Treatment of autoimmune disorders such as psoriasis, rheumatoid arthritis, and Crohn's disease with so called biologics (selective immunomodulatory drugs) has become a standard way to treat severe or recalcitrant forms of these diseases. In particular, antagonists of tumour necrosis factor α (TNF-α) have been proved to be highly efficacious. In this review we summarise the current knowledge on problems associated with treatment with biologics, with particular emphasis on TNF-α inhibitors and autoantibody development. We also discuss the possible increased risk of lymphoma.
Background
Monoclonal antibodies and fusion proteins have become an important group of drugs (known as biologic drugs) for treatment of chronic autoimmune disorders.w1 Advances in antibody engineering and new techniques have allowed the generation of fusion proteins and chimeric, humanised, and fully humanised monoclonal antibodies. Fourteen biologic drugs have already been approved in the United States (by the US Food and Drug Administration (FDA)) and in other countries; over 70 are in late stage clinical trials (at least phase II) and over 1000 in preclinical development.w2
Treatment with these agents is well established for patients with rheumatoid arthritis and Crohn's disease, and in recent years a major focus of research into new biologics was psoriasis, an inflammatory T cell mediated skin disease. In particular, inhibitors of TNF-α were found to be very effective in the treatment of chronic plaque-type psoriasis, pustular psoriasis, and psoriatic arthritis.w3-w5 The mechanism of action of these drugs is either binding free TNF-α to the soluble receptor-like fusion protein etanercept (Enbrel) or direct inhibition of TNF-α bioactivity with the monoclonal antibodies infliximab (chimeric, Remicade) or adalimumab (fully human, Humira).1-5 w6 Other biologics targeting activated T cells and/or their migration into the skin are alefacept (Amevive) and efalizumab (Raptiva). Alefacept is a dimeric fusion protein consisting of a CD2-binding portion of human leukocyte function antigen-3 (LFA-3) linked to the Fc portion of human IgG1; it has been approved by the FDA for treating psoriasis. Efalizumab is a humanised monoclonal antibody directed against the CD11a subunit of leukocyte function antigen-1 (LFA-1) approved by the FDA as well as in European countries.6,7 w7
Summary points
Immunomodulatory biologic drugs are increasingly being used in the treatment of chronic autoimmune disorders such as psoriasis, rheumatoid arthritis, and Crohn's disease
The clinical efficacy and tolerability of these new agents has proved to be favourable
The use of biologics is associated with the development of antibodies against the substance (and/or of autoantibodies) with varying incidence
Concomitant treatment with immunosuppressive agents such as methotrexate seems to lower the incidence of autoantibody formation
The clinical relevance of antibodies to the biologics or of autoantibodies remains unclear
Many patients receiving these new therapies often respond rapidly to them and get a lot of clinical benefit. An increasing number of reports are focusing on side effects and problems of long term safety that require further examination (box 1). One of the major problems during treatment with anti-TNF-α agents has been the reactivation of tuberculosis and the possible development of other opportunistic infections.w8-w11 Infusion and injection reactions are the most frequently reported side effects during treatment2,8 w12 and cause about 15% of treatment discontinuations.w13 w14 Malignancies such as lymphoma and other haematological disorders have also been reported, as well as demyelinating diseases or worsening of congenital heart failure.9-11 w15-w18
Two other important problems associated with biologics are their immunogenic potential and the risk of autoimmune reactions. Autoantibodies after treatment with TNF-α inhibitors have often been observed, although their clinical relevance remains unclear.2,3,12-15 The formation of antibodies against the drugs themselves has also been noted and may effect thesafety and pharmacokinetics of the respective treatment.16-19
Box 1: Side effects of anti-TNF-α treatment
Infections (tuberculosis (especially extrapulmonary and miliary tuberculosis); other opportunistic infections (histoplasmosis, listeriosis, aspergillosis, pneumocystosis); sepsis)
Malignancies (lymphoma)
Other haematological disorders (aplastic anaemia, pancytopenia)
Demyelinating disorders/neuropathy (multiple sclerosis, optical neuritis)
Worsening of congestive heart failure
Occurrence of autoantibodies and autoimmunity (human anti-chimeric antibodies, antinuclear antibodies, anti-doublestranded DNA antibodies, anti-phospholipid antibodies, lupus or lupus-like syndrome)
Infusion/injection and hypersensitivity reactions
Box 2: Relation between antibody formation and treatment
Lower doses of therapeutic antibodies are more immunogenic than higher doses18,19
Single or episodic administration leads to a higher incidence of antibody formation18
Patients with Crohn's disease who received infusions every eight weeks during the ACCENT 1 trial showed significantly higher treatment responses and remission rates and a lower incidence of antibodies to infliximab compared with those who received only a single infusion19
A greater number of infusions and a higher total dose of infliximab may be associated with the development of new autoantibodiesw22
Long term data for the use of alefacept or efalizumab are lacking, but during clinical trials efalizumab was associated with a low incidence of serious adverse events (few cases of immune mediated thrombocytopenia), and no increased risk of serious infections or malignancies compared with placebo was observed.7 For alefacept the incidence of malignancies during clinical trials was slightly increased compared with placebo, as was the incidence of serious infections (www.rxlist.com).
