Abstract
To elucidate the genetic architecture of amyotrophic lateral sclerosis (ALS) and find associated loci, we assembled a custom imputation reference panel from whole-genome-sequenced patients with ALS and matched controls (n = 1,861). Through imputation and mixed-model association analysis in 12,577 cases and 23,475 controls, combined with 2,579 cases and 2,767 controls in an independent replication cohort, we fine-mapped a new risk locus on chromosome 21 and identified C21orf2 as a gene associated with ALS risk. In addition, we identified MOBP and SCFD1 as new associated risk loci. We established evidence of ALS being a complex genetic trait with a polygenic architecture. Furthermore, we estimated the SNP-based heritability at 8.5%, with a distinct and important role for low-frequency variants (frequency 1–10%). This study motivates the interrogation of larger samples with full genome coverage to identify rare causal variants that underpin ALS risk.
ALS is a fatal neurodegenerative disease that affects 1 in 400 people, with death occurring within 3 to 5 years of the onset of symptoms1. Twin-based studies estimate heritability to be around 65%, and 5–10% of patients with ALS have a positive family history1,2. Both of these features are indicative of an important genetic component in ALS etiology. Following initial discovery of a risk-associated C9orf72 locus in ALS genome-wide association studies (GWAS)3–5, identification of a pathogenic hexanucleotide-repeat expansion in this locus revolutionized the field of ALS genetics and biology6,7. The majority of ALS heritability, however, remains unexplained, and only two additional risk loci have since been identified robustly3,8.
To discover new genetic risk loci and elucidate the genetic architecture of ALS, we genotyped 7,763 new cases and 4,669 controls and additionally collected genotype data from published GWAS of ALS. In total, we analyzed 14,791 cases and 26,898 controls from 41 cohorts (Supplementary Table 1 and Supplementary Note). We combined these cohorts on the basis of genotyping platform and nationality to form 27 case–control strata. In total, 12,577 cases and 23,475 controls passed quality control (Online Methods and Supplementary Tables 2–5).
For imputation purposes, we obtained high-coverage (~43.7×) whole-genome sequencing data from 1,246 patients with ALS and 615 controls from the Netherlands (Online Methods and Supplementary Fig. 1). After quality control, we constructed a reference panel including 18,741,510 single-nucleotide variants (SNVs). Imputing this custom reference panel into Dutch ALS cases considerably increased the imputation accuracy for low-frequency variants (minor allele frequency (MAF) = 0.5–10%) in comparison to commonly used reference panels from 1000 Genomes Project Phase 1 (ref. 9) and Genome of the Netherlands10 (Fig. 1a). Improvement was also observed when imputing into ALS cases from the UK (Fig. 1b). To benefit from the global diversity of haplotypes, the custom and 1000 Genomes Project panels were combined, which further improved imputation. Given these results, we used the merged reference panel to impute all strata in our study.
Figure 1.
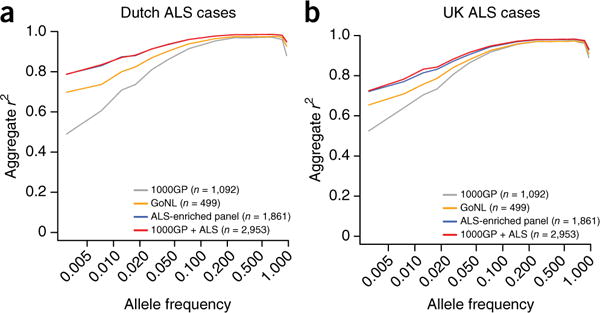
Comparison of imputation accuracy. (a,b) Aggregate r2 values between imputed and sequenced genotypes on chromosome 20 are shown when using different reference panels for imputation. Allele frequencies were calculated from the Dutch samples included in the Genome of the Netherlands (GoNL) cohort. The highest imputation accuracy was achieved when imputing from the merged custom and 1000 Genomes Project (1000GP) panel. The difference in accuracy was most pronounced for low-frequency alleles (frequency 0.5–10%) in ALS cases from both the Netherlands (a) and the UK (b).
