Abstract
Purpose
Breast cancers have a poorer prognosis if estrogen receptor expression was lost during recurrence. It is unclear whether this conversion is cell autonomous or whether it can be promoted by the microenvironment during cancer dormancy. We explored the ability of marrow-derived stromal cell lines to arrest co-cultured breast cancer cells and suppress estrogen receptor alpha (ER) expression during arrest, facilitating the emergence of estrogen-independent breast cancer clones.
Methods
Cancer cell growth, ER protein, microRNA and mRNA levels were measured in breast cancer cell lines exposed to conditioned medium from marrow stromal lines in the presence and absence of estrogen and of signaling pathway modulators.
Results
We demonstrate that paracrine signaling from the stromal cell line HS5 downregulated ER in T47D and MCF7 breast cancer cells. This occurred at the mRNA level and also through decreased ER protein stability. Additionally, conditioned medium (CM) from HS5 arrested the breast cancer cells in G0/G1 in part through interleukin-1 (IL1) and inhibited cancer cell growth despite activation of proliferative pathways (Erk and AKT) by the CM. Similar findings were observed for CM from the hFOB 1.19 osteoblastic cell line but not from two other fibroblastic marrow lines, HS27A and KM101. HS5-CM inhibition of MCF7 proliferation could not be restored by exogenous ER, but was restored by the IL1-antagonist IL1RA. In the presence of IL1RA, HS5-CM activation of AKT and Erk enabled the outgrowth of breast cancer cells with suppressed ER that were fulvestrant-resistant and estrogen-independent.
Conclusions
We conclude that marrow-derived stromal cells can destabilize estrogen receptor protein to convert the ER status of growth-arrested ER+ breast cancer cell lines. The balance between stromal pro- and anti-proliferative signals controlled the switch from a dormant phenotype to estrogen-independent cancer cell growth.
Introduction
Roughly 15% of metastatic estrogen-receptor alpha (hereafter ER)–positive tumors convert to ER-negative status[1] with a resultant negative prognosis[2]. One study of disseminated tumor cells (DTC) in bone marrow aspirates identified only 28% concordance between ER expression in primary tumors with that in DTC, suggesting that dormant micrometastases may lose ER expression at an even higher rate [3]. It is unclear whether this change in ER-status is cell-autonomous or is directed or sustained by signals in the microenvironment.
Conversion of cancer cells to ER-negativity may arise during cancer dormancy. Stromal cells have been reported to induce dormancy of metastatic breast cancer cells, as recently reported in a mouse model[4]. In a model of dormancy using MCF7 cells in ovariectomized mice, Ogba et. al., reported that 25–30% of micro-metastatic cells had converted to an ER-negative[5] status.
We hypothesized that ER-negative clones could be induced by paracrine signals from bone marrow stromal cells concurrent with growth arrest. ER loss can occur through transcription-associated proteolysis [6], through epigenetic silencing [7] or transcriptional repression [8] of the ESR1 gene, through ubiquitin- or NEDD8-controlled proteasomal degradation [9] [10] [11] or through translational inhibition by microRNAs [12]. Paracrine control of estrogen receptor alpha levels has been reported to occur through exosomal transfer of inhibitory microRNAs [13] or through hormones inhibiting ESR1 transcription (for review, [14]). The importance of the microenvironment as a mediator of ER-expression was recently underscored by the finding that intraductal of ER-positive cancer cells in a xenograft model preserved ER levels and a luminal phenotype whereas fat pad injection suppressed ER expression and promoted a basal-like phenotype [15].
HS5 cells are fibroblastic stromal cells derived from bone marrow[16, 17] that have been used to model cancer dormancy. The HS5 line is CD146-negative and expresses the CDCP1 CUB-domain protein, similar to a subset of marrow cells that are prevalent on biopsies [18]. That phenotypic similarity indicates that HS5 functionality may overlap the activity of a physiological subset of marrow stroma. HS5 have been used in combination with osteoblast and endothelial cells in a 3-D matrix to generate a growth inhibitory niche that suppressed the proliferation of co-cultured luminal and basal breast cancer cell lines[19]. Secreted components from that mixture of cells were sufficient to inhibit growth[19]. HS5 cells also arrested T47 and MDA-MB-231 cells in spheroid co-culture [20]. In prostate cancer cells, paracrine BMP7 signaling by HS5 cells induced a dormant state, although a paracrine anti-proliferative effect of this stromal line on breast cancer cells has not been reported.
We report that HS5 stromal cells suppressed estrogen receptor expression at multiple levels in MCF7 and T47D breast cancer cells and provided both pro- and anti-proliferative paracrine signals that functioned independently of ER expression. Blockade of the growth suppressive stromal signaling pathway unmasked stromal promotion of estrogen-independent growth and anti-estrogen resistance in breast cancer cells. These results support a model through which marrow stroma direct the emergence of estrogen-negative metastases discordant with primary tumors.
Methods and Materials
Cells
MCF7, MDA-MB-231, HS5, HS27A, hFOB 1.19 (hFOB) cells were obtained from ATCC; T47D and BT474 breast cancer cells were obtained from Dr. Steffi Oesterreich (University of Pittsburgh). Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2mM L-Glutamine, 100 IU penicillin and 100μg/ml streptomycin (P/S). HS27A cells (from ATCC) and KM101 [21] obtained from Michael Epperly (University of Pittsburgh) were initially carried in RPMI and then adapted to DMEM supplemented with 10% FBS, 2mM L-Glutamine, 100 IU penicillin and 100μg/ml streptomycin (P/S) prior to CM collection. MCF7 cells expressing tetracycline-inducible HA-tagged estrogen receptor or mCherry (from Dr. Sarat Chandarlapaty, Memorial Sloan Kettering Cancer Center) and hFOB cells were maintained in DMEM/F12 without phenol-red, supplemented with 10% fetal bovine serum (FBS), 2mM L-Glutamine and P/S. Tet-inducible cells had G418 (100 ug/ml) and hygromycin (100 ug/ml) added. hFOB cells were incubated at 34°C, all the other cell-lines were incubated at 37°C. Fresh aliquots of cells were thawed for use every 2–3 months. Cells were routinely tested for mycoplasma. Conditioned medium was prepared as described in Supplemental Methods. Tandem repeat analysis was performed by Arizona Research Labs verifying MCF7, MDA-MB-231, HS5, HS27, T47D, hFOB 1.19 cells.
Reagents
Cytokines and pathways inhibitors used are described in Supplementary Table 1. Antibodies used are listed in Supplementary Table 2.
Cell proliferation assay
Breast cancer cells were plated in 96-well plates (black plate, clear bottom, Costar) at a low density (200–1000 cells/well in 100μl medium) to highlight solitary cell behavior. Indicated treatments were replenished every 3 days. HS5-CM was added to 30% final volume based on pilot titrations for maximal effect, except where stated otherwise; an equivalent volume of complete medium or 30% CM from other stromal lines as indicated was added to control wells. In pilot experiments, there was no difference between addition of complete medium or of MCF7-CM to experimental controls. Cell proliferation was measured at day 7 unless otherwise noted (for cells grown in complete growth medium) or day 14 (for cells in estrogen free medium) using a fluorescence-based cell proliferation kit (CyQUANT, Life Technologies) following the manufacturer’s instructions. These time points were optimal for a linear response of the cyquant assay when few cells are seeded. Fluorescence intensity (indicating cell numbers) was measured with a fluorescence 96-well plate reader (Beckman) at wavelength of 480 to 52 0nm. Each experimental replicate was done with 3–6 technical replicates.
Conditioned medium preparation
HS5 stromal cells at about 80% confluence cultured in T-75 flasks were fed with 10mL fresh complete growth medium. After 3 days, this conditioned medium was harvested and spin at 1000×g for 5min then filtered with 0.22μm filter to removed cell debris. For estrogen-free conditioned medium, cells were washed with PBS 3 times and then fed with 10mL fresh DMEM (without phenol-red) supplemented with 5% charcoal stripped serum. Conditioned medium was then aliquoted and stored at −80°C for future use. CM was generally used at 1 ml of conditioned medium per 800,000 HS5 cells. For experiment 2D, CM from each stromal line was adjusted to 1 ml of conditioned medium per 500,000 cells. As needed, CM aliquots were adjusted with culture medium to this concentration prior to freezing. Aliquots were thawed once and then discarded.
