Abstract
TMEM16A (ANO1), the pore-forming subunit of calcium-activated chloride channels, regulates several physiological and pathophysiological processes such as smooth muscle contraction, cardiac and neuronal excitability, salivary secretion, tumour growth, and cancer progression. Gating of TMEM16A is complex because it involves the interplay between increases in intracellular calcium concentration ([Ca2+]i), membrane depolarization, extracellular Cl− or permeant anions, and intracellular protons. Our goal here was to understand how these variables regulate TMEM16A gating and to explain four observations. a) TMEM16A is activated by voltage in the absence of intracellular Ca2+. b) The Cl− conductance is decreased after reducing extracellular Cl− concentration ([Cl−]o). c) ICl is regulated by physiological concentrations of [Cl−]o. d) In cells dialyzed with 0.2 µM [Ca2+]i, Cl− has a bimodal effect: at [Cl−]o < 30 mM TMEM16A current activates with a monoexponential time course, but above 30 mM [Cl−]o ICl activation displays fast and slow kinetics. To explain the contribution of Vm, Ca2+ and Cl− to gating, we developed a 12-state Markov chain model. This model explains TMEM16A activation as a sequential, direct, and Vm-dependent binding of two Ca2+ ions coupled to a Vm-dependent binding of an external Cl− ion, with Vm-dependent transitions between states. Our model predicts that extracellular Cl− does not alter the apparent Ca2+ affinity of TMEM16A, which we corroborated experimentally. Rather, extracellular Cl− acts by stabilizing the open configuration induced by Ca2+ and by contributing to the Vm dependence of activation.
Keywords: Chloride channel, kinetics, patch clamp, permeation, gating, mathematical modelling
INTRODUCTION
Cl− movements through Ca2+ activated chloride (Cl−) channels (CaCCs) play important roles in many cellular functions such as regulation of smooth muscle contraction, regulation of cardiac and neuronal excitability, salivary secretion, and tumour growth and progression in many types of cancer [13, 21, 29, 40]. TMEM16A (ANO1) and TMEM16B (ANO2) are the pore-forming subunits of CaCCs [6, 45, 50]. The gating mechanism of these channels is quite complex. Gating requires increases in intracellular calcium concentration ([Ca2+]i), but also involves membrane depolarization, extracellular Cl− or permeant anions, and intracellular protons. Ca2+-dependent activation is coupled to Vm [27, 35, 49] and is regulated by intracellular protons that compete for Ca2+ binding [1, 8]. When [Ca2+]i is in the range of 0.1 – 0.8 µM, channel gating is strongly Vm-dependent, and the I–Vm relationships show outward rectification. Such Vm-dependence is lost when [Ca2+]i >5 µM. Hence, the dose-response curve to [Ca2+]i displays weak Vm-dependence [2, 49, 52]. This Vm-dependence is explained by a voltage-dependent Ca2+ dissociation [49]. We have shown that TMEM16A and TMEM16B exhibit fast and slow gating modes [11]. Both modes depend on intracellular Ca2+, membrane voltage (Vm) and the extracellular Cl− concentration ([Cl−]o). Apart from the Ca2+-dependent activation, TMEM16A can also be activated by strong depolarizations in the absence of intracellular Ca2+ [49].
We assume that direct binding of Ca2+ activates TMEM16A [2, 3, 27]. This idea is supported by the following observations: a) injection of Ca2+, Ba2+ or Sr2+ into Xenopus oocytes activates CaCC [32]; b) TMEM16A in excised patches or liposomes can be activated by Ca2+, Ba2+ or Sr2+ [34, 46, 49]; c) mutating residues E702, E705, E730 and D734 located in the Ca2+ binding pocket of TMEM16A decrease the Ca2+ sensitivity 100 – 1000-fold [47, 52]; d) the x-ray structure of the Nectria haematococca TMEM16 homolog (nhTMEM16) shows the presence of two Ca2+ ions bound to a site located within the membrane [5]; e) changing the nhTMEM16 Ca2+-coordinating residues significantly reduces the functional Ca2+ sensitivity, which strongly indicates these residues are part of a high-affinity Ca2+ binding site [5, 34, 47]; and f) vertebrate TMEM16A is activated in a cooperative manner by Ca2+ [49]. Also, other regions of TMEM16A including the N-terminus and the first intracellular loop have been shown to contribute to Ca2+ sensitivity [17, 49]. Thus, the existing evidence strongly suggests that TMEM16A is directly activated by binding at least two Ca2+ ions.
The role of permeant ions in channel gating has increasingly been recognized. Gating of Cl− channels such as ClC-2, volume-sensitive channels, and CFTR are reported to be dependent on permeant anion [23, 25, 41, 51]. CaCCs are not the exception. Anions with a higher permeability than Cl− such as SCN−, I−, and NO−3 when applied from the extracellular side promote opening and accelerate the opening rate while slowing down the closing rate in CaCCs [4, 36, 38, 49]. In addition, these anions decrease the EC50 for Ca2+ both in Xenopus oocyte CaCCs as well as heterologous-expressed TMEM16A and TMEM16B [4, 38, 39]. These observations suggest gating and ion permeation are coupled in TMEM16A channels.
The rather complex gating of TMEM16 channels cannot be understood without the help of a model. In this work, we developed a 12-state Markov chain model to explain the TMEM16A gating mechanism. The model reproduces the complex TMEM16A activation in response to Vm, Ca2+ and different [Cl−]o. The model also predicts that extracellular Cl− does not alter the channel’s affinity for Ca2+. Further experiments corroborated this prediction. We propose that TMEM16A is activated by sequential, direct, Vm-dependent binding of two Ca2+ ions coupled by a Vm-dependent binding of one external Cl− ion.
MATERIALS AND METHODS
Culture of HEK 293 cells and expression of TMEM16A
Human embryonic kidney 293 cells (HEK293) were cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco BRL, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum, 1% gentamicin and 1% L-glutamine at 37 C° in a 95% O2/5% CO2 atmosphere. Stable cell lines were developed by transfecting a cDNA encoding the mouse TMEM16A (ac variant) sub-cloned into the bi-cistronic expression vector pIRES2-EGFP (Clontech, Mountain View, CA, USA.). The PolyFect transfection reagent (Qiagen, Valencia, CA, USA) was used according to the manufacturer’s specification. After 24 h, the cells were transferred to 24-well plates at low density in DMEM medium supplemented with 1 mg/ml G418 (Sigma- Aldrich Co. St. Louis, MO, USA.). G418-resistant transformants were identified by EGFP fluorescence and expanded. Stable cell lines were maintained in medium supplemented with G418 at 500 µg/ml. Cells were seeded at low density on 5 mm circular coverslips before electrophysiological recordings.
Recording solutions
Table 1 lists the composition of external solutions (ES) and internal solutions (IS) used to record the Cl− current activated by intracellular Ca2+ or Vm (ICl). IS with buffered Ca2+ was prepared using EGTA and Ca2+; the free [Ca2+]i was estimated using MAXCHELATOR (maxchelator.stanford.edu). ES-140Cl and IS-40Cl/0.2Ca are control solutions. An IS with 5 µM [Ca2+]i (IS-40Cl/5Ca) was used to activate fully TMEM16A and record ICl with long depolarizations in cells bathed with ES-140Cl. To analyse the activation of TMEM16A in zero [Ca2+]i we used IS-40Cl/0Ca containing 25.2 mM EGTA and 50 mM HEPES. The Cl− dependence of activation was studied using ES containing 140, 109, 70.5, 30, 10 or 1.5 mM [Cl−]o. The pH of each solution was adjusted to 7.3 with TEA-OH or NaOH. ES was made hypertonic relative to the internal solutions to avoid activation of volume-sensitive Cl− channels present in HEK293 cells [23]. Osmolarity was adjusted by adding D-mannitol and measured using the vapour pressure point method (VAPRO, Wescor Inc., South Logan, UT, USA). All chemicals were purchased from Sigma-Aldrich (Co. St. Louis, MO, USA.).
Table 1. Recording solutions.
External solutions with different [Cl−] were prepared by mixing solutions ES-136Cl−, ES-10Cl− and ES-1.5Cl− to obtain the required Cl− concentration.
| External solutions (mM) | |||||
|---|---|---|---|---|---|
| Compound | ES-140Cl | ES-140Na | ES-10Cl | ES-1.5Cl | ES-30Cl |
| TEA-Cl | 139 | - | 9 | 0.5 | 29 |
| NaCl | 139 | - | - | ||
| CaCl2 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| HEPES | 20 | 20 | 20 | 20 | 20 |
| D-Mannitol | 100 | 100 | 300 | 320 | 330 |
| Osmolarity (mosm/kg) | 390 | 390 | 340 | 340 | 390 |
| Total [Cl−] | 140 | 140 | 10 | 1.5 | 30 |
| Internal solutions (mM) | |||||||
|---|---|---|---|---|---|---|---|
| Compound | IS-40Cl/0Ca | IS-40Cl/0.2Ca | IS-40Cl/0.8Ca | IS-40Cl/1.3Ca | IS-40Cl/5Ca | IS-40Cl/12Ca | IS-83Cl/0.2Ca |
| TEA-Cl | 40 | 30 | 17 | 27.4 | 11.8 | 2 | 72.5 |
| CaCl2 | - | 5.2 | 11.5 | 6.3 | 14.1 | 19 | 5.2 |
| HEPES | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| D-Mannitol | 90.2 | 85 | 150 | 154 | 85 | 85 | - |
| EGTA-TEA | 25.2 | 25.2 | 25 | - | - | 25.2 | |
| HEDTA | - | - | - | 25 | 25 | 25 | - |
| Osmolarity (mosm/kg) | 290 | 290 | 290 | 290 | 290 | 290 | 288 |
| Free [Ca2+] (µM) | 0 | 0.2 | 0.8 | 1.3 | 5 | 12 | 0.2 |
| Total [Cl−] | 40 | 40 | 40 | 40 | 40 | 40 | 83 |
Electrophysiological recordings
We record ICl at room temperature using the whole cell configuration of the patch clamp technique. Cells were held at −60 mV and the Vm was changed stepwise from −100 mV to +160 mV or +180 mV in 20 mV increments, and then returned to −60 mV or −100 mV. Pulse duration varied between 0.5 to 20 s. ICl was recorded with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA); currents were filtered at 5 kHz and digitized at 10 kHz for 0.5 s pulses. For 20 s pulses, the currents were filtered at 1 kHz and digitized at 2 kHz using pClamp software. Errors due to liquid junction potentials (−4.8 to −6.6 mV for our recording solutions) were minimized by using a 0.5 M KCl agar-bridge to ground the recording chamber and not corrected.