We review here the latest safety data of etanercept, infliximab, adalimumab, with a specific focus on antibody formation and development of autoimmune disorders and lymphoma. We also review the published literature on the safety of alefacept and efalizumab in the treatment of psoriasis.
Sources and selection criteria
We searched Medline and obtained original articles through the library service. We also used relevant pages found through an internet search using Google and included data from the Annual European Congress of Rheumatology held in Berlin in June 2004.
Induction of antibodies against biologics
Incidence of antibody formation
Immune responses to biological products have been reported for many approved therapeutics.w19 The formation of antibodies against infliximab is an emerging issue in the treatment of patients with Crohn's disease and rheumatoid arthritis (box 2). Whether these antibodies can attenuate the efficacy of treatment or whether they have no detectable effect on the activity of the product is unclear. Further clinical trials are needed to investigate the possible relation between these antibodies and the long term efficacy of infliximab.17-19 w20 w21
Baert et al identified antibodies against infliximab in 61% of their patients with Crohn's disease.17 A concentration of ≥ 8.0 μg/ml was associated with a shorter duration of response and a higher risk of infusion reactions, which were generally mild to moderate. In other trials, antibodies to infliximab were reported in up to 44% (table 1), and infusion reactions as well as the loss of an initial response were found to be strongly related to the amount of antibodies detected.18,19 w20 w21. These antibodies seemed to be specific for infliximab and did not crossreact with other therapeutic antibodies.w23
Table 1.
Studies showing incidence of antibodies to infliximab
| Patient cohort | Incidence of antibodies to infliximab | |
|---|---|---|
| Baert et al17 |
125 patients with Crohn's disease |
61% after fifth infusion (43% v 75% with or without concomitant immunosuppressants respectively); >40% after first infusion |
| Farrell et al18 |
53 patients with Crohn's disease |
36% (24% v 63% with or without concomitant immunosuppressants respectively) |
| Hanauer et al19 |
233 patients with Crohn's disease at week 54 |
27% (38%, 11%, 8% respectively for episodic treatment; scheduled (every 8 weeks) dose of 5 mg/kg; scheduled (every 8 weeks) dose of 10 mg/kg |
| Maini et alw24 |
428 patients with rheumatoid arthritis treated with methotrexate |
8% |
| Wolbink et alw21 | 50 patients with rheumatoid arthritis (44 taking concomitant methotrexate) | 44% |
Mechanisms of antibody induction
Several reasons could account for the different rates of autoantibodies found in clinical trials. Concomitant treatment with hydrocortisone significantly reduced the frequency and serum levels of antibodies against infliximab.18 Other immunosuppressive agents like methotrexate and azathioprine also seem to prevent the formation of antibodies against infliximab.17,18 Controversial results have been published, however, and controlled trials will have to assess which immunosuppressive agent provides the best protection against the development of these antibodies.w21 w24
Most of the patients with rheumatoid arthritis were treated with concomitant immunosuppressants, whereas less than half of the patients with Crohn's disease were given additional treatment with these agents. Therefore, the dosing regimen and concomitant medication is often difficult to compare between different cohorts of patients and might account for the varying incidence of antibody formation reported.
