Summary
The bar is high to improve on current combination antiretroviral therapy (ART), now highly effective, safe, and simple. However antibodies that bind the HIV envelope are able to uniquely target the virus as it seeks to enter new target cells, or as it is expressed from previously infected cells. Further, the use of antibodies against HIV as a therapeutic may offer advantages. Antibodies can have long half-lives, and are being considered as partners for long-acting antiretrovirals for use in therapy or prevention of HIV infection. Early studies in animal models and in clinical trials suggest that such antibodies can have antiviral activity but, as with small molecule antiretrovirals, the issues of viral escape and resistance will have to be addressed.
Most promising, however, are the unique properties of anti-HIV antibodies: the potential ability to opsonize viral particles, to direct antibody-dependent cellular cytotoxicity (ADCC) against actively infected cells, and ultimately the ability to direct the clearance of HIV-infected cells by effector cells of the immune system. These distinctive activities suggest that HIV antibodies and their derivatives may play an important role in the next frontier of HIV therapeutics, the effort to develop treatments that could lead to an HIV cure.
Keywords: HIV, monoclonal antibodies, ADCC, entry inhibition, cure
HIV replication and current antiretroviral therapy
An examination of the lifecycle of HIV-1 informs the discussion of new approaches to antiretroviral therapy (ART), in the context of the array of small molecule inhibitors that already provide remarkably effective treatment for HIV-1 infection. HIV infection is characterized by cycles of virus production and reinfection, a process that occurs optimally within activated CD4+ T lymphocytes. Viral expression begins with the transcription and translation of early, regulatory viral gene products from the integrated proviral genome within the infected cell. This leads to a cascade of expression of late, structural viral proteins, and the assembly and budding of infectious viral particles (1).
Virions then spread within the host to infect new, susceptible target cells. The process of infection can occur directly by cell-to-cell spread in vitro, but how often this occurs in vivo is unknown (2). Predominantly, HIV particles enter new host cells via direct contact across the “immunological synapse” of an infected cell apposed to a target cell, or once the budded virion has travelled free from the producer cell. In either case, the HIV particle first engages the CD4 receptor in a weak interaction with the HIV envelope glycoprotein that docks the virion at the target cell. Then a second interaction of HIV envelope with a cellular chemokine receptor, principally the CCR5 receptor, induces a conformation shift in the HIV envelope structure that allows a fusion event to occur between the viral and cellular membranes (3).
Viral fusion with the target cell membrane then allows the deposition of the viral nucleocapsid within the newly infected cell, which delivers the HIV genome in its RNA form, along with molecules of HIV reverse transcriptase (RT) and integrase, in the cellular cytoplasm. HIV RT then co-opts cellular nucleosides and directs transcription of viral RNA into double-stranded linear DNA copies of the HIV genome. Viral integrase then forms a pre-integration complex with the HIV DNA and travels through nuclear pores to find a site for integration into the host genome. This is a vulnerable time for the infection process, as reverse transcription of the HIV genome must be complete and accurate in the face of the host apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (ApoBEC) factors, which induce hypermutation of the incoming viral RNA genome as it is reversed transcribed in to viral DNA. Further, the new viral DNA genome must rapidly achieve successful integration before it falls prey to degradation by host nucleases, or anneals in an auto-ligation event that recreates a dead-end circular viral genome.
HIV infection is then irreversibly established within the new target cell by a surviving viral genome that has entered the host genome without being marked by lethal hypermutation. Viral gene expression may then proceed in the cascade that leads to production of a new swarm of virions, or the viral genome may lapse in to a state of latency. Overall, while the establishment of durable viral latency is a rare event, and robust viral expression may ensue in most cells immediately following infection, a significant number of newly infected cells—perhaps those infected while in a less activated state—may express viral particles after a delay or over a prolonged period of time (4).
Viral replication leads, directly or indirectly, to the loss of CD4+ T cells and immune dysfunction. Poised to interrupt this relentless process that gradually leads to fatal immunodeficiency in most infected humans, is an arsenal of more than three dozen Food and Drug Administration–approved drugs and co-formulations available for treatment of HIV-1 infection. These small molecule antiretroviral drugs can be divided into classes based on their antiviral molecular mechanism: (1) nucleoside-analog and non–nucleoside-analog reverse transcriptase inhibitors (NRTIs and NNRTIs), (2) integrase inhibitors, (3) protease inhibitors, (4) fusion inhibitors, and (5) coreceptor antagonists. The vast majority of HIV-infected people on ART around the world receive combination therapy using drugs from the first three classes that target the viral RT, integrase, or protease enzymes. These widely used inhibitors target the HIV lifecycle after viral entry and before viral particles are expressed (NNRTIs, NRTIs, and integrase and protease inhibitors).
Once combination ART (cART) is employed in a combination potent enough to ablate ongoing viral replication and prevent the emergence of drug resistance, an event documented clinically by the suppression of plasma viremia to fewer than 50 copies of HIV-1 RNA/µl, immune recovery often ensues, and AIDS-related clinical events become rare. The ongoing implementation of ART throughout the world has been one of the greatest success stories of modern medicine. However, current ART has burdens that include the cost of drugs and medical care, adherence to lifelong therapy, the potential for the emergence of drug-resistant HIV if adherence is not maintained, and the potential long-term toxicities of small-molecule drugs.