The effects of [Cl−]o on TMEM16A activation were determined by measuring the magnitude of tail currents (Itail) at −100 mV after a depolarising test pulse to +120 mV whose duration was varied from 0 to 3 s. The initial magnitude of Itail after each pulse and each [Cl−]o was used to calculate the tail conductance (Gtail) as a function of time and [Cl−]o using Equation 1.
| Equation 1 |
where Vrev is the reversal potential of ICl. G(t,[Cl−]o) was then normalized to the conductance obtained at the end of a 3 s test pulse with 140 mM Cl−. The resulting normalized G vs time curve represented the time course of TMEM16A activation at +120 mV at each [Cl−]o. Dose-response curves were constructed using recordings obtained from patches excised from cells stably expressing TMEM16A. Patches were held at the desired Vm and exposed to increasing [Ca2+]i ranging from 0 to 12 µM (Table 1) using a Fast-Step Perfusion System (VC-77SP Warner Instruments, Hamden, CT, USA). In between each [Ca2+]i test solution, a control solution containing 0 Ca2+ and 25.2 mM EGTA was applied to check for changes in seal resistance. ICl was recorded with an Axopatch 200B amplifier, filtered at 1 kHz and digitized at 10 kHz.
Analysis and modelling
The magnitude of ICl at each Vm was converted to conductance using Equation 1. Normalization was carried out using either G+120mV or Gmax to obtain GNorm (G/Gmax or G/ G+120mV), which is proportional to the apparent open probability of the channel. We extracted the time constants by fitting the data to a mono or bi-exponential function of the form:
| Equation 2 |
where y0 is the value of y when t→∞, t is the time, τ is a time constant, a and b are the contribution of fast and slow processes, and τf and τs are the fast and slow time constants [11], respectively. The dose-response curves were constructed using normalized currents; only patches with stable basal current obtained with 0 [Ca2+]i were used. In each case the basal current was subtracted to obtain the magnitude of the current activated by a given [Ca2+]i. The currents obtained from a particular patch were normalized to the maximum current estimated using equation:
| Equation 3 |
where Imax is the estimated maximum current, EC50 is the [Ca2+]i needed to obtain Imax=0.5, and nH is the Hill coefficient. To search for a kinetic scheme able to describe the time- and Vm-dependencies of ICl collected under a variety of experimental conditions we used Markov chain kinetic models [9] because they provide a good description of gating properties for different channels [24, 26, 28, 43, 48]. Different models with various discrete closed (C) and open (O) states were built using IChMASCOT software [10, 42]. Experimental and model ICl vs Vm, GNorm vs [Ca2+]i, and EC50 were compared. Finally, by computing the probability of occupation of each of the kinetic states as a function of time we aimed to find the activation route(s) of TMEM16A. In the model, a given transition was controlled by forward and backward rate constants, which were Vm-dependent in most cases:
| Equation 4 |
where kf and kr are forward and backward rate constants, respectively, kf,0 and kr,0 are the values of kf and kr at Vm=0, zf, zr are the apparent gating charges associated with a given transition, F is the Faraday constant, R is gas constant, and T is the temperature. The most likely values of the rate constant parameters at Vm = 0 mV for a given model were obtained by best-fits as described in [12]. For that purpose, the squared differences between experimental and modelled values were minimized as follows:
| Equation 5 |
and
| Equation 6 |
where N = number of different data sets included in the global fitting procedure; Mi = number of independent experimental observations in the dataset i; Eij = experimental values of a given observation, Sij = modelled values of a given observation; Wi = normalized weight factor for specific dataset; wi = arbitrary weight factor for the measurement i; and Eimax = maximal experimental value for the dataset i. The global fit was repeated 15 times. The resulting values were averaged and then used for the final fit to obtain the values reported in Table 2. Subsequently, we simulated the steady-state activation properties of TMEM16A using the IonChannelLab software [44] along with the rate constants previously determined with IChMASCOT. The experimental conditions to be tested such as [Ca2+]i, [Cl−]o, [Cl−]i, and Vm were included. To determine the most likely activation pathway, we calculated the probability (P) that TMEM16A stayed in a given state of a chosen gating kinetic model. For that purpose, the differential equations describing the time dependence of P (Equations 1 – 12 in Supplemental material) were numerically integrated using the Gear’s BDF method. P was calculated for 20 s depolarizations with 0.2 µM [Ca2+]i, 140 mM and 30 mM [Cl−]o and 40 mM [Cl−]i and for 1 s depolarizations with 0 µM [Ca2+]i, 140 mM and 30 mM [Cl−]o and 40 mM [Cl−]i using the rate constants listed in Table 2.
Table 2. Parameter values associated with the rate constants of Scheme IV.
(A) Figures listed were obtained from the global fit of TMEM16A data shown in Figures 5 and 6. z values are the apparent charge. (B) The relationship between forward and backward rate constants and weight factors l, L, h, H, m and M—which take into account the presence of Ca2+ in a given state. Grey boxes include weight factor values obtained from the global fit. Values shown in Italics and parenthesis (A and B) are averaged values obtained during the first 15 fit trials and were used as a seed for the final global fit. All listed parameter values were used for simulations of Scheme IV shown in Figures 7, 8 and 9.
| A | |||||||
| Rate Constant | Expression | Parameter | Parameter Values At 0 mV | ||||
| α1 |
|
α1,0 (s−1) | 0.0077 (0.00803±0.00064) | ||||
| zα | 0 (0±0) | ||||||
| β1 |
|
β1,0 (s−1) | 917.1288 (875.07±51.77) | ||||
| zβ | 0.0064 (0.01133±0.0021) | ||||||
| kO1 |
|
kO1,0(s−1 µM−1) | 597.9439 (583.58±14.41) | ||||
| z1 | 0 (0±0) | ||||||
| kO2 |
|
kO2,0 (s−1) | 2.8530 (2.8547±0.0189) | ||||
| z2 | 0.1684 (0.166±0.00118) | ||||||
| αCl1 |
|
k1,0 (s−1) | 1.8872 (1.9349±0.0708) | ||||
| zl1 | 0.1111 (0.1141±0.00403) | ||||||
| βCl1 |
|
k2,0 (s−1) | 5955.783 (5769.96±207.83) | ||||
| zl2 | 0.3291 (0.3272±0.00346) | ||||||
| kCCl1 |
|
k+Cl,0(s−1 mM−1) | 1.143×10−6 (1.14×10−6±9.17×10−8) | ||||
| z+Cl | 0.1986 (0.2112±0.0238) | ||||||
| kCCl2 |
|
k−Cl,0 (s−1) | 0.0009 (8.52×10−4±1.28×10−5) | ||||
| z−Cl,0 | 0.0427 (0.04847±0.0098) | ||||||
| kOCl1 |
|
k+Ol,0 (s−1 mM−1) | 1.1947 (1.1495±0.0459) | ||||
| z+Ol | 0.6485 (0.65±0.0026) | ||||||
| kOCl2 |
|
k−Ol,0 | 3.4987 (3.443±0.1096) | ||||
| z−Ol | 0.0300 (0.0308±0.00054) | ||||||
| B | |||||||
| α2 = lα1 | α3 = l2α1 | β2 = Lβ1 | β3 = L2β1 | ||||
| kCCl3 = hkCCl1 | kCCl5 = h2kCCl1 | kCCl4 = HkCCl2 | kCCl6 = H2kCCl2 | ||||
| kOCl3 = mkOCl1 | kOCl5 = m2kOCl1 | kOCl4 = MkOCl2 | kOCl6 = M2kOCl2 | ||||
|
|
|
|
kO2Cl = kO2 | ||||
|
|
|
βCl2 = hLβCl1 | βCl3 = (hL)2βCl1 | ||||
| kC1 = kO1 |
|
l = 41.6411 (42.04±1.7) | m= 0.0102 (0.010±0.00023) | h= 0.3367 (0.342±0.018) | |||
| L= 0.1284 (0.134±0.004) | M= 0.0632 (0.064±0.001) | H= 14.2956 (14.2±0.70) | |||||
Figures and fits were constructed using Origin (Origin Lab, Northampton, MA). Data were plotted as mean ± SEM of n (number of independent experiments). Dashed black lines and arrows in each Figure indicate ICl=0. Where necessary, a Student t-test was used to evaluate statistically significant differences between data sets at P<0.05; as indicated by the * symbol.
RESULTS
Here we characterized the effects of Vm and [Cl−]o on TMEM16A gating. We used these data to build a kinetic model to explain the contribution of Vm, Ca2+, and Cl− to TMEM16A gating. Finally, using the model we predict the most likely pathway taken by the channel during activation.
