Summary
Rituximab-containing salvage chemotherapy followed by high-dose therapy and autologous stem cell transplant (ASCT) in chemosensitive patients remains the standard of care for patients with relapsed and refractory diffuse large B-cell lymphoma (DLBCL). However, its role in those patients achieving less than a complete response to first-line therapy (primary refractory disease) in the rituximab era is not well defined. We reviewed the outcomes of 82 transplant-eligible patients with primary refractory DLBCL who underwent salvage therapy with the intent of administering high-dose therapy and ASCT to patients achieving chemosensitive remission. The estimated 3-year overall and progression-free survival for all patients was 38% and 29%, respectively, and 65% and 60% respectively for patients proceeding to stem cell transplant. Long-term remission was achieved in 45% of patients achieving a partial response (PR) to initial induction therapy and <20% of patients with stable or progression of disease following initial therapy. These results suggest that salvage chemotherapy with the intent of subsequent high-dose therapy and ASCT remains a feasible strategy in certain patients with primary refractory DLBCL, particularly for those achieving a PR to frontline therapy. The primary barrier to curative therapy in patients with primary refractory disease is resistance to salvage therapy, and future studies should be aimed towards increasing the response rate in this population.
Keywords: lymphoma, DLBCL, refractory, transplant, rituximab
Approximately 10–15% of patients with diffuse large B-cell lymphoma DLBCL treated with R-CHOP [rituximab, cyclophosphamide, hydroxydaunomycin (doxorubicin), Oncovin (vincristine), prednisone] fail to achieve a complete response (CR) and have documented persistent disease at the completion of first-line therapy (Coiffier et al, 2002; Feugier et al, 2005). This has been referred to as primary refractory disease (Philip et al, 1987; Gribben et al, 1989; Vose et al, 1993, 2001; Kewalramani et al, 2000; Hamlin et al, 2003) although it has also been defined as patients achieving less than a PR to front-line therapy (Mills et al, 1995; Villela et al, 2001), patients achieving less than a PR to front-line therapy, or patients progressing within 3 months of completion of treatment (Telio et al, 2012; Hitz et al, 2015). The standard treatment for patients with DLBCL in first relapse is rituximab combined with salvage chemotherapy (ST). For those achieving either a PR or CR to ST, consolidation with high–dose therapy and autologous stem cell transplant (HDT/ASCT) is the standard of care (Philip et al, 1995). Data from two randomized studies and one registry study in the rituximab era suggest that 40–50% of patients are ultimately cured with this strategy (Vose et al, 2001; Gisselbrecht et al, 2010; Crump et al, 2014); however, a minority of patients in these studies had primary refractory disease. Earlier data demonstrated few long-term survivors amongst patients with primary refractory disease to CHOP (Philip et al, 1987; Villela et al, 2001; Martin et al, 2008; Hitz et al, 2015) with the benefit of HDT/ASCT restricted to the few patients who achieved a chemosensitive response to salvage chemotherapy (Stiff et al, 1998; Vose et al, 2001). A retrospective analysis of 85 transplant-eligible patients with primary refractory disease following CHOP chemotherapy who were treated at the Memorial Sloan Kettering Cancer Center (MSKCC) with ICE (ifosfamide, carboplatin, etoposide) chemotherapy demonstrated a 3-year event-free and overall survival of 25% and 22%, respectively (Kewalramani et al, 2000). The outcomes of transplant-eligible patients with primary refractory DLBCL in the rituximab era remain unknown. Furthermore, whether the outcomes of all patients who fail to achieve a CR to first-line therapy for DLBCL have equivalent outcomes with salvage therapy or whether outcomes vary depending on chemosensitivity to frontline therapy, has not been established.
We reviewed patients with a diagnosis of DLBCL between 2002 and 2014 and identified 82 patients with primary refractory disease, described as failure to achieve CR to front-line, rituximab- and anthracycline-containing regimens, and who were subsequently treated with ST with intent to consolidate with HDT/ASCT. We assessed outcomes for these patients, including progression-free (PFS), event-free (EFS) and overall survival (OS), and evaluated the effect of key clinicopathological characteristics, including the second-line International Prognostic Index (IPI) score, nature of response to primary therapy (primary partial responders or primary progressors), response to salvage therapy, and histological classification of DLBCL, on outcomes.
Materials and methods
Patients
Transplant-eligible patients with DLBCL that achieved less than a CR to initial rituximab and anthracycline-containing regimens between 2002 and 2014 were identified. Patients with histological diagnoses of DLBCL, grey zone lymphoma, B-cell lymphoma unclassifiable, and transformed low-grade lymphoma were included. We excluded patients with primary mediastinal large B-cell lymphoma, as these patients are known to have distinct disease biology and superior outcomes as compared with DLBCL-not otherwise specified (NOS) (Rosenwald et al, 2003; Dunleavy et al, 2013). Transplant eligibility was determined by the treating oncologist. Patients not treated with standard salvage platinum and anti-CD20 containing regimens and patients deemed ineligible for HDT/ASCT at the initiation of ST were excluded. Primary refractory disease was defined as a failure to achieve a CR with a front-line regimen containing rituximab and an anthracycline. Determination of refractory disease was based on radiographic (17%) or biopsy proven (83%) progression at the end of therapy; patients who had persistent disease documented by imaging rather than biopsy, had both clear radiographic progression of disease [defined as an increase in the size of old sites or new sites of disease by computed tomography (CT) or maximum standardized uptake value (SUVmax) > 10 on positron emission tomography (PET)] and also had sites of disease involvement inaccessible outside of an open surgical procedure. Patients with primary refractory disease were further sub-classified as either primary partial responders (PR to initial therapy) or primary progressors (minimal or no response to initial therapy); 86% of primary partial responders and 81% of primary progressors underwent biopsy to document refractory disease. Approval for this retrospective review was obtained from the Institutional Review and Privacy Board at MSKCC.