Immune responses to therapeutic antibodies are also influenced by the construct type (murine, chimeric, humanised, or human).w25 There are no standardised assays for measuring the amount of antibodies, and the sensitivity and specificity of the assays used are often not fully reported.w25 Analysis can be performed only in samples in which no infliximab is present because the drug interferes with the measurement and leads to a lower rate of antibodies detected.w26 Antibody measurement during the above mentioned studies was performed with a commercial assay from Prometheus Laboratories or with a non-commercial Centocor assay. To date, no studies have compared both assay characteristics.
Humanised monoclonal antibodies such as efalizumab and fully human therapeutic molecules such as etanercept and alefacept or adalimumab have to be further investigated in relation to the induction of antibody formation. Recent studies suggest that antibodies against etanercept occur in about 16% of cases (leaflet in etanercept package). The overall incidence of antibodies against adalimumab during three clinical trials was about 5% but differed remarkably according to the concomitant medication used (leaflet in adalimumab package).16 Under monotherapy with adalimumab 12% of the patients tested positive for antibodies against the drug16 whereas in the trials where additional methotrexate was used2,20 only 1% of the patients developed these antibodies. However, in the latter trials one of the patients treated with placebo also tested positive for anti-adalimumab antibodies, which may point to problems with detection and sensitivity of the assay system, thus possibly affecting the validity of the data.
In the combined safety database of the two biologics approved for the treatment of psoriasis vulgaris only, an overall incidence of antibodies against alefacept was seen in about 3% of cases, and development of antibodies against efalizumab occurred in about 6.3%. Antibody development did not correlate with a worse outcome or significant changes in the incidence of adverse events (www.rxlist.com).
Autoimmunity
The development of antinuclear antibodies and autoimmune disorders has been observed after treatment with TNF-α inhibitors (box 3, table 2).12,15 To our knowledge, no autoimmunity related phenomena after treatment with alefacept or efalizumab have been reported. Besides its important role as a proinflammatory cytokine, TNF-α also causes serious immunosuppressive effects by regulating antigen-presenting cell functions and apoptosis of potentially autoreactive T cells.w27 Therefore, antagonising TNF-α and its suppressive effects may lead to the development or unmasking of autoimmune diseases.21,22 It remains unclear whether the detection of anti-doublestranded DNA antibodies is predictive of an increased risk of developing lupus-like syndrome or systemic lupus erythematosus. Box 4 gives details of trials of autoimmunity during treatment with TNF-α inhibitors.
Table 2.
Autoimmunity associated with TNF-α inhibitors
|
Incidence (%) |
No of patients with lupus-like syndrome or systemic lupus erythematosus |
|||
|---|---|---|---|---|
| Study | Cohort | New positive antinuclear antibodies | Anti-doublestranded DNA antibodies | |
|
Infliximab |
||||
| Vermeire et al12 |
125 patients with Crohn's disease |
49.6 |
32.5 |
2 |
| Charles et al13 |
156 patients with rheumatoid arthritis |
24 |
14 |
1 |
| Bobbio-Pallavicini et al14 |
30 patients with rheumatoid arthritis |
30 |
16.7 |
0 |
| De Rycke et al15 |
97 patients with rheumatoid arthritis or spondylarthropathy |
45.3 |
13.4 |
0 |
|
Adalimumab |
||||
| Weinblatt et al2 |
271 patients with rheumatoid arthritis |
11 |
3.9 |
0 |
| Furst et al3 | 636 patients with rheumatoid arthritis | 26.5 | 12.5 | 0 |
Box 3: Autoimmune diseases and autoantibodies that may occur during treatment with TNF-α inhibitors
Lupus-like syndrome
Leukocytoclastic vasculitis
Systemic lupus erythematosus
Antinuclear antibodies
Anti-doublestranded DNA
Case reports of drug induced lupus after treatment with infliximab and etanercept have been published22 w28-w31 and a French national survey found 12 cases of systemic lupus erythematosus after anti-TNF-α therapy (in about 7000 patients treated) for inflammatory arthritis.23 Despite the relatively high incidence of anti-doublestranded DNA antibodies, the frequency of clinical lupus related to anti-TNF-α treatment is very low. No evidence exists that patients who develop new autoantibodies are at significantly increased risk of developing drug induced lupus, but the long term impact remains unclear.21,24 The risk of developing self limiting autoimmune syndromes seems in most cases to be small compared with the impressive clinical benefit the patients experience with these drugs. Nevertheless these findings underline the need for further monitoring of autoantibody production in patients treated with TNF-α antagonists over longer periods. Physicians therefore need to look for signs and symptoms of autoimmune disorders when using TNF-α antagonists.