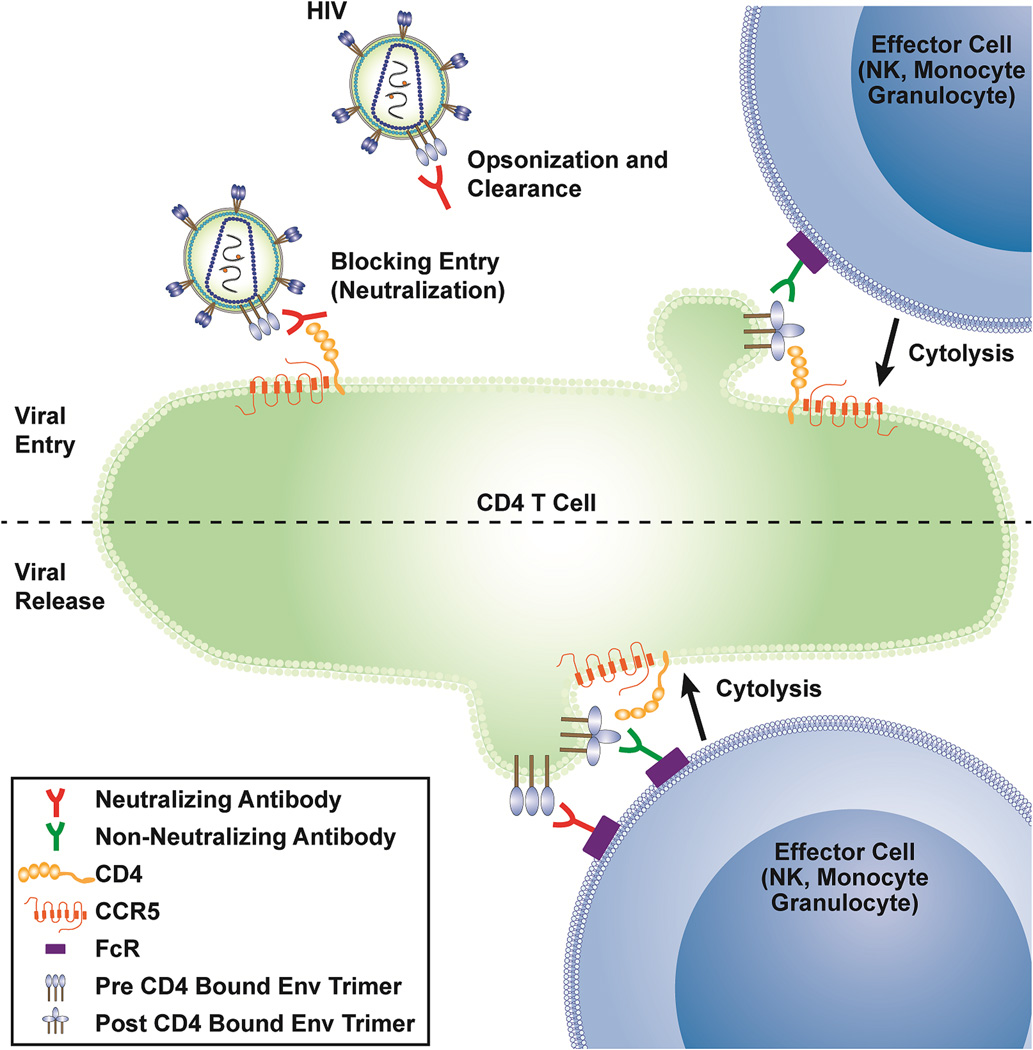
It is in this context that we may consider the possible benefits and challenges of humoral ART using broadly neutralizing anti-HIV antibodies (bnAbs). Therapy with bnAbs uniquely targets the viral lifecycle at its two poles: viral entry and viral particle expression (Figure 1). Of note, these two stages of HIV-1 lifecycle can be targeted by monoclonal antibodies (mAbs) recognizing the CD4 inducible (CD4i) constant region 1 and 2 (C1/C2) and the gp41 cluster I region that are non-neutralizing Abs (non-nAb) and that will be further described below. Therefore, both bnAbs and non-nAbs can be used to generate molecules that can target infected cells. While fusion inhibitors and coreceptor antagonists block viral entry, these drugs have thus far found limited use in the clinic. Fusion inhibitors have not gained traction in the clinic largely due to manufacturing cost and the challenges of frequent subcutaneous injection required for their use. Coreceptor antagonists, mAbs, and mAb-based molecules share the potential benefit of blocking infection at an early stage, which prevents the transcription of viral DNA or viral proteins within the target cells, though clinical data has yet to bear this out.
Figure 1. Potential antiviral activity of neutralizing and non-neutralizing antibodies.
Shown are potential sites of activity within the life cycle of HIV for neutralizing antibodies (opsonization or neutralization of free virus, ADCC of cells expressing HIV envelope during virus budding) or non-neutralizing antibodies (ADCC of cells during virus entry after envelope binding to CD4, ADCC of cells expressing HIV envelope during virus budding - if CD4 is encountered).
As biologics, mAbs-based therapeutics might spare HIV-infected patients the risk of long-term end-organ toxicities, although antibody therapy may be accompanied by long-term risks as yet unappreciated. Further, as mAbs and their derivatives may be engineered to have very durable pharmacokinetic profiles, or even be delivered via cellular or genetic therapies, they have the potential to ameliorate the challenges of lifelong adherence to therapy administration (5). However, as will be discussed in detail, bnAbs will have to contend with the capacity of HIV to evolve resistance to therapeutic agents, in this case via the same plasticity of HIV envelope structure that allows the virus to escape the antiviral immune response. So, like standard antiretrovirals, mAbs will most likely have to be administered as carefully tested, effective combinatorial therapy.
Persistent HIV infection despite effective ART
HIV-1 infection remains incurable despite the maintenance of viremia suppression, the prevention of drug resistance, and durable clinical benefits of standard ART. Persistent HIV infection despite ART is also marked by mild but persistent abnormalities of the immune system, and bnAbs might contribute to efforts to eradicate infection, or more fully resolve the effects of HIV infection on immune function.
Latent and persistent infection by a small population of quiescent, replication-competent proviruses is founded within a long-lived population of memory T cells, capable of reigniting new rounds of infection if therapy is interrupted. This latent pool of virus is established within days of infection and is unaffected by the antiviral immune response or current therapy (6,7). The latent pool is maintained in part by the quiescent state of the infected cell, the enforcement of proviral latency by host cellular factors, and the long lifespan of such cells. Homeostatic proliferation of latently infected cells may also contribute to latent infection (8,9). HIV DNA integrants are enriched in or near host genes associated with cell cycle control, suggesting that the integration of HIV-1 into such sites could lead to proliferation of these latently infected cells (10,11). Although one study examining a limited number of integrants suggests that these proliferating integrants are defective (12), a recent clinical observation calls this conclusion into question (13). Should rare latently infected cells transiently display viral proteins while undergoing proliferation, anti-HIV antibodies with antibody-dependent cell-mediated cytotoxic (ADCC) activity could allow clearance of these persistently infected cells. Indeed, some evidence suggests that residual viremia observed with suppressive ART may originate from viral species in the resting CD4+ T cell reservoir (14).
The potential contribution of residual virus replication despite ongoing ART remains controversial in some quarters. Multiple controlled studies of therapy intensification have failed to demonstrate an effect of any of the current antiretroviral agents on low-level viremia, suggesting that such viremia is generated by cells infected prior to the implementation of therapy, and not through residual replication (15–21). Two studies of integrase inhibitor intensification found transient changes in forms of HIV 2-LTR DNA or reductions in immune activation (22,23). And one study found HIV sequence evolution within lymph node tissue in patients treated for 6 months (24), a relatively short period of time after initiating ART. However, other studies found no evidence of viral evolution in plasma or tissues following years of suppression of viremia (25,26). If bnAbs can provide a novel and additional antiviral effect, acting at a novel step of the viral lifecycle and/or possessing unique pharmacokinetic properties, residual replication—if it exists—might be impacted.
HIV bnAbs as therapy for cure or control
In addition to increasing the efficacy, safety, and ease of administration of current ART, bnAbs have the potential to extend beyond the therapeutic potential of small molecule inhibitors of viral targets. Antibodies differ from small-molecule drugs that interfere with viral replication in that antibodies have the potential to affect the half-lives of both free virus and infected cells. As part of a novel combination approach to disrupt latency and clear persistent infection now under expanded study in several research groups, bnAbs could play a role in inducing the clearance of infected cells—both recently infected cells and long-lived latently infected cells. The goal of such approaches would first be the induction of a state of viral remission -- defined as the absence of viremia, infectiousness, and immune dysfunction despite the interruption of standard ART -- and, ultimately, the cure of HIV infection.