In total, we imputed 8,697,640 variants passing quality control into the 27 strata and tested the strata separately for association with ALS risk by logistic regression. We then included the results in an inverse-variance-weighted, fixed-effects meta-analysis, which identified four loci associated at genome-wide significance (P < 5 × 10−8) (Fig. 2a). The previously reported C9orf72 (rs3849943)3–5,8, UNC13A (rs12608932)3,5 and SARM1 (rs35714695)8 loci all reached genome-wide significance, as did a new association for a nonsynonymous variant in C21orf2 (rs75087725, P = 8.7 × 10−11; Supplementary Tables 6–10). This variant was present on only 10 haplotypes in the 1000 Genomes Project reference panel (MAF = 1.3%), whereas it was present on 62 haplotypes in our custom reference panel (MAF = 1.7%). As a result, more strata passed quality control for this variant by passing the allele frequency threshold of 1% (Supplementary Table 11). This result demonstrates the benefit of the merged reference panel with ALS-specific content, which improved imputation and resulted in the identification of a genome-wide significant association.
Figure 2.
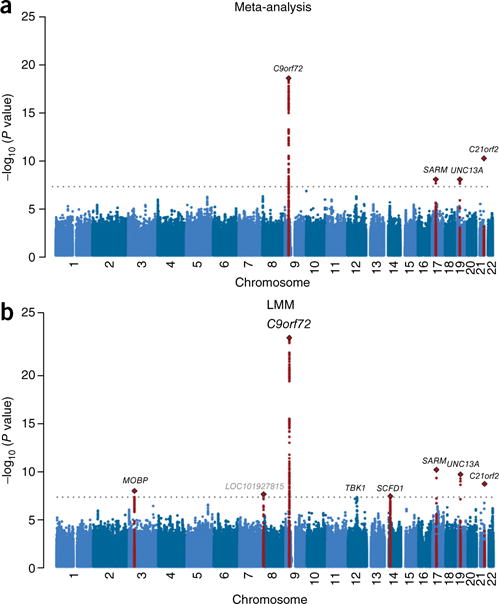
Meta-analysis and LMM associations. (a) Manhattan plot for the meta-analysis results. This approach yielded four genome-wide-significant associations. The associated SNP in C21orf2 is a nonsynonymous variant not found to be associated in previous GWAS. (b) Manhattan plot for the LMM results. This analysis yielded three loci in addition to those identified by meta-analysis with associations that reached genome-wide significance (MOBP, LOC101927815 and SCFD1). The association for SNPs in the previously identified ALS risk gene TBK1 approached genome-wide significance (P = 6.6 × 10−8). As the C21orf2 SNP was removed from a Swedish stratum because of MAF <1%, this SNP was tested separately, but it is presented here together with all SNPs with MAF >1% in all strata. LOC101927815 is shown in gray because the association for this locus could not be replicated. Loci are labeled by the name of the nearest gene. The dotted lines correspond to the significance threshold of P = 5 × 10−8.
Linear mixed models (LMMs) can improve power while controlling for sample structure11, which would be particularly important in our study that included a large number of imperfectly balanced strata. Even though LMM analysis for ascertained case–control data potentially results in a small loss of power in comparison to meta-analysis11, we judged the advantage of combining all strata while controlling the false positive rate to be more important than this potential loss and therefore jointly analyzed all strata in an LMM to identify additional risk loci. There was no overall inflation of the LMM test statistics in comparison to the meta-analysis test statistics (Supplementary Fig. 2). We observed modest inflation of test statistics in the quantile–quantile plot (λGC = 1.12, λ1,000 = 1.01; Supplementary Fig. 3). LD score regression yielded an intercept of 1.10 (standard error of 7.8 × 10−3). Although an LD score regression intercept higher than 1.0 can indicate the presence of residual population stratification, which is fully corrected for in an LMM, this can also reflect a distinct genetic architecture where most causal variants are rare or a noninfinitesimal architecture12. The LMM identified all four genome-wide-significant associations from the meta-analysis. Furthermore, three additional loci—MOBP at 3p22.1 (rs616147), SCFD1 at 14q12 (rs10139154) and a long noncoding RNA at 8p23.2 (rs7813314)— were associated at genome-wide significance (Fig. 2b, Table 1 and Supplementary Tables 12–14). SNPs in the MOBP locus have been reported to be associated in a GWAS on progressive supranuclear palsy (PSP)13 and to act as a modifier for survival in frontotemporal dementia (FTD)14. The putative pleiotropic effects of variants in this locus suggest that ALS, FTD and PSP share a neurodegenerative pathway. We also found that rs74654358 at 12q14.2 in the TBK1 gene approximated genome-wide significance (MAF = 4.9%, odds ratio (OR) = 1.21 for the A allele, P = 6.6 × 10−8). This gene was recently identified as an ALS risk gene through exome sequencing15,16.
Table 1.