Charcoal stripping
Conditioned medium was charcoal stripped as indicated by addition of dextran-coated charcoal (Sigma C6241) added at 20 mg charcoal/ml media followed by gentle rocking overnight at 4° C. Subsequently, samples were spun for 15 minutes at 2000 g and collected supernatant filtered through a 0.22 micron syringe filter.
Cell cycle analysis
Cell cycle analysis was conducted to show the relative proportion of MCF7 cells in each of the sub G1, G0/G1, S, G2/M phases in cells pre-synchronized with 1uM nocodazole for 24 hrs. MCF-7 cells were grown in the presence or absence of 50% HS5-CM or KM101-CM for 24 hours. Cells were then harvested, fixed in ice-cold 70% ethanol and stored at 4° C. Fixed cells were stained for flow cytometric analysis with PI/RNase staining buffer, (BD Biosciences San Jose, CA) and analyzed using a BD Accuri C6 flow cytometer and accompanying software (BD Biosciences, San Jose, CA).
Estrogen-independent growth
To measure estrogen-independent growth, breast cancer cells were incubated for 2–3 days in estrogen-depleted medium consisting of phenol-red-free DMEM (Life Technologies, Grand Island NY, catalogue number 31053-028) containing 5% charcoal-stripped fetal bovine serum (Sigma F6765) and 1% penicillin-streptomycin. Cells were then harvested with phenol-red free trypsin and plated into 96-well plates for Cyquant assays or 24 well plates for crystal violet staining. For experiments measuring estrogen-independent growth in the presence of IL1RA and/or HS5-CM, these were added fresh every 3–4 days. For estrogen-free experiments, HS5 cells were grown in phenol red-free charcoal stripped medium for 2–3 days prior to harvesting of HS5-CM. HS5-CM was added to 30% final volume and equivalent medium was added to control wells.
Exosome preparation
Exosomes were prepared using differential centrifugation as described[22, 23] with modifications as noted in Supplemental Methods, and were confirmed for enrichment in the exosomal marker TSG101.
Western blotting and immunoprecipitations
Standard techniques were used; immunoprecitations were performed using the Pierce Co-Immunoprecipitation kit (Cat: 26149, Thermo-Fisher, Rockford, IL) according to manufacturers instructions. For additional information, see Supplemental Methods.
Luciferase assay
MCF7 cells were co-transfected with renilla plasmid and luciferase reporter plasmids either 3× ERE TATA luc or ERα 3′-UTR-luc as noted in text or pGL3-basic vector control. Luciferase assays used the Promega Dual-Luciferase Reporter Assay System. (see Supplemental Methods).
Immunofluorescence
MCF7 cells grown on glass coverslips were treated with control media or 30 % HS5-CM for 24 hours. Cells were fixed in 2% paraformaldehyde, permeabilized with 0.1% Triton X-100 and blocked in PBS + 2% BSA. Cells were incubated with 1:1000 anti-Estrogen Receptor alpha antibody [6F11] (abcam 9269, Cambridge, MA) at room temperature, washed, and incubated with goat anti-mouse Alexa Fluor 555 secondary antibody (Life Technologies, Eugene OR). Nuclei were counter-stained with Hoechst (Sigma, B-2883) and coverslips mounted with gelvatol. HS5, HS27 and KM101 were grown on glass coverslips, fixed and processed as above. Cells were stained with 0.25ug/ml Anti-Human IGF-1 antibody (500-P11, PeproTech, Rocky Hill, NJ) and 10ug/ml Anti-Human IL-1β antibody (MAB201, R&D Systems, Minneapolis, MN) followed by goat anti-rabbit Alexa Fluor 555 and goat anti-mouse Alexa Fluor 488 secondary antibodies (Life Technologies, Eugene OR).
ER decay curve
MCF7 cells were seeded in 6-well plates at a density of 200,000 cells/well in 2ml complete growth medium. After 24h, cells were washed once with PBS and re-fed with 2ml fresh complete medium with or without 30% HS5-CM and treated with 10μM cycloheximide. Cells were harvested at different time points as indicated with 60μl laemmli buffer and prepared for Western Blotting. Blots were stained with ponceau and then probed with ERα. Images were scanned and protein bands were quantitated with ImageJ. Relative ERα amounts were normalized to corresponding ponceau bands; graphed values were normalized to the average value at time point 0.
Estrogen-independent growth
MCF7, T47D and HS5 cells were treated in estrogen-depleted medium consisting of phenol-red-free DMEM and charcoal-stripped fetal bovine serum for these experiments as detailed in Supplemental Methods.
Statistical analysis
Statistical analysis was conducted using tests as indicated, with results considered significant at the p<0.05 level. Binary comparisons used Student’s t-test; comparisons of experiments with 3 or more conditions used one-way ANOVA as indicated, using Tukey’s post-test to control the Type I error rate in subsequent pairwise comparisons. For comparison of different stromal CM effects, ER protein decay rates and for comparison of ICI dose response in the presence and absence of HS5-CM and IL1RA, mixed effects models were used for analysis as noted in figure legends and detailed in Supplementary Statistical Methods.
Results
HS5 and hFOB cells inhibit growth of breast cancer cell lines through IL-1 signaling
In order to study whether growth-suppressive stroma comprise a favorable niche for loss of cancer cell estrogen receptor, we studied HS5 and other marrow stromal lines as mediators of quiescence and of shifts in estrogen-expression in cancer cells. The HS5 marrow-derived stromal line has been used to model dormancy in breast and prostate cancer [19, 24] and shares surface marker (CD105, CD73, CD44, CD90, data not shown) with mesenchmal stem cells (MSC’s), also expressing a rarer marker (CDCP1) seen in a subset of marrow cells localized around bone [18].
While cell-cell contact with HS5 has been shown to inhibit breast cancer cell growth, we tested whether HS5 conditioned medium (HS5-CM) had growth-inhibitory activity by measuring the effect of HS5-CM on growth of three ER-positive cell lines using a fluorescence-based cell proliferation assay of breast cancer cells seeded at low density to highlight solitary cell behavior (Cyquant, see methods). Using this sensitive measure of growth inhibition, the HS5-CM was shown to inhibit the growth of MCF-7, BT474 and T47D cells (Figure 1A). Growth arrest was reversible, in that withdrawal of HS5-CM for 6 days allowed a resumption of growth, while maintaining cell sensitivity to re-added CM. (Supplementary Figure S1).
Figure 1. HS5-CM induces G1-phase growth arrest of ER-positive cell lines.
A. Growth at 7 days of breast cancer cells in the presence or absence of 30% HS5-CM. 400 cells were seeded with exception of T47D (1000 cells). Mean and standard deviation of 3 independent experiments (except T47D, n=5) is shown. Experiments are graphed together and normalized to control (no HS5-CM) for each cell line for clarity. Growth in arbitrary units (a.u.) representing Cyquant assay fluorescence, *p<0.05, **p<0.01 by T-Test. B. HS5-CM inhibitory effects dominate over KM101 CM stimulatory effects. MCF7 cells were exposed to 40% MCF7 CM, 20%MCF7+20%HS5-CM, 20% MCF7 + 20% KM101 CM, or to 20% HS% +20% KM101 CM. Growth over 7 days in arbitrary units (a.u.) in Cyquant assays (mean+/−SEM, n=2). *p<0.05; **p<0.01; ***p<0.001 by one way ANOVA and Tukey’s post-test. C. Induction of p21-cdk2 complex formation by HS5-CM. MCF7 immunoprecipitates and immunodepleted lysates as indicated. In each experiment, lysates were harvested 20 hours after HS5-CM addition. D. Suppression of Rb hyperphosphoryation in MCF7 and T47D cells by HS5-CM. E. HS5-CM coordinately activates p38, Erk and AKT, manifested by increase in phosphorylation. Total and phosphorylated protein levels are from MCF7 cells harvested 24 hours after treatment with control medium or 30% HS5-CM as indicated (representative of 4 or more experiments. F. HS5-CM activates AKT through the IGF1/insulin axis. MCF7 cells were incubated with medium, HS5-CM or the IGF1R/IR-inhibitor OSI-906 as indicated for 20 hours then harvested.