Activation of TMEM16A in the absence of intracellular Ca2+
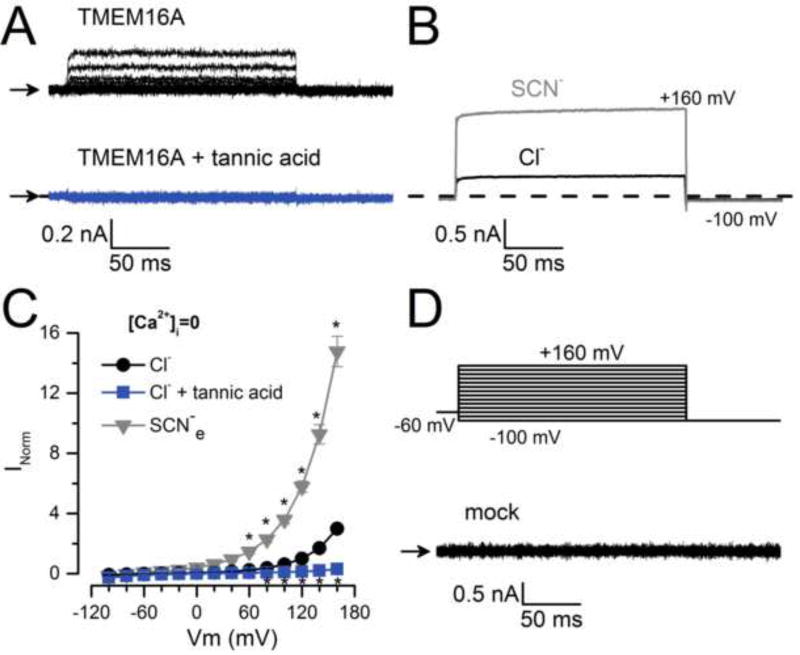
Under physiological conditions, CaCCs are activated by depolarization and increases in [Ca2+]i [2, 27, 31, 35]. However, TMEM16A can be activated even when no Ca2+ is present on the cytosolic side [49]. Here we recorded whole cell Cl− currents (ICl) in the absence of intracellular Ca2+ (with 25.2 mM EGTA). ICl displays fast onset kinetics and lacks an inward tail (Itail) current upon repolarization to −100 mV (Figure 1A, black traces). The current is inhibited with 100 µM tannic acid, a TMEM16A blocker (Figure 1A, bottom panel). To strengthen the assertion that the current was carried through TMEM16A in the absence of intracellular Ca2+, we replaced external Cl− with SCN−, a more permeable anion that accelerates opening and retards channel closing [36, 49]. Figure 1B shows representative current traces recorded at +160 mV from a cell bathed first with 140 mM Cl− then 140 mM SCN−. This manoeuvre increased the current (Figures 1B and 1C, triangles), consistent with SCN− permeating through TMEM16A. However, we could not determine a definite reversal potential of the current in zero [Ca2+]i to calculate the permeability ratio PSCN/PCl. In contrast, ICl was negligible at every Vm in cells transfected with an empty vector (Figure 1D). These results indicate that the ICl recorded in zero Ca2+ is flowing through TMEM16A activated by Vm and not through some other endogenous conductance.
Fig. 1. Activation of TMEM16A in zero intracellular Ca2+.
(A) Representative ICl recordings (n=5) obtained from HEK293 cells expressing TMEM16A, bathed in a solution containing 0 (black traces) or 100 µM tannic acid (grey traces). (B) Representative current traces (n=5) obtained from the same cell bathed in 140 mM Cl− (black) or 140 mM SCN− (grey). Currents evoked by pulses to +160 mV followed by repolarization to −100 mV. (C) ICl−Vm relationships from cells bathed in [Cl−]o=140 mM (circles), [Cl−]o=140 mM + 100 µM of tannic acid (squares), or 140 mM SCN− (triangles). For each cell, whole cell currents were normalized to the current magnitude recorded at +120 mV from control cells bathed in [Cl−]o=140 mM. Normalized currents from different cells were averaged and plotted. (D) Lack of ICl (n=6) in mock transfected cells dialyzed with a solution containing 40 mM [Cl−]i. In A and D the [Cl−]o was 140 mM and the ICl were generated using the protocol shown in D. In all experiments, cells were dialyzed with 0 µM [Ca2+]i +40 mM [Cl−]i (IS-40Cl/0Ca).
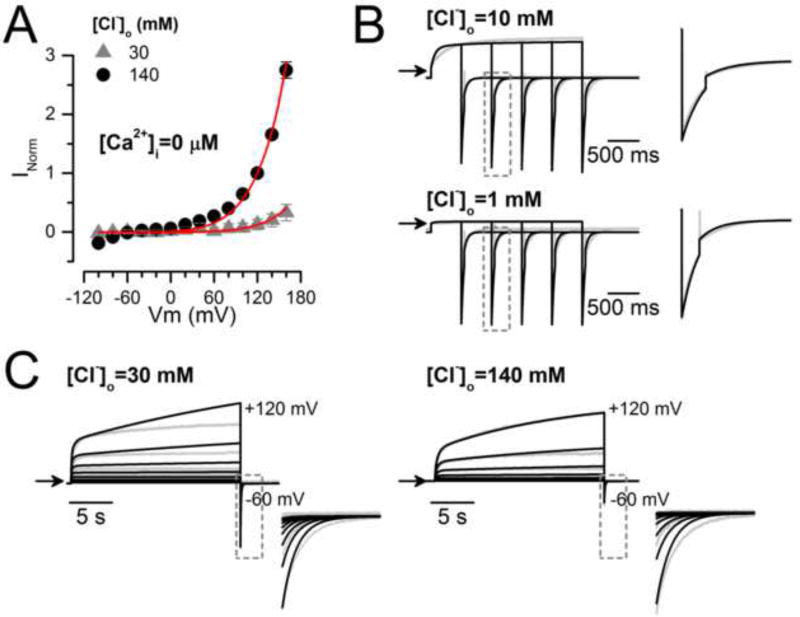
Cl−-dependence of TMEM16A gating
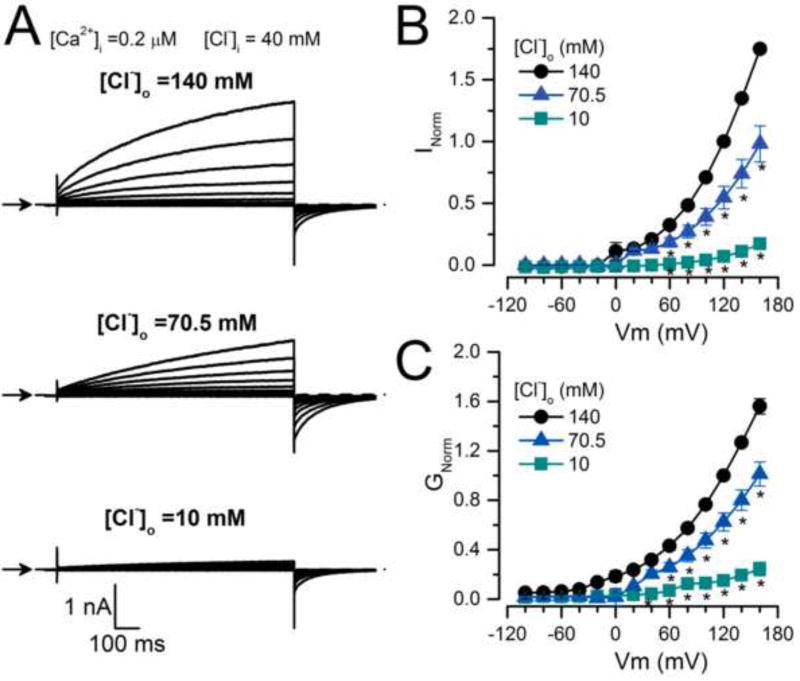
To analyse the effect of extracellular Cl− we recorded ICl at different [Cl−]o. Figure 2A shows examples of ICl recorded from the same cell dialyzed with an IS containing 0.2 µM [Ca2+]i, 40 mM [Cl−]i, that was sequentially exposed to 140 mM, 70 mM and 10 mM [Cl−]o. The ICl magnitude decreased when [Cl−]o was reduced. This is summarized in the ICl vs Vm curves in Figure 2B. Since reduction of [Cl−]o from 140 mM to 10 mM increased the driving force (Vm−Vrev) from −92.6 ± 2.5 mV to −146.2 ± 4.6 mV, we expected a 1.6-fold increase in Itail amplitude at −100 mV. However, Itail in 10 mM Cl− did not increase; instead it fell drastically; the mean Itail_10mM/Itail_140mM ratio was 0.78 ± 0.05 (n=7). The time constant values of Itail at −100 mV after the pre-pulse to +160 mV were 47 ± 4 ms and 43.7 ± 3.1 ms with 140 mM and 10 mM [Cl−]o, respectively, indicating no change in kinetics. When the conductance was calculated using the current magnitude at the end of each pulse and normalized using the value at +120mV (GNorm), we observed a 5-fold conductance drop at each Vm (Figure 2C) when [Cl−]o was reduced from 140 mM to 10 mM. Thus, at low external [Cl−]o the apparent open probability of TMEM16A declines.
Fig. 2. TMEM16A chloride conductance depends on [Cl−]o.
(A) Representative ICl recordings (n=7) obtained from the same cell sequentially bathed in ES containing 140, 70.5 and 10 mM [Cl−]o and dialyzed with an IS containing 0.2 µM [Ca2+]i + 40 mM [Cl−]i (IS-40.5Cl/0.2Ca). Cells were held at −60 mV, then stepped for 0.5 s to voltages ranging from −100 mV to +160 mV in 20 mV increments and repolarised to −100 mV. Note that Itail at −100 mV did not increase despite the increased driving force for Cl− (i.e. driving force = −92.6 ± 2.5 mV with [Cl−]o 140 mM and −146.2 ± 4.6 mV with 10 mM [Cl]o). (B) ICl vs Vm relationships at the indicated [Cl−]o. (C) GNorm vs Vm curves at the indicated [Cl−]o. ICl and G were normalized to either ICl or G obtained at +120 mV with [Cl−]o=140 mM. External solutions with different [Cl−]o were prepared by mixing ES-136Cl and ES-1.5Cl.
To determine the effects of [Cl−]o on time-dependent activation we recorded Itail from the same cell sequentially exposed to 140, 109, 70, 30, 10 and 1.5 mM. The cells were dialyzed with an IS containing 0.2 µM Ca2+ and 40 mM Cl−. Figure 3A shows representative recordings. Tail currents were obtained at −100 mV after a +120 mV depolarization as shown in the voltage protocol. In this case, we lengthened the depolarization from 0.5 s to 3 s in 0.5 s increments. At 140 mM [Cl−]o the Itail amplitude (indicated by arrows) increased with longer pulses. In contrast, with 10 mM [Cl−]o the Itail magnitude was smaller. This finding could be explained if gating is dependent on [Cl−]o. In Figure 3B, GNorm at the indicated [Cl−]o is plotted as a function of pulse duration. The graph shows that decreasing the [Cl−]o from 140 mM to 10 mM produces a 3- fold reduction in GNorm. A 5-fold decrease in GNorm is observed when comparing 140 mM with 1.5 mM [Cl]o. At [Cl−]o ≤ 30 mM, GNorm levelled off and the GNorm vs time curves were described by a single exponential function (solid lines) with τ = 293.7 ± 17 ms and 334 ± 19.6 ms for 1.5 mM and 10 mM [Cl−]o, respectively. However, with [Cl−]o >30 mM, only the initial part (time ≤ 0.5 s) of the curves was described by a single exponential because as pulse duration increased GNorm rose in an almost linear fashion. τ = 368.2 ± 23 ms and 353.4 ± 23.1 ms for 109 mM and 140 mM [Cl−]o, respectively.