Clearing residual HIV infection that remains despite effective and prolonged suppression of viral replication by ART presents a unique challenge for the immune system and immunotherapeutics. In the current paradigms of therapies to disrupt latency and clear persistent infection, the targets for clearance are rare populations of cells, induced to express HIV proteins by a new class of therapeutic termed latency reversing agents. These latently infected cells may also express viral antigens for a very limited period of time, and in low quantity, presenting a challenge to any native or engineered immune response.
Following the reactivation of latent HIV, viral antigens are presented on the surface of the cell and thus could be targeted by antibodies or antibody-derived molecules. Immunotoxins, which are bifunctional chimeric proteins consisting of a targeting portion, such as an antibody or a ligand, and a toxin effector domain, have provided proof of concept for this approach (27). Initial clinical trials in HIV disease using immunotoxins failed to have a sustained impact on immunological or clinical markers (28), likely due in part to the inability to recognize a breadth of Env targets. In the bone-marrow-liver (BLT) humanized mouse model an immunotoxin effectively eliminated HIV-infected cells persisting despite ART (29).
BnAbs have by definition exceptional breadth across multiple viral isolates. As will be discussed in detail in the following sections, bnAbs have been shown to be potent in vitro, and can protect against or suppress active infection in animal models of HIV infection (30–32,34). Recently, one bnAbs were shown to have antiviral activity in phase I human clinical trials (35, 58). BnAb-mediated immunotherapies may leverage the activity of the host immune system, bringing to bear both innate and adaptive immune responses (36). As antibodies interact via Fc receptors on natural killer (NK) cells and phagocytes, these effector cells may then mediate the clearance of infected cells (37). Furthermore, these infected cell populations may be widely distributed across anatomical compartments, and in many patients the HIV-specific immune response may have waned in the absence of recent antigen exposure, and/or may be dysfunctional or depleted by HIV disease. Therefore, a novel and robust immune response may be necessary to detect and clear cells producing low-level viremia, and quiescently infected cells induced to leave the latent state. In this setting, bnAbs, particularly those that also had or were engineered to possess ADCC activity, may be of great value.
In vivo evidence for antiretroviral activity of bnAbs
The preceding sections detail the compelling rationale for why bnAbs would have an in vivo antiviral effect and should be tested for such activity. One approach to assess bnAb activity in vivo is to compare the level of viremia and the speed of viral escape from bnAbs in individuals who have, or have not, generated bnAbs during natural HIV infection. Seminal studies from the 1990s showed that serum from most HIV-infected subjects failed to substantially neutralize the concurrent virus circulating in plasma (38,39). Studying subjects longitudinally from seroconversion further demonstrated that autologous serum virus neutralization could occur early after infection, but was always accompanied by rapid escape and replacement of the viral quasispecies by viruses resistant to autologous serum neutralization, and there were minimal to no discernable changes in viral load associated with these changes (38–42). More recent data indicate that broad serum neutralization in chronic infection is associated with higher, rather than lower, viral loads, and is further characterized by evidence of ongoing virus escape from the neutralizing Ab (nAb) response (43–45). While some have posited a lack of inverse correlation between serum nAb activity and plasma viral load as evidence for absent in vivo antiviral activity of serum neutralizing antibodies, the evidence for rapid viral escape from the nAb response in all of the studies argues for exactly the opposite: bnAbs have a direct antiviral effect on replicating virus, which serves as the selecting pressure for escape. If the antibodies were not detrimental to circulating virus, there would be no evidence of escape. Collectively, the data suggest that the virus can always stay a step ahead of the nAb response during natural infection, probably because of a low barrier to resistance of the virus to each subsequent broadening mutation in the antibody repertoire. The question then becomes whether exogenously administered bnAbs can more effectively inhibit virus replication than nAbs that develop naturally in response to virus escape. Multiple studies in animal models and human clinical trials have begun to address these questions.
Humanized mouse studies
Initial experiments to test the in vivo therapeutic effect of bnAbs were carried out in humanized mice, and demonstrated only limited antiviral effect (46,47). This is probably due to several factors. First, the nAbs used in early studies (b12, 2F5, 2G12) were not nearly as potent or broad as more recently described bnAbs. Second, the Hu-PBL-SCID model shows generally greater variability in HIV replication than some of the more sophisticated mouse models that incorporate human stem cells, thymus, and/or liver tissues in reconstituting the mice. As a result, single nAb therapy was generally ineffective in Hu-PBL-SCID mice (46,47), though combinations of nAbs occasionally led to sustained viral suppression. In the majority of cases, however, viral suppression by nAbs—if it occurred—was transient and associated with the rapid selection of viruses that escaped the MAb therapy. These early studies gave a good indication that neutralizing nAbs could have an antiviral effect in vivo, but that the barrier to resistance (at least to early nAbs) was low.
With the description of more bnAbs, it became important to test them for antiviral activity in vivo, and humanized mice were the most easily accessible models. These recent studies have incorporated more physiologic humanized mouse models than were used in earlier studies (30,48,49). The collective findings show that when there is adequate potency of a nAb against the circulating strain of HIV, there will be a measurable effect on viral replication; the more potent the antibody, the more substantial the antiviral effect (30). The barrier to resistance also was found to be important. In general single antibodies had no or only transient effects on virus replication, where combinations of antibodies proved to have a more persistent antiviral effect and prolonged time to resistance development (30,48,49). While none of the studies addressed the mechanism of the antiviral effect, a recent study where HIV-infected cells were infused into humanized mice, in the presence or absence of bnAbs, suggests that enhanced clearance of virus-infected cells is one possible mechanism by which bnAbs exert their antiviral effect in vivo (37). This latter finding suggests that efforts to improve effector function of existing bnAbs may lead to improved antiviral effects.
Nonhuman primate studies
A number of experiments assessing the in vivo antiviral activity of bnAbs have been performed in nonhuman primates (NHP). Initial studies using the anti-CD4 mAb 5A8 showed an antiviral effect in monkeys infected with simian immunodeficiency virus (SIVmac), but were limited by the rapid development of anti-mouse antibodies within monkeys to whom 5A8 was passively administered (50,51). In contrast, more recent studies with human bnAbs have shown rather dramatic antiviral effects of individual and combinations of passively administered antibodies (33,34) in simian/human immunodeficiency virus (SHIV)–infected monkeys. Antiviral effects in excess of 3 logs of plasma virus suppression were seen, and the virus tended to rebound after antibody concentrations dropped, though in some monkeys with low baseline plasma virus load, persistent viral suppression was observed even after antibody levels dropped below a therapeutic concentration (33). SHIV resistance to antibodies to the glycan V3 site developed in monkeys given 10–1074 (34) but not PGT121 (33), possibly reflecting different barriers to resistance to these two antibodies in vivo. While Barouch et al observed a sharp decline in cell-associated cellular SHIV DNA during antibody therapy (33), this should not be interpreted as indicating a direct effect of the bnAbs in killing of SHIV-infected cells. Standard ART in humans also leads to a decline in cell-associated viral DNA, reflecting the fact that most viral replication in vivo occurs in recently activated and infected CD4 T cells that express proviral DNA as part of the viral life cycle (52,53).