We compared the effect on MCF7 growth of conditioned medium from HS-5 cells with CM from an equal number of KM101 cells [21], another marrow-derived fibroblastic line that expresses (data not shown) surface markers CD73- and CD105. In contrast to HS5-CM, the CM from KM101 cells increased cancer cell growth. However, when growth-stimulatory CM was mixed with HS5-CM, the mixture inhibited MCF7 growth (Figure 1B). The dominant suppressive effect of HS5-CM on growth indicated an active function rather than absence of stimulatory signals, suggesting that HS5 could model stromal components that enforce the cellular dormancy of bone metastases.
Growth inhibition was associated with formation of p21-cdk2 complexes (Figure 1C) and decreased Rb phosphorylation (Figure 1D).
Dormant tumor cells have been characterized to have a low pErk1/2 to phospho-p38 ratio [25–27] and HS5 cell phosphorylation of p38 in co-cultured cancer cells has been linked previously to growth arrest by HS5 [19]. We observed durable upregulation of phospho-p38 in MCF7 cells exposed to HS5-CM (Figure 1E), however p38 activity was not required for HS5 to inhibit growth (Supplementary Figure S2). Moreover, HS5-CM also increased phospho-Erk such that the pErk1/2 to phospho-p38 ratio was unaltered with growth arrest. In addition to pErk, HS5-CM also activated the AKT growth/survival pathway, durably phosphorylating AKT. AKT phosphorylation was durable out to 7 days when growth assays were harvested (data not shown). Upregulation of AKT by HS5-CM could be inhibited by the IGF1/insulin receptor blocker OSI-906, implicating IGF1 as the paracrine activator of AKT (Figure 1F).
Given that growth arrest was sustained despite activation of signaling pathways associated with proliferation and survival, we sought to determine factors in the CM responsible for inhibiting MCF7 cell growth. HS5-CM has been previously reported to inhibit PC3 prostate cell proliferation via BMP7 in a Noggin-sensitive manner[24]. However, we noted no reversal of HS5-CM suppression of MCF7 growth when using Noggin to block BMPs (Supplementary Figure S3).
We assayed the HS5-CM for the expression of 39 cytokines as candidate growth inhibitors using Luminex assays. Of these, HS5-CM cytokines at ng/ml concentrations that were highly (>50x) enriched compared with KM101 CM included GCSF, CXCL1, IL6, IL8, CCL2, CCL7, GMCSF; these did not inhibit MCF7 growth (data not shown)). HS5-CM also contained IL1 (α, 120 pg/ml; β, 8 pg/ml) that has been reported to have growth suppressive effects against MCF7[28].
In order to determine whether IL1 signaling accounted for the antiproliferative effect of HS5-CM, we tested whether the IL1 antagonist IL1RA restored MCF7 growth in HS5-CM. IL1RA partially reversed growth suppression (Figure 2A, D) when added at a level that prevented both HS5-CM and IL1β from inducing lipocalin 2 (LCN2, a known target of IL1β[29] (Supplementary Figure S4A, B)). The co-addition of IL1RA and HS5-CM increased cell cycling as manifest by fewer cells in G0/G1 and more cells in G2/M phase (Figure 2B). IL1RA decreased HS5-CM induction of p21 and restored Rb hyperphosphorylation in HS5-CM treated cells. Although IL1RA increased cell cycling, it did not prevent HS5 phosphorylation of p38, further indicating that growth inhibition was not maintained by p38 stress signaling (Supplemental Figure S4C).
Figure 2. HS5-CM suppression of MCF7 growth is dependent on IL1.
A. IL1RA reversal of HS5-CM mediated growth suppression as shown by crystal violet staining. MCF7 cells were stained on 15 day post indicated treatment. B. Blockade of IL1 signaling by IL1RA partially reverses cell cycle effects of HS5-CM. Cells were treated with 1uM nocodazole for 24 hrs prior to analysis. Data are expressed as mean +/− SD of 3 independent experiments (day 5 n=2, day 7 n=1). There was no significant difference between any of the groups in the sub G1 phase. In S phase only the comparison between control and HS5-CM treated cells was significant, p=0.040. All groups except for control compared to IL1 RA were significantly different in the G0/G1 and G2/M phases (one way Anova, Tukey post-test correction). C. Immunofluorescence detection of IL1β (green) and IGF-1 (red) in stromal cells. D. Effect of stromal CM’s on MCF7 growth, with or without IL1-signaling blockade. Cells were treated on days 0 and 3 with IL1 β (100 pg/ml) or with 30% CM from indicated stromal cells (adjusted to 1 ml CM per 500K stromal cells) with or without IL1RA (50 ng/ml) as indicated. 3–4 independent experiments shown, see Supplemental methods for analysis. E. Growth at 7 days of MCF7 or MDA-MB-231 cells exposed to HS5- or hFOB CM (20%) or medium (DMEM, DMEM/F12 respectively) alone, or to 100pg/ml or 100 ng/ml IL1β. Comparisons for each cell line by 1-way ANOVA with Tukey’s correction for multiple comparisons. Two independent experiments. *p<0 .05, **p<0.01, ***p<0.001.
We measured whether other bone marrow stromal cell CM’s inhibited MCF7 growth in a IL1RA-sensitive way. These included CM from KM101 (above), the hFOB1.1.9 osteoblastic cell line [30] that had been combined with HS5 and HUVEC cells in a biomatrix rendering embedded breast cancer cells dormant in vivo [19], and the HS27 cell line that had been co-derived from marrow with HS5 but differs in its cytokine profile and ability to support hematopoietic progenitor cells [16, 17]. Figure 2C demonstrates that each of these cell lines express IGF1 but that only HS5 and hFOB expressed IL1β.
The expression of IL1β correlated with the ability of stromal-CM to suppress MCF7 growth. Figure 2D demonstrates that recombinant IL1β as well as HS5-CM and hFOB-CM inhibited MCF7 growth, whereas KM101- and HS27-CM did not. Suppressive CM was partially reversed by IL1RA, confirming IL1 as contributer to paracrine growth inhibition by both HS5 and hFOB. Figure 2E demonstrates that sensitivity to IL1β distinguishes the HS5-CM-suppressed MCF7 cells from non-suppressed MDA-MB-231 cells.
Estrogen receptor-alpha protein destabilization by conditioned medium from HS5 cells
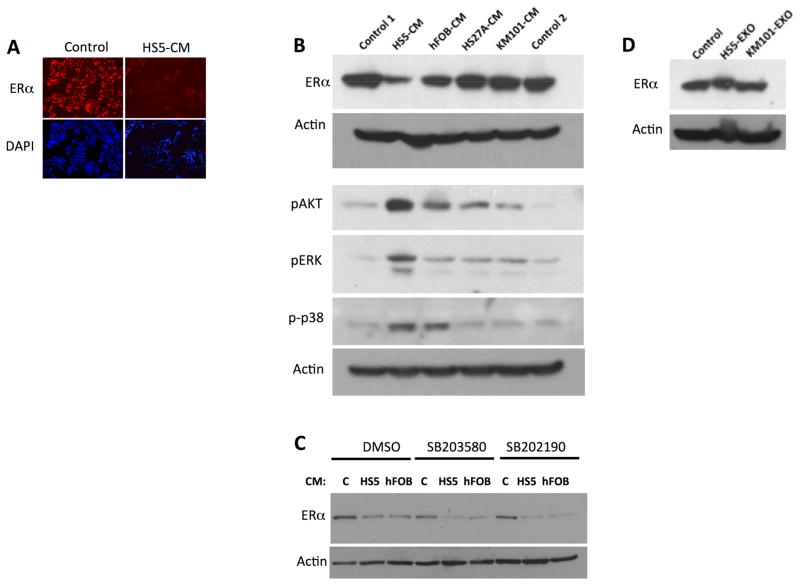
Having established that the HS5 secretome was growth-inhibitory, we proceeded to investigate its effect on ER expression. We measured whether HS5-CM inhibited the expression of ER, comparing it to CM from the other stromal cell lines used in growth assays. Figure 3A demonstrates loss of ER by immunofluorescence and 3B by immunoblotting in MCF7 cells exposed for 16 hours to HS5-CM. ER protein levels were decreased on average 9.9-fold by HS5-CM (n=11) and to a lesser extent (2.9-fold, n=4) by hFOB-CM. Notably, hFOB-CM caused phosphorylation of Akt, pErk and p38 in MCF7, similarly to HS5-CM (Figure 3A and Supplemental figure S8D). Conditioned medium from KM101 and HS27A cell lines had minimal effect on ER levels in MCF7. The KM101- and HS27A-CM did not phosphorylate p38 (Figure 3A), unlike the CM’s that suppressed ER. This suggested that the p38 pathway could be involved in stromal suppression of ER. However, neither the SB20358 nor SB202190 p38 inhibitors (added 10 minutes prior to CM at 10 uM) prevented ER suppression by HS5-CM (Figure 3C). This indicated that p38 pathway activation was unlikely to mediate ER suppression.