Fig 3. Extracellular Cl− regulates TMEM16A gating.
(A) ICl recordings from the same cell bathed in 140 mM (left) or 10 mM [Cl−]o (right). ICl was recorded using the Vm protocol shown in the inset. The duration of the test pulse increased from 0 to 3 s in 0.5 s steps. Arrows indicate the peak current amplitudes at −100 mV. (B) The magnitude of Itail recorded as in A was used to determine the conductance at each time and [Cl−]o (n=5). GNorm was calculated using the G obtained with [Cl−]o=140 mM and a 3s depolarization. Note that a single exponential can not describe the entire time course (continuous lines) when [Cl−]o ≥ 30 mM. (C) GNorm(t) plots obtained from a cell stimulated with a 20 s depolarization to +140 mV and dialyzed with 0.2 µM Ca2+ (IS-40.5Cl/0.2Ca). The cell was exposed to 140 mM (left, ES-140Cl) and then to 30 mM (right, ES-30Cl) [Cl−]o. ICl was converted to G(t) and then normalized using the G value at +140 mV to determine GNorm(t) at each [Cl−]o (n=4–8). GNorm(t) plots were fit with a bi-exponential function (continuous lines) to obtain τs, τf and fractional contribution of each component. Note that the y-scale bar is 1 and 0.1 for 140 and 30 mM Cl−, respectively. (D) TMEM16A-ICl recorded from cells dialyzed with 5 µM [Ca2+]i (IS-40Cl/5Ca) and depolarized to +100 mV. ICl from each cell was normalized to ICl obtained with a 20 s depolarization and then normalized values were averaged (n=8). The continuous line is the fit to a mono-exponential function.
In cells bathed with a solution containing 140 mM or 30 mM [Cl−]o we extended the duration of the Vm pulses to 20 s and found that ICl displayed a second mode of gating, something we previously reported [11]. Figure 3C shows that the conductance was reduced nearly 22-fold upon switching from 140 mM to 30 mM [Cl]o (note the scale bar is 10-times smaller for 30 mM [Cl]o). At 140 mM [Cl−]o (left) the GNorm(t) at +140 mV continuously increased for the entire 20 s pulse duration. The time course had a fast (τf = 448.1 ± 26.3 ms) onset followed by a slower rise (τs = 19.1 ± 3.8 s). The contributions of the fast and slow components were 0.17 ± 0.04 and 0.83 ± 0.04, respectively. With 30 mM [Cl−]o, GNorm(t) was described by a τf of 368.6 ± 55.5 ms and τs of 6.2 ± 1.5 s; however, the fractional contribution of the fast and slow phases were reversed: 0.92 ± 0.1 and 0.08 ± 0.1, respectively. These observations reinforce the conclusion that TMEM16A gating depends on [Cl−]o. We next asked if the Cl− effect on gating would also occur in the presence of high [Ca2+]i, a condition that maximally activates TMEM16A [49]. To test this, we recorded ICl from cells dialyzed with 5 µM [Ca2+]i. Figure 3D shows a trace obtained by averaging the current from 8 cells that were bathed in 140 mM [Cl−]o and depolarized to +100 mV for 20 s and then repolarizsed to −60 mV. Under these conditions, ICl did not display dual gating, instead the current had a monoexponential time course with τ of 3.7 ± 0.36 s. Taken together, our data demonstrate that TMEM16A gating is dependent on external Cl− but the Cl− effects are attenuated when high [Ca2+]i activates the channel.
Kinetic analysis of TMEM16A gating
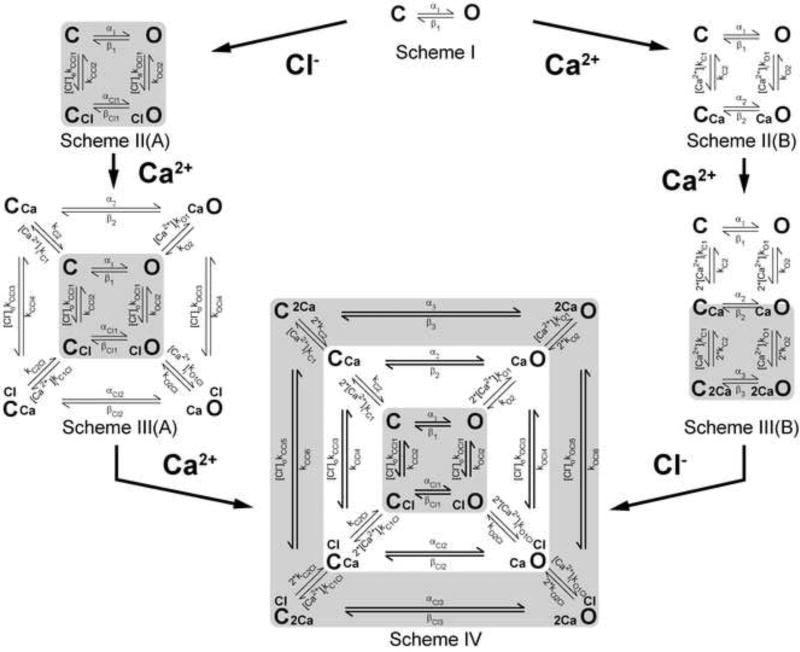
The data in Figures 1, 2 and 3 as well as evidence cited in the Introduction highlight the complexity of TMEM16A activation. In an effort to have an integrated view of the activation mechanism we constructed a kinetic model that takes into account the Vm, Ca2+, and [Cl−]o dependencies of TMEM16A. Scheme IV in Figure 4 presents a 12-state model with 26 free parameters that satisfactorily reproduce the Vm, Ca2+ and Cl− dependencies of channel activation. The model was built based on the following experimental data and assumptions:
TMEM16A can be activated by Vm in the absence of intracellular Ca2+ and the presence of extracellular Cl− as shown in Figure 1 (see also [49]).
Intracellular Cl− plays no role in TMEM16A activation. This observation is based on experiments where the Vm-dependence of TMEM16A was nearly identical in the presence of 40 and 88 mM [Cl−]i (data not shown).
TMEM16A can be activated in a cooperative way by Vm, intracellular Ca2+, and Cl− (Figures 2 and 3).
The interaction between intracellular Ca2+ and TMEM16A is a Vm-dependent process [49].
Binding of at least 2 Ca2+ ions activates TMEM16A as suggested by dose-response curves and structural data [5, 17, 35, 49, 52].
Binding of the two Ca2+ ions occurs in a sequential manner with the same single site-affinity.
Activation of TMEM16A by Vm in cells dialyzed with 0 µM [Ca2+]i (Figure 1) was described as a simple closed-open (C ⇆ O) transition. This condition is represented by Scheme I in Figure 4 where α1 and β1 are forward and backward rate constants (Equation 4) that change exponentially with Vm [15, 16].
Fig 4. Kinetic model employed to explain the Vm, Ca2+, and Cl− contributions to TMEM16A gating.
Scheme I represents Vm-dependent transitions in the absence of intracellular Ca2+ and external Cl−. C and O are closed and open states, and α1 and β1 are Vm-dependent rate constants. Scheme II(A) represents the transitions in the presence of one external Cl− when [Ca2+]i =0. [Cl−]okCCl1 and [Cl−]okOCl1 are rate constants controlling Cl− association to C and O configurations, while kCCl2, kOCl2 rate constants control Cl− dissociation. Scheme III(A) results after binding of one Ca2+ to states represented in Scheme II(A). The [Ca2+]ikO1, [Ca2+]ikO1Cl, [Ca2+]ikC1Cl and [Ca2+]ikC1 are Ca2+ association rate constants while kO2, kO2Cl, kC2Cl and kC1 are Ca2+ dissociation rate constants. The subscript Cl indicates rate constants associated with Cl−-occupied transitions. Scheme II(B) represents transitions resulting after binding of one intracellular Ca2+ to C and O states depicted in Scheme I. [Ca2+]ikC1, [Ca2+]ikO1 are rate constants controlling Ca2+ association to C and O configurations, while kC2, kO2 rate constants control Ca2+ dissociation. Scheme III(B) represents the transitions resulting after binding of an additional Ca2+ to states shown in Scheme II(B). Association and dissociation of the second Ca2+ was assumed to be same as for the first Ca2+ binding reaction. Scheme IV results after binding of an additional Ca2+ to states shown in Scheme III(A). The association and dissociation rate constants were the same as that for the first Ca2+ binding (left pathway). Alternatively, Scheme IV could be achieved after binding of one external Cl− to states shown in Scheme III(B) with forward rate constants [Cl−]okCCl5, [Cl−]o kCCl3, [Cl−]okCCl1, [Cl−]okOCl1, [Cl−]okOCl3 and [Cl−]okOCl5 and backward rate constants kCCl6, kCCl4, kCCl2, kOCl2, kOCl4 and kOCl6. Concentric kinetic cycles with states connected by wider arrows indicate cycles that did not satisfy microscopic reversibility. Table 2A shows the Equations for each rate constant.
In the presence of Cl−, a channel dwelling in C or O states can bind Cl− and move into CCl and ClO states (see Scheme II(A) in Figure 4). The rate constants [Cl−]okCCl1, kCCl2, [Cl−]okOCl1, and kOCl2 control the C ⇆ CCl and O ⇆ ClO transitions in the presence of external Cl−, while αCl1 and βCl1 control the CCl ⇆ ClO transitions. A subsequent increase in intracellular Ca2+ will drive the channel into one of the following states: C ⇆ CCa, O ⇆ CaO, CCl ⇆ ClCCa and ClO ⇆ ClOCa (see Scheme III(A), Figure 4). These transitions are controlled by forward rate constants [Ca2+]ikC1, [Ca2+]ikC1Cl, [Ca2+]ikO1Cl and [Ca2+]ikO1 and backward rate constants kC2, kC2Cl, kO2Cl and kO2 (described by Equation 4). Previous fast perfusion experiments show that upon Ca2+ removal the current deactivates in a Vm-dependent manner [49]. Therefore, only the rate constants controlling unbinding of Ca2+, kO2, kC2, kC2Cl and kO2Cl are Vm-dependent. The rate constants controlling Ca2+-binding are Vm-independent. Depending on their permeability anions can facilitate channel activation [36] and decrease the amount of Ca2+ needed to produce half-maximum activation [4, 39]. Based on this we introduced an effect of Ca2+ on the rate constants (kCCl and kOCl) controlling the C ⇆ CCl and O ⇆ ClO transitions. This effect of Ca2+ is represented in the model by weight parameters l, L, h, H, m and M. Table 2B lists the relationship between rate constants and weight factors. Finally, the Ca2+ dose-response curves suggest that at least two Ca2+ are needed to activate TMEM16A [2, 27, 35, 47, 49, 52]. The binding of an additional Ca2+ to the channels leads to Scheme IV. In this new scheme we presuppose that: 1) the open and closed state can bind Ca2+ with different affinity, and 2) within each state both Ca2+ ions bind with the same single site affinity.