The substantial antiviral effect of bnAbs in NHPs raises the question of whether they would be an effective adjunctive therapy in a cure strategy. Early therapy during acute infection has been shown to limit the development of the latent reservoir, thereby decreasing the barrier to cure (54). A study was therefore performed to determine if bnAbs would have an impact on virus load during acute infection, and whether this would further reduce the latent reservoir over what ART alone could accomplish (55). NHPs were treated with a single bnAb (VRC01), a combination of bnAbs (VRC07-523 and PGT121), or ART alone starting 10 days after IV infection with SHIV162P3. At 21 days, monkeys in all three arms received ART. The antiviral effect in the combination bnAb arm was identical to the ART alone arm, and there was no difference in time to full viral suppression, cell-associated DNA, or time to rebound when ART was stopped. These data suggest that bnAb therapy, at least during acute SHIV infection, does not have a greater effect on the latent reservoir than ART alone.
Human clinical trials
The true antiviral effect of bnAbs will be determined in human clinical trials, several of which have already occurred. In an attempt to determine the direct antiviral effect of bnAbs on HIV replication, bnAbs have been given to several HIV-infected individuals not on ART, and the impact on virus replication has been measured. The initial studies to look for a direct antiviral effect of antibodies in humans were performed with the anti-CD4 molecule ibalizumab (56,57). Only a modest (up to 1 log) and transient decrease in plasma viremia was observed before antiviral resistance arose. In two more recent studies that tested CD4 binding site (CD4bs) mAbs (3BNC117 and VRC01), they were shown to have a greater, but mostly still transient, effect on plasma virus load (approximately 1–2.5 log decrease) (35,58). While subjects with low baseline plasma virus loads had more sustained viral control, the selection of MAb-resistant variants was variable. Subjects with pre-existing resistance had little to no antiviral response. Among individuals with sensitive virus quasispecies, resistance frequently became apparent as the virus load rose before plasma antibody levels declined to sub-therapeutic levels (58). Some individuals, however, maintained viral suppression until antibody levels dropped, and the rebounding virus remained sensitive to the infused bnAb (35).
Another way to assess the antiviral effect of bnAbs is to assess their ability to maintain virus suppression in individuals whose virus replication is already suppressed by ART, but who undergo an analytic treatment interruption. In this setting there may be minimal virus replication initially, and it may therefore be less likely that resistance to the bnAb(s) will arise. In the first studies to test this approach, a cocktail of neutralizing mAbs (2G12, 2F5, and 4E10) was tested for its ability to halt or delay rebound viremia during analytical treatment interruption (59,60). This antibody combination had limited effect on delaying virus rebound in these two studies. Of interest, only 2G12 appeared to consistently select for resistant viral strains within rebounding virus, suggesting that, of the three antibodies tested in the cocktail, it was the only one imposing a substantial antiviral effect. Two recent publications employed the CD4bs mAbs 3BNC117 and VRC01 in similar strategies to maintain suppression during ATI (61; Chun, NEJM, 2016 - pending). Both studies demonstrated a delay in time to virus rebound, but resistance was either present or developed in a high enough percentage of participants to indicate that the barrier to resistance to these single bnAb products is too low for them to serve as single agents for maintaining virus suppression.
Future directions for utilization of bnAbs to treat HIV-1 infection
Much remains unknown about the potential therapeutic usage of bnAbs. First and foremost, the actual in vivo antiviral mechanism (or mechanisms, as there may be more than one) is largely unknown. Do bnAbs act solely as entry inhibitors, or do they also act through their ability to opsonize and promote clearance of plasma virus? While it is clear that they can mediate ADCC and other effector mechanisms against virus-infected cells in vitro, what is the evidence that such mechanisms operate in vivo? The answer to this latter question will be crucial to the application of bnAbs in HIV cure strategies.
To date, the evidence suggests that basic antiviral drug therapy paradigms can be applied to therapy with bnAbs. Individual bnAbs will vary in potency and ease of generating resistance. However, as has been evident with all antiretrovirals, even the most broad and potent bnAb will probably prove ineffective as a single agent. Combinations of bnAbs with non-overlapping resistance patterns will likely be required to achieve an acceptable therapeutic and resistance profile for clinical development. Their long half-lives in vivo (approximately 14 days) make them attractive candidates for maintenance therapy (62). The introduction of long half-life mutations in the Fc region could further extend their in vivo half-life (63), making them good candidates to combine with long-acting integrase inhibitors for maintenance therapy.
The development of mAbs to treat multiple human diseases has exploded in the last several years. It is therefore not surprising that antibody therapy against HIV infection is showing some promise. However, there remains much to learn and much to do before routine clinical use of bnAb therapy becomes a reality.
Non-neutralizing antibodies
Non-neutralizing Abs (non-nAbs) can play a significant role in protection from infections caused by smallpox (64,65), Sindbis (66,67), yellow fever (68), influenza virus (69), Ebola virus (70), and Epstein-Barr virus–related cancer manifestation (71), supporting the rationale that non-nAbs hold great potential to be harnessed by an antiviral vaccine regimen. Among the non-neutralizing functions, Ab can provide protection from viral infection by mediating virolysis, phagocytosis, or ADCC through recruitment of complement factors and/or engaging Fc-receptor (FcR)–bearing cells. Interestingly, the ability of antibody to mediate these polyfunctional activities has been correlated with vaccine-induced protection from SHIV in the NHP model (72) and linked to control of virus replication in HIV-1–infected elite controllers (73). ADCC responses that are mainly directed against the envelope glycoprotein have also been reported to control acute SIV infection in vaccinated NHP (74), to prevent HIV-1 mother-to-infant transmission (75), and to prevent disease progression in HIV-1–infected children (76) and adults (77–80). Furthermore, the importance of non-nAbs functions is also supported by the results of the RV144 trial, which was the only trial, out of six phase III clinical studies performed to date, that showed some level of efficacy (81).