Discovery and replication of new genome-wide significant loci
| Discovery
|
Replication
|
Combined
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | MAFcases | MAFcontrols | OR | Pmeta | PLMM | MAFcases | MAFcontrols | OR | P | Pcombined | I2 |
| rs75087725 | 0.02 | 0.01 | 1.45 | 8.65 × 10−11 | 2.65 × 10−9 | 0.02 | 0.01 | 1.65 | 3.89 × 10−3 | 3.08 × 10−10 | 0.00* |
| rs616147 | 0.30 | 0.28 | 1.10 | 4.14 × 10−5 | 1.43 × 10−8 | 0.31 | 0.28 | 1.13 | 2.35 × 10−3 | 4.19 × 10−10 | 0.00* |
| rs10139154 | 0.34 | 0.31 | 1.09 | 1.92 × 10−5 | 4.95 × 10−8 | 0.33 | 0.31 | 1.06 | 9.55 × 10−2 | 3.45 × 10−8 | 0.05* |
| rs7813314 | 0.09 | 0.10 | 0.87 | 7.46 × 10−7 | 3.14 × 10−8 | 0.12 | 0.10 | 1.17 | 7.75 × 10−3 | 1.05 × 10−5 | 0.80** |
Genome-wide-significant loci from the discovery phase including 12,557 cases and 23,475 controls were directly genotyped and tested for association in the replication phase including 2,579 cases and 2,767 controls. The three top associated SNPs in the MOBP (rs616147), SCFD1 (rs10139154) and C21orf2 (rs75087725) loci replicated with associations in the same direction as in the discovery phase and an association in the combined analysis that exceeded that in the discovery phase. Cochrane’s Q test,
P > 0.1,
P = 4.0 × 10−6.
MAF, minor allele frequency; OR, odds ratio, Pmeta, meta-analysis P value; PLMM, linear mixed-model P value; Pcombined, P value from meta-analysis of the associations in the discovery and replication phase.
In the replication phase, we genotyped the newly discovered associated SNPs in nine independent replication cohorts, totaling 2,579 cases and 2,767 controls. In these cohorts, we replicated the signals for the C21orf2, MOBP and SCFD1 loci, with lower P values in the combined analysis than in the discovery phase (combined P value = 3.08 × 10−10, 4.19 × 10−10 and 3.45 × 10−8 for rs75087725, rs616147 and rs10139154, respectively; Table 1 and Supplementary Fig. 4)17. The combined signal for rs7813314 was less significant because the effects for the discovery and replication phases were in opposite directions, indicating non-replication. Although replication yielded an effect estimate for rs10139154 similar to that obtained in the discovery phase, this effect was not statistically significant (P = 0.09) in the replication phase alone. This lack of significance reflects the limited sample size of our replication phase, a feature that is inherent to studies of ALS because of its low prevalence. Even larger sample sizes are warranted to replicate this signal robustly.
There was no evidence of residual association in each locus after conditioning on the top SNP, indicating that all the risk loci are independent signals. Apart from the C9orf72, UNC13A and SARM1 loci, we found no evidence of associations previously described in smaller GWAS (Supplementary Table 15).
The association of the low-frequency nonsynonymous SNP in C21orf2 suggested that this gene could be directly involved in ALS risk. Indeed, we found no evidence that linkage disequilibrium (LD) between this SNP and sequenced variants beyond the boundaries of C21orf2 explained the association of this locus (Supplementary Fig. 5). In addition, we investigated the burden of rare coding mutations in C21orf2 in a set of whole-genome-sequenced cases (n = 2,562) and controls (n = 1,138). After quality control, these variants were tested for association using pooled association tests for rare variants and applying correction for population structure (tests T5 and T1 for alleles with 5% and 1% frequency, respectively; Supplementary Note). This approach demonstrated an excess of nonsynonymous and loss-of-function mutations in C21orf2 among ALS cases that persisted after conditioning on rs75087725 (PT5 = 9.2 × 10−5, PT1 = 0.01; Supplementary Fig. 6), further supporting the notion that C21orf2 contributes to ALS risk.
