Abstract
Adenosine is a determinant of metabolic control of organ function increasing oxygen supply through the A2 class of adenosine receptors and reducing oxygen demand through A1 adenosine receptors (A1AR). In the kidney, activation of A1AR in afferent glomerular arterioles has been suggested to contribute to tubuloglomerular feedback (TGF), the vasoconstriction elicited by elevations in [NaCl] in the macula densa region of the nephron. To further elucidate the role of A1AR in TGF, we have generated mice in which the entire A1AR coding sequence was deleted by homologous recombination. Homozygous A1AR mutants that do not express A1AR mRNA transcripts and do not respond to A1AR agonists are viable and without gross anatomical abnormalities. Plasma and urinary electrolytes were not different between genotypes. Likewise, arterial blood pressure, heart rates, and glomerular filtration rates were indistinguishable between A1AR+/+, A1AR+/−, and A1AR−/− mice. TGF responses to an increase in loop of Henle flow rate from 0 to 30 nl/min, whether determined as change of stop flow pressure or early proximal flow rate, were completely abolished in A1AR−/− mice (stop flow pressure response, −6.8 ± 0.55 mmHg and −0.4 ± 0.2 in A1AR+/+ and A1AR−/− mice; early proximal flow rate response, −3.4 ± 0.4 nl/min and +0.02 ± 0.3 nl/min in A1AR+/+ and A1AR−/− mice). Absence of TGF responses in A1AR-deficient mice suggests that adenosine is a required constituent of the juxtaglomerular signaling pathway. A1AR null mutant mice are a promising tool to study the functional role of A1AR in different target tissues.
Adenosine is a purine nucleoside that is formed by intracellular or extracellular breakdown of adenine nucleotides, or by the hydrolysis of S-adenosyl-L-homocysteine. Because of the ubiquitous nature of adenine nucleotides, all cells are possible sources of adenosine. Adenosine that is formed in the cytosol can cross the cell membrane via a nucleoside transporter to enter the interstitial space (1). At least four G protein-coupled cell-surface receptors of the P1 class of purinoceptors (A1, A2A, A2B, A3) mediate the biological effects of adenosine (2, 3). A1 adenosine receptors (A1AR) are integral membrane proteins of ≈37 kDa with seven transmembrane-spanning domains. A1AR are primarily coupled to adenyl cyclase via inhibitory Gi proteins, but they also signal through activation of phospholipase C (2–4). A1AR are expressed at highest levels in brain, spinal chord, testes, and adipose tissue. At lower levels, they are also found in heart and kidney (5). In general, adenosine acting through A1AR tends to protect tissues by reducing oxygen demand.
Localization studies using in situ hybridization and reverse transcription (RT)-PCR indicate that A1AR mRNA in the kidney is expressed predominantly in glomerular afferent arterioles and juxtaglomerular granular cells, but expression of the receptor also exists in tubular segments, especially in thick ascending limbs and collecting ducts (6, 7). Studies using selective A1AR agonists and antagonists indicate that A1AR in afferent arterioles mediate vasoconstriction and inhibition of renin secretion. Specifically, adenosine has been implicated as a mediator of the local pathway that is initiated by a change in NaCl transport across macula densa cells and that affects afferent arteriolar tone and renin secretion (8, 9). Furthermore, tubular A1AR appear to modify tubular NaCl absorption, even though the direction and localization of this action is somewhat controversial.
The study of mice with targeted gene deletions has become a tool that complements and extends the conclusions reached from the application of pharmacological interventions. To further examine the role of A1AR, we have generated an A1AR-deficient mouse strain by homologous recombination methods. We report results from studies in these mice that focus on the role of A1AR in juxtaglomerular control of afferent arteriolar tone. Our data show that TGF, the vasoconstriction resulting from an increase in macula densa NaCl concentration, is abolished in A1AR knockout mice. This observation appears to establish that A1AR are required for TGF responsiveness and that adenosine therefore acts as the major mediator of the TGF response. Because homozygous A1AR null mice are viable and without gross behavioral or anatomic abnormalities, studies in these mice promise to provide further insights into the role of A1AR in central nervous, cardiac, and other organ functions.
Methods
Generation of A1AR Knockout Mice.