Although anti-HIV-1 envelope-specific non-nAb functions have been correlated with reduced infection risk in RV144, the previously discussed sterilizing protective attributes observed for the bnAbs have thus far not been recapitulated when non-nAbs have been evaluated in passive protection studies using NHP models (91–94). Similarly, a subsequent NHP passive protection study conducted by Moog and collaborators indicated that the vaginal application of the non-nAbs 246-D and 4B3 directed against the principal immune-domain on gp41 did not protect against high dose vaginal SHIV-162P3 challenge, whereas bnAb treatment protected a significant number of animals (92). Nonetheless, animals treated with the combination of non-nAbs were capable of controlling viremia relative to the infected controls. Recent studies evaluated the protective effects of A32 (94,95) and 7B2 (93,97,98), non-nAbs directed against CD4 inducible epitopes (99) reported in the C1/C2 region and the gp41 cluster I epitopes, respectively. The passive infusion studies conducted with these two mAbs demonstrated that they did not prevent infection from challenge with high dose SHIV1BaL, but were able to limit the number of transmitted/founder (T/F) HIV-1 variants that established infection (93). A common limitation of these studies the reliance on the high dose challenge to evaluate the protective effect of non-nAbs. The amount of virus present in a typical high dose challenge far exceeds the average 0.42×104 copies/ml (range 0.01–6.9×104) of virus present in the semen of untreated HIV-1 infected individuals (100). It is therefore possible that a protective non-nAb response, such as the ADCC observed in RV144, might be confined to prevention of transmission by a low dose challenge. This possibility requires testing; however, the limited half-life of human mAbs upon infusion and restriction of the number of infusions that can be administered due to human mAb immunogenicity in NHP currently prevents performance of appropriate multiple low-dose challenge studies. A recent study aimed at circumventing these difficulties proved that it may be a difficult task. Fuchs and colleagues used a recombinant adeno-associated viral vector (rAAV) to deliver the 5L7 anti-SIV non-nAb directed against the envelope glycoproteins into rhesus monkeys before repeated marginal-dose challenge (101). One of the five animals treated with the rAAV-mAb did not become infected after six exposures; moreover, the peak as well as virus set point in the other animals were significantly lower than control groups in this study. The highest ADCC response was observed in the protected animal. Unfortunately, the anti-SIV mAb delivered by rAAV proved to be as immunogenic as the IgG in other studies, since 9 of the 12 animals tested positive for anti-IgG within 4 weeks of administration of the rAAV-mAbs.
The experiments conducted thus far in NHP model using non-nAbs have not directly addressed the ability of this class of mAbs to perform as immune therapeutics and either control virus replication or impact the reservoir of latently infected cells, but they suggested that these mAbs can target infected cells by engaging the Fc-R-bearing cells. Therefore, similarly to nAbs, the non-nAbs can be a platform to generate molecules to be used as immune therapeutics in HIV-1 infection.
Bispecific antibodies and HIV-1 infection
Bispecific antibodies (bsAbs) are usually defined as molecules engineered to combine two antigen-binding variable fragments (Fv) of immunoglobulins in order to recognize two separate antigens (103). These molecules can therefore simultaneously interact with two different antigens presented on the surface of an individual cell or on two distinct cells. Since the first design of bsAb, a large body of literature now reports several combinations of bispecific and even trispecific Abs that can be used to diagnose or treat several human diseases including, but not limited to, infectious diseases and cancer (104).
The two major goals of designing these molecules have been to increase the breadth of antigens recognized by a monospecific mAb, and/or to engage antigen-expressing cells while simultaneously recruiting cytotoxic effector cells. Both types of constructs can be divided into two major classes depending upon the presence of the antibody Fc region or lack thereof.
In the pursuit of preventing and treating HIV-1 infection, bsAbs were initially designed to target free HIV-1 virions or HIV-1–infected cells based on gp41-specific Abs and demonstrated to mediate: 1) virus neutralization (105); 2) neutralization and ADCC (106); and 3) redirection of CD3+ T cells against HIV-1–infected or latently infected cells lines (107).
More recently, a different approach has increased the bsAbs neutralizing activity. Sun and collaborators designed a bsAb (iMabm36) by linking the anti-CD4 Ab ibalizumab (iMab) to two copies of the single domain CD4-inducible epitope m36Ab (108). The iMabm36 was demonstrated to have higher neutralizing capacity than the m36 alone, and the capablity of neutralizing 83% of 118 pseudoviruses with an IC50<0.1µg/ml. A clinical trial is currently evaluating the potency of this bsAb in the current formulation for treating HIV-1 infection, and new proteins will be generated to target the pool of latently infected cells.
To increase the potency and breadth of bnAbs against HIV-1, four independent bsAbs were developed using the following combinations of bnAbs: VRC07x10e8, VRC07xPGT121, VRC07xPG9-16, and 10e8xPG9-16 (109). The most promising bispecific combination was represented by the VRC07xPG9-16 Ab that neutralized >84% pseudoviruses with IC50<1µg/ml and a 3– to 37-fold increased potency against 8 of 10 pseudoviruses included in this panel compared to the individual mAbs. Of note, this bispecific Ab neutralized 10 pseudoviruses resistant to VRC07 (n=5) and PG9-16 (n=5) mAb when tested alone. Additional NHP studies revealed no difference in the pharmacokinetics between the parental and bispecific Abs, further indicating the advance in designing bsAbs that could be used for treatment and/or cure of HIV-1 infection.
Novel engineered bispecific molecules: BiTEs vs DARTS
Bispecific Abs have also been designed to recognize the antigenic structure of interest on the surface of target cells with one arm and engage functional receptors of the effector cells with the second arm. These new molecules lack the Fc region of the immunoglobulin. The first generation of these molecules was termed bispecific T cell engagers (BiTEs), and was developed to treat CD19+ B cell lymphoma. BiTEs consist of a protein composed of two single chain antibodies joined by a single polypeptide linker (110). This bs Ab used an anti-CD19 arm linked to an anti-CD3 arm (bscCD19xCD3) that could provide potent engagement of the CD3+CD4+ and CD3+CD8+ T cell subsets directed against the human B cell lymphoma (111). Four additional BiTEs with different specificities have already been moved into phase I clinical studies to treat several malignancies (104). The second generation of BiTEs was named dual-affinity re-targeting (DART™) molecules (112). In the DART™, the heavy and light chains of the two antigen binding specificities of the diabody were separated into two separate polypeptide chains. The DART™ was additionally stabilized by the addition of a C-terminal disulfide bridge. A side-by-side in vitro comparison of CD19xCD3 BiTE and DART™ proteins based on the mouse anti-human CD3 and mouse anti-human CD19 mAb blinatumab indicated that the more rigid conformation of DART™ compared to BiTE proteins could provide a higher maximal activity level, half-maximal concentration, and induction of T cell activation (113). Prompted by these initial observations in developing immune therapeutics for cancer treatment, new BiTE and DART™ molecules have been designed to tackle the treatments of HIV-1 infection. The first HIV-specific BiTE molecule, VRC07- αCD3, was engineered based on the CD4bs-specific VRC07 bnAb (114) combined with an anti-CD3 arm to engage cytotoxic CD8 T cells (115). This protein was not only able to direct the cytotoxic activity of resting CD8+ T cells against a cell line that represent a model for constitutive and inducible HIV-1 expression, but also to recognize latently infected cells present in the peripheral blood mononuclear cells (PBMCs) from HIV-1 infected individuals under suppressive cART. This latter activity resulted in a decreased in vitro frequency of CD4+ T cells harboring provirus after 48 hours in vitro incubation of the target CD4+ and effector CD8+ T cells in presence of the VRC07-αCD3.