In an effort to fine-map the other loci to pinpoint susceptibility genes, we searched for SNPs in these loci with cis expression quantitative trait locus (cis-eQTL) effects observed in brain and other tissues (Supplementary Table 16 and Supplementary Note)18. We found overlap with previously identified brain cis-eQTLs for five regions (Supplementary Fig. 7, Supplementary Table 17 and Supplementary Data Set). In the C9orf72 locus, we found that proxies of rs3849943 (LD r2 = 0.21–0.56) only had a brain cis-eQTL effect on C9orf72 (minimal P = 5.27 × 10−7), which harbors the hexanucleotide-repeat expansion that drives this GWAS signal. Additionally, we found that rs12608932 and its proxies in the UNC13A locus had an exon-level cis-eQTL effect on KCNN1 in frontal cortex (P = 1.15 × 10−3)19. Another overlap was observed in the SARM1 locus where rs35714695 and its proxies had the strongest exon-level cis-eQTL effect on POLDIP2 in multiple brain tissues (P = 2.32 × 10−3). In the SCFD1 locus, rs10139154 and its proxies had a cis-eQTL effect on SCFD1 in cerebellar tissue (P = 7.71 × 10−4). For the MOBP locus, rs1768208 and its proxies had a cis-eQTL effect on RPSA (P = 7.71 × 10−4).
To describe the genetic architecture of ALS, we generated polygenic scores, which can be used to predict phenotypes for traits with a poly-genic architecture20. We calculated SNP effects using an LMM in 18 of the 27 strata and subsequently assessed predictive ability in the other 9 independent strata. This analysis showed that a significant albeit modest proportion of the phenotypic variance could be explained by all SNPs (Nagelkerke r2 = 0.44%, r2 = 0.15% on the liability scale, P = 2.7 × 10−10; Supplementary Fig. 8). This finding adds to the existing evidence that ALS is a complex genetic trait with a polygenic architecture. To further quantify the contribution of common SNPs to ALS risk, we estimated SNP-based heritability using three approaches, all assuming a population baseline risk of 0.25% (ref. 21). GCTA-REML estimated the SNP-based heritability at 8.5% (s.e.m. = 0.5%). Haseman–Elston regression yielded a very similar estimate of 7.9%, and LD score regression estimated the SNP-based heritability at 8.2% (s.e.m. = 0.5%). The heritability estimates for each chromosome were significantly correlated with chromosome length (r2 = 0.46, P = 4.9 × 10−4; Fig. 3a), again indicative of a polygenic architecture in ALS.
Figure 3.
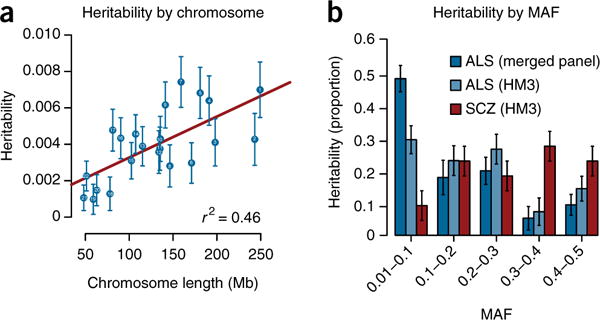
Partitioned heritability. (a) Heritability estimates for each chromosome were significantly correlated with chromosome length (P = 4.9 × 10−4). (b) For ALS, there was a clear trend where more heritability was explained by the low-frequency alleles. This effect was still observed when, for a fair comparison between ALS and a previous study partitioning heritability for schizophrenia (SCZ) using identical methods22, SNPs present in HapMap 3 (HM3) were included. Error bars correspond to standard errors.
We found that the genome-wide-significant loci only explained 0.2% of heritability, and the bulk of the heritability (8.3%, s.e.m. = 0.3%) was thus captured by SNPs with associations below genome-wide significance. This finding implies that many genetic risk variants have yet to be discovered. Understanding where these unidentified risk variants remain across the allele frequency spectrum will inform the design of future studies to identify these variants. We therefore estimated heritability partitioned by MAF. Furthermore, we contrasted these results with those for common polygenic traits studied in GWAS such as schizophrenia. We observed a clear trend indicating that most variance is explained by low-frequency SNPs (Fig. 3b). Exclusion of the C9orf72 locus, which harbors the rare pathogenic repeat expansion, and the other genome-wide-significant loci did not affect this trend (Supplementary Fig. 9). This architecture is different from that expected for common polygenic traits and reflects a polygenic rare variant architecture observed in simulations22.
To gain better insight into the biological pathways that explain the associated loci found in this study, we looked for enriched pathways using DEPICT23. This analysis identified SNAP receptor (SNARE) activity as the only enriched category (false discovery rate (FDR) < 0.05; Supplementary Fig. 10). SNARE complexes have a central role in neurotransmitter release and synaptic function24, which are both perturbed in ALS25.