Alternatively, when both intracellular Ca2+ and Cl− are present, a channel dwelling between C and O states (Scheme I) can bind two Ca2+ ions to move into Schemes II(B) and III(B), respectively. The transitions between C and O states are controlled by rate constants [Ca2+]ikC1, kC2, [Ca2+]ikO1 and kO2. Next binding of one external Cl− to channels in Scheme III(B) leads to Scheme IV. Note that binding of just one Cl− by channels represented in Scheme II(B) leads to Scheme III(A). Since Cl− permeates through the channel, then we do not expect that all cycles of Scheme IV will satisfy microscopic reversibility. Cycles represented with states connected by wider arrows did not satisfy the reversibility principle.
The models depicted by Schemes III(A) and IV take into account the contribution of Vm, Ca2+, and Cl− to gating and hence can be used to describe the properties of TMEM16A. To test them we performed a global fit of data describing the Vm, Ca2+, and Cl− dependence of gating. The experimental data used in the global fits included:
ICl vs Vm curves obtained from cells dialyzed with 0 Ca2+ and 40 mM Cl− and bathed in 140 mM or 30 mM Cl−.
Mean ICl traces obtained from cell dialyzed with [Ca2+]i = 0, 0.2 and 1 µM and stepped to Vm ranging from −100 mV to 160 mV.
Representative ICl traces obtained by fast perfusion of 20 µM [Ca2+]i to excised patches held at Vm from −100 mV to 100 mV in 20 mV increments.
Mean ICl recordings obtained from cells sequentially exposed to 1 mM, 10 mM and 140mM [Cl−]o.
Mean ICl recordings obtained from cells dialyzed with 0.2 µM [Ca2+]i and 40 mM [Cl−]i while bathed in 140 mM or 30 mM [Cl−]o and depolarized to −20 mV to +120 mM for 20 s.
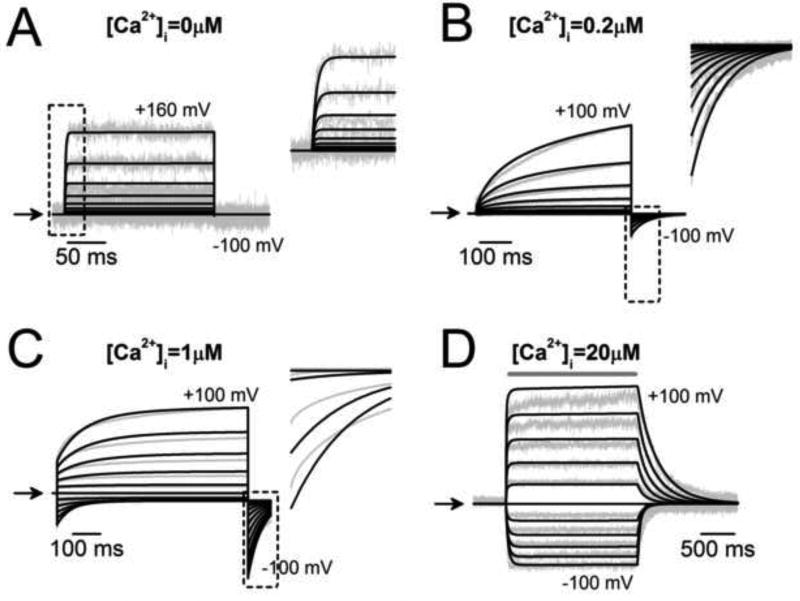
The inclusion of such a wide range of data in the global fit constrained the value for the rate constants and ensured the robustness of our model. The model depicted in Scheme III(A), which included only one Ca2+ bound, partially reproduced TMEM16A activation. It was not able to describe activation at very high [Ca2+]i, the kinetics of tail currents in cells dialyzed with 1 µM Ca2+, or ICl generated with 20 s pulses from cells exposed to 30 mM Cl− (Supplemental Figures 1 and 2). In contrast, Scheme IV, which included two Ca2+ ions bound, described the data reasonably well. The results are shown in Figures 5 and 6 as solid black traces (fits) superimposed on the experimental data (grey lines). Figures 5 A, B and C show fits of Scheme IV to ICl recorded from different cells dialyzed with 0, 0.2 and 1 µM [Ca2+]i. Figure 5D displays the fits of ICl generated by rapid perfusion with 20 µM [Ca2+]i to inside-out patches held from −100 mV to +100 mV. The model fit the entire traces nicely. The model was able to fit the fast onset of the currents recorded at 0 [Ca2+]i (inset in A), the fast tail currents recorded with 0.2 µM Ca2+ (inset in B) and the fast on and off time courses of the current activated with 20 µM Ca2+. However, the model did not describe well the tail current recorded with 1 µM Ca2+ (inset in C). Similarly, Scheme IV was able to fit the ICl−Vm relationships obtained from cells exposed to 30 and then 140 mM [Cl−]o in the absence of intracellular Ca2+ (Figure 6A, grey and black circles) and the Itail recorded from different cells exposed to 10 and 1 mM [Cl−]o, dialyzed with 0.2 µM [Ca2+]i and 40 mM [Cl−]i (Figure 6B). Scheme IV also fitted the mean ICl recorded with 20 s depolarizations from cells exposed to 30 mM and 140 mM [Cl−]o and dialyzed with 0.2 µM [Ca2+]i and 40 mM [Cl−]i (Figure 6C). Here we can see that the model described quite well ICl recorded in the presence of 140 mM Cl− but not 30 mM Cl−. Fast components, such as the tail currents (right insets in B and C) at both [Cl−] were well described. The overall global fit goodness of the model with the binding of Ca2+ to two sites with the same affinity (kO2/ kO1), given by the regression coefficient (R2) associated with the minimization process had a value of 0.98 (Equation 5). We also tested the alternative model that considers different Ca2+ affinities for the two Ca2+ binding sites: kO2/ kO1 for the first site and c* kO2/ kO1, for the second site, where c is a constant parameter. This model fits the data with the same R2=0.98 as that obtained with Scheme IV with two sites with the same affinity. However, the binding affinities only varied by a factor of 0.61and the Vm-dependence of EC50 was indistinguishable from that obtained assuming the same affinity. Therefore, we consider that Scheme IV with two Ca2+ binding sites with the same affinity was a reasonable model of TMEM16A and can be used to obtain the rate constant parameters and the apparent charge (z) values for Scheme IV. Table 2 lists these values. The z values indicate that most of the Vm-dependence is conferred by: a) the charge transferred during the Ca2+ unbinding steps controlled by ko2 (z=0.168), b) the transition CCl ⇆ OCl controlled by αCl1 (z=0.11) and βCl1 (z=0.32), and c) the Cl− binding steps to C or O conformations controlled by kCCl1 (z=0.2) and kOCl (z=0.65), respectively.
Fig 5. Global fit using Scheme IV to TMEM16A recordings obtained at different Vm and [Ca2+]i.
Best fits were performed with the IChMASCOT software; solid lines are fits and grey lines are data. (A–C) Fits to ICl recordings obtained between −100 mV to +100 mV (20 mV increments) from cells dialyzed with 0 (A), 0.2 (B), or 1 (C) µM [Ca2+]i. D Best fits to data obtained by fast perfusion with 20 µM [Ca2+]i to an inside-out patch held at Vm between −100 mV to +100 mV. The insets in A, B and C show magnified fits to fast phases of ICl enclosed in black squares. ICl shown in A and B were recorded from cells dialyzed with [Cl−]i = 40 mM; C and D were from cells exposed to symmetrical 140 mM Cl−.
Fig 6. Global fit using Scheme IV to TMEM16A recordings obtained at different [Cl−]o.
Grey lines are data and solid lines are best fits to: (A) I−Vm relationships obtained with 30 mM and 140 mM [Cl−]o from cells dialyzed with a zero Ca2+ solution; (B) ICl recorded from the same cell exposed to 10 mM and 1 mM [Cl−]o, dialyzed with 0.2 µM [Ca2+]i and stimulated with the Vm protocol shown in Figure 3A; (C) ICl recorded from cells exposed to 30 mM and 140 mM [Cl−]o, dialyzed with 0.2 µM [Ca2+]i and stimulated with 20 s pulses from −20 mV to +120 mV. Data in B and C were obtained from cells dialyzed with [Cl−]i = 40 mM. The right insets in B and C show magnified fits to fast phases of Itail enclosed in grey squares. The R2 value for the global fits shown in Figures 5 and 6 was 0.98.