Using the structure of DART™ molecules, several different HIV-1–specific DARTs have recently been generated and tested in vitro for their ability to recruit CD8+ cytotoxic T cells to recognize autologous infected cells. In the germinal study conducted by Sung and collaborators, the HIV-1–specific arm of the DART was based on the A32 and 7B2 non-nAbs recognizing the highly conserved regions of the CD4i C1/C2 and gp41 cluster I envelope regions (116). The second study investigated an additional group of DARTs based on the N332-glycan PGT121 (117), V1/V2 PGT145 (118), CD4bs VRC01 (119,120), and MPER 10e8 (121) bnAbs generated using the same CD3 arm (122). All these molecules were demonstrated to have antiviral activity at nanogram levels and, therefore, suitable for in vivo utilization. Analyses of these molecules indicated that they can retain the breadth of the mAbs, can recruit resting CD8+ T cells to eliminate infected cells, and can reduce virus recovery when combined with latency-reversing agents capable of re-activating virus replication in PBMC samples obtained from HIV-1–infected individuals. Of interest, DARTs based on the bnAbs also retained their ability to neutralize the virus (122). In both studies, paired combinations of different DARTs did not reveal antagonistic effects and instead suggest that additive effects could be obtained.
It has been discussed that utilization of latency-reversing agents in combination with cART alone does not impact the size of the pool of latently infected cells due to several combined factors: 1) the concentration of cART may not be sufficient at the active site of virus replication in the germinal centers; 2) virus replication may not provide sufficient activating signals to the antigen-specific CD8 T cells; 3) escape mutant selected during the ontogeny of the adaptive immune responses may not be recognized by the existing cellular and humoral responses. The development of BiTEs and DARTs could overcome these hurdles, and future clinical evaluation of these molecules is already planned.
Future direction in the clinical applications of HIV-1 natural and engineered antibodies
Initial clinical studies revealed that utilization of individual bnAbs might face limitations due to the presence of circulating HIV-1 isolates already resistant to their recognition or to the appearance of escape mutants during mAb treatment. The absence of a single “magic bullet” to treat HIV-1 infection should not be a surprising observation, but it has spurred a new phase of pre-clinical research to address these initial concerns.
In vitro studies of BiTEs and DARTs have provided initial promising results, but more has to be done to address their half-life, safety, and efficacy in clinical settings. It seems reasonable to envision that the development of a cocktail of these molecules could also be necessary based on the potential presence of HIV-1 isolate that are already resistant to or may become resistant during treatment.
Acknowledgments
GF has filed patent applications on 2 mAb and related molecules described in this review.
We thank Dr. Ashley Morgan for editorial assistance and Brenda Hartman for graphics support. This work was supported by the Intramural Research Program, Vaccine Research Center, NIAID (R.A.K.). Additional funding was from UM1-AI100645-01 from NIAID (G.F.), UM1 AI126619 from NIAID, NIDA, NIMH, and NINDS (D.M.M.), P30-AI50410 (UNC CFAR, NIAID), and from the Collaboration for AIDS Vaccine Discovery grant OPP1032325 from the Bill and Melinda Gates Foundation (R.A.K.). We thank all the HIV+ participants for their critical contributions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest statement:
DM and RK have no conflicts of interest to disclose.
References
- 1.Freed EO. HIV-1 replication. Somat Cell Mol Genet. 2001;26(1–6):13–33. doi: 10.1023/a:1021070512287. [DOI] [PubMed] [Google Scholar]
- 2.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Micro. 2008;6(11):815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 3.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93(5):681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 4.Palmer S, Maldarelli F, Weigand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deal CE, Balazs AB. Vectored antibody gene delivery for the prevention or treatment of HIV infection. Curr Opin HIV AIDS. 2015;10(3):190–197. doi: 10.1097/COH.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archin NM, Vaidya NK, Kuruc JD, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A. 2012;109(24):9523–9528. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun TW, Engel D, Berrey MM, et al. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95(15):8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosque A, Famiglietti M, Weyrich AS, Goulston C, Planelles V. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 2011;7(10):e1002288. doi: 10.1371/journal.ppat.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner TA, McLaughlin S, Garg K, et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345(6196):570–573. doi: 10.1126/science.1256304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldarelli F, Wu X, Simonetti FR, et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345(6193):179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn LB, Silva IT, Oliveira TY, et al. HIV-1 integration landscape during latent and active infection. Cell. 2015;160(3):420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonetti FR, et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci U S A. 2016;113(7):1883–1888. doi: 10.1073/pnas.1522675113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson JA, Archin NM, Ince W, et al. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J Virol. 2011;85(10):5220–5223. doi: 10.1128/JVI.00284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi RT, Bosch RJ, Aga E, et al. No evidence for decay of the latent reservoir in HIV-1-infected patients receiving intensive enfuvirtide-containing antiretroviral therapy. J Infect Dis. 2010;201(2):293–296. doi: 10.1086/649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi RT, Coombs RW, Chan ES, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59(3):229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammer SM, Ribaudo H, Bassett R, et al. A randomized, placebo-controlled trial of abacavir intensification in HIV-1-infected adults with virologic suppression on a protease inhibitor-containing regimen. HIV Clin Trials. 2010;11(6):312–324. doi: 10.1310/hct1106-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMahon D, Jones J, Wiegand A, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50(6):912–919. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinoso JB, Kim SY, Wiegand AM, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106(23):9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallejo A, Gutierrez C, Hernandez-Novoa B, et al. The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS. 2012;26(15):1885–1894. doi: 10.1097/QAD.0b013e3283584521. [DOI] [PubMed] [Google Scholar]
- 21.Cillo AR, Hilldorfer BB, Lalama CM, et al. Virologic and immunologic effects of adding maraviroc to suppressive antiretroviral therapy in individuals with suboptimal CD4+ T-cell recovery. AIDS. 2015;29(16):2121–2129. doi: 10.1097/QAD.0000000000000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 23.Llibre JM, Buzón MJ, Massanella M, et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antivir Ther. 2012;17(2):355–364. doi: 10.3851/IMP1917. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzo-Redondo R, Fryer HR, Bedford T, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530(7588):51–56. doi: 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kearney MF, Spindler J, Shao W, et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 2014;10(3):e1004010. doi: 10.1371/journal.ppat.1004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Josefsson L, von Stockenstrom S, Faria NR, et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A. 2013;110(51):E4987–E4996. doi: 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pincus SH, Wehrly K, Cole R, et al. In vitro effects of anti-HIV immunotoxins directed against multiple epitopes on HIV type 1 envelope glycoprotein 160. AIDS Res Hum Retroviruses. 1996;12(11):1041–1051. doi: 10.1089/aid.1996.12.1041. [DOI] [PubMed] [Google Scholar]