The gene encoding the mouse A1AR was cloned by screening a BAC 129/SvJ embryonic stem (ES) genomic library (Genome Systems, St. Louis) using a 786-bp probe generated by PCR on the basis of homology between human, rat, and partial mouse cDNA sequences. A targeting vector was constructed designed to delete the entire coding sequence and to replace it with lacZ and neomycin resistance gene cassettes. The targeting vector was linearized with AscI and transfected into R1 embryonic stem cells (129 × 1/129 S1 hybrid) by electroporation (stem cell facility of the University of Michigan). Two positive ES clones were identified by PCR screening of 480 neomycin-resistant colonies. Correct targeting was confirmed by Southern hybridization, and chromosome counts demonstrated euploidy. The verified ES cells were injected into blastocysts from C57BL/6 mice and implanted into pseudopregnant foster females. Only one line yielded viable chimeras that were crossed with C57BL/6J females to yield heterozygous (A1AR+/−) animals. Heterozygous progeny were intercrossed to obtain homozygous mutants and wild-type controls, as determined by PCR on tail DNA. For genotyping, tail DNA was isolated and tested for the presence of wild-type and mutant genes, with A1AR and (Neo-R) specific primers generating PCR products of 444 bp from the wild-type allele and 457 bp from the mutant allele. Primer sequences were sense A1AR, 5′-GTACATCTCGGCCTTCCAGG-3′; antisense A1AR, 5′-GAGAATACCTGGCTGACTAG-3′; sense neo-R, 5′-ACAACAGACAATCGGCTGCTCTGATG-3′; and antisense neo-R, 5′-TGCGCGCCTTGAGCCTGGCGAAC-3′ (10).
RT-PCR.
Total RNA was isolated from kidneys by using TRI reagent. cDNA was synthesized by Moloney murine leukemia virus reverse transcriptase (Superscript, BRL). Amplification of A1AR cDNA by conventional RT-PCR was performed as described previously (11). For quantitative RT-PCR, oligonucleotides were chosen by using PRIMER EXPRESS 1.0 (Applied Biosystems) with probe being positioned at an exon–intron junction. Sequences of oligonucleotides for A1AR are sense, 5′-CAGAGCTCCATCCTGGCTCT-3′; antisense, 5′-CGCTGAGTCACCACTGTCTTG-3′; and probe, 5′-(FAM)CGGTACCTCCGAGTCAAGATCCCTCTCC(TAMRA)-3′. PCR amplification with TagMan Universal PCR Master Mix was performed by using the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Cycling conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 repeats of 95°C for 0.15 min and 60°C for 1 min. Parallel real time RT-PCR amplification of β-actin was performed. Relative abundance of A1AR PCR products, normalized by β-actin, was calculated from threshold cycle numbers, ΔCT (A1AR-β-actin), according to the manufacturer's paradigm.
Blood Pressure, Heart Rate, and Urine and Plasma Chemistry.
Animals were maintained on standard rodent diet and tap water. Blood pressure and heart rate in conscious mice were determined by tail cuff manometry (Visitech Systems, Apex, NC). Animals were conditioned by placing them into the holding device twice per day on 3 consecutive days. Systolic blood pressure represents the average of measurements on 6 consecutive days, each consisting of the mean of 50 individual cycles. Measurements were performed between 10 and 11 a.m.
Electrolytes were measured in plasma separated from 0.3–0.6-ml samples of heparinized blood collected immediately after anesthesia by puncturing the vena cava. Urine was collected for 24 h in metabolic cages. Urine Na, K, Cl, and osmolarity were measured with standard methods.
Glomerular Filtration Rate.
Female mice were anesthetized with 100 mg/kg inactin i.p. and 100 mg/kg ketamine i.m., and catheters were placed into the jugular vein, femoral artery, and bladder. Animals were infused with 125I-iothalamate (Questcor Pharmaceuticals, Carlsbad, CA) at ≈5 μCi/h (1 Ci = 37 GBq). After 30 min of equilibration, a blood sample was taken, and three 15-min collections of bladder urine were made. Blood samples were collected in heparinized 5-μl microcaps. 125I-iothalamate radioactivities were measured in a gamma counter in duplicates by using 0.5-μl samples of plasma and urine.