Eligibility for salvage therapy
All patients were staged according to standard Ann Arbor criteria (Lister et al, 1989). All patients had adequate cardiac function (left ventricular ejection fraction >50%) and renal function (serum creatinine ≤132·6 μmol/l or creatinine clearance ≥60 ml/min or higher) to undergo platinum-based chemotherapy.
Salvage chemotherapy and response criteria
ST was administered as previously described (Kewalramani et al, 2004; el Gnaoui et al, 2007; Gisselbrecht et al, 2010; Matasar et al, 2013; Crump et al, 2014). PET-CT was performed within 4 weeks of initiation of ST and either PET-CT or CT of the chest, abdomen, and pelvis was performed within 4 weeks of completion of the third cycle of ST. Response criteria were per the International Harmonization Project (IHP) when pre- and post- ST PET were available, otherwise by the sum of the product of the diameters (SPD) on CT scan as per the International Working Group (Cheson et al, 1999, 2007) and were compared to the reference imaging study performed following completion of first-line therapy.
High-dose therapy and autologous stem cell transplantation
Patients deemed to have chemosensitive disease by their treating oncologist were eligible for HDT/ASCT. Granulocyte colony-stimulating factor mobilized peripheral blood progenitor cells (PBPC) were typically collected on recovery from the third cycle of ST; a minority of patients required second mobilization attempts or the addition of plerixafor to augment stem cell yield. All patients undergoing HDT/ASCT were required to have adequate pulmonary function (diffusion capacity greater than 50% of predicted) and liver function (serum bilirubin level < 34·2 μmol/l). The choice of the conditioning regimen depended on the patient’s age, the extent of prior therapy, and the clinical trials active at the time of transplantation. Pre-transplant involved field radiotherapy (IFRT) was administered at the discretion of the primary physician, typically in the setting of refractory disease that was confined to a single radiation port.
Statistical analysis
The endpoints of interest, PFS, EFS and OS, were assessed from the first day of ST in the intention-to-treat cohort and day of transplant for the transplanted subset analysis. PFS was defined from the starting point until progression or death, and patients who were alive and progression-free at the end of the study period were censored at the date of last available follow-up. EFS was defined from the starting point until treatment failure, relapse, death from all causes, toxicity due to ST or HDT/ASCT, or secondary malignancy, whichever came first, and patients who did not experience any of these events were censored at the date of last follow-up. OS was defined from the starting point until death, and patients who did not die during the study period were censored at the date of last follow-up. All three endpoints were analysed using the Kaplan-Meier method and Cox proportional hazards models (Cox, 1972).
Factors known at the time of initiation of ST that were selected as possible prognostic indicators of PFS or OS included: age (younger than 60 years vs. 60 years or older), gender, histological classification (DLBCL-NOS vs. transformed vs. other), cell-of-origin [germinal centre B-cell (GCB) vs. non-GCB by Hans criteria (Hans et al, 2004)], primary treatment response (partial responders vs. primary progressors), Ann Arbor stage (I/II vs. III/IV), disease bulk (less than 5 cm vs. 5–10 cm. vs. more than 10 cm in longest axis), lactate dehydrogenase (LDH; normal vs. elevated), Karnofsky Performance Status (KPS; less than 80 vs. 80 or greater), number of extranodal sites of disease (1 or fewer vs. 2 or more), bone marrow involvement, second-line IPI score (low vs. low-intermediate vs. high-intermediate vs. high), and second line age-adjusted IPI (aaIPI; low vs. low-intermediate vs. high-intermediate/high (Lerner et al, 2007). Factors that were significant in univariate analysis (P < 0·05) were candidates for a multivariate model, which was created using a stepwise backward selection procedure (Mantel, 1966). Analysis of PET response to salvage therapy by IHP as a prognostic indicator of PFS or OS was analysed separately on the cohort of patients who underwent PET restaging. The overall response rate (ORR) was calculated as a proportion, including an exact 95% confidence interval (CI), and was compared with primary treatment response using a chi-squared test. All statistical tests were two-sided, and 5% was set as the level of significance. Statistical analyses were performed using R 3.2.0 (R Core Team 2015), including the ‘survival’ and ‘Hmisc’ packages.
Results
Patient characteristics
Pre-ST treatment characteristics of the 82 patients are shown in Tables I and II. Median age was 57 (range 25–73) years; 37% were older than 60 years and 61% were male. With respect to histology, 73% of patients had DLBCL-NOS, 13% had transformed low-grade B cell lymphoma, 7% had T-cell rich B-cell lymphoma, 4% had grey zone lymphoma and 2% had B-cell lymphoma unclassifiable. With respect to initial treatment response, 34% of patients were partial responders whereas 66% were primary progressors. An elevated LDH, KPS <80 and Ann Arbor stage III–IV disease were seen at relapse in 68%, 20% and 62% of patients, respectively. Bulky disease (>10 cm) and multiple (≥2) extranodal sites of disease were present in 17% and 43% of patients. An elevated second-line IPI (≥3) and aaIPI (≥3) were seen in 49% and 55% of patients, respectively.
Table I.
Patient characteristics before salvage chemotherapy.