Although the biological role of C21orf2, a conserved leucine-rich-repeat protein, remains poorly characterized, this protein is part of the ciliome and is required for the formation and/or maintenance of primary cilia26. Defects in primary cilia are associated with various neurological disorders, and cilia numbers are decreased in mice expressing the Gly93Ala mutant of human SOD1, a well-characterized ALS model27. C21orf2 has also been localized to mitochondria in immune cells28 and is part of the interactome of the protein product of NEK1, which has previously been associated with ALS15. Both proteins seem to be involved in DNA repair mechanisms29. Although future studies are needed to dissect the function of C21orf2 in ALS pathophysiology, we speculate that defects in C21orf2 may lead to primary cilium and/or mitochondrial dysfunction or inefficient DNA repair and thereby result in adult-onset disease. The other associated loci will require more extensive studies to fine-map causal variants. SARM1 has been suggested to be a susceptibility gene for ALS, mainly because of its role in Wallerian degeneration and its interaction with UNC13A8,30. Although these are indeed interesting observations, the brain cis-eQTL effect for SNPs in this locus on POLDIP2 suggests that POLDIP2 and not SARM1 could in fact be the causal gene in this locus. Similarly, KCNN1, which encodes a neuronal potassium channel involved in neuronal excitability, could be the causal gene either through a direct eQTL effect or rare variants in LD with the associated SNP in UNC13A.
In conclusion, we have identified a key role for rare variation in ALS and discovered SNPs in new complex loci. Our study therefore informs future study design in ALS genetics, promoting the combination of larger sample sizes, full genome coverage and targeted genome editing experiments, leveraged together to fine-map new loci, identify rare causal variants and thereby elucidate the biology of ALS.
ONLINE METHODS
The software packages used, their version, web source and references are described in Supplementary Table 18.
GWAS discovery phase and quality control
Details on the acquired genotype data from previously published GWAS are described in Supplementary Table 1. Methods for case and control ascertainment for each cohort are described in the Supplementary Note. All cases and controls gave written informed consent, and the relevant institutional review boards approved this study. To obtain genotype data for newly genotyped individuals, genomic DNA was hybridized to the Illumina OmniExpress array according to the manufacturer’s protocol. Subsequent quality control included (i) removing low-quality SNPs and individuals from each cohort, (ii) combining unbalanced cohorts on the basis of nationality and genotyping platform to form case–control strata, (iii) removing low-quality SNPs, related individuals and population outliers per stratum and (iv) calculating genomic inflation factors per stratum. More details are described in the Supplementary Note and Supplementary Figure 11. The number of SNPs and individuals failing each quality control step per cohort and stratum is displayed in Supplementary Tables 2–5.
Whole-genome sequencing (custom reference panel)
Individuals were whole-genome sequenced on the Illumina HiSeq 2500 platform using PCR-free library preparation and 100-bp paired-end sequencing, yielding a minimum of 35× coverage. Reads were aligned to the hg19 human genome build, and after variant calling (Isaac variant caller) additional SNV and sample quality control was performed (Supplementary Fig. 12 and Supplementary Note). Individuals in our custom reference panel were also included in the GWAS in strata sNL2, sNL3 and sNL4.
Merging reference panels
All high-quality calls in the custom reference panel were phased using SHAPEIT2 software. After checking strand and allele inconsistencies, both the 1000 Genomes Project reference panel (release 05-21-2011)31 and custom reference panel were imputed up to the union of their variants as described previously32. Variants with inconsistent allele frequencies between the two panels were removed.
Imputation accuracy performance
To compare the imputation accuracy between different reference panels, 109 unrelated ALS cases of Dutch ancestry sequenced by Complete Genomics and 67 ALS cases from the UK sequenced by Illumina were selected as a test panel. All variants not present on the Illumina Omni1M array were masked, and the SNVs on chromosome 20 were subsequently imputed back using four different reference panels (1000 Genomes Project, GoNL, custom panel and merged panel). Concordance between the imputed alleles and sequenced alleles was assessed in each allele frequency bin where allele frequencies were calculated from the Dutch samples included in the Genome of the Netherlands cohort.
GWAS imputation
Prephasing was performed for each stratum using SHAPEIT2 with the 1000 Genomes Project phase 1 (release 05-21-2011) hap-lotypes31 as a reference panel. Subsequently, strata were imputed up to the merged reference panel in 5-Mb chunks using IMPUTE2. Imputed variants with a MAF <1% or INFO score <0.3 were excluded from further analysis. Variants with allele frequency differences between strata, defined as deviating by >10 s.d. from the normalized mean allele frequency difference between those strata and an absolute difference >5%, were excluded because they are likely to represent sequencing or genotyping artifacts. Imputation concordance scores for cases and controls were compared to assess biases in imputation accuracy (Supplementary Table 19).