Micropuncture Studies.
Experiments were performed in male A1AR+/+, A1AR+/−, and A1AR−/− littermates derived from mating of heterozygous animals. Mice (3–4 months old) were anesthetized with 100 mg/kg inactin i.p. and 100 mg/kg ketamine i.m. Cannulas were placed in trachea and femoral artery for measurement of arterial blood pressure and femoral vein for an i.v. maintenance infusion of 2.25 g/dl BSA in saline at a rate of 0.35 ml/h. The left kidney was placed in a lucite cup. Stop flow pressure as an index of glomerular capillary pressure was determined during loop perfusions at 0 and 30 nl/min as described previously (12). To assess the response of nephron filtration rate to a change in loop perfusion rate, timed collections of early proximal flow rate were performed for 2–3 min in front of a tubular wax block while the loop was perfused at 30 or 0 nl/min. Sample volumes were derived from fluid column length in constant bore capillaries. The sequence of the flow change was randomized. The following perfusion fluid was used (in mM/liter): 136 NaCl/4 NaHCO3/4 KCl/2 CaCl2/7.5 urea and 100 mg/100 ml FD&C green (Keystone Scientific, Bellefonte, PA).
Results
The mouse A1AR gene consists of two exons and spans ≈5 kb. The coding region is derived from both exons (Fig. 1). The encoded A1AR protein has a molecular mass of 36,700 daltons and consists of 326 aa. The amino acid identity of the rat, human, and mouse homologues is 87% overall and 92% in the transmembrane domains. Targeted disruption of the mouse A1AR gene in ES cells and correct insertion of the targeting construct was verified by Southern blot analysis of HindIII and SacI digests of genomic DNA from ES clones identified by PCR screening (Fig. 1B).
Figure 1.
(A) Organization of the mouse A1AR gene, the targeting vector, and the mutant allele after recombination. Boxes in black and gray indicate exons and coding regions; relevant restriction sites and location of 5′ and 3′ probes are shown. Herpes simplex virus thymidine kinase (TK) and lacZ were from the pNZTK2 vector (R. D. Palmiter, Howard Hughes Medical Institute, Univ. of Washington, Seattle); the neomycin resistance cassette flanked by two loxP sites (Fl Neo) and driven by the phosphoglycerate kinase promoter were from the Biomedical Core Facilities (Univ. of Michigan, Ann Arbor, MI). (B) Southern blots on HindIII and SacI digests of recombinant ES cell clone DNA showing predicted wild-type and recombinant fragments as indicated by arrows.
Crossings of mice heterozygous for the A1AR mutation yielded offspring with the expected Mendelian distribution. In a group of 170 animals, the percentage of wild-type, heterozygous, and homozygous mice was 25%, 54%, and 21%. The female to male ratio (in %) was 44:56 in A1AR+/+, 48:52 in A1AR+/−, and 50:50 in A1AR−/−. Absence of A1AR mRNA in kidney tissue was confirmed by conventional RT-PCR (Fig. 2A). Furthermore, quantitative RT-PCR was performed to compare mRNA levels in wild-type and heterozygous A1AR mutant mice. A1AR mRNA abundance corrected for β-actin as determined from the threshold cycle number was approximately half in A1AR+/− compared with A1AR+/+ mice (Fig. 2B). Average threshold cycle (CT) numbers for A1AR were 23.28 ± 0.6 (+/+, n = 3), 24.36 ± 0.28 (+/−, n = 3), 40 ± 0 (−/−, n = 2; CT value is indistinguishable from water control); values for β-actin are 15.02 ± 0.3 (+/+, n = 3), 15.05 ± 0.08 (+/−, n = 3), and 15.0 ± 0.1 (−/−, n = 2). Functional evidence for the absence of A1AR was obtained by determination of the blood pressure and heart rate response to the A1AR agonist N-cyclohexyladenosine in anesthetized animals. i.v. injection of 35 and 175 μg of cyclohexyladenosine caused a dose-dependent fall in arterial blood pressure and heart rate in wild-type mice but had no effect in A1AR−/− mice (Fig. 3).
Figure 2.