| All patients | Primary partial responders | Primary progressors | |
|---|---|---|---|
| Total patients, | 82 | 28 | 54 |
| Age, years; median (range) | 57 (25–73) | ||
| >60 years | 30 (37%) | 5 (18%) | 25 (46%) |
| >70 years | 5 (6%) | 0 (0%) | 5 (9%) |
| Gender | |||
| Female | 32 (39%) | 11 (39%) | 21 (39%) |
| Male | 50 (61%) | 17 (61%) | 33 (61%) |
| Histology | |||
| DLBCL | 60 (73%) | 23 (82%) | 37 (69%) |
| Non-GCB subtype | 25 (30%) | 10 (36%) | 15 (28%) |
| GCB subtype | 35 (43%) | 13 (46%) | 22 (41%) |
| Transformed low-grade BCL | 11 (13%) | 3 (11%) | 8 (15%) |
| T cell-rich BCL | 6 (7%) | 1 (4%) | 5 (9%) |
| Grey zone lymphoma | 3 (4%) | 1 (4%) | 2 (4%) |
| BCL unclassifiable | 2 (2%) | 0 (0%) | 2 (4%) |
| Initial treatment | |||
| R-CHOP | 63 (77%) | 21 (75%) | 42 (78%) |
| R-EPOCH | 9 (11%) | 2 (7%) | 7 (13%) |
| R-CHOP+R-ICE | 4 (5%) | 2 (7%) | 2 (4%) |
| Other | 6 (7%) | 3 (11%) | 3 (6%) |
| Initial response | |||
| Partial responder | 28 (34%) | 28 (100%) | 0 (0%) |
| Primary progressor | 54 (66%) | 0 (0%) | 54 (100%) |
| Disease bulk at progression | |||
| <5 cm | 40 (49%) | 18 (64%) | 22 (41%) |
| 5–10 cm | 27 (33%) | 7 (25%) | 20 (37%) |
| >10 cm | 14 (17%) | 3 (11%) | 11 (20%) |
| BM involvement at progression | |||
| Yes | 7 (9%) | 1 (4%) | 6 (11%) |
| No | 57 (70%) | 23 (82%) | 34 (63%) |
| Not performed | 18 (22%) | 4 (14%) | 14 (25%) |
| Ann Arbor stage at progression | |||
| 1 | 7 (9%) | 2 (7%) | 5 (9%) |
| 2 | 24 (29%) | 10 (36%) | 14 (25%) |
| 3 | 4 (5%) | 3 (11%) | 1 (2%) |
| 4 | 47 (57%) | 13 (46%) | 34 (63%) |
| LDH at progression | |||
| Within normal limits | 26 (32%) | 11 (39%) | 15 (28%) |
| Elevated | 56 (68%) | 17 (61%) | 39 (72%) |
| KPS at relapse | |||
| 80+ | 66 (80%) | 26 (93%) | 40 (74%) |
| <80 | 16 (20%) | 2 (7%) | 14 (26%) |
| Extranodal sites at progression | |||
| 0 | 22 (27%) | 9 (32%) | 13 (24%) |
| 1 | 25 (30%) | 11 (39%) | 14 (26%) |
| 2+ | 35 (43%) | 8 (29%) | 27 (50%) |
| Second-line IPI | |||
| Low | 25 (30%) | 11 (39%) | 14 (26%) |
| Low-intermediate | 17 (21%) | 8 (29%) | 9 (17%) |
| High-intermediate | 25 (30%) | 8 (29%) | 17 (31%) |
| High | 15 (18%) | 1 (4%) | 14 (26%) |
| Second-line aaIPI | |||
| Low | 14 (17%) | 7 (25%) | 7 (13%) |
| Low-intermediate | 23 (28%) | 9 (32%) | 14 (26%) |
| High-intermediate | 35 (43%) | 10 (36%) | 25 (46%) |
| High | 10 (12%) | 2 (7%) | 8 (15%) |
All values are given as n (%) unless otherwise indicated.
aaIPI, age-adjusted International Prognostic Index; BCL, B cell lymphoma; BM, bone marrow; DLBCL, diffuse large B cell lymphoma; GCB, germinal centre B cell; IPI, International Prognostic Index; KPS, Karnofsky performance score; LDH, lactate dehydrogenase;R-CHOP, rituximab, cyclophosphamide, hydroxydaunomycin (doxorubicin), Oncovin (vincristine), prednisone; R-EPOCH, rituximab, etoposide, prednisone, Oncovin, cyclophosphamide, hydroxydaunomycin; R-ICE, rituximab, ifosfamide, carboplatin, etoposide.
Table II.
Salvage chemotherapy characteristics and response.
| All patients | Primary partial responders | Primary progressors | |
|---|---|---|---|
| Salvage therapy | |||
| R-ICE | 58 (71%) | 22 (79%) | 36 (67%) |
| R-DHAP | 6 (7%) | 2 (7%) | 4 (7%) |
| R-DHAX | 6 (7%) | 1 (4%) | 5 (9%) |
| O-DHAP | 9 (11%) | 2 (7%) | 7 (13%) |
| Other | 3 (4%) | 1 (4%) | 2 (4%) |
| Response to therapy | |||
| CR | 15 (18%) | 7 (25%) | 8 (15%) |
| PR | 31 (38%) | 12 (43%) | 19 (35%) |
| SD | 10 (12%) | 3 (11%) | 7 (13%) |
| PD | 26 (32%) | 6 (21%) | 20 (37%) |
All values are given as n (%).
CR, complete response; O-DHAP, ofatumumab, dexamethasone, high-dose cytarabine, cisplatin; PD, progressive disease; PR, partial response; R-DHAP, rituximab, dexamethasone, high-dose cytarabine, cisplatin; R-DHAX, rituximab, dexamethasone, high-dose cytarabine, oxaliplatin; R-ICE, rituximab, ifosfamide, carboplatin, etoposide; SD, stable disease.
Response to salvage chemotherapy
71% of patients received ICE and rituximab (R-ICE) and 26% received DHAP (dexamethasone, high-dose cytarabine, cisplatin) or DHAX (dexamethasone, high-dose cytarabine, oxaliplatin) chemotherapy in combination with rituximab or ofatumumab (Table II). Sixty-three (77%) of the 82 patients completed the total planned number of cycles of ST. Of the 19 patients who did not complete all planned cycles, 16 had progression of disease during therapy, usually detected by interim restaging CT scan performed after two cycles of ST. Three patients did not complete three cycles due to toxicity; one patient developed ifosfamide neurotoxicity and was switched from R-ICE to R-EPOCH (rituximab, etoposide, prednisone, Oncovin, cyclophosphamide, hydroxydaunomycin). Two patients suffered fatal infectious complications during ST (cytomegalovirus pneumonitis and septic shock). The ORR to ST was 56% (95% CI 45–67%), with 18% achieving a CR and 38% achieving a PR. Primary partial responders had a non-statistically significant higher ORR and CR rate as compared to primary progressors (ORR 68 vs. 50%, P = 0·19, CR 25 vs. 15%, P = 0·41). There was no difference in response rates between patients who received ofatumumab and rituximab, as has been previously reported (Matasar et al, 2013; van Imhoff et al, 2014). There was also no difference in response rates between histological subtypes.
