi: 10.1155/2012/976314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McMahon EM, Andersen DK, Feldman JM, Schanberg SM. Methamphetamine-induced insulin release. Science. 1971;174:66–8. doi: 10.1126/science.174.4004.66. [DOI] [PubMed] [Google Scholar]
- 63.Banks ML, Buzard SK, Gehret CM, Monroy AN, Kenaston MA, Mills EM, Sprague JE. Pharmacodynamic characterization of insulin on MDMA-induced thermogenesis. Eur J Pharmacol. 2009;615:257–261. doi: 10.1016/j.ejphar.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stratton SJ, Rogers C, Brickett K, Gruzinski G. Factors associated with sudden death of individuals requiring restraint for excited delirium. Am J Emerg Med. 2001;19:187–91. doi: 10.1053/ajem.2001.22665. [DOI] [PubMed] [Google Scholar]