Meta-analysis
Logistic regression was performed on imputed genotype dosages under an additive model using SNPTEST software. On the basis of scree plots, one to four principal components were included per stratum. These results were then combined in an inverse-variance-weighted, fixed-effect meta-analysis using METAL. No marked heterogeneity across strata was observed as the Cochrane’s Q test statistics did not deviate from the null distribution (λ = 0.96). Therefore, no SNPs were removed owing to excessive heterogeneity. The genomic inflation factor was calculated, and the quantile–quantile plot is provided in Supplementary Figure 3a.
Linear mixed model
All strata were combined including SNPs that passed quality control in every stratum. Subsequently, genetic relationship matrices (GRMs) were calculated for each chromosome including all SNPs using the Genome-Wide Complex Trait Analysis (GCTA) software package. Each SNP was then tested in an LMM including a GRM composed of all chromosomes excluding the target chromosome (leave one chromosome out, LOCO). The genomic inflation factor was calculated, and the quantile–quantile plot is provided as Supplementary Figure 3b.
Replication
For the replication phase, independent ALS cases and controls from Australia, Belgium, France, Germany, Ireland, Italy, the Netherlands and Turkey that were not used in the discovery phase were included. A pre-designed TaqMan genotyping assay was used to replicate rs75087725 and rs616147. Sanger sequencing was performed to replicate rs10139154 and rs7813314 (Supplementary Table 20 and Supplementary Note). All genotypes were tested in a logistic regression per country and subsequently underwent meta-analysis.
Rare variant analysis in C21orf2
The burden of nonsynonymous rare variants in C21orf2 was assessed in whole-genome sequencing data obtained from ALS cases and controls from the Netherlands, Belgium, Ireland, the UK and the United States. After quality control, the burden of nonsynonymous and loss-of-function mutations in C21orf2 was tested for association in each country and meta-analysis was subsequently performed. More details are provided in the Supplementary Note and Supplementary Figure 13.
Polygenic risk scores
To assess the predictive accuracy of polygenic risk scores in an independent data set, SNP weights were assigned on the basis of the LMM (GCTA-LOCO) analysis in 18 of 27 strata. SNPs in high LD (r2 >0.5) in a 250-kb window were clumped. Subsequently, polygenic risk scores for cases and controls in the nine independent strata were calculated on the basis of their genotype dosages using PLINK v1.9. To obtain the Nagelkerke r2 and corresponding P values, these scores were then regressed on their true phenotype in a logistic regression where (on the basis of scree plots) the first three principal components, sex and stratum were included as covariates.
SNP-based heritability estimates
GCTA-REML
GRMs were calculated using GCTA software including genotype dosages passing quality control in all strata. On the basis of the diagonal of the GRM, individuals representing subpopulations that contained an abundance of rare alleles (diagonal values mean ±2 s.d.) were removed (Supplementary Fig. 14a). Pairs where relatedness (off-diagonal) exceeded 0.05 were removed as well (Supplementary Fig. 14b). The eigenvectors for the first ten principal components were included as fixed effects to account for more subtle population structure. The prevalence of ALS was defined as the lifetime morbid risk for ALS (that is, 1 in 400)21. To estimate the SNP-based heritability for all non-genome-wide-significant SNPs, the genotypes for the SNPs reaching genome-wide significance were modeled as fixed effects. The variance explained by the GRM therefore reflects the SNP-based heritability of all non-genome-wide-significant SNPs. SNP-based heritability partitioned by chromosome or MAF was calculated by including multiple GRMs, calculated on SNPs from each chromosome or in the respective frequency bin, in one model.
Haseman–Elston regression
The phenotype correlation–genotype correlation (PCGC) regression software package was used to calculate heritability on the basis of the Haseman–Elston regression including the eigenvectors for the first ten principal components as covariates. The prevalence was again defined as the lifetime morbid risk (1 in 400).
LD score regression
Summary statistics from GCTA-LOCO and LD scores calculated from European individuals in 1000 Genomes Project were used for LD score regression. Associated SNPs (P < 5 × 10−8) and variants not in HapMap 3 were excluded. Considering adequate correction for population structure and distant relatedness in the LMM, the intercept was constrained to 1.0 (ref. 12).
Biological pathway analysis (DEPICT)
Functional interpretation of associated GWAS loci was carried out using DEPICT, using locus definition based on 1000 Genomes Project Phase 1 data. This method prioritizes genes in the affected loci and predicts involved pathways, biological processes and tissues, using gene co-regulation data from 77,840 expression arrays. Three separate analyses were performed for GWAS loci reaching P = 1 × 10−4, P = 1 × 10−5 or P = 1 × 10−6. One thousand permutations were used for adjusting the nominal enrichment P values for biases and additionally 200 permutations were used for FDR calculation.