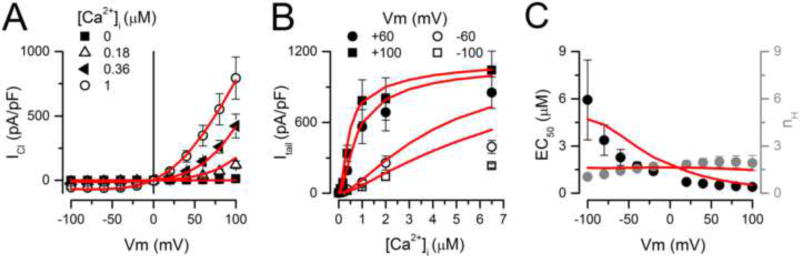
If these global fits are a realistic representation of TMEM16A gating, then Scheme IV plus the set of parameter values listed in Table 2 should be able to predict the quasi-steady-state properties of TMEM16A. These properties include ICl−Vm curves, Ca2+ dose-response curves, and the Vm-dependence of EC50 and the Hill coefficient. We chose these fingerprint properties because they have been well characterized by different laboratories [2, 19, 27, 31, 32, 35]. Our approach consisted of simulating TMEM16A currents under different [Ca2+]i and Vm using IonChannelLab software [44] as described in Methods. Then we used the simulated ICl to construct ICl−Vm curves and Ca2+ dose-response curves. To make a stringent comparison, we compared the simulated properties to previously published data [49]. Data was from cells dialyzed with [Ca2+]i ranging from 0 to 6.5 µM, and stimulated with 0.7 s pulses from −100 mM to +100 mV. Figure 7 shows superimposed experimental data (black symbols) and model predictions (solid lines) for ICl vs Vm relationships at the indicated [Ca2+]i (Figure 7A), dose-response curves to [Ca2+]i at different Vm (Figure 7B), and Vm-dependence of EC50 and the Hill coefficient nH (Figure 7C). These properties of TMEM16A, except the EC50, were adequately predicted by Scheme IV and its associated rate constants. Nevertheless, the EC50 value predicted by the model at +60 mV/140 mM [Cl−]o was 0.85 µM, which is within the range of values determined experimentally [2, 17, 22, 35, 49].
Fig 7. Scheme IV mimics the ICl vs Vm, dose-response curve to [Ca2+]i and the EC50/nH vs Vm relationships for TMEM16A channels.
Black symbols correspond to experimental data. Solid lines are simulations using Scheme IV. (A) Current Density vs Vm relationships. (B) Current Density as a function of [Ca2+]i at the indicated Vm. (C) EC50 for TMEM16A Ca2+ activation and the Hill coefficient nH vs Vm. EC50 and nH values were obtained from B. The experimental data was collected using [Cl−]i=[Cl−]o= 140 mM and 0.7 s depolarizations. The same conditions were used in the simulations. All simulations were carried out using the IonChannelLab software and the rate constants listed in Table 2. R2 = 0.95.
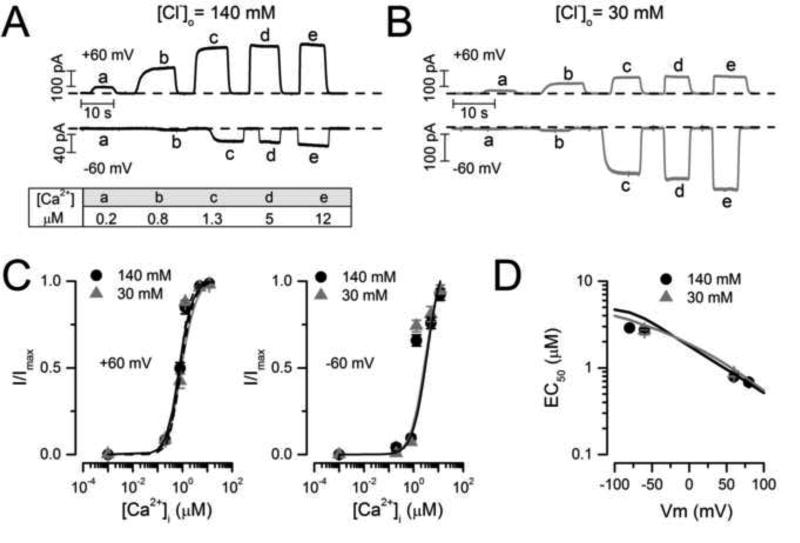
Finally, it has been shown that anions more permeable than Cl− modify CaCC and TMEM16A gating [36, 49]. Importantly, anions more permeable than Cl− increase the apparent Ca2+ sensitivity of TMEM16A and TMEM16B [4, 39]. In this work, we show that increasing [Cl−]o facilitates gating, but whether or not this effect is due to an increased Ca2+ sensitivity is unknown. Therefore, we used the model to predict whether extracellular Cl− regulates TMEM16A gating by altering its Ca2+ or voltage sensitivity. Dose-response curves to Ca2+ were calculated using Scheme IV with 140 mM or 30 mM [Cl−]o and Vm = +60/−60 mV. The model predicts that neither the apparent Ca2+ sensitivity nor the Vm-dependence of EC50 are altered by decreasing [Cl−]o. To determine whether this prediction is true, we determined the dose-response curves to Ca2+ for TMEM16A using inside-out patches held at ± 60 and ± 80 mV in 140 mM or 30 mM [Cl−]o. Figures 8A and 8B show typical raw ICl traces obtained from two different patches bathed in 140 mM (A) and 30 mM (B) Cl− while the [Ca2+]i was changed between zero and the concentrations indicated by the small letters. Upward deflections are records obtained at +60 mV while downward deflections are records obtained at −60 mV. Clearly ICl at +60 mV saturated when [Ca2+]i was around 5–12 µM regardless of the [Cl−]o present. At −60 mV, ICl nearly saturated with 12 µM [Ca2+]i. Figure 8C shows the resulting dose-response curves at +60 mV (left) and at −60 mV (right). The data (black and grey circles) nicely matched the values predicted by Scheme IV (solid lines). Furthermore, the predicted Vm-dependence of EC50 was also matched by the experimental data (continuous lines and symbols in Figure 8D). The EC50 value of 0.85 µM predicted by the model at +60 mV and 140 mM [Cl−]o was similar to those experimentally reported: 1.5, 1.3, 0.4, 1.5, and 0.79 ± 0.05 µM [27, 46, 48, 49, this work]. Taken together these results demonstrate that external Cl− does not alter Ca2+-sensitivity in TMEM16A. The fact that our kinetic model predicted this result is a good indication of the model robustness and that the model includes critical information about the mechanism of TMEM16A gating.
Fig 8. The apparent Ca2+ sensitivity of TMEM16A was not altered by lowering the [Cl−]o.
A–B Typical ICl recordings from inside-out patches held at ± 60 mV and exposed to 140 mM (A) or 30 mM [Cl−]o (B). ICl were activated by applying 0, 0.2 (a), 0.8 (b), 1.3 (c), 5 (d) and 12 µM [Ca2+]i (e). Dashed lines indicate ICl obtained with 0 [Ca2+]i and 25.2 mM EGTA. (C–D) Dose-response and EC50 vs Vm curves (symbols) obtained from experimental data like that shown in A and B were identical to the predictions made with Scheme IV at 30 (grey lines) and 140 (black lines) mM [Cl−]o.
Activation pathways
Since Scheme IV was able to reproduce the activation properties of TMEM16A, we decided to apply the model to determine what would be the most likely pathway(s) followed by TMEM16A during the activation process. For this purpose, we calculated the probability (P) that TMEM16A visited each of the 12 states when intracellular Ca2+, external Cl−, and Vm were varied (Supplemental material). To obtain a gating pathway we determined the most likely state to be occupied once the channel visited a given state. For example, when the channel visits state C, it can go to O, CCl or CCa states. Our P value calculations indicated that state CCa was the most likely state to be occupied. We repeated this strategy to find the next most probable state to be visited until the channel reached ClO2Ca. First, we consider activation of TMEM16A by a 0.5 s depolarization to +120 mV followed by repolarization to −60 mV when [Ca2+]i = 0, [Cl−]i = 40 mM and [Cl−]o changed between 140 mM and 30 mM. This condition is represented by the pink kinetic scheme box in Figure 9C. The time-dependent P values for states C, O, CCl, and ClO are shown in Figure 9A. PC, PO, PCCl and PClO values were 0.782, 0, 0.217 and 0.0008, when [Cl−]o was 140 mM. After external Cl− was decreased to 30 mM PC increased to 0.941, while PCCl and PClO fell to 0.057 and 0.0002, respectively. Thus, in the absence of intracellular Ca2+ the most likely gating route is C → CCl → ClO (Figure 9C, blue and pink arrows).
Fig 9. TMEM16A activation pathways predicted by Scheme IV.
(A) Time dependent PC, PO, PCCl and PClO when [Cl−]o was 140 mM (blue lines) or 30 mM (red lines) while [Ca2+]i and [Cl−]i were 0 M and 40 mM, respectively. Note that PO was ≈ 0 at both [Cl−]. (B) Time dependent PC, PCCl, PCCa, PClCCa, PClOCa, PC2Ca, PO2Ca, and PClO2Ca are shown in rectangles coloured according to the colours of the layers of Scheme IV presented in panel C. Left column: [Ca2+]i = 0.2 µM, [Cl−]o/[Cl−]i=140/40 mM. Right column: [Ca2+]i = 0.2 µM, [Cl−]o/[Cl−]i=30/40 mM. PO, PClO, POCa, and PClC2Ca are not shown for clarity, their values were ≈ 0. P values were calculated by numerically integrating the differential equations (supplemental material) under the following conditions: [Cl−]o =140 mM or 30 mM, [Cl−]i = 40 mM, [Ca2+]i = 0 (A), 0.2 (B) µM, and 20 s pulse to +140 mV followed by repolarization to −60 mV. The set of rate constants listed in Table 2 was used to obtain the time dependence of occupation probability. (C) TMEM16A activation pathways according to probability of occupation. Blue and pink arrows indicate the pathway in the absence of intracellular Ca2+ while [Cl−]o was 140 mM or 30 mM, respectively. Black, grey, green and light green arrows indicate the activation pathways when intracellular Ca2+ was 0.2 µM while [Cl−]o was 140 mM (black and green lines) or 30 mM (grey and light green lines). [Cl−]i was set at 40 mM. Red button = Ca2+, blue donut = Cl−.