Figure 3. Down-regulation of Estrogen Receptor by CM from HS5 and hFOB stromal cells.
A. Immunofluorescent stain of MCF7 cells exposed to control medium or to HS5-CM. ER immunostaining (above); Hoechst stain shown below. B. Down-regulation of ER in MCF7 by HS5-CM and hFOB CM and not by KM101, or HS27A. Two exposures of ER immunoblotting shown. Cells were incubated with 30% CM for 16 hours before harvest. CM’s and medium controls (1, DMEM, 10% serum; 2, DMEM/F12 10% serum) were added to 30% v/v. Actin loading control shown for each of two parallel gels. C. Vehicle control (v/v, DMSO) and p38 inhibitors at 10uM did not prevent ER downregulation by HS5 and hFOB CM. 20 hour incubation of MCF7 with CM. D. PBS control or 10μg/ml of exosomes from HS5 and from (control) KM101 had no effect on ER expression.
Because exosomes from fibroblasts in primary ER-negative tumors have been reported to suppress ER expression [13], we also tested whether exosomes from HS5 mediated the observed down-regulation of ERs. Figure 3D demonstrates that HS5 exosomes (HS5 exo) did not mediate ER suppression.
We tested whether HS5-CM destabilized ER alpha protein. The ability of the proteasome inhibitor MG132 to restore ER protein expression in HS5-CM-treated breast cancer cells (Figure 4A) indicated that the stability of the estrogen receptor protein could be compromised by the stromal cell CM. Figure 4B demonstrates increased ubiquitination of immunoprecipitated estrogen receptor from MCF7 cells exposed to HS5-CM, compared to control medium. Consistent with this, HS5-CM exposure increased high molecular weight forms evident in long exposure of ER immunoblots (Supplemental Figure S5a). Global proteasome activity did not change with HS5-CM exposure (Supplemental Figure S5b). We measured MCF7 ER levels in the presence of cycloheximide with or without HS5-CM to determine decay rates. Destabilization of ER by HS5-CM is shown in Figure 4C (representative Western blots in Figures 4D and S5). The degradation rate is compatible with a decrease in the calculated half-life of ER from 10.72 hours (95% confidence interval (9.19,12.59) in the absence of HS5-CM to 3.76 hours (95% confidence interval 3.44, 4.00) in the presence of HS5-CM (p=0.008, mixed effects analysis of covariance model). In addition to tripling the degradation rate of ER protein, HS5-CM also lowered the level of MCF7 mRNA (3.9-fold by qPCR). Unlike growth arrest, the inhibition of ER transcript did not require IL1 (Figure 4E).
Figure 4. Promotion of ER turnover by HS5-CM.
A. Suppression of ER by HS5-CM was reversed by MG132 (5ug/ml). B. Anti-ubiquitin immunoblot of control- or HS5-CM exposed MCF7 cell lysates immunoprecipitated with Ig or anti-ER as noted. C. Decay curve for ER in control or HS5-CM. Time after cycloheximide as indicated, see text for details. Mean and SD of 4 experiments (2 for time points 0.5 and 8 hours). Difference between the change in ER/Ponceau over time between control and HS5-CM-treated cells was assessed by a mixed effects analysis of covariance model, as detailed in Supplementary Statistical Methods. D. Example blot from cycloheximide study. E. Inhibition of ER mRNA by HS5-CM. Representative RT-PCR of 3 endpoint and 2 qPCR experimental replicates.
It was conceivable the estrogen or estrogenic compounds produced by HS5 cells could explain the enhanced ER degradation in cells exposed to HS5-CM. However, ER levels continued to be suppressed by HS5-CM subjected to charcoal stripping to remove estrogens (data not shown). Moreover, transcription-associated ER phosphorylation (S118) was decreased by HS5-CM treatment and the addition to MCF7 of HS5-CM did not activate expression of a 3X-ERE-luc reporter sensitive to ligand activation of ER signaling (Supplementary Figure S6).
Estrogen receptor suppression does not depend on HS5-upregulated microRNAs
Fibroblasts from ER-negative tumors have been found to inhibit expression of ER in MCF7 by exosomal transfer of mir221/222 [13], and miR 221/222 expression in MDA-MB-268 breast cancer line suppresses ER translation [12]. Exosomal miR transfer in HS5-CM was unlikely because HS-5 exosomes did not suppress ER. However, we found that miR222 was elevated 2-fold in MCF7 cells exposed to HS5-CM (Supplementary Table 3), raising the prospect that miRs upregulated by HS5-CM contributed to ER loss independently of ubiquitin-mediated turnover. In fact, HS5-CM lowered by ~35% the expression of a luciferase reporter under the control of the ER 3′-UTR that is the site of miR action. However, inhibition of miR222 and other HS5-upregulated miRs (miR 132, 29a, 29b) did not prevent HS5-CM from suppressing ER, indicating that miRs, if involved, were a minor contributor to this paracrine down-regulation of ER (Supplementary Figure S7). In addition to blocking these microRNAs, inhibitors of multiple pathways that have been linked to ER down-regulation were tested but did not reverse ER loss in MCF7exposed to HS5 and/or hFOB CM (Supplemental Figure S8).
Uncoupling of growth arrest and ER down-regulation
A reasonable presumption is that ER down-regulation accounted for the growth arrest of MCF7 cells by HS5-CM. Given that HS5-CM suppressed growth largely through IL1, it would follow that IL1 contributed to ER loss and that forced ER expression would prevent arrest by HS5 CM or IL1. We found, however that addition of IL1 did not suppress ER protein (Figure 5B). Moreover, IL1RA restored growth to HS5-CM-treated cells (Figure 2) without re-expression of ER (Figure 5A, B, Supplementary Figure S8). IL1RA did not alter induction of phospho-Akt, Erk or p38 by HS5- or hFOB-CM (Figure 5C); our data link those pathways to HS5-CM effects on growth (below) but not to ER loss (Supplemental Figure 8D) in MCF7. In order to determine whether exogenous ER could rescue cells from HS5-mediated growth arrest, we stably transfected MCF7 cells with a GFP-ER fusion protein. The GFP-positive population expressed ER at a high level, with continued expression after HS5-treatment. Nonetheless, these ER-expressing cells also were growth-arrested when exposed to the conditioned medium (Figure 5D). We confirmed that ER-expression could not rescue growth in HS5-CM treated MCF7 in experiments using tetracycline-inducible HA-tagged ER (Figures 5E,F). These findings indicated that a decrease in ER was not necessary or sufficient for HS5-mediated growth arrest.
Figure 5. ER expression level is unrelated to MCF7 growth suppression by HS5-CM.