PET scans were performed on 70 (85%) of patients. In patients in whom PET data was available, the ORR by PET was 70%, with 26% achieving a PET CR (defined as Deauville 1–3) and 44% achieving a PET PR (defined as Deauville 4). Primary partial responders had a non-statistically significant higher ORR and CR rate by PET as compared to primary progressors (ORR 80 vs. 64%, P = 0·27, CR 35 vs. 20%, P = 0·16).
HDT and ASCT
Thirty-seven (45%) patients proceeded to consolidative stem cell transplant; 33 of whom underwent ASCT and four patients underwent allogeneic stem cell transplant at the discretion of their providers. Of the patients undergoing ASCT, the overwhelming majority (88%) had chemosensitive disease with 27% achieving a CR and 61% achieving a PR. Four patients with stable disease proceeded to HDT/ASCT; all of these patients had improvement in disease burden but failed to meet the criteria for PR and all received radiotherapy to persistently involved sites sides prior to transplant. Thirteen patients who achieved a chemosensitive remission did not proceed to ASCT in first remission; 11 of these patients experienced rapid progression of disease between their restaging imaging study and admission for planned ASCT. One patient underwent allogeneic SCT following definitive IFRT to areas of chemorefractory disease, and in one patient ASCT was deferred due to poor functional status.
The characteristics and conditioning regimens for patients proceeding to HDT/ASCT are listed in Table III. The majority (97%) of patients received one of three conditioning regimens: BEAM (carmustine, etoposide, cytarabine, melphalan) (70%), CBV (cyclophosphamide, carmustine, etoposide) (8%), or IE (ifosfamide, etoposide, total body irradiation) (8%). Eighteen (55%) of the patients who underwent HDT/ASCT received hyperfractionated IFRT prior to transplant. There was one (2·7%) possible transplantation-related death, due to peri-transplant neutropenic sepsis with subsequent multi-organ failure. An additional three patients (total of 4, or 12%) of 33 patients died within 100 days of stem cell reinfusion, all due to diffuse progression of disease.
Table III.
Characteristics and conditioning regimens for patients who underwent HDT/ASCT.
| Patients | Primary partial responders | Primary progressors | |
|---|---|---|---|
| Total patients, n | 33 | 17 | 16 |
| Age, years; median (range) | 57 (25–72) | ||
| >60 years | 9 (27%) | 3 (18%) | 6 (38%) |
| >70 years | 2 (6%) | 0 (0%) | 2 (13%) |
| Response to therapy | |||
| CR | 9 (27%) | 6 (35%) | 3 (19%) |
| PR | 20 (61%) | 9 (53%) | 11 (69%) |
| SD | 4 (12%) | 2 (12%) | 2 (13%) |
| Hyperfractionated IFRT prior to ASCT | 18 (55%) | 12 (71%) | 6 (38%) |
| Conditioning regimen | |||
| BEAM | 26 (79%) | 12 (71%) | 14 (88%) |
| IE | 3 (9%) | 3 (18%) | 0 (0%) |
| CBV | 3 (9%) | 1 (6%) | 2 (13%) |
| Other | 1 (3%) | 1 (6%) | 0 (0%) |
All values are given as n (%) unless otherwise indicated.
ASCT, autologous stem cell transplantation; BEAM, BCNU (carmustine), etoposide, cytarabine, melphalan; IFRT, involved field radiotherapy; CBV-M, cyclophosphamide, BCNU (carmustine), etoposide; CR, complete response; HDT/ASCT, high dose therapy/autologous stem cell transplant; IE, ifosfamide, etoposide; PR, partial response; SD, stable disease.
Overall and progression-free survival
Median follow-up for survivors was 33 months. The estimated 3-year OS and PFS were 38% and 29% respectively (Fig 1). Primary partial responders to initial therapy had a statistically superior OS and PFS as compared to primary progressors (P = 0·006 for OS and 0·008 for PFS) (Fig 2); 49% of primary partial responders were progression free at 3 years compared to 17% of primary progressors. The PFS of patients differed significantly by response to salvage therapy as determined by PET; patients with a Deauville 1–3 response had an estimated 3-year PFS of 68%, as compared to 30% in patients with a Deauville 4 response and 0% in patients with a Deauville 5 response (P < 0·001) (Fig 3).
Fig 1.
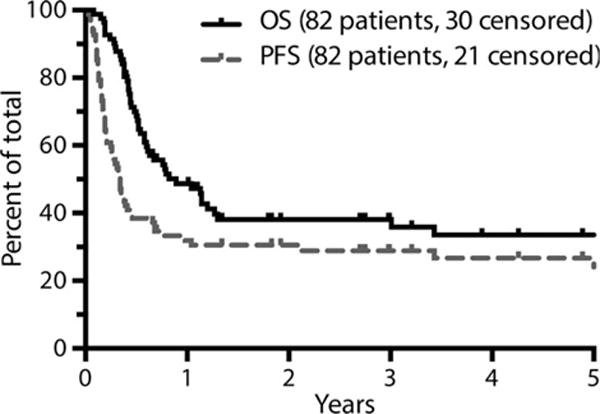
Survival analysis by intention-to-treat (N = 82). Figures are truncated at 5 years. OS, overall survival; PFS, progression-free survival.
Fig 2.
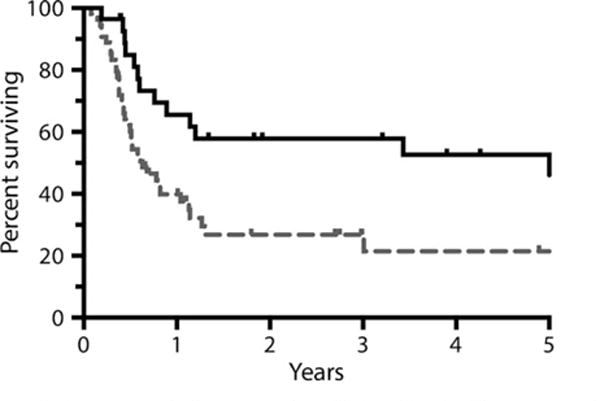
Overall survival by response to initial therapy (N = 82). Figure is truncated at 5 years.