- 28.Davey RT, Jr, Boenning CM, Herpin BR, et al. Use of recombinant soluble CD4 Pseudomonas exotoxin, a novel immunotoxin, for treatment of persons infected with human immunodeficiency virus. J Infect Dis. 1994;170(5):1180–1188. doi: 10.1093/infdis/170.5.1180. [DOI] [PubMed] [Google Scholar]
- 29.Denton PW, Long JM, Wietgrefe SW, et al. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog. 2014;10(1):e1003872. doi: 10.1371/journal.ppat.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein F, Halper-Stromberg A, Horwitz JA, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horwitz JA, Halper-Stromberg A, Mouquet H, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A. 2013;110(41):16538–13543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietzsch J, Gruell H, Bournazos S, et al. A mouse model for HIV-1 entry. Proc Natl Acad Sci U S A. 2012;109(39):15859–15864. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barouch DH, Whitney JB, Moldt B, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shingai M, Nishimura Y, Klein F, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503(7475):277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caskey M, Klein F, Lorenzi JC, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bournazos S, Ravetch JV. Fcgamma receptor pathways during active and passive immunization. Immunol Rev. 2015;268(1):88–103. doi: 10.1111/imr.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu CL, Murakowski DK, Bournazos S, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352(6288):1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frost SD, Wrin T, Smith DM, et al. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci U S A. 2005;102(51):18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100(7):4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert J, Abrahamsson B, Nagy K, et al. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990;4(2):107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Arendrup M, Sönnerborg A, Svennerholm B, et al. Neutralizing antibody response during human immunodeficiency virus type 1 infection: type and group specificity and viral escape. J Gen Virol. 1993;74(Pt 5):855–863. doi: 10.1099/0022-1317-74-5-855. [DOI] [PubMed] [Google Scholar]
- 42.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 43.Doria-Rose NA, Klein RM, Daniels MG, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010;84(3):1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Wang C, O’Dell S, et al. Selection pressure on HIV-1 envelope by broadly neutralizing antibodies to the conserved CD4-binding site. J Virol. 2012;86(10):5844–5856. doi: 10.1128/JVI.07139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto T, Lynch RM, Gautam R, et al. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Sci Transl Med. 2015;7(298):298ra120. doi: 10.1126/scitranslmed.aab3964. [DOI] [PubMed] [Google Scholar]
- 46.Andrus L, Prince AM, Bernal I, et al. Passive immunization with a human immunodeficiency virus type 1-neutralizing monoclonal antibody in Hu-PBL-SCID mice: isolation of a neutralization escape variant. J Infect Dis. 1998;177(4):889–897. doi: 10.1086/515251. [DOI] [PubMed] [Google Scholar]
- 47.Poignard P, Sabbe R, Picchio GR, et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999;10(4):431–438. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- 48.Freund NT, Horwitz JA, Nogueira L, et al. A new glycan-dependent CD4-binding site neutralizing antibody exerts pressure on HIV-1 in vivo. PLoS Pathog. 2015;11(10):e1005238. doi: 10.1371/journal.ppat.1005238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoddart CA, Galkina SA, Joshi P, et al. Efficacy of broadly neutralizing monoclonal antibody PG16 in HIV-infected humanized mice. Virology. 2014;462–463:115–125. doi: 10.1016/j.virol.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reimann KA, Burkly LC, Burrus B, Waite BC, Lord CI, Letvin NL. In vivo administration to rhesus monkeys of a CD4-specific monoclonal antibody capable of blocking AIDS virus replication. AIDS Res Hum Retroviruses. 1993;9(3):199–207. doi: 10.1089/aid.1993.9.199. [DOI] [PubMed] [Google Scholar]
- 51.Reimann KA, Cate RL, Wu Y, et al. In vivo administration of CD4-specific monoclonal antibody: effect on provirus load in rhesus monkeys chronically infected with the simian immunodeficiency virus of macaques. AIDS Res Hum Retroviruses. 1995;11(4):517–525. doi: 10.1089/aid.1995.11.517. [DOI] [PubMed] [Google Scholar]
- 52.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 53.Wei X, Ghosh SK, Taylor ME, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 54.Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7(3):e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolton DL, Pegu A, Wang K, et al. Human immunodeficiency virus type 1 monoclonal antibodies suppress acute simian-human immunodeficiency virus viremia and limit seeding of cell-associated viral reservoirs. J Virol. 2015;90(3):1321–1332. doi: 10.1128/JVI.02454-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobson JM, Kuritzkes DR, Godofsky E, et al. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 2009;53(2):450–457. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuritzkes DR, Jacobson J, Powderly WG, et al. Antiretroviral activity of the anti-CD4 monoclonal antibody TNX-355 in patients infected with HIV type 1. J Infect Dis. 2004;189(2):286–291. doi: 10.1086/380802. [DOI] [PubMed] [Google Scholar]
- 58.Lynch RM, Boritz E, Coates EE, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7(319):319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PubMed] [Google Scholar]
- 59.Mehandru S, Vcelar B, Wrin T, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007;81(20):11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trkola A, Kuster H, Rusert P, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11(6):615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 61.Scheid JF, Horwitz JA, Bar-On Y, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613):556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ledgerwood JE, Coates EE, Yamshchikov G, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182(3):289–301. doi: 10.1111/cei.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ko SY, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zinkernagel RM, Oldstone MB. Cells that express viral antigens but lack H-2 determinants are not lysed by immune thymus-derived lymphocytes but are lysed by other antiviral immune attack mechanisms. Proc Natl Acad Sci U S A. 1976;73(10):3666–3670. doi: 10.1073/pnas.73.10.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perrin LH, Zinkernagel RM, Oldstone MB. Immune response in humans after vaccination with vaccinia virus: generation of a virus-specific cytotoxic activity by human peripheral lymphocytes. J Exp Med. 1977;146(4):949–969. doi: 10.1084/jem.146.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmaljohn AL, Johnson ED, Dalrymple JM, Cole GA. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- 67.Schmaljohn AL, Kokubun KM, Cole GA. Protective monoclonal antibodies define maturational and pH-dependent antigenic changes in Sindbis virus E1 glycoprotein. Virology. 1983;130(1):144–154. doi: 10.1016/0042-6822(83)90124-1. [DOI] [PubMed] [Google Scholar]
- 68.Schlesinger JJ, Brandriss MW, Walsh EE. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J Immunol. 1985;135(4):2805–2809. [PubMed] [Google Scholar]
- 69.Hashimoto G, Wright PF, Karzon DT. Antibody-dependent cell-mediated cytotoxicity against influenza virus-infected cells. J Infect Dis. 1983;148(5):785–794. doi: 10.1093/infdis/148.5.785. [DOI] [PubMed] [Google Scholar]
- 70.Wilson JA, Hevey M, Bakken R, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287(5458):1664–1666. doi: 10.1126/science.287.5458.1664. [DOI] [PubMed] [Google Scholar]
- 71.Mathew GD, Qualtiere LF, Neel HB, Pearson GR. IgA antibody, antibody-dependent cellular cytotoxicity and prognosis in patients with nasopharyngeal carcinoma. Int J Cancer. 1981;27(2):175–180. doi: 10.1002/ijc.2910270208. [DOI] [PubMed] [Google Scholar]
- 72.Barouch DH, Alter G, Broge T, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349(6245):320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ackerman ME, Mikhailova A, Brown EP, et al. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 2016;12(1):e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gómez-Román VR, Patterson LJ, Venzon D, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol. 2005;174(4):2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 75.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 2012;8(6):e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe. 2015;17(4):500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baum LL, Cassutt KJ, Knigge K, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157(5):2168–2173. [PubMed] [Google Scholar]