A. Suppression of ER by HS5-CM was not reversed by IL1 blockade. MCF7 or T47D cells were incubated for 24 hours with HS5-CM with or without IL1RA as indicated. B. HS5-CM suppressed ER in MCF7 cells whereas IL1 (100 pg/ml) did not. C. Downstream phosphorylation by ER-suppressive HS5- and hFOB CM is not altered by IL1 blockade. MCF7 were treated for 20 hours with the indicated CM. FF, foreskin fibroblast line; these non-marrow fibroblasts lacked the paracrine pAKT/pERK/p38-stimulating ability of HS5 and hFOB. Ponceau stain shows immunoblot loading over an area spanning the probed bands. D. Exogenous expression of ER did not prevent HS5-CM induced growth suppression. Parental MCF7 cells (Control) or flow-sorted stable clones expressing GFP or GFP-ER were incubated with or without HS5-CM in 7-day Cyquant assays (technical replicates of one experiment shown). E. MCF7 cells stably expressing tetracycline-inducible, HA-tagged wild-type estrogen receptor alpha (wt ER-inducible) or vector control (mCherry-inducible) were exposed to doxycycline (Doxy, 0.5 ug/ml) followed by medium alone or HS5-CM (30%) as indicated; Cyquant assessment of growth at 4 days. F. WB demonstrates induction of ER concurrent with experiment “E” with sustained exogenous HA-tagged ER expression in the presence of HS5-CM.
Estrogen-independence of HS5-CM-primed luminal breast cancer cell lines rescued from IL1 growth suppression
Because ER expression remained low in HS5-CM-treated cancer cells in which IL1RA rescued growth, we investigated whether these rescued cells exhibited a reduced dependence on estrogen.
We assayed the growth in estrogen-depleted medium of MCF7 and T47D cells in the presence or absence of IL1RA and/or of CM from HS5 cultured in estrogen-depleted medium. The cells exposed to HS5-CM combined with IL1RA had a growth advantage in the absence of estrogen (Supplemental Figure S9). Under conditions of estrogen depletion, HS5-CM repressed ER and activated MEK/ERK, AKT and p38 MAPK pathways (Figure 6A), similarly to findings in estrogen-replete culture (Figure 1E). To further investigate the requirement for AKT, p38 and ERK activation in stromal CM promotion of MCF7 estrogen-independent growth, we added pathway inhibitors to MCF7 cells exposed to HS5-CM and IL1RA under estrogen-free conditions. This is shown by crystal violet staining (Figure 6B) and quantitation of staining in Figure 6C. The AKT-inhibitor perifostine prevented MCF7 outgrowth under these conditions. We additionally tested whether the IGF1 inhibitor OSI-906 (linsitinib) that blocked AKT activation also prevented MCF7 growth in HS5-exposed, IL1-blocked MCF7. It appeared that IGF1/insulin signaling was required for HS5-CM to augment growth of MCF7 in the absence of estrogen. MEK/ERK signaling was also involved in the promotion of estrogen-independent growth, given that the MEK inhibitor U0126 prevented the stimulation of growth. In contrast, p38 was not required for outgrowth of the estrogen-independent cells since IL1RA-augmented growth was unencumbered by the p38-inhibitor SB203580 (Figure 6C).
Figure 6. HS5-CM supports estrogen-independent growth when IL1 is blocked.
A. Effect of IL1RA and HS5-CM on indicated proteins in estrogen-free medium; cells were pre-incubated in estrogen-free medium for 2 days and treated as indicated for 2 days before harvest. B. Crystal violet staining of MCF7 growth (4000 cells seeded) under estrogen free conditions with or without HS5-CM, IL1RA, or both, in the presence of inhibitors directed at AKT (perifostine, 5 uM), MEK/ERK (U0126), p38 (SB203580, 2 uM) or IGF1R (OSI906, 1 uM) pathways. C. Quantitation of crystal violet staining, experiments as in B; HS5-CM plus IL1RA indicated by HI. Analysis by mixed effects ANOVA, mean+/− SEM shown, n=8 independent experiments; pairwise comparisons between treatments were adjusted for multiplicity by Westfall’s method (see Supplemental methods). *p<0 .05, **p<0.01, ***p<0.001
Fulvestrant-resistance of stromal CM-primed luminal breast cancer cell lines rescued from IL1 growth suppression
Given the enhanced ability of HS5-CM/IL1RA-treated MCF7 and T47D cells to grow in estrogen-depleted conditions, we explored whether these treatments sustained growth in the presence of the ER degrader ICI182,780 (fulvestrant) used for cancer recurrence or for progression through antiestrogen therapy. Cells cultured in complete medium were seeded for growth assays in the presence of vehicle, HS5-CM, IL1RA or HS5-CM and IL1RA. A dose-response assay for growth was performed using fulvestrant (ICI). The addition of IL1RA to HS5-CM increased growth as expected. ICI was less effective against T47D and MCF7 cells when they were exposed to HS5-CM or to the combination of HS5-CM and IL1RA as was evident by the significantly blunted dose-response slope relative to control (no additions) or to IL1RA alone (Figure 7). The data indicate that ER suppression in HS5-CM cells was sufficient to undermine responses to ER-targeted treatment when the cells were released from growth suppression by IL1RA.
Figure 7. Fulvestrant resistance in cells growing in HS5-CM and IL1RA.
MCF7 and T47D cells were cultured for 14 days in the presence or absence of IL1RA (50 ng/ml) or 30% HS5-CM with indicated concentrations of ICI 182,780 (ICI). Growth was measured using Cyquant assays. ICI stock was serially diluted so that equivalent ICI volume was added to each well. Mean and SD shown for 4 independent experiments. The rates of change were characterized by a nonlinear mixed effects model, as described in the Supplementary Statistical Methods. N.S., non-significant, *p<0.05, ***p<0.001.
Discussion
This manuscript reports increased degradation of ER protein in breast cancer cells exposed to the secretome of HS5 stromal cells, a cell line with functional [16, 17] and phenotypic [18] similarities to marrow stromal cells present in biopsies. Cancer cells exposed to this conditioned medium were growth arrested. Unexpectedly, the growth arrest did not result from ER loss, but was mediated primarily by IL1 in the CM. Our results would support the possibility that ER-negative metastases arising from ER-expressing disseminated tumor cells undergo conversion in response to paracrine signals while arrested in the bone microenvironment. Given the prognostic importance of ER-loss in recurrent disease[2], it is important to distinguish cell autonomous versus microenvironment-mediated contributions to this phenotypic shift.
Conditioned medium from HS5 cells increased ubiquitination of ER in MCF7 cells and lowered ER levels in a proteasome-dependent manner. We have noted upregulation of multiple proteasome component genes in a preliminary investigation of the HS5-CM- induced transcriptome (data not shown) and considered whether proteosomal activity was globally increased. However, no difference in 20S proteasome activity occurred with MCF7 treatment by HS5-CM. Investigation of ER-specific ubiquitination/degradation pathways is underway.
ER loss was augmented by a decrease in ER mRNA in MCF7 cells after HS5-CM treatment. We have investigated whether cytokines that have been reported to inhibit transcription of the ER gene—EGF[31], IGF-1[32], Oncostatin M[33], FGF7 [34], TGFβ [35], BMP7[36] or IL1 [37, 38]--mediated the inhibitory effect of HS5-CM on ER but could not link the ER loss to any of these cytokines using neutralizing antibodies or specific inhibitors. We recently became aware that while this project was underway, Lang et. al. reported loss of ER-positive fluorescent staining in MCF7 cells exposed to HS5 co-culture in microfluidics channels under estrogen-free conditions [39]. They ascribed ER loss to paracrine activation of ER signaling. This is less likely under our conditions in which HS5-CM lowered transcription-associated ER-S118 phosphorylation (in contrast to [39]), did not activate an ERE-reporter in MCF7 and suppressed ER whether HS5-CM was charcoal-stripped or not, or medium contained estrogen or not.
Concurrent with ER loss, ER-positive breast cancer cell lines (MCF7 and T47D) underwent growth arrest when exposed to CM from HS5 and hFOB cells. This extends prior reports that mixed HS5/hFOB/breast cancer cell co-culture inhibited breast cancer growth[19] [20].