Risk of lymphoma development
Another problem that has been noted after treatment with TNF-α antagonists is the development of malignancies such as lymphoma (box 5). Several population studies during the past 20 years have shown an increased rate of lymphoproliferative disorders—particularly non-Hodgkin's lymphoma—in patients with rheumatoid arthritis (and to a lesser degree in patients with Crohn's disease and psoriasis) compared with the general population.w32-w35 There is a known relation between the use of immunosuppressive treatments and the development of lymphoproliferative malignancies,w36 and concern about an increased risk of lymphoma after treatment with TNF-α antagonists has been raised.w37 w38 An FDA meeting in March 2003 about the safety of new drugs for rheumatoid arthritis, examined data of patients with rheumatoid arthritis being treated with infliximab, etanercept, and adalimumab (controlled clinical trials and post-marketing experience) and estimated the risk of lymphoma and neoplasia development among those patients (www.rheumatology.org/publications/hotline/0303TNFL.asp?aud=mem). This examination found an increased risk of lymphoma (standard incidence ratio 2.3 to 6.4). Interdrug comparison was impossible owing to different trial designs and patient characteristics. As a result of this evaluation, a warning concerning malignancy has been added to the labelling for all therapeutic anti-TNF-α agents (letter from Centocor, October 2004).
Figure 1.
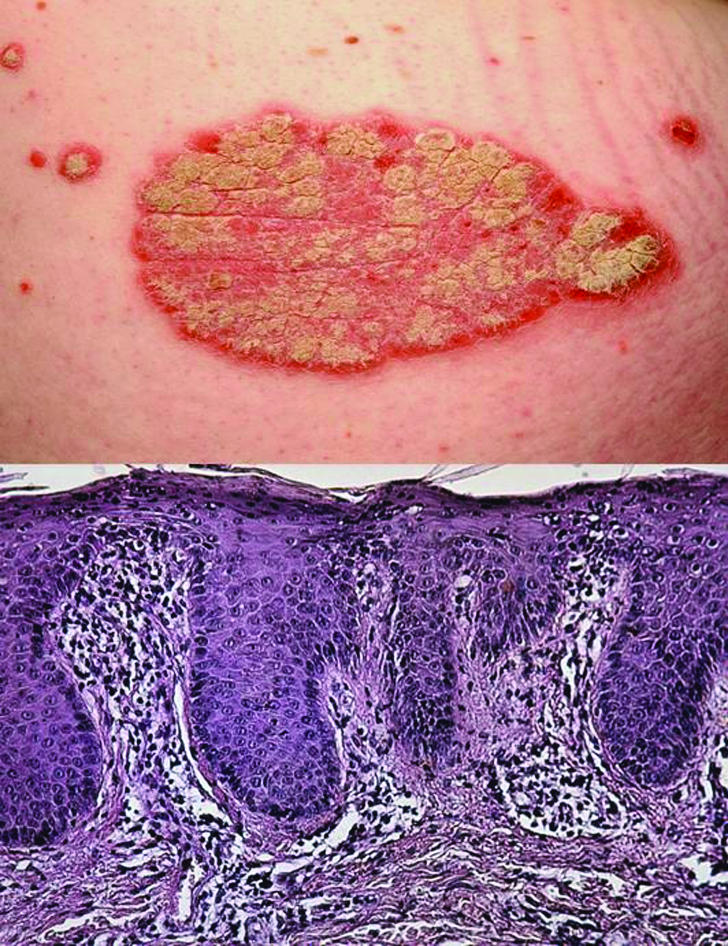
Psoriasis vulgaris: Top: typical erythemato-squamous plaque. Bottom: microscopic cross sectional view of plaque, showing elongated epidermal rete ridges and a dense inflammatory infiltrate
Box 4: Studies on autoimmunity during treatment with TNF-α inhibitors
Prospective clinical study of autoimmunity in 125 patients with Crohn's disease treated with infliximab12
The cumulative prevalence of antinuclear antibodies was 56.8% (71 patients) after a follow up of 24 months, compared with 7.2% (nine patients) at baseline
In most patients the antibodies emerged shortly after the first infusion; these antibodies were associated with the development of butterfly or papulosquamous skin rashes
Fourteen of the antinuclear antibody-positive patients developed skin manifestations, compared with only two in the group of patients without development of autoantibodies
Assessment of the incidence of anti-doublestranded DNA antibodies after infliximab treatment with or without concomitant methotrexate in a placebo controlled trial with 156 patients with rheumatoid arthritis13
The incidence of positive antinuclear antibody titres increased from 29% to 53%
Anti-doublestranded DNA antibodies occurred in 14% of the patients treated with infliximab (0% at baseline); one of the patients developed a reversible lupus-like syndrome
None of the patients receiving placebo treatment tested positive for anti-doublestranded DNA antibodies
Concomitant methotrexate did not significantly change the incidence of anti-doublestranded DNA antibodies
Two large clinical trials with adalimumab in rheumatoid arthritis patients2,3