Fig 3.

Progression-free survival as determined by PET response (N = 82). Figure is truncated at 5 years.
With respect to patients proceeding to ASCT, median follow-up for survivors from the date of SCT was 37 months. The estimated 3-year OS and PFS of patients proceeding to transplant was 65% and 60% respectively (Fig 4). There were insufficient events to perform a univariate or multivariate analysis of risk factors in the transplanted cohort. However, patients with early stage disease who received pre-ASCT IFRT had a superior PFS to those who did not receive IFRT (data not shown).
Fig 4.
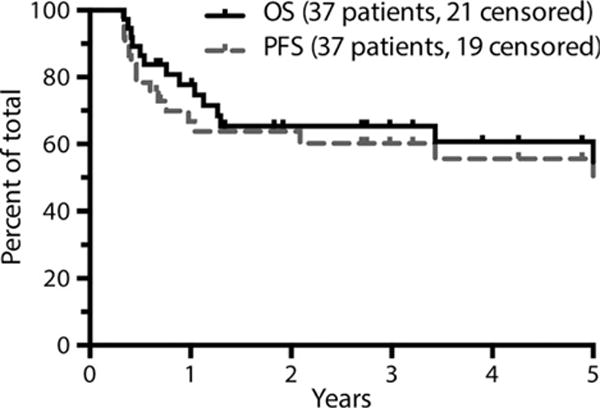
Survival analysis of patients who underwent transplantation (N = 37). Figure is truncated at 5 years. OS, overall survival; PFS, progression-free survival.
Nine of 45 patients who did not undergo consolidative transplant after first ST are alive and two remain free from disease progression at the time of last follow-up. Only two patients who did not proceed to HDT/ASCT following first ST remain alive after 2 years of follow-up. One patient achieved a chemosensitive remission to third-line R-EPOCH and IFRT following progression of disease to R-ICE therapy and subsequently underwent HDT/ASCT. The other patient failed to collect adequate stem cells for HDT/ASCT. He was treated with IFRT to the right adrenal gland and relapsed 7 months later in the central nervous system.
Prognostic factors
Within the ITT cohort, univariate analysis revealed that age ≥60 years, primary progressive disease, bulk >10 cm (vs. <5 cm), elevated LDH, KPS <80, multiple extranodal sites of disease, and an elevated second-line IPI were associated with inferior PFS. With respect to histological subtype, there was no difference between GCB and non-GCB subtypes of DLBCL with respect to PFS. There was no association between PFS with gender, stage, or bone marrow involvement. Multivariate analysis revealed KPS <80 and an elevated LDH to be independently associated with PFS; patients with either or both of these risk factors had a hazard ratio of 3·01 and 4·52 for disease progression, respectively, as compared to patients with neither of these risk factors (Fig 5; Table IV). The independent association of primary progressive disease with PFS approached statistical significance in a multivariate analysis (hazard ratio 1·82 versus partial responders, P = 0·057).
Fig 5.
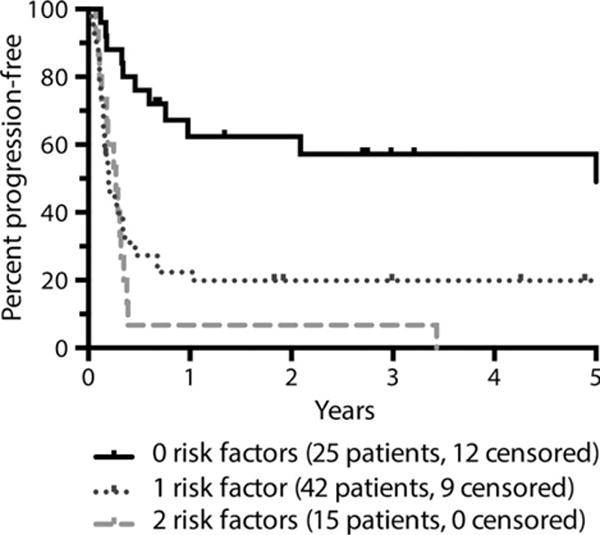
Progression-free survival as determined by presence or absence of impaired Karnofsky performance score and/or elevated lactate dehydrogenase (N = 82). Figure is truncated at 5 years. RF, risk factor.
Table IV.
Number of risk factors associated with PFS in multivariate analysis (N = 82).
| Factor | HR | 95% CI |
|---|---|---|
| Number of PFS risk factors (ref = 0, N = 25) | ||
| (KPS <80 or LDH elevated, N = 42) | 3·01 | (1·57, 5·76) |
| (KPS <80 and LDH elevated, N = 15) | 4·52 | (2·09, 9·79) |
95% CI, 95% confidence interval; HR, Hazard ratio; KPS, Karnofsky performance score; LDH, lactate dehydrogenase; PFS, progression-free survival.
Discussion
Optimal management of patients with primary refractory DLBCL has remained uncharacterized for a variety of reasons. There are no randomized trials restricted to patients with primary refractory DLBCL; all prospective studies have included patients with both relapsed and refractory disease. The definition of primary refractory disease has been variable, with most retrospective analyses including both patients with true refractory disease and patients with early relapse. Finally, the efficacy of HDT/ASCT in patients with primary refractory disease remains unclear. Prior studies, including results from the Autologous Blood and Marrow Transplant Registry (ABMTR) and the Southwest Oncology Group (SWOG) have suggested that HDT/ASCT may be beneficial in patients with chemosensitive disease (Stiff et al, 1998; Vose et al, 2001), but the patients in these studies did not receive rituximab as part of front-line therapy.