In the growth-arrested state, exposure to the stromal CM primed cells for future growth by activating the MEK/ERK and AKT pathways in the cancer cells. These pathways support ER-mediated proliferation and are typically activated through alternative means in cells escaping dormancy or anti-estrogen treatment[40], [41, 42] [43]. In our study, these activated pathways were necessary to increase estrogen-independent growth of the MCF7 cells. Cancer cell outgrowth in estrogen-depleted conditions therefore only required blockade of anti-proliferative stromal signals and paracrine activation of MEK/ERK and AKT signaling and so did not require re-expression of ER.
The stromal CM support of estrogen-independent MCF7 or T47D growth that we observed is compatible with in vivo studies in which co-injection of cancer-activated stromal fibroblasts[44] or of a mesenchymal stem cell subset[45] with MCF7 rendered the cells hormone-independent in xenograft models.
A limitation of this in vitro study is that these in vitro results do not clarify why ER loss only occurs in a subset of bone metastatic cancer. It is possible that cells lose ER during the metastatic process rather than dormancy, seeding ER-negative cells from ER-heterogeneous primary tumor or losing ER during epithelial mesenchymal transition early in metastasis [8]. ER could be lost because of cancer-autonomous mutations (e.g. p53 [46]) at a point in the metastatic process. Alternatively, cancer cells that convert could have associated stochastically with a distinct subset of stromal cells that induce ER loss. We examining whether surface markers shared by ER-suppressing (HS5, hFOB) but not non-suppressing (KM101, HS27A) stromal cell lines are indicators of a suppressive capability. Ultimately, it will be necessary to identify the marrow stromal subset sharing the HS5 phenotype and function and demonstrate its co-localization with ER-negative but not ER-positive metastatic cells in clinical samples.
We showed that growth arrest involved paracrine IL1 from HS5 or from the pre-osteoblastic stromal line hFOB. IL1 has been variably reported to inhibit[28, 38, 47] or to promote[48]) MCF7 growth. In our study, the ability of the IL1 receptor antagonist (IL1RA) to reverse growth arrest confirmed IL1 as a paracrine inhibitor of cancer cell growth by HS5 and by hFOB stromal cells. The osteoblastic niche supports quiescent hematopoietic stem cells and is targeted for colonization by competing cancer cells [49]. Replicative marking of melanoma [50] and myeloma [51] cells has shown osteoblasts to support rare quiescent cancer cells. It has been established that metastatic breast cancer cells home to the osteoblastic niche and that it can provide a suitable environment for growth [43]. Whether disseminated breast cancer cells exploit this niche during dormancy and whether localized IL1 is engaged in the commitment to dormancy or whether stromal IL1 signals are inhibited by growth stimuli in vivo will require further study.
The growth suppressive role of IL1 in HS5-CM that we report adds to the findings of other (p38, ALK5-dependent) growth inhibitory pathways activated during cell-cell contact of HS5 cells with SUM159 breast cancer cells[19]. Our inhibitor experiments appeared to rule out p38- as a mediator of the paracrine suppression of MCF7 or T47D growth by HS5. Although a low phospho-Erk/phospho-p38 ratio has been validated as a determinant of dormancy in such in vitro models and subsequently in vivo [25], we demonstrated that stromal-derived IL1 is capable of arresting cells in which both pErk and p-p38 are high. Additionally, high levels of phospho-p38 did not prevent cycling when IL1 was blocked. Given that we focused on paracrine signaling, it is possible that cell-cell interactions engage additional pathways such that p38 becomes a necessary and dominant mediator of IL1 effects or that IL1 is uncoupled from growth control.
Growth arrest was not secondary to down-regulation of ER in the MCF7 or T47D cells exposed to HS5-CM. Although one report has indicated that high dose IL1 (~10× the concentration in HS5-CM) inhibited growth and lowered ER by 40% in MCF7 [37, 38], our data showed that IL1 contributed to growth inhibition but was not involved in ER down-regulation. ER remained low when IL1 signaling was blocked. Exogenous ER could not rescue growth whereas IL1 receptor antagonist could, indicating ER-independence of growth arrest. It is notable that ER down-regulation did not contribute to growth arrest, suggesting that disseminated cancer cells do not depend on anti-ER treatment to exit the cell cycle in inhibitory microenvironments.
IL1 receptors and IL1/IL1RA cytokines have been identified in the primary tumor microenvironment in human breast cancer [52]. IL1 has been associated with either a pro-tumorigenic- or anti-tumorigenic inflammatory environment in vivo. For instance, tumor growth of MCF xenograft implants stably expressing IL1α exceeds that of controls [53] and knockout mice for IL1α and β are resistant to syngeneic melanoma angiogenesis and spread [54]. In contrast, exogenous IL1alpha and β inhibit syngeneic tumor growth [55–57].
It is possible that IL1 contributes to quiescence of disseminated cancer cells in bone (as opposed to BMP-mediated quiescence that was shown to occur in lung but not bone metastases [58, 59]), and that loss of IL1 signaling facilitates emergence from quiescence. Although stromal cell and hematopoietic cell production of IL1 ex vivo has been described[60], there is little information on IL1 expression by stroma in the endosteal niche targeted by bone metastatic breast cancer. Our data demonstrating that stromal IL1 is antiproliferative is consistent with prolonged relapse-free survival associated with high IL1β-expressing tumors in 1802 ER+ breast cancer patients[61] (IL1b, 39402_at, using the online survival tool kmplot.com/breast, accessed 12/5/2015). A suppressive role for IL1 in human breast cancer relapse is also supported by a recent report that stromal IL1β expression is correlated with longer relapse-free and overall survival in breast carcinomas [62]. An analysis of women with favorable Mammoprint profiles of primary breast tumors found IL1β and IL1R1 to be associated with non-recurrence [63]. A caveat is that the applicability of IL1 expression levels in tumors to that in the microenvironment of disseminated cancer cells is tenuous.
The HS5 and hFOB stromal cells produced not only growth inhibitory IL-1 but also IGF1 that selects for bone metastatic clones [64] and activates AKT, a driver of micrometastases [43]. IGF1, pERK and pAKT were required for outgrowth of (ER-suppressed) clones but only did so if suppressive IL1 was blocked. The dominant effect of IL1 raises the possibility that IL1 is a contributor to dormancy of disseminated luminal breast cancer cells in bone. In that case, forced exit from dormancy using IL1RA could render otherwise chemoresistant, dormant cells sensitive to therapy. Mouse models have shown IL1RA to be anti-tumorigenic; on the other hand, high circulating IL1RA has been observed in cancer patients and linked to tumor load (reviewed in [65, 66]). We note that pretreatment with IL1RA enhanced melanoma sensitivity to chemotherapy in mice [67]. Clinical trials using IL1RA (anakinra, Kineret) as adjuvant therapy in metastatic breast (NCT01802970) or other (NCT01624766, NCT02550327, NCT02090101) cancers are under way.
It would be interesting if our finding of a both pro- and anti-oncogenic paracrine signaling from a bone marrow stromal line recapitulates an in vivo function of HS5-like stromal cells in the disseminated tumor cell setting. In such a case, the growth-arrested state would represent a “yin-yang” situation in which latent oncogenic capabilities masked by dormancy, and new phenotypes (such as ER suppression) can manifest once growth is initiated.
Supplementary Material
Acknowledgments
This project was supported by NIH grant R01CA154728 (RAS). The project used the UPCI Biostatistics Facility and Cancer Proteomics Facility: Luminex Core that are supported in part by award P30CA047904. We thank Drs. Steffi Oesterreich and Tim Burns (University of Pittsburgh) for helpful comments, Dr. Vera Donnenberg for assistance with flow cytometry and for HFF1 foreskin fibroblasts, and Lucas Santana-Santos for his work on the microRNA analysis algorithm. In addition, we appreciate the generosity of Didier Picard (Universite de Geneve) for the gift of the ER-3′-UTR reporter plasmid; Dr. Sarat Chandarlapaty (Sloan Kettering Cancer Center) for MCF7 cells stably expressing inducible estrogen receptor or vector (mCherry) control; and University of Pittsburgh investigators Steffi Oesterreich for T47D cells, Michael Epperly for KM101 cells and Adrian Lee for phospho-IGF1R antibody. The 3× ERE TATA luc was a gift from Donald McDonnell (Addgene plasmid # 11354) and pEGFP-C1-ER alpha was a gift from Michael Mancini (Addgene plasmid # 28230). We thank Yingjian Li (University of Pittsburgh) for assistance in exosome preparation. The use of the fluorescence plate reader in the laboratory of Dr. Ben van Houten (University of Pittsburgh) is also appreciated.