(A) RT-PCR of kidney mRNA by using A1AR and β-actin-specific primers in the presence and absence of reverse transcriptase. A1AR RT-PCR products were absent in mRNA from A1AR−/− mice. (B) Abundance of A1AR mRNA as determined by quantitative RT-PCR (data are means of measurements on three individual RNA samples).
Figure 3.
Effect of the A1AR agonist cyclohexyladenosine (CHA) in two different doses (35 and 175 ng) on heart rate (Upper) and systolic blood pressure (Lower) in A1AR+/+ (n = 10) and A1AR−/− mice (n = 12).
A1AR knockout mice did not show gross morphological abnormalities and did not obviously differ from wild-type animals in appearance, fertility, behavior, and growth. The increase in body weight and kidney weight within the first 6 months of life was the same between A1AR+/+ and A1AR−/− mice. Moreover, there was no difference in the linear relationship between body weight and kidney weight in A1AR+/+ and A1AR−/− mice. Sections of perfusion-fixed kidneys from A1AR+/+ and A1AR−/− mice did not reveal differences in histological appearance at the light microscopical level.
Systolic blood pressure and heart rate measurements in conscious mice by using the tail cuff method showed an average systolic pressure of 115 ± 4 (n = 6) and 107 ± 2.8 mmHg (n = 8) in wild-type and A1AR−/− mice, respectively (P = 0.15). Heart rates averaged 623 ± 9 and 602 ± 17 in A1AR+/+ and A1AR−/− mice (P = 0.31). In anesthetized mice, systolic arterial pressure and heart rate averaged 113 ± 3.2 mmHg and 526 ± 35 (n = 10) in wild-type, and 106 ± 3.4 mmHg and 528 ± 25 (n = 12) in A1AR knockout mice (P = 0.19 and 0.96, respectively).
There were no significant differences in plasma Na, K, Cl, creatinine, urea, and glucose concentrations between A1AR+/+, A1AR+/−, and A1AR−/− mice (Table 1). Similarly, electrolyte concentrations and urinary osmotic concentration in 24-h urine collections made in metabolic cages were not different between wild-type and A1AR mutant animals (Table 2).
Table 1.
Plasma concentration of sodium, potassium, chloride, creatinine, urea, and glucose in wild-type, heterozygous, and homozygous A1AR littermates
| Plasma | Na, mEq/liter | K, mEq/liter | Cl, mEq/liter | Creatinine, mg/dl | Urea, mg/dl | Glucose, mg/dl |
|---|---|---|---|---|---|---|
| A1R+/+ | 150.3 ± 3.3 | 2.56 ± .26 | 123.6 ± 2 | 0.13 ± .1 | 25.7 ± 3.9 | 244 ± 43 |
| A1R+/− | 151.3 ± 2.7 | 2.53 ± .27 | 121.5 ± 2.9 | 0.13 ± .1 | 21.3 ± 1.6 | 199 ± 21 |
| A1R−/− | 154.2 ± 4.1 | 2.6 ± .29 | 122 ± 2.4 | 0.2 ± .05 | 26.8 ± 5.4 | 264 ± 48 |
n = 6 for all three genotypes.
Table 2.
Urine concentration of sodium and potassium, urine osmolarity, and urine flow in wild-type and A1AR−/− mice
| Urine | Na, mEq/liter | K, mEq/liter | Cl, mEq/liter | Uosm, mOsm/liter | UV, ml/24 h |
|---|---|---|---|---|---|
| A1AR+/+ | 221 ± 40 | 349 ± 42 | 242 ± 39 | 2813 ± 365 | 0.6 ± 0.2 |
| A1AR−/− | 174 ± 11 | 279 ± 17 | 189 ± 13 | 2414 ± 121 | 1.2 ± 0.3 |
Urine collected in metabolic cages; n = 4 (A1AR+/+), n = 8 (A1AR−/−). None of the differences between A1AR+/+ and A1AR−/− mice were significant (range of P values from 0.09 for K to 0.27 for UV).