Subsequently, P values were calculated when [Ca2+]i was 0.2 µM, [Cl−]o was either 140 or 30 mM, [Cl−]i = 40 mM, and Vm was +120 mV during 20 s followed by repolarization to −60 mV. These experimental conditions are the same as the ones used for collecting data shown in Figure 3C (right) and data collected using short depolarizations. Figure 9B shows P values for states in the inner circle (pink), middle circle (white), and outer circle (grey), respectively. When [Cl−]o was 140 mM (left column), the depolarization induced an abrupt decrease of PC, followed by a further exponential decrease. Such decrease was mirrored by an abrupt increase and then exponential decrease in PCCa. At the same time, PClOCa and PC2Ca increased little, and PO2Ca increased transiently. Notably, PClO2Ca increased steadily reaching 0.4 by the end of the 20 s depolarization. The same trend was observed when [Cl−]o was 30 mM, albeit the magnitude of P for each state declined. Interestingly, during the first 2 s into the depolarization PClC decreased but then increased in a time-dependent manner. In summary, in the presence of 0.2 µM [Ca2+]i and 140 mM or 30 mM [Cl−]o, TMEM16A activation proceeded following two pathways. At early times (<2 s), the route C → CCa → C2Ca → O2Ca → ClO2Ca (black arrow) was preferred. However, as depolarization continued, the route C → ClC → ClCCa → ClOCa → ClO2Ca (green arrow) also contributed to the total current. The effect of lowering [Cl−]o to 30 mM was to decrease the contribution of both routes to the total current (grey and light green arrows). From these calculations, we observed that the greatest contribution to the total current is given by the active states CaOCl, 2CaO and 2CaOCl. Therefore the net effect of increasing chloride from 30 to 140 mM was to increase PCaOCl and P2CaOCl.
DISCUSSION
CaCC gating is a complex process; it involves the combined action of Vm, Ca2+ and Cl−. Several kinetic models have been previously proposed to explain the Vm and Ca2+-dependence of CaCC gating [2, 20, 27]. However, these early models assumed an initial Ca2+-dependent C ⇆ CCa transition and did not consider a C ⇆ O transition in the absence of Ca2+ or the role of external Cl− in gating. These models predict an open probability of zero at any Vm if [Ca2+]i = 0, which is not the case for TMEM16A [49]. In this work, we present an alternative kinetic model that satisfactorily accounts for the Vm, Ca2+, and Cl−-dependent gating of TMEM16A channel. We propose that the gating of the TMEM16A Cl− channel is caused by consecutive direct Vm-dependent binding of two intracellular Ca2+ ions, coupled to a Vm-dependent binding of external Cl−. External Cl− facilitates gating by binding to a Ca2+-free closed state and favouring the transition to Ca2+-bound open states. By means of facilitating the 2CaO ⇆ 2CaOCl transition, external Cl− induces the slow gating [11]. In our model (Scheme IV) the direct Ca2+-binding is controlled by forward rate constants that are independent of voltage and Vm-dependent backward rate constants. The off-rate constant measured from simulations with the model predicted that Ca2+ remains attached to the channel at depolarized voltages. This coincides with experimental data showing that at depolarized Vm the rate of current decay—due to Ca2+ washout—is very slow compared to that observed at negative Vm. Also, increasing the [Ca2+]i decreases the off rate of whole cell currents at negative potentials [2, 27, 52]. Such data show that Ca2+ unbinding is a Vmdependent phenomenon [19, 27, 35, 49, 52]. Thus, the open state is stabilized when Ca2+ is high, and/or Vm is depolarized.
The model is not perfect. It cannot describe well Itail recorded with 1 µM Ca2+ and ICl recorded in the presence of 30 mM Cl− using 20 s depolarizations. Also, the EC50 is not adequately predicted by Scheme IV. There are different possibilities to explain these discrepancies. The rate constants are relatively simple; they are exponential functions of Vm and depended linearly on [Cl−]o. However, such dependence could be state dependent and/or be more complicated. The weight factors (l, L, h, H, m, and M) are assumed to be constant and we considered that only one Cl− ion is needed affect channel gating without affecting the single channel conductance. These assumptions may not hold under all experimental conditions. Finally, for simplicity, we assumed that Ca2+ binds to two sites with a single binding affinity which we call single-site affinity. This may not be the case as suggested by mutagenesis experiments which show that mutations located in different parts of the protein alter Ca2+ sensitivity [17, 47, 49, 52]. Inadequacies of one or all these variables would hinder the ability of the model to reproduce the data. Refining the model to include the exact number of Ca2+ binding sites, their binding affinity and allosteric interactions with Cl− would be more realistic as more structural data becomes available. In addition, our model ignores the permeation process. Including this property would be necessary to understand the effects of pore occupancy by permeant anions on channel gating [4, 36, 39]. Despite these limitations, Scheme IV predicted no change in the apparent Ca2+ affinity of TMEM16A when [Cl−]o was lowered from 140 mM to 30 mM, and this prediction was successfully corroborated experimentally. The model also predicts that a small fraction of the charge (z2 = 0.17) is transferred during the Ca2+ unbinding steps. This number agrees with the small Vm-dependence of the dose-response curves to [Ca2+]i [2, 22, 35, 49]. We consider that Scheme IV was effective because a large data set was used to extract the rate constant parameters. This tactic largely constrained the possible values of the rate constant parameters and contributed to enhance the robustness of the model.
The effect of external Cl− on CaCCs is maybe more complex than previously anticipated since our data show that varying external Cl− alters both the fast and slow gating modes of TMEM16A and TMEM16B [11]. In addition, our model-dependent calculations using the rate constants listed in Table 2 revealed that external Cl− ion plays a significant role on Vm-dependent activation in the absence of Ca2+. Under this condition, there is a substantial amount of charge transferred during the Cl− binding steps to either C or O conformations as indicated by z+Cl = 0.2 and z+Ol = 0.65 (Table 2A). The binding of one Cl− to the C conformation of the channel prompts its conversion into ClC and subsequently the ClO state. The later transition is also accompanied by charge transferred as indicated by zl1 = 0.11 and zl2 = 0.33 (Table 2). The ClO state would enable Cl− conductance in the absence of intracellular Ca2+. Furthermore, when Ca2+ is bound to the channel the external Cl− favours the 2CaO ⇆ 2CaOCl transition and so the slow component of channel gating arises. In fact, by increasing [Cl−]o from 30 mM to 140 mM in the presence of 0.2 µM [Ca2+]i we observed an increase in P2CaOCl + PCaOCl from 0.08 to 0.22, suggesting that Ca2+ remains bound to the channel for a longer time. Also, an increased occupation of the 2CaOCl state implies a longer dwell time of the channel in this state. High [Cl−]o shifted to negative values the Vm-dependence of occupation for the 2CaOCl state such that at a given Vm the probability of occupation is increased with high [Cl−]o. Despite these changes, the overall apparent affinity of TMEM16A for Ca2+ was not altered. However, previous experimental observations suggest a coupling of the permeation and gating mechanisms. For example, highly permeant anions alter the kinetics and EC50 as described for CaCC from mouse parotid acinar cells, Xenopus oocytes and TMEM16B [4, 36, 38, 39]. These data can be reconciled considering that TMEM16A may have two anion binding sites as previously suggested [38] and the external anions might alter TMEM16A gating by at least two mechanisms. One mechanism may involve permeation and gating, while another mechanism may affect only gating, similar to what we describe here. Our idea describes the effect of external Cl− onTMEM16A based on what has been proposed for other Cl− channels. For example, in CLC-0 and CLC-1 external anions have a profound facilitating effect on gating [7, 14, 37] and in CLC-2 Vm-dependence is conferred by permeant anions that occupy and then exit the pore [25, 41]. Thus, the role of permeant anions in gating the Cl− channels may be more important that thought in the past. More research is needed to establish the connection between permeation and gating.
TMEM16A gating shares some similarities with BK channel gating. For example, both channels display coupling between Vm and Ca2+ binding, both channels are activated by Vm alone, and increments in [Ca2+]i lead to a leftward shift of the GNorm(Vm) curve. However, there are also differences: BK has an intrinsic Vm sensor and TMEM16A does not, Ba2+ activates BK channels by interacting with the Ca2+ binding site but Ba2+ also binds and blocks the conduction pathway of BK channels while Ba2+ only activates TMEM16A [24, 33, 34, 49, 52, 53]. Allosteric models have been successful in explaining the interaction between Vm and Ca2+ in the gating of BK channels [24]. Although TMEM16A gating is not exactly like the gating of BK channels, an allosteric model should be considered for TMEM16A channel.
Our findings could be relevant to TMEM16A function under physiological conditions. For example, the model could be helpful to understand the behaviour of TMEM16A during agonist-stimulated Ca2+-dependent fluid secretion. Secretory epithelial cells response to agonist include an oscillatory membrane potential accompanied by oscillations in the intracellular [Ca2+]i [30]. Furthermore, during fluid and electrolyte secretion by acinar cells, [Cl−]i decreases from about 61 to 29 mM [18] due to Cl− exit through TMEM16A channels [40]. Under these conditions, Cl− would transiently increase on the extracellular side which would favour activation of TMEM16A to sustain secretion. Although the TMEM16A activation pattern may very complex under physiological conditions, it can be understood using Scheme IV.
In summary, we propose a TMEM16A gating mechanism caused by consecutive direct Vm-dependent binding of two intracellular Ca2+ ions, coupled to a Vm-dependent binding of one external Cl−.
Supplementary Material
Acknowledgments
The authors thank C.Y. Hernandez-Carballo and Yuan Yuan Cui for excellent technical assistance and Kuai Yu for providing ANO1 patch clamp records for analysis. This work was supported by grants 219949 (CONACyT, Mexico) to JA. HCH is supported by grants from the National Institutes of Health GM60448, AR067786, and EY11482. JAC-V and JJ De J-P are recipients of a Graduate Student Fellowship from CONACyT, Mexico (229968 and 234820). SCR is a recipient of a Postdoctoral Fellowship from CONACyT, Mexico (256034-290807).