In our cohort of patients with primary refractory DLBCL, primary refractory was strictly defined as patients failing to achieve CR to first-line anthracycline-based chemoimmunotherapy and having either primary partial response or primary progression. The ORR to salvage regimens of was 56% with a CR rate of 18%. This is comparable to published subgroup analyses of response rates in patients with refractory disease or early relapse; for example, in the Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study, the overall response rate in patients with refractory or early relapsed (<12 months) disease was 46% (Gisselbrecht et al, 2010).
Approximately 38% of our patients with primary refractory disease are alive, and 29% are progression-free 3 years from initiation of ST. Our outcomes are somewhat favourable compared to existing data regarding patients with primary refractory DLBCL in the rituximab era. However, our cohort includes only patients treated with standard ST and intent to proceed to autologous transplant in chemosensitive patients, whereas most studies showing a comparatively poor outcome in primary refractory disease included patients treated with palliative intent (Villela et al, 2001; Vose et al, 2001; Telio et al, 2012; Rovira et al, 2015).
Our data demonstrate that outcomes of certain patients who are able to tolerate salvage therapy are similar to patients with relapsed disease. In particular, patients with a PR or better to front-line therapy had outcomes similar to historical relapsed patients, with about 45% achieving long-term remissions. Conversely, patients with primary progressive disease were cured in less than 20% of cases; this result is similar to outcomes of patients in the CORAL study with relapsed disease less than 12 months following rituximab-containing initial regimens (Gisselbrecht et al, 2010). Our data from patients undergoing restaging PET-CT after salvage chemotherapy suggests that patients who achieve a PET CR to first salvage are cured in nearly 70% of cases whereas only 30% of PET-avid chemosensitive patients achieve long-term remissions. These results are similar to data in the relapsed setting in which nearly 80% of PET-negative and 50% of PET-avid chemosensitive patients were cured with a similar strategy (Sauter et al, 2015).
Our data further suggest that HDT/ASCT is a safe and effective consolidation strategy in this population. Patients with chemosensitive disease who proceeded to HDT/ASCT had a 3-year OS of 65% and PFS of 60%, with only one transplant-related death. Our results are consistent with a recent report suggesting that early failure of frontline rituximab-based chemoimmunotherapy did not predict futility of ASCT, with a 3-year OS of 59% (Hamadani et al, 2014). Our rate of long-term disease control without significant transplant-related morbidity compares favourably with reduced-intensity conditioning allogeneic transplant, which, in a similar setting, resulted in a 3-year OS of 38% but was accompanied by grade 2 or higher GVHD in more than 40% of cases (Glass et al, 2014). Of note, the outcomes of patients who did not achieve a remission-adequate response to first ST and proceed to transplant were uniformly poor. These results are consistent with the overall poor outcomes of patients who do not respond to second-ST, with median OS of well less than 1 year (Elstrom et al, 2010) and few long-term survivors (Ardeshna et al, 2005), and differs from patients with relapsed disease, many of whom can be cured with third-line salvage chemotherapy and ASCT (van den Neste et al, 2016). Our data strongly suggest that patients with primary refractory disease who do not enjoy an adequate response to first ST are unlikely to achieve a chemosensitive remission with conventional strategies and underscores the need for novel therapies aimed at improving the response rate to first ST in this population. However, our data also demonstrates that patients with a PR or better to front-line therapy have outcome similar to those with relapsed disease and should not be excluded from any clinical trials enrolling relapsed patients.
A number of univariate factors were prognostically significant in our cohort. Patients with a PR to induction therapy, as well as younger patients and patients with a low secondary IPI, demonstrated favourable outcomes, whereas elderly patients and patients with an elevated second-line IPI had comparatively poor outcomes. This is similar to patients with relapsed DLBCL, in whom the second-line age-adjusted IPI is associated with long-term outcomes, largely through its association with response to ST (Hamlin et al, 2003). Elderly patients with an elevated second-line IPI (and particularly those with an elevated LDH) derive limited benefit from conventional strategies and may benefit from novel therapeutic approaches. Notably, cell of origin was not found to be associated with outcomes, consistent with its lack of prognostic value in relapsed disease (Moskowitz et al, 2005; Costa et al, 2008).
In summary, this is the largest reported series of patients with primary refractory DLBCL treated with curative intent in the rituximab era. ST with consolidative ASCT leads to a durable remission in about 45% of patients with a PR to front-line therapy, but in fewer than 20% of patients with primary progressive disease. Patients with chemosensitive disease proceeding to ASCT achieve long-term remission in 60% of cases. This data suggests that transplant-eligible patients with DLBCL and a PR or better to frontline therapy should be treated with standard salvage regimens and consolidation with HDT/ASCT in patients with chemosensitive disease, as their outcomes are similar to patients with relapsed disease. Patients with primary progressive DLBCL are less chemosensitive to conventional ST and we would recommend investigational strategies in this population to increase the likelihood of proceeding to transplant. Patients with an elevated LDH or poor performance status also respond poorly to conventional ST and investigational approaches may also be more appropriate in this setting.
Acknowledgments
SV and CHM designed the study, analysed the data and wrote the manuscript draft. SV and NG collected the data for analysis. Statistical analysis was performed by KMW and ZZ. CSS, MJM and AJD critically reviewed the manuscript drafts, approved the final version, and made the decision to submit the manuscript for publication. The research of SV was partially supported by an NIH Training Grant T32-CA009207-36A1. The research of KMW and ZZ was partly supported by an NIH Core Grant P30 CA008748.
Footnotes
No authors declare competing financial interests.