Footnotes
None of the authors have conflicts of interest with any material in this report.
References
- 1.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, Oudejans J, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12(5):R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoefnagel LD, Moelans CB, Meijer SL, van Slooten HJ, Wesseling P, Wesseling J, Westenend PJ, Bart J, Seldenrijk CA, Nagtegaal ID, et al. Prognostic value of estrogen receptor alpha and progesterone receptor conversion in distant breast cancer metastases. Cancer. 2012;118(20):4929–4935. doi: 10.1002/cncr.27518. [DOI] [PubMed] [Google Scholar]
- 3.Fehm T, Krawczyk N, Solomayer EF, Becker-Pergola G, Durr-Storzer S, Neubauer H, Seeger H, Staebler A, Wallwiener D, Becker S. ERalpha-status of disseminated tumour cells in bone marrow of primary breast cancer patients. Breast Cancer Res. 2008;10(5):R76. doi: 10.1186/bcr2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 5.Ogba N, Manning NG, Bliesner BS, Ambler SK, Haughian JM, Pinto MP, Jedlicka P, Joensuu K, Heikkila P, Horwitz KB. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res. 2014;16(6):489. doi: 10.1186/s13058-014-0489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Slingerland JM. Links between oestrogen receptor activation and proteolysis: relevance to hormone-regulated cancer therapy. Nat Rev Cancer. 2014;14(1):26–38. doi: 10.1038/nrc3622. [DOI] [PubMed] [Google Scholar]
- 7.Macaluso M, Montanari M, Noto PB, Gregorio V, Bronner C, Giordano A. Epigenetic modulation of estrogen receptor-alpha by pRb family proteins: a novel mechanism in breast cancer. Cancer Res. 2007;67(16):7731–7737. doi: 10.1158/0008-5472.CAN-07-1476. [DOI] [PubMed] [Google Scholar]
- 8.Dhasarathy A, Kajita M, Wade PA. The transcription factor snail mediates epithelial to mesenchymal transitions by repression of estrogen receptor-alpha. Mol Endocrinol. 2007;21(12):2907–2918. doi: 10.1210/me.2007-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan X, Zhou T, Tai YH, Wang C, Zhao J, Cao Y, Chen Y, Zhang PJ, Yu M, Zhen C, et al. Elevated expression of CUEDC2 protein confers endocrine resistance in breast cancer. Nat Med. 2011;17(6):708–714. doi: 10.1038/nm.2369. [DOI] [PubMed] [Google Scholar]
- 10.Fan M, Bigsby RM, Nephew KP. The NEDD8 pathway is required for proteasome-mediated degradation of human estrogen receptor (ER)-alpha and essential for the antiproliferative activity of ICI 182,780 in ERalpha-positive breast cancer cells. Mol Endocrinol. 2003;17(3):356–365. doi: 10.1210/me.2002-0323. [DOI] [PubMed] [Google Scholar]
- 11.Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci U S A. 1999;96(5):1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klinge CM. miRNAs and estrogen action. Trends Endocrinol Metab. 2012;23(5):223–233. doi: 10.1016/j.tem.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah SH, Miller P, Garcia-Contreras M, Ao Z, Machlin L, Issa E, El-Ashry D. Hierarchical paracrine interaction of breast cancer associated fibroblasts with cancer cells via hMAPK-microRNAs to drive ER-negative breast cancer phenotype. Cancer Biol Ther. 2015;16(11):1671–1681. doi: 10.1080/15384047.2015.1071742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol. 2004;24(11):4605–4612. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sflomos G, Dormoy V, Metsalu T, Jeitziner R, Battista L, Scabia V, Raffoul W, Delaloye JF, Treboux A, Fiche M, et al. A Preclinical Model for ERalpha-Positive Breast Cancer Points to the Epithelial Microenvironment as Determinant of Luminal Phenotype and Hormone Response. Cancer Cell. 2016;29(3):407–422. doi: 10.1016/j.ccell.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85(4):997–1005. [PubMed] [Google Scholar]
- 17.Iwata M, Sandstrom RS, Delrow JJ, Stamatoyannopoulos JA, Torok-Storb B. Functionally and phenotypically distinct subpopulations of marrow stromal cells are fibroblast in origin and induce different fates in peripheral blood monocytes. Stem Cells Dev. 2014;23(7):729–740. doi: 10.1089/scd.2013.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwata M, Torok-Storb B, Wayner EA, Carter WG. CDCP1 identifies a CD146 negative subset of marrow fibroblasts involved with cytokine production. PLoS One. 2014;9(10):e109304. doi: 10.1371/journal.pone.0109304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, Mariotti V, Buchupalli B, Foster K, Bonnet D, et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 2013;73(23):6886–6899. doi: 10.1158/0008-5472.CAN-13-0991. [DOI] [PubMed] [Google Scholar]
- 20.Cavnar SP, Rickelmann AD, Meguiar KF, Xiao A, Dosch J, Leung BM, Cai Lesher-Perez S, Chitta S, Luker KE, Takayama S, et al. Modeling selective elimination of quiescent cancer cells from bone marrow. Neoplasia. 2015;17(8):625–633. doi: 10.1016/j.neo.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FitzGerald TJ, Santucci MA, Harigaya K, Woda B, McKenna M, Sakakeeny MA, Pierce JH, Kase K, Holland CA, Greenberger JS. Radiosensitivity of permanent human bone marrow stromal cell lines: effect of dose rate. Int J Radiat Oncol Biol Phys. 1988;15(5):1153–1159. doi: 10.1016/0360-3016(88)90198-8. [DOI] [PubMed] [Google Scholar]
- 22.Kruger S, Abd Elmageed ZY, Hawke DH, Worner PM, Jansen DA, Abdel-Mageed AB, Alt EU, Izadpanah R. Molecular characterization of exosome-like vesicles from breast cancer cells. BMC Cancer. 2014;14:44. doi: 10.1186/1471-2407-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shelke GV, Lasser C, Gho YS, Lotvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208(13):2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63(7):1684–1695. [PubMed] [Google Scholar]
- 26.Chery L, Lam HM, Coleman I, Lakely B, Coleman R, Larson S, Aguirre-Ghiso JA, Xia J, Gulati R, Nelson PS, et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget. 2014;5(20):9939–9951. doi: 10.18632/oncotarget.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA. ERK1/2 and p38alpha/beta signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res. 2011;17(18):5850–5857. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paciotti GF, Tamarkin L. Interleukin-1 directly regulates hormone-dependent human breast cancer cell proliferation in vitro. Mol Endocrinol. 1988;2(5):459–464. doi: 10.1210/mend-2-5-459. [DOI] [PubMed] [Google Scholar]
- 29.Cowland JB, Muta T, Borregaard N. IL-1beta-specific up-regulation of neutrophil gelatinase-associated lipocalin is controlled by IkappaB-zeta. J Immunol. 2006;176(9):5559–5566. doi: 10.4049/jimmunol.176.9.5559. [DOI] [PubMed] [Google Scholar]
- 30.Harris SA, Enger RJ, Riggs BL, Spelsberg TC. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J Bone Miner Res. 1995;10(2):178–186. doi: 10.1002/jbmr.5650100203. [DOI] [PubMed] [Google Scholar]
- 31.Stoica A, Saceda M, Doraiswamy VL, Coleman C, Martin MB. Regulation of estrogen receptor-alpha gene expression by epidermal growth factor. J Endocrinol. 2000;165(2):371–378. doi: 10.1677/joe.0.1650371. [DOI] [PubMed] [Google Scholar]
- 32.Stoica A, Saceda M, Fakhro A, Joyner M, Martin MB. Role of insulin-like growth factor-I in regulating estrogen receptor-alpha gene expression. J Cell Biochem. 2000;76(4):605–614. doi: 10.1002/(sici)1097-4644(20000315)76:4<605::aid-jcb9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 33.West NR, Murphy LC, Watson PH. Oncostatin M suppresses oestrogen receptor-alpha expression and is associated with poor outcome in human breast cancer. Endocr Relat Cancer. 2012;19(2):181–195. doi: 10.1530/ERC-11-0326. [DOI] [PubMed] [Google Scholar]