These showed a higher rate for new antinuclear antibodies in patients treated with adalimumab than in those treated with placebo (3.9% v zero)
New anti-doublestranded DNA antibodies were detected in 12.5% of patients in the adalimumab group and in 1% of the placebo group
Box 5: Risk of malignancies after treatment with TNF-α inhibitors
Until 2002, 26 cases of malignancy after treatment with TNF-α inhibitors had been reported to the FDA, of which 18 were after treatment with etanercept (19 per 100 000 people treated with etanercept) and eight after treatment with infliximab (6.6 per 100 000 people treated with infliximab)9; 14 cases occurred within two months of treatment
In randomised controlled clinical trials with adalimumab, 10 cases of lymphoma had been identified by March 2003 (www.rxlist.com).
A few cases of lymphoma after treatment with alefacept and efalizumab have also been reported (www.rxlist.com).
The possibility that TNF-α inhibitors may accelerate the development of lymphomas in patients receiving long term immunosuppressive treatment with ciclosporin or methotrexate (agents often used as first or second line treatment) has also been discussed.w39 How safe TNF-α inhibitors are when used long term is unclear, but patients should be monitored closely for development of lymphoma (especially as lymphoma can present with an atypical clinical picturew40) until the potential risk is better understood.
Additional educational resources
Websites
MedWatch (www.fda.gov/medwatch/index.html)—The Food and Drug Administration's programme for safety information and for reporting adverse events
Pharmacy Benefits Management Strategic Healthcare Group (www.vapbm.org/PBM/drugmonograph.htm)—Drug monographs and recommendations for practitioners based on current medical evidence and expert opinion from clinicians
Internet drug index (www.rxlist.com)—Information for both consumers and medical professionals about, for example, side effects, precautions, contraindications
Reviews
Hyrich KL, Silman AJ, Watson DK, Symmons DP. Anti-tumour necrosis factor alpha therapy in rheumatoid arthritis: an update on safety. Ann Rheum Dis 2004;63: 1538-43.
Hanauer SB. Efficacy and safety of tumor necrosis factor antagonists in Crohn's disease: overview of randomized clinical studies. Rev Gastroenterol Disord 2004;4(suppl 3): S18-24.
Symmons DP, Silman AJ. Anti-tumor necrosis factor alpha therapy and the risk of lymphoma in rheumatoid arthritis: no clear answer. Arthritis Rheum 2004;50: 1703-6.
Supplementary Material
 Extra references (w1-w40) are on bmj.com
Extra references (w1-w40) are on bmj.com
Contributors: UM conceived the idea for this article, provided basic background literature, and corrected the draft version of the manuscript. SR did a thorough literature search and wrote the draft manuscript. UM is the guarantor.
Competing interests: UM is a consultant for or has received travel grants or honorariums from BiogenIdec (the manufacturer of alefacept), Centocor (the manufacturer of infliximab), Essex/Schering Plough, Fujisawa, Fumapharm, GlaxoSmithKline, Hermal, Leo Pharmaceuticals, Merz Pharma, Mölnlycke Healthcare, Novartis, Schering, Serono (the manufacturer of efalizumab), and Wyeth (the manufacturer of etanercept).