References
- Ardeshna KM, Kakouros N, Qian W, Powell MG, Saini N, D’Sa S, Mackinnon S, Hoskin PJ, Goldstone AH, Linch DC. Conventional second-line salvage chemotherapy regimens are not warranted in patients with malignant lymphomas who have progressive disease after first-line salvage therapy regimens. British Journal of Haematology. 2005;130:363–372. doi: 10.1111/j.1365-2141.2005.05603.x. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised response criteria for malignant lymphoma. Journal of Clinical Oncology. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, van den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. New England Journal of Medicine. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Costa LJ, Feldman AL, Micallef IN, Inwards DJ, Johnston PB, Porrata LF, Ansell SM. Germinal center B (GCB) and non-GCB cell-like diffuse large B cell lymphomas have similar outcomes following autologous haematopoietic stem cell transplantation. British Journal of Haematology. 2008;142:404–412. doi: 10.1111/j.1365-2141.2008.07207.x. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B-Statistical Methodology. 1972;34:187–220. [Google Scholar]
- Crump M, Kuruvilla J, Couban S, Macdonald DA, Kukreti V, Kouroukis CT, Rubinger M, Buckstein R, Imrie KR, Federico M, di Renzo N, Howson-Jan K, Baetz T, Kaizer L, Voralia M, Olney HJ, Turner AR, Sussman J, Hay AE, Djurfeldt MS, Meyer RM, Chen BE, Shepherd LE. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. Journal of Clinical Oncology. 2014;32:3490–3496. doi: 10.1200/JCO.2013.53.9593. [DOI] [PubMed] [Google Scholar]
- Dunleavy K, Pittaluga S, Maeda LS, Advani R, Chen CC, Hessler J, Steinberg SM, Grant C, Wright G, Varma G, Staudt LM, Jaffe ES, Wilson WH. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. New England Journal of Medicine. 2013;368:1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrom RL, Martin P, Ostrow K, Barrientos J, Chadburn A, Furman R, Ruan J, Shore T, Schuster M, Cerchietti L, Melnick A, Coleman M, Leonard JP. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clinical Lymphoma, Myeloma & Leukemia. 2010;10:192–196. doi: 10.3816/CLML.2010.n.030. [DOI] [PubMed] [Google Scholar]
- Feugier P, van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, Christian B, Lepage E, Tilly H, Morschhauser F, Gaulard P, Salles G, Bosly A, Gisselbrecht C, Reyes F, Coiffier B. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. Journal of Clinical Oncology. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht C, Glass B, Mounier N, Gill SD, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, Briere J, Moskowitz CH, Schmitz N. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of Clinical Oncology. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass B, Hasenkamp J, Wulf G, Dreger P, Pfreundschuh M, Gramatzki M, Silling G, Wilhelm C, Zeis M, Gorlitz A, Pfeiffer S, Hilgers R, Truemper L, Schmitz N. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncology. 2014;15:757–766. doi: 10.1016/S1470-2045(14)70161-5. [DOI] [PubMed] [Google Scholar]
- el Gnaoui T, Dupuis J, Belhadj K, Jais JP, Rahmouni A, Copie-Bergman C, Gaillard I, Divine M, Tabah-Fisch I, Reyes F, Haioun C. Rituximab, gemcitabine and oxaliplatin: an effective salvage regimen for patients with relapsed or refractory B-cell lymphoma not candidates for high-dose therapy. Annals of Oncology. 2007;18:1363–1368. doi: 10.1093/annonc/mdm133. [DOI] [PubMed] [Google Scholar]
- Gribben JG, Goldstone AH, Linch DC, Taghipour G, McMillan AK, Souhami RL, Earl H, Richards JD. Effectiveness of high-dose combination chemotherapy and autologous bone marrow transplantation for patients with non-Hodgkin’s lymphomas who are still responsive to conventional-dose therapy. Journal of Clinical Oncology. 1989;7:1621–1629. doi: 10.1200/JCO.1989.7.11.1621. [DOI] [PubMed] [Google Scholar]
- Hamadani M, Hari PN, Zhang Y, Carreras J, Akpek G, Aljurf MD, Ayala E, Bachanova V, Chen AI, Chen YB, Costa LJ, Fenske TS, Freytes CO, Ganguly S, Hertzberg MS, Holmberg LA, Inwards DJ, Kamble RT, Kanfer EJ, Lazarus HM, Marks DI, Nishihori T, Olsson R, Reddy NM, Rizzieri DA, Savani BN, Solh M, Vose JM, Wirk B, Maloney DG, Smith SM, Montoto S, Saber W, Alpdogan O, Cashen A, Dandoy C, Finke R, Gale R, Gibson J, Hsu JW, Janakiraman N, Laughlin MJ, Lill M, Cairo MS, Munker R, Rowlings PA, Schouten HC, Shea TC, Stiff PJ, Waller EK. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2014;20:1729–1736. doi: 10.1016/j.bbmt.2014.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D, Noy A, Portlock CS, Straus DJ, Yahalom J, Nimer SD, Moskowitz CH. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102:1989–1996. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Hitz F, Connors JM, Gascoyne RD, Hoskins P, Moccia A, Savage KJ, Sehn LH, Shenkier T, Villa D, Klasa R. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Annals of Hematology. 2015;94:1839–1843. doi: 10.1007/s00277-015-2467-z. [DOI] [PubMed] [Google Scholar]
- van Imhoff GW, McMillan A, Matasar MJ, Radford J, Ardeshna KM, Kuliczkowski K, Kim W, Hong XN, Goerloev JS, Davies A, Barrigon MDC, Ogura M, Fennessy M, Liao QM, van der Holt B, Lisby S, Lin TS, Hagenbeek A. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the Orcharrd Study (OMB110928) Blood. 2014;124:630. doi: 10.1200/JCO.2016.69.0198. [DOI] [PubMed] [Google Scholar]
- Kewalramani T, Zelenetz AD, Hedrick EE, Donnelly GB, Hunte S, Priovolos AC, Qin J, Lyons NC, Yahalom J, Nimer SD, Moskowitz CH. High-dose chemoradiotherapy and autologous stem cell transplantation for patients with primary refractory aggressive non-Hodgkin lymphoma: an intention-to-treat analysis. Blood. 2000;96:2399–2404. [PubMed] [Google Scholar]
- Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, O’Connor O, Filippa DA, Teruya-Feldstein J, Gencarelli A, Qin J, Waxman A, Yahalom J, Moskowitz CH. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- Lerner RE, Thomas W, Defor TE, Weisdorf DJ, Burns LJ. The International Prognostic Index assessed at relapse predicts outcomes of autologous transplantation for diffuse large-cell non-Hodgkin’s lymphoma in second complete or partial remission. Biology of Blood and Marrow Transplantation. 2007;13:486–492. doi: 10.1016/j.bbmt.2006.12.452. [DOI] [PubMed] [Google Scholar]
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, Rosenberg SA, Coltman CA, Tubiana M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. Journal of Clinical Oncology. 1989;7:1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–170. [PubMed] [Google Scholar]
- Martin A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, Andreu R, Salar A, Garcia-Sanchez P, Vazquez L, Nistal S, Requena MJ, Donato EM, Gonzalez JA, Leon A, Ruiz C, Grande C, Gonzalez-Barca E, Caballero MD. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93:1829–1836. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]
- Matasar MJ, Czuczman MS, Rodriguez MA, Fennessy M, Shea TC, Spitzer G, Lossos IS, Kharfan-Dabaja MA, Joyce R, Fayad L, Henkel K, Liao Q, Edvardsen K, Jewell RC, Fecteau D, Singh RP, Lisby S, Moskowitz CH. Ofatumumab in combination with ICE or DHAP chemotherapy in relapsed or refractory intermediate grade B-cell lymphoma. Blood. 2013;122:499–506. doi: 10.1182/blood-2012-12-472027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin’s lymphoma. Journal of Clinical Oncology. 1995;13:588–595. doi: 10.1200/JCO.1995.13.3.588. [DOI] [PubMed] [Google Scholar]
- Moskowitz CH, Zelenetz AD, Kewalramani T, Hamlin P, Lessac-Chenen S, Houldsworth J, Olshen A, Chaganti R, Nimer S, Teruya-Feldstein J. Cell of origin, germinal center versus nongerminal center, determined by immunohistochemistry on tissue microarray, does not correlate with outcome in patients with relapsed and refractory DLBCL. Blood. 2005;106:3383–3385. doi: 10.1182/blood-2005-04-1603. [DOI] [PubMed] [Google Scholar]
- van den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, Milpied N, Radford J, Ketterer N, Shpilberg O, Duhrsen U, Ma D, Briere J, Thieblemont C, Salles G, Moskowitz CH, Glass B, Gisselbrecht C. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplantation. 2016;51:51–57. doi: 10.1038/bmt.2015.213. [DOI] [PubMed] [Google Scholar]
- Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, Colombat P, Goldstone AH, Gorin NC, Flesh M, Laporte JP, Maraninchi D, Pico J, Bosly A, Anderson C, Schots R, Biron P, Cabanillas F, Dicke K. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. New England Journal of Medicine. 1987;316:1493–1498. doi: 10.1056/NEJM198706113162401. [DOI] [PubMed] [Google Scholar]
- Philip T, Guglielmi C, Hagenbeek A, Somers R, van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL, Coiffier B, Biron P, Mandelli F, Chauvin F. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. New England Journal of Medicine. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. Available at: http://www.R-project.org/[Accessed [Accessed 23 February 2016]. [Google Scholar]
- Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, Leblanc M, Chiorazzi M, Zhao H, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Staudt LM. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. Journal of Experimental Medicine. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira J, Valera A, Colomo L, Setoain X, Rodriguez S, Martinez-Trillos A, Gine E, Dlouhy I, Magnano L, Gaya A, Martinez D, Martinez A, Campo E, Lopez-Guillermo A. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Annals of Hematology. 2015;94:803–812. doi: 10.1007/s00277-014-2271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, Hilden P, Devlin SM, Zelenetz AD, Moskowitz CH. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015;125:2579–2581. doi: 10.1182/blood-2014-10-606939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiff PJ, Dahlberg S, Forman SJ, McCall AR, Horning SJ, Nademanee AP, Blume KG, Leblanc M, Fisher RI. Autologous bone marrow transplantation for patients with relapsed or refractory diffuse aggressive non-Hodgkin’s lymphoma: value of augmented preparative regimens–a Southwest Oncology Group trial. Journal of Clinical Oncology. 1998;16:48–55. doi: 10.1200/JCO.1998.16.1.48. [DOI] [PubMed] [Google Scholar]
- Telio D, Fernandes K, Ma C, Tsang R, Keating A, Crump M, Kuruvilla J. Salvage chemotherapy and autologous stem cell transplant in primary refractory diffuse large B-cell lymphoma: outcomes and prognostic factors. Leukaemia & Lymphoma. 2012;53:836–841. doi: 10.3109/10428194.2011.643404. [DOI] [PubMed] [Google Scholar]
- Villela L, Lopez-Guillermo A, Montoto S, Rives S, Bosch F, Perales M, Ferrer A, Esteve J, Colomo L, Campo E, Montserrat E. Prognostic features and outcome in patients with diffuse large B-cell lymphoma who do not achieve a complete response to first-line regimens. Cancer. 2001;91:1557–1562. [PubMed] [Google Scholar]
- Vose JM, Anderson JR, Kessinger A, Bierman PJ, Coccia P, Reed EC, Gordon B, Armitage JO. High-dose chemotherapy and autologous hematopoietic stem-cell transplantation for aggressive non-Hodgkin’s lymphoma. Journal of Clinical Oncology. 1993;11:1846–1851. doi: 10.1200/JCO.1993.11.10.1846. [DOI] [PubMed] [Google Scholar]
- Vose JM, Zhang MJ, Rowlings PA, Lazarus HM, Bolwell BJ, Freytes CO, Pavlovsky S, Keating A, Yanes B, van Besien K, Armitage JO, Horowitz MM, Autologous B. Autologous transplantation for diffuse aggressive non-Hodgkin’s lymphoma in patients never achieving remission: a report from the Autologous Blood and Marrow Transplant Registry. Journal of Clinical Oncology. 2001;19:406–413. doi: 10.1200/JCO.2001.19.2.406. [DOI] [PubMed] [Google Scholar]