- 78.Forthal DN, Landucci G, Haubrich R, et al. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis. 1999;180(4):1338–1341. doi: 10.1086/314988. [DOI] [PubMed] [Google Scholar]
- 79.Lambotte O, Pollara J, Boufassa F, et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS ONE. 2013;8(9):e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung AW, Navis M, Isitman G, et al. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr. 2011;58(2):127–131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 82.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonsignori M, Pollara J, Moody MA, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86(21):11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pollara J, Bonsignori M, Moody MA, et al. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol. 2014;88(14):7715–7726. doi: 10.1128/JVI.00156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu P, Williams LD, Shen X, et al. Capacity for infectious HIV-1 virion capture differs by envelope antibody specificity. J Virol. 2014;88(9):5165–5170. doi: 10.1128/JVI.03765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tomaras GD, Ferrari G, Shen X, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A. 2013;110(22):9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yates NL, Liao H-X, Fong Y, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med. 2014;6(228):228ra39. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung AW, Ghebremichael M, Robinson H, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med. 2014;6(228):228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 89.Li SS, Gilbert PB, Tomaras GD, et al. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. J Clin Invest. 2014;124(9):3879–3790. doi: 10.1172/JCI75539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robinson JE, Elliott DH, Martin EA, Micken K, Rosenberg ES. High frequencies of antibody responses to CD4 induced epitopes in HIV infected patients started on HAART during acute infection. Hum Antibodies. 2005;14(3–4):115–121. [PubMed] [Google Scholar]
- 91.Burton DR, Hessell AJ, Keele BF, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011;108(27):11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moog C, Dereuddre-Bosquet N, Teillaud J-L, et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2013;7(1):46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 93.Santra S, Tomaras GD, Warrier R, et al. Human non-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques. PLoS Pathog. 2015;11(8):e1005042. doi: 10.1371/journal.ppat.1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dugast A-S, Chan Y, Hoffner M, et al. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PLoS ONE. 2014;9(5):e97229. doi: 10.1371/journal.pone.0097229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moore JP, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70(3):1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Acharya P, Tolbert WD, Gohain N, et al. Structural definition of an antibody-dependent cellular cytotoxicity response implicated in reduced risk for HIV-1 infection. J Virol. 2014;88(21):12895–12906. doi: 10.1128/JVI.02194-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pincus SH, Fang H, Wilkinson RA, et al. In vivo efficacy of anti-glycoprotein 41, but not anti-glycoprotein 120, immunotoxins in a mouse model of HIV infection. J Immunol. 2003;170(4):2236–2241. doi: 10.4049/jimmunol.170.4.2236. [DOI] [PubMed] [Google Scholar]
- 98.Craig RB, Summa CM, Corti M, Pincus SH. Anti-HIV double variable domain immunoglobulins binding both gp41 and gp120 for targeted delivery of immunoconjugates. PLoS ONE. 2012;7(10):e46778. doi: 10.1371/journal.pone.0046778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69(9):5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Butler DM, Smith DM, Cachay ER, et al. Herpes simplex virus 2 serostatus and viral loads of HIV-1 in blood and semen as risk factors for HIV transmission among men who have sex with men. AIDS. 2008;22(13):1667–1671. doi: 10.1097/QAD.0b013e32830bfed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fuchs SP, Martinez-Navio JM, Piatak M, Lifson JD, Gao G, Desrosiers RC. AAV-delivered antibody mediates significant protective effects against SIVmac239 challenge in the absence of neutralizing activity. PLoS Pathog. 2015;11(8):e1005090. doi: 10.1371/journal.ppat.1005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Florese RH, Van Rompay KKA, Aldrich K, et al. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177(6):4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 103.Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997;15(2):159–163. doi: 10.1038/nbt0297-159. [DOI] [PubMed] [Google Scholar]
- 104.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discovery Today. 2015;20(7):838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 105.Mabondzo A, Aussage P, Bartholeyns J, et al. Bispecific antibody targeting of human immunodeficiency virus type 1 (HIV-1) glycoprotein 41 to human macrophages through the Fc IgG receptor I mediates neutralizing effects in HIV-1 infection. J Infect Dis. 1992;166(1):93–99. doi: 10.1093/infdis/166.1.93. [DOI] [PubMed] [Google Scholar]
- 106.Mabondzo A, Boussin F, Raoul H, et al. Antibody-dependent cellular cytotoxicity and neutralization of human immunodeficiency virus type 1 by high affinity cross-linking of gp41 to human macrophage Fc IgG receptor using bispecific antibody. J Gen Virol. 1994;75(Pt 6):1451–1456. doi: 10.1099/0022-1317-75-6-1451. [DOI] [PubMed] [Google Scholar]
- 107.Yin S, Okada N, Okada H. Elimination of latently HIV-1-infected cells by lymphoblasts armed with bifunctional antibody. Microbiol Immunol. 2001;45(1):101–108. doi: 10.1111/j.1348-0421.2001.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 108.Sun M, Pace CS, Yao X, et al. Rational design and characterization of the novel, broad and potent bispecific HIV-1 neutralizing antibody iMabm36. J Acquir Immune Defic Syndr. 2014;66(5):473–483. doi: 10.1097/QAI.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Asokan M, Rudicell RS, Louder M, et al. Bispecific antibodies targeting different epitopes on the HIV-1 envelope exhibit broad and potent neutralization. J Virol. 2015;89(24):12501–12512. doi: 10.1128/JVI.02097-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bird RE, Walker BW. Single chain antibody variable regions. Trends Biotechnol. 1991;9(4):132–137. doi: 10.1016/0167-7799(91)90044-i. [DOI] [PubMed] [Google Scholar]
- 111.Dreier T, Lorenczewski G, Brandl C, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100(6):690–697. doi: 10.1002/ijc.10557. [DOI] [PubMed] [Google Scholar]
- 112.Johnson S, Burke S, Huang L, et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol. 2010;399(3):436–449. doi: 10.1016/j.jmb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 113.Moore PA, Zhang W, Rainey GJ, et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011;117(17):4542–4551. doi: 10.1182/blood-2010-09-306449. [DOI] [PubMed] [Google Scholar]
- 114.Diskin R, Scheid JF, Marcovecchio PM, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334(6060):1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pegu A, Asokan M, Wu L, et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun. 2015;6:8447. doi: 10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sung JAM, Pickeral J, Liu L, et al. Dual-affinity re-targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Invest. 2015;125(11):4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walker LM, Huber M, Doores KJ, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McLellan JS, Pancera M, Carrico C, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–344. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu X, Yang Z-Y, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang J, Ofek G, Laub L, et al. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491(7424):406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sloan DD, Lam C-YK, Irrinki A, et al. Targeting HIV reservoir in infected CD4 T cells by dual-affinity re-targeting molecules (DARTs) that bind HIV envelope and recruit cytotoxic T cells. PLoS Pathog. 2015;11(11):e1005233. doi: 10.1371/journal.ppat.1005233. [DOI] [PMC free article] [PubMed] [Google Scholar]