References
- 1.Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 2004;51: 534-42. [DOI] [PubMed] [Google Scholar]
- 2.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48: 35-45. [DOI] [PubMed] [Google Scholar]
- 3.Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (safety trial of adalimumab in rheumatoid arthritis). J Rheumatol 2003;30: 2563-71. [PubMed] [Google Scholar]
- 4.Gottlieb AB, Matheson RT, Lowe N, Krueger GG, Kang S, Goffe BS, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol 2003;139: 1627-32. [DOI] [PubMed] [Google Scholar]
- 5.Leonardi CL, Powers JL, Matheson RT, Goffe BS, Zitnik R, Wang A, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003;349: 2014-22. [DOI] [PubMed] [Google Scholar]
- 6.Krueger GG, Papp KA, Stough DB, Loven KH, Gulliver WP, Ellis CN. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol 2002;47: 821-33. [DOI] [PubMed] [Google Scholar]
- 7.Lebwohl M, Tyring SK, Hamilton TK, Toth D, Glazer S, Tawfik NH, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med 2003;349: 2004-13. [DOI] [PubMed] [Google Scholar]
- 8.Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340: 253-9. [DOI] [PubMed] [Google Scholar]
- 9.Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twentysix cases reported to the Food and Drug Administration. Arthritis Rheum 2002;46: 3151-8. [DOI] [PubMed] [Google Scholar]
- 10.Antoni C, Braun J. Side effects of anti-TNF therapy: current knowledge. Clin Exp Rheumatol 2002;20: S152-7. [PubMed] [Google Scholar]
- 11.Weisman MH. What are the risks of biologic therapy in rheumatoid arthritis? An update on safety. J Rheumatol Suppl 2002;65: 33-8. [PubMed] [Google Scholar]
- 12.Vermeire S, Noman M, van Assche G, Baert F, van Steen K, Esters N, et al. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn's disease: a prospective cohort study. Gastroenterology 2003;125: 32-9. [DOI] [PubMed] [Google Scholar]
- 13.Charles PJ, Smeenk RJ, de Jong J, Feldmann M, Maini RN. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials. Arthritis Rheum 2000;43: 2383-90. [DOI] [PubMed] [Google Scholar]
- 14.Bobbio-Pallavicini F, Alpini C, Caporali R, Avalle S, Bugatti S, Montecucco C. Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment. Arthritis Res Ther 2004;6: R264-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Rycke L, Kruithof E, van Damme N, Hoffman IE, van den BN, van den BF, et al. Antinuclear antibodies following infliximab treatment in patients with rheumatoid arthritis or spondylarthropathy. Arthritis Rheum 2003;48: 1015-23. [DOI] [PubMed] [Google Scholar]
- 16.Van de Putte LB, Atkins C, Malaise M, Sany J, Russell AS, van Riel PL, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis 2004;63: 508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baert F, Noman M, Vermeire S, van Assche G, D'Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003;348: 601-8. [DOI] [PubMed] [Google Scholar]
- 18.Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology 2003;124: 917-24. [DOI] [PubMed] [Google Scholar]
- 19.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359: 1541-9. [DOI] [PubMed] [Google Scholar]
- 20.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50: 1400-11. [DOI] [PubMed] [Google Scholar]
- 21.Ferraro-Peyret C, Coury F, Tebib JG, Bienvenu J, Fabien N. Infliximab therapy in rheumatoid arthritis and ankylosing spondylitis-induced specific antinuclear and antiphospholipid autoantibodies without autoimmune clinical manifestations: a two-year prospective study. Arthritis Res Ther 2004;6: R535-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stratigos AJ, Antoniou C, Stamathioudaki S, Avgerinou G, Tsega A, Katsambas AD. Discoid lupus erythematosus-like eruption induced by infliximab. Clin Exp Dermatol 2004;29: 150-3. [DOI] [PubMed] [Google Scholar]
- 23.De Bandt MJ, Sibilia J, Le Loet X, Prouzeau S, Fautrel B, Marcelli C, et al. Systemic lupus erythematosus induced by anti-TNF-alpha therapy: a French national survey. Ann Rheum Dis 2004;63(suppl 28): 213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furst DE, Breedveld FC, Kalden JR, Smolen JS, Burmester GR, Bijlsma JW, et al. Updated consensus statement on biological agents, specifically tumour necrosis factor alpha (TNFalpha) blocking agents and interleukin-1 receptor antagonist (IL-1ra), for the treatment of rheumatic diseases. Ann Rheum Dis 2004;63(suppl 2): ii2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