Glomerular filtration rate (GFR) averaged 460 ± 69 μl/min and 463.5 ± 44 μl/min in female wild-type and A1AR−/− mice, respectively (n = 6 for +/+ and n = 9 for −/−). As shown in Fig. 4, there was no change in GFR over the 45-min observation period. Average age, kidney weight, and body weight was 3.1 ± 0.1 months, 273 ± 8 mg, and 22.3 ± 0.8 g for A1AR+/+, and 3.3 ± 0.3 months, 264 ± 12 mg, and 22.1 ± 0.9 g for A1AR−/− animals. GFR expressed per gram of kidney weight averaged 1706 ± 275 μl/min in wild-type and 1750 ± 138 μl/min in A1AR−/− mice.
Figure 4.
Average GFR in three successive 15-min clearance periods in six female A1AR+/+ and nine female A1AR−/− mice. Dotted lines indicate mean GFR for the 45-min observation period. Vertical lines show SEM.
Mean stop flow pressure (PSF) in male wild-type mice averaged 35.8 ± 0.9 mmHg falling to 28.7 ± 1 mmHg when loop flow was increased to 30 nl/min (P < 0.0001). In A1AR+/− mice, PSF averaged 36.2 ± 1.2 mmHg, and an increase in loop flow reduced PSF to 33.2 ± 1.4 mmHg (P = 0.002). In A1AR knockout mice, PSF averaged 36 ± 0.8 mmHg at zero loop flow and 35.6 ± 0.8 mmHg at a loop flow of 30 nl/min (P = 0.06). Examples of original recordings of PSF in a wild-type and A1AR knockout mouse are shown in Fig. 5. Thus, although there was no significant difference in baseline PSF between genotypes, PSF at the elevated flow rate increased with the reduction in A1AR copy number. Thus, the usually observed flow-induced decrease in glomerular capillary pressure did not occur in the A1AR-deficient mice (Fig. 6).
Figure 5.
Recording of stop flow pressure (PSF, upper traces) and arterial pressure (AP, lower traces) in an A1AR+/+ and an A1AR−/− mouse. Black boxes indicate periods of loop of Henle perfusion at 30 nl/min (the gray box represents a flow of 10 nl/min).
Figure 6.
Mean stop flow pressure at loop of Henle flow rates of 0 and 30 nl/min in A1AR+/+, A1AR+/−, and A1AR−/− mice. Vertical bars are SEM.
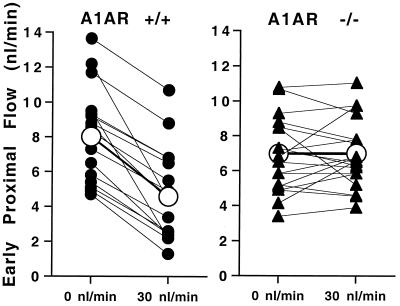
To verify that the absence of changes in glomerular capillary pressure was paralleled by absent responses of nephron filtration rate (SNGFR), we determined the response of early proximal flow rate (EPFR) to a saturating increase in loop flow rate. EPFR closely correlates with SNGFR because of the early puncture site, and it has therefore been used as an index of SNGFR in numerous rat studies (13). EPFR at loop flows of 0 and 30 nl/min averaged 8 ± 0.7 nl/min and 4.6 ± 0.6 nl/min, respectively, in A1AR+/+ mice. In A1AR knockouts, EPFR was 6.98 ± 0.5 nl/min at zero flow and 7 ± 0.42 nl/min at a flow of 30 nl/min. Data from individual nephrons are shown in Fig. 7. Flow-induced decrements of 3.4 ± 0.4 nl/min (−43%) in A1AR+/+ mice and −0.02 ± 0.3 nl/min (+1%) in A1AR−/− mice indicate that the normal NaCl-dependent reduction in SNGFR is completely lacking in A1AR-deficient mice.
Figure 7.
Early proximal flow rate during loop of Henle perfusion at 0 and 30 nl/min in A1AR+/+ and A1AR−/− mice. Lines connect measurements in the same tubule. Closed symbols are data from individual nephrons; open symbols are mean values.
Discussion
We have generated a mutant mouse strain in which the expression of the A1AR gene was disrupted by homologous recombination. The present study reports our attempt to assess the effect of A1AR deficiency on renal function. A1AR in the kidney are expressed most predominantly on renal afferent arterioles, but the receptor mRNA has also been identified at lower levels in a number of renal tubular segments, including thick ascending limbs and collecting ducts. Commensurate with