Supplementary Material
Acknowledgments
The work of the contributing groups was supported by various grants from governmental and charitable bodies. Details are provided in the Supplementary Note.
Footnotes
Accession codes. The GWAS summary statistics and sequenced variants are publicly available through the Project MinE data browser at http://databrowser.projectmine.com/.
Note: Any Supplementary Information and Source Data files are available in the online version of the paper.
AUTHOR CONTRIBUTIONS
A.V., N.T., K.L., B.R., K.V., M.R.-G., B.K., J.Z., L.L., L.D.G., S.M., F.S., V.M., M.d.C., S. Pinto, J.S.M., R.R.-G., M.P., S. Chandran, S. Colville, R.S., K.E.M., P.J.S., J.H., R.W.O., A. Pittman, K.S., P.F., A. Malaspina, S.T., S. Petri, S. Abdulla, C.D., M.S., T. Meyer, R.A.O., K.A.S., M.W.-P., C.L.-H., V.M.V.D., J.Q.T., L.E., L. McCluskey, A.N.B., Y.P., T. Meitinger, P.L., M.R.-B., C.R.A., C. Maurel, G. Bensimon, B.L., A.B., C.A.M.P., S.S.-D., A.D., N.W.W., L.T., W.L., A.F., M.R., S. Cichon, M.M.N., P.A., C. Tzourio, J.-F.D., A.G.U., F.R., K.E., A.H., C. Curtis, H.M.B., A.J.v.d.K., M.d.V., A.G., M.W., C.E.S., B.N.S., O.P., C. Cereda, R.D.B., G.P.C., S.D’A., C.B., G.S., L. Mazzini, V.P., C.G., C. Tiloca, A.R., A. Calvo, C. Moglia, M.B., S. Arcuti, R.C., C.Z., C.L., S. Penco, N.R., A. Padovani, M.F., B.M., R.J.S., PARALS Registry, SLALOM Group, SLAP Registry, FALS Sequencing Consortium, SLAGEN Consortium, NNIPPS Study Group, I.B., G.A.N., D.B.R., R.P., M.C.K., J.G., O.W.W., T.R., B.S., I.K., C.A.H., P.N.L., F.C., A. Chìo, E.B., E.P., R.T., G.L., J.P., A.C.L., J.H.W., W.R., P.V.D., L.F., T.P., R.H.B., J.D.G., J.E.L., O. Hardiman, P.M.A., P.C., P.V., V.S., M.A.v.E., A.A.-C., L.H.v.d.B. and J.H.V. were involved in phenotyping, sample collection and management. W.v.R., A.S., A.M.D., R.L.M., F.P.D., R.A.A.v.d.S., P.T.C.v.D., G.H.P.T., M.K., A.M.B., W.S., A.R.J., K.P.K., I.F., A.V., N.T., R.D.S., W.J.B., A.V., K.V., M.R.-G., B.K., L.L., S. Abdulla, K.S., E.P., F.P.D., J.M., C. Curtis, G. Breen, A.A.-C. and J.H.V. prepared DNA and performed SNP array hybridizations. W.v.R., S.L.P., K.P.K., K.L., A.M.D., P.T.C.v.D., G.H.P.T., K.R.v.E., P.I.W.d.B. and J.H.V. were involved in the next-generation sequencing analyses. W.v.R., K.R.v.E., A. Menelaou, P.I.W.d.B., A.A.-C. and J.H.V. performed the imputation. W.v.R., A.S., F.P.D., R.L.M., S.L.P., S.d.J., I.F., N.T., W.S., A.R.J., K.P.K., K.R.v.E., K.S., H.M.B., P.I.W.d.B., M.A.v.E., C.M.L., G. Breen, A.A.-C., L.H.v.d.B. and J.H.V. performed GWAS analyses. W.v.R., A.M.D., R.A.A.v.d.S., R.L.M., C.R.A., M.K., A.M.B., R.D.S., E.P.M., J.A.F., C. Tunca, H.H., K.Z., P.C., P.V. and J.H.V. performed the replication analyses. W.v.R., A.S., R.L.M., M.R.R., J.Y., N.R.W., P.M.V., C.M.L., A.A.-C. and J.H.V. performed polygenic risk scoring and heritability analyses. S.d.J., U.V., L.F., T.H.P., W.v.R., O. Harschnitz, G. Breen, R.J.P. and J.H.V. performed biological pathway analyses. U.V., L.F., W.v.R. and J.H.V. performed eQTL analyses. W.v.R., A.S., A.A.-C., L.H.v.d.B. and J.H.V. prepared the manuscript with contributions from all authors. A.A.-C., L.H.v.d.B. and J.H.V. directed the study.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- 2.Al-Chalabi A, et al. An estimate of amyotrophic lateral sclerosis heritability using twin data. J Neurol Neurosurg Psychiatry. 2010;81:1324–1326. doi: 10.1136/jnnp.2010.207464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Es MA, et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet. 2009;41:1083–1087. doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