- 34.Chang HL, Sugimoto Y, Liu S, Ye W, Wang LS, Huang YW, Lin YC. Keratinocyte growth factor (KGF) induces tamoxifen (Tam) resistance in human breast cancer MCF-7 cells. Anticancer Res. 2006;26(3A):1773–1784. [PubMed] [Google Scholar]
- 35.Petrel TA, Brueggemeier RW. Increased proteasome-dependent degradation of estrogen receptor-alpha by TGF-beta1 in breast cancer cell lines. J Cell Biochem. 2003;88(1):181–190. doi: 10.1002/jcb.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi M, Otsuka F, Miyoshi T, Otani H, Goto J, Yamashita M, Ogura T, Makino H, Doihara H. Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation. J Endocrinol. 2008;199(3):445–455. doi: 10.1677/JOE-08-0226. [DOI] [PubMed] [Google Scholar]
- 37.Danforth DN, Jr, Sgagias MK. Interleukin 1 alpha blocks estradiol-stimulated growth and down-regulates the estrogen receptor in MCF-7 breast cancer cells in vitro. Cancer Res. 1991;51(5):1488–1493. [PubMed] [Google Scholar]
- 38.Danforth DN, Jr, Sgagias MK. Interleukin-1 alpha and interleukin-6 act additively to inhibit growth of MCF-7 breast cancer cells in vitro. Cancer Res. 1993;53(7):1538–1545. [PubMed] [Google Scholar]
- 39.Lang JD, Berry SM, Powers GL, Beebe DJ, Alarid ET. Hormonally responsive breast cancer cells in a microfluidic co-culture model as a sensor of microenvironmental activity. Integr Biol (Camb) 2013;5(5):807–816. doi: 10.1039/c3ib20265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keshamouni VG, Mattingly RR, Reddy KB. Mechanism of 17-beta-estradiol-induced Erk1/2 activation in breast cancer cells. A role for HER2 AND PKC-delta. J Biol Chem. 2002;277(25):22558–22565. doi: 10.1074/jbc.M202351200. [DOI] [PubMed] [Google Scholar]
- 41.Svensson S, Jirstrom K, Ryden L, Roos G, Emdin S, Ostrowski MC, Landberg G. ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene. 2005;24(27):4370–4379. doi: 10.1038/sj.onc.1208626. [DOI] [PubMed] [Google Scholar]
- 42.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1(9):707–717. [PubMed] [Google Scholar]
- 43.Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, Zhao H, Zhao Z, Du S, Tao J, et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell. 2015;27(2):193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto MP, Badtke MM, Dudevoir ML, Harrell JC, Jacobsen BM, Horwitz KB. Vascular endothelial growth factor secreted by activated stroma enhances angiogenesis and hormone-independent growth of estrogen receptor-positive breast cancer. Cancer Res. 2010;70(7):2655–2664. doi: 10.1158/0008-5472.CAN-09-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, Tilghman SL, Salvo VA, Fonseca JP, Lacey MR, et al. Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat. 2010;121(2):293–300. doi: 10.1007/s10549-009-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs-Young R, Shirley SH, Lambertz I, Colby JK, Tian J, Johnston D, Gimenez-Conti IB, Donehower LA, Conti CJ, Hursting SD. P53 genotype as a determinant of ER expression and tamoxifen response in the MMTV-Wnt-1 model of mammary carcinogenesis. Breast Cancer Res Treat. 2011;130(2):399–408. doi: 10.1007/s10549-010-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sgagias MK, Kasid A, Danforth DN., Jr Interleukin-1 alpha and tumor necrosis factor-alpha (TNF alpha) inhibit growth and induce TNF messenger RNA in MCF-7 human breast cancer cells. Mol Endocrinol. 1991;5(11):1740–1747. doi: 10.1210/mend-5-11-1740. [DOI] [PubMed] [Google Scholar]
- 48.Sosnoski DM, Norgard RJ, Grove CD, Foster SJ, Mastro AM. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin Exp Metastasis. 2015;32(4):335–344. doi: 10.1007/s10585-015-9710-9. [DOI] [PubMed] [Google Scholar]
- 49.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z, Orlowski RZ, Wang M, Kwak L, McCarty N. Osteoblastic niche supports the growth of quiescent multiple myeloma cells. Blood. 2014;123(14):2204–2208. doi: 10.1182/blood-2013-07-517136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawson MA, McDonald MM, Kovacic N, Hua Khoo W, Terry RL, Down J, Kaplan W, Paton-Hough J, Fellows C, Pettitt JA, et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat Commun. 2015;6:8983. doi: 10.1038/ncomms9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, Barrows G, Kreutzer DL. The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol. 2003;23(2):269–284. [PubMed] [Google Scholar]
- 53.Kumar S, Kishimoto H, Chua HL, Badve S, Miller KD, Bigsby RM, Nakshatri H. Interleukin-1 alpha promotes tumor growth and cachexia in MCF-7 xenograft model of breast cancer. Am J Pathol. 2003;163(6):2531–2541. doi: 10.1016/s0002-9440(10)63608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100(5):2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saito M, Fan D, Lachman LB. Antitumor effects of liposomal IL1 alpha and TNF alpha against the pulmonary metastases of the B16F10 murine melanoma in syngeneic mice. Clin Exp Metastasis. 1995;13(4):249–259. doi: 10.1007/BF00133480. [DOI] [PubMed] [Google Scholar]
- 56.Pezzella KM, Neville ME, Huang JJ. In vivo inhibition of tumor growth of B16 melanoma by recombinant interleukin 1 beta. I. Tumor inhibition parallels lymphocyte-activating factor activity of interleukin 1 beta proteins. Cytokine. 1990;2(5):357–362. doi: 10.1016/1043-4666(90)90066-3. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura S, Nakata K, Kashimoto S, Yoshida H, Yamada M. Antitumor effect of recombinant human interleukin 1 alpha against murine syngeneic tumors. Jpn J Cancer Res. 1986;77(8):767–773. [PubMed] [Google Scholar]
- 58.Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH, Giancotti FG. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150(4):764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez J, Zhang XH. BMP/Coco antagonism as a deterministic factor of metastasis dormancy in lung. Breast Cancer Res. 2013;15(1):302. doi: 10.1186/bcr3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bodo M, Baroni T, Tabilio A. Haematopoietic and stromal stem cell regulation by extracellular matrix components and growth factors. J Stem Cells. 2009;4(1):57–69. [PubMed] [Google Scholar]
- 61.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez-Garcia B, Eiro N, Miranda MA, Cid S, Gonzalez LO, Dominguez F, Vizoso FJ. Prognostic significance of inflammatory factors expression by stroma from breast carcinomas. Carcinogenesis. 2016;37(8):768–776. doi: 10.1093/carcin/bgw062. [DOI] [PubMed] [Google Scholar]
- 63.Mittempergher L, Saghatchian M, Wolf DM, Michiels S, Canisius S, Dessen P, Delaloge S, Lazar V, Benz SC, Tursz T, et al. A gene signature for late distant metastasis in breast cancer identifies a potential mechanism of late recurrences. Mol Oncol. 2013;7(5):987–999. doi: 10.1016/j.molonc.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang XH, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, Smid M, Foekens JA, Massague J. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell. 2013;154(5):1060–1073. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48. doi: 10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29(2):317–329. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Triozzi PL, Aldrich W. Effects of interleukin-1 receptor antagonist and chemotherapy on host-tumor interactions in established melanoma. Anticancer Res. 2010;30(2):345–354. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.