References
- 1.Arreola J, Melvin JE, Begenisich T. Inhibition of Ca2+-dependent Cl− Channels from Secretory Epithelial Cells by Low Internal pH. J Membr Biol. 1995;147:95–104. doi: 10.1007/BF00235400. [DOI] [PubMed] [Google Scholar]
- 2.Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J Gen Physiol. 1996;108:35–47. doi: 10.1085/jgp.108.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arreola J, Reyes JP, Rosales Saavedra T, Pérez Cornejo P. Chloride channels activated by intracellular ligands. In: James K, Ceri D, editors. Ion Channels: From Structure to Function. Oxford University Press; 2010. pp. 506–511. [Google Scholar]
- 4.Betto G, Cherian OL, Pifferi S, Cenedese V, Boccaccio A, Menini A. Interactions between permeation and gating in the TMEM16B/anoctamin2 calcium-activated chloride channel. J Gen Physiol. 2014;143:703–18. doi: 10.1085/jgp.201411182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature. 2014;516:207–212. doi: 10.1038/nature13984. [DOI] [PubMed] [Google Scholar]
- 6.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJV. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science (80-) 2008;322:590–4. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 7.Chen TY, Miller C. Nonequilibrium gating and voltage dependence of the ClC-0 Cl- channel. J Gen Physiol. 1996;108:237–250. doi: 10.1085/jgp.108.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun H, Cho H, Choi J, Lee J, Kim S, Kim H, Oh U. Protons inhibit anoctamin 1 by competing with calcium. Cell Calcium. 2015;5:431–41. doi: 10.1016/j.ceca.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Colquhoun D, Hawkes AG. Relaxation and Fluctuations of Membrane Currents that Flow through Drug-Pperated Channels. Proc R Soc Lond B Biol Sci. 1977;199:231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- 10.Covarrubias M, Bhattacharji A, De Santiago-Castillo JA, Dougherty K, Kaulin YA, Na-Phuket TR, Wang G. The Neuronal Kv4 Channel Complex. Neurochem Res. 2008;33:1558–1567. doi: 10.1007/s11064-008-9650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz Rangel S, De Jesús Pérez JJ, Contreras Vite JA, Pérez Cornejo P, Hartzell H, Arreola J. Gating modes of calcium-activated chloride channels TMEM16A and TMEM16B. J Physiol. 2015;24:5283–98. doi: 10.1113/JP271256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougherty K, De Santiago-Castillo J a, Covarrubias M. Gating Charge immobilization in Kv4.2 Channels: The Basis of Closed-State Inactivation. J Gen Physiol. 2008;131:257–73. doi: 10.1085/jgp.200709938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, Rock JR, Harfe BD, Henson BJ, Kunzelmann K, Schreiber R, Seethala RS, Egloff AM, Chen X, Lui VW, Grandis JR, Gollin SM. TMEM16A Induces MAPK and Contributes Directly to Tumorigenesis and Cancer Progression. Cancer Res. 2012;72:3270–3281. doi: 10.1158/0008-5472.CAN-12-0475-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engh AM, Faraldo-Gomez JD, Maduke M. The Mechanism of Fast-Gate Opening in ClC-0. J Gen Physiol. 2007;130:335–349. doi: 10.1085/jgp.200709759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyring H. The Activated Complex in Chemical Reactions. J Chem Phys. 1935;445:107 –115. doi: 10.1063/1.1749604. [DOI] [Google Scholar]
- 16.Eyring H. The Activated Complex and the Absolute Rate of Chemical Reactions. Chem Rev. 1935;17:65–77. doi: 10.1021/cr60056a006. [DOI] [Google Scholar]
- 17.Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, Pagani F, Galietta LJV. Regulation of TMEM16A Chloride Channel Properties by Alternative Splicing. J Biol Chem. 2009;284:33360–8. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foskett JK. [Ca2+]i modulation of Cl- content controls cell volume in single salivary acinar cells during fluid secretion. Am J Physiol. 1990;259:C998–C1004. doi: 10.1152/ajpcell.1990.259.6.C998. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Hernandez JM, Stühmer W, Parekh AB. Calcium dependence and distribution of calcium-activated chloride channels in Xenopus oocytes. J Physiol. 1997;502(Pt 3):569–74. doi: 10.1111/j.1469-7793.1997.569bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haase A, Hartung K. Activation and inactivation kinetics of a Ca2+-activated Cl− current: photolytic Ca2+ concentration and voltage jump experiments. Pflugers Arch. 2006;452:81–90. doi: 10.1007/s00424-005-0004-y. [DOI] [PubMed] [Google Scholar]
- 21.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 22.Hartzell HC, Qu Z. Chloride Currents in Acutely Isolated Xenopus Retinal Pigment Epithelial Cells. J Physiol. 2003;549:453–69. doi: 10.1113/jphysiol.2003.040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández-Carballo CY, De Santiago-Castillo JA, Rosales-Saavedra T, Pérez-Cornejo P, Arreola J. Control of volume-sensitive chloride channel inactivation by the coupled action of intracellular chloride and extracellular protons. Pflugers Arch Eur J Physiol. 2010;460:633–644. doi: 10.1007/s00424-010-0842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Jesús Pérez JJ, Castro-Chong A, Shieh R, Hernández Carballo CY, de Santiago Castillos JA, Arreola J. Gating the glutamate gate of CLC-2 chloride channel by pore occupancy. J Gen Physiol. 2016;1:25–37. doi: 10.1085/jgp.201511424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korn SJ, Horn R. Statistical discrimination of fractal and markov models of single-channel gating. Biophys J. 1988;54:871–877. doi: 10.1016/S0006-3495(88)83023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuruma A, Hartzell HC. Bimodal Control of a Ca2+-Cctivated Cl− Channel by Different Ca2+ Signals. J Gen Physiol. 2000;115:59–80. doi: 10.1085/jgp.115.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–7. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Large WA, Wang Q. Characteristics and physiological role of the Ca(2+)-activated Clconductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. doi: C435–C454. [DOI] [PubMed] [Google Scholar]
- 30.Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445–469. doi: 10.1146/annurev.physiol.67.041703.084745. [DOI] [PubMed] [Google Scholar]
- 31.Miledi R. A Calcium-Dependent Transient Outward Current in Xenopus laevis Oocytes. Proc R Soc Lond B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- 32.Miledi R, Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984;357:173–83. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neyton J, Miller C. Potassium blocks barium permeation through a calcium-activated potassium channel. J Gen Physiol. 1988;92:549–67. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni YL, Kuan AS, Chen TY. Activation and Inhibition of TMEM16A Calcium-Activated Chloride Channels. PLoS One. 2014;9:e86734. doi: 10.1371/journal.pone.0086734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilius B, Prenen J, Voets T, Van Den Bremt K, Eggermont J, Droogmans G. Kinetic and pharmacological properties of the calcium-activated chloride-current in macrovascular endothelial cells. Cell Calcium. 1997;22:53–63. doi: 10.1016/S0143-4160(97)90089-0. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Cornejo P, De Santiago JA, Arreola J. Permeant Anions Control Gating of Calcium-dependent Chloride Channels. J Membr Biol. 2004;198:125–33. doi: 10.1007/s00232-004-0659-x. [DOI] [PubMed] [Google Scholar]
- 37.Pusch M, Ludewig U, Rehfeldt A, Jentsch TJ. Gating of the voltage-dependent chloride channel ClC-0 by the permeant anion. Nature. 1995;373:527–531. doi: 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- 38.Qu Z, Hartzell HC. Anion Permeation in Ca2+-Activated Cl− Phannels. J Gen Physiol. 2000;116:825–844. doi: 10.1085/jgp.116.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes JP, López-Rodríguez A, Espino-Saldaña AE, Huanosta-Gutiérrez A, Miledi R, Martínez-Torres A. Anion permeation in calcium-activated chloride channels formed by TMEM16A from Xenopus tropicalis. Pflugers Arch Eur J Physiol. 2014;466:1769–1777. doi: 10.1007/s00424-013-1415-9. [DOI] [PubMed] [Google Scholar]
- 40.Romanenko VG, Catalán MA, Brown DA, Putzier I, Hartzell HC, Marmorstein AD, Gonzalez-Begne M, Rock JR, Harfe BD, Melvin JE. Tmem16A encodes the Ca2+-activated Cl− channel in Mouse Submandibular Salivary Gland Acinar Cells. J Biol Chem. 2010;285:12990–13001. doi: 10.1074/jbc.M109.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sánchez-Rodríguez JE, De Santiago-Castillo JA, Arreola J. Permeant anions contribute to voltage dependence of ClC-2 chloride channel by interacting with the protopore gate. J Physiol. 2010;588:2545–56. doi: 10.1113/jphysiol.2010.189175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sánchez-Rodríguez JE, De Santiago-Castillo JA, Contreras-Vite JA, Nieto-Delgado PG, Castro-Chong A, Arreola J. Sequential interaction of chloride and proton ions with the fast gate steer the voltage-dependent gating in ClC-2 chloride channels. J Physiol. 2012;590:4239–53. doi: 10.1113/jphysiol.2012.232660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sansom MS, Ball FG, Kerry CJ, McGee R, Ramsey RL, Usherwood PNR. Markov, fractal, diffusion, and related models of ion channel gating: A comparison with experimental data from two ion channels. Biophys J. 1989;56:1229–1243. doi: 10.1016/S0006-3495(89)82770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santiago-Castillo JA, Covarrubias M, Sánchez-Rodríguez JE, Perez-Cornejo P, Arreola J. Simulating complex ion channel kinetics with IonChannelLab. Channels (Austin) 2010;4:422–8. doi: 10.4161/chan.4.5.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression Cloning of TMEM16A as a Calcium- Activated Chloride Channel Subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terashima H, Picollo A, Accardi A. Purified TMEM16A is sufficient to form Ca2+-activated Cl− channels. Proc Natl Acad Sci U S A. 2013;110:19354–9. doi: 10.1073/pnas.1312014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tien J, Peters CJ, Wong XM, Cheng T, Jan YN, Jan LY, Yang H. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. Elife. 2014;3:1–19. doi: 10.7554/eLife.02772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandenberg CA, Bezanilla F. A sodium channel gating model based on single channel, macroscopic ionic, and gating currents in the squid giant axon. Biophys J. 1991;60:1511–1533. doi: 10.1016/S0006-3495(91)82186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao Q, Yu K, Perez-Cornejo P, Cui Y, Arreola J, Hartzell HC. Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc Natl Acad Sci U S A. 2011;108:8891–8896. doi: 10.1073/pnas.1102147108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim W-S, Park SP, Lee J, Lee B, Kim B-M, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–5. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 51.Yeh HI, Yeh JT, Hwang TC. Modulation of CFTR gating by permeant ions. J Gen Physiol. 2015;145:47–60. doi: 10.1085/jgp.201411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu K, Duran C, Qu Z, Cui YY, Hartzell HC. Explaining Calcium-Dependent Gating of Anoctamin-1 Chloride Channels Requires a Revised Topology. Circ Res. 2012;110:990–999. doi: 10.1161/CIRCRESAHA.112.264440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Y, Zeng XH, Lingle CJ. Barium ions selectively activate BK channels via the Ca2+-bowl site. Proc Natl Acad Sci. 2012;109:11413–11418. doi: 10.1073/pnas.1204444109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.