- 4.Laaksovirta H, et al. Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol. 2010;9:978–985. doi: 10.1016/S1474-4422(10)70184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shatunov A, et al. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol. 2010;9:986–994. doi: 10.1016/S1474-4422(10)70197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9orf72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renton AE, et al. A hexanucleotide repeat expansion in C9orf72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogh I, et al. A genome-wide association meta-analysis identifies a novel locus at 17q11.2 associated with sporadic amyotrophic lateral sclerosis. Hum Mol Genet. 2014;23:2220–2231. doi: 10.1093/hmg/ddt587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genome of the Netherlands Consortium. Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat Genet. 2014;46:818–825. doi: 10.1038/ng.3021. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Zaitlen NA, Goddard ME, Visscher PM, Price AL. Advantages and pitfalls in the application of mixed-model association methods. Nat Genet. 2014;46:100–106. doi: 10.1038/ng.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Höglinger GU, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin DJ, et al. Myelin oligodendrocyte basic protein and prognosis in behavioral-variant frontotemporal dementia. Neurology. 2014;83:502–509. doi: 10.1212/WNL.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirulli ET, et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347:1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freischmidt A, et al. Haploinsufficiency of TBK1 causes familial ALS and frontotemporal dementia. Nat Neurosci. 2015;18:631–636. doi: 10.1038/nn.4000. [DOI] [PubMed] [Google Scholar]
- 17.Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–213. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- 18.Nicolae DL, et al. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6:e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramasamy A, et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014;17:1418–1428. doi: 10.1038/nn.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wray NR, et al. Pitfalls of predicting complex traits from SNPs. Nat Rev Genet. 2013;14:507–515. doi: 10.1038/nrg3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston CA, et al. Amyotrophic lateral sclerosis in an urban setting: a population based study of inner city London. J Neurol. 2006;253:1642–1643. doi: 10.1007/s00415-006-0195-y. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012;44:247–250. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pers TH, et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun. 2015;6:5890. doi: 10.1038/ncomms6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramakrishnan NA, Drescher MJ, Drescher DG. The SNARE complex in neuronal and sensory cells. Mol Cell Neurosci. 2012;50:58–69. doi: 10.1016/j.mcn.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:616–630. doi: 10.1038/nrneurol.2011.152. [DOI] [PubMed] [Google Scholar]
- 26.Lai CK, et al. Functional characterization of putative cilia genes by high-content analysis. Mol Biol Cell. 2011;22:1104–1119. doi: 10.1091/mbc.E10-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Peterson R, Turnbull J. Adenylyl cyclase type 3, a marker of primary cilia, is reduced in primary cell culture and in lumbar spinal cord in situ in G93A SOD1 mice. BMC Neurosci. 2011;12:71. doi: 10.1186/1471-2202-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krohn K, et al. Immunochemical characterization of a novel mitochondrially located protein encoded by a nuclear gene within the DFNB8/10 critical region on 21q22.3. Biochem Biophys Res Commun. 1997;238:806–810. doi: 10.1006/bbrc.1997.7352. [DOI] [PubMed] [Google Scholar]
- 29.Fang X, et al. The NEK1 interactor, C21orf2, is required for efficient DNA damage repair. Acta Biochim Biophys Sin (Shanghai) 2015;47:834–841. doi: 10.1093/abbs/gmv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vérièpe J, Fossouo L, Parker JA. Neurodegeneration in C elegans models of ALS requires TIR-1/Sarm1 immune pathway activation in neurons. Nat Commun. 2015;6:7319. doi: 10.1038/ncomms8319. [DOI] [PubMed] [Google Scholar]
- 31.Delaneau O, et al. Integrating sequence and array data to create an improved 1000 Genomes Project haplotype reference panel. Nat Commun. 2014;5:3934. doi: 10.1038/ncomms4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3 (Bethesda) 2011;1:457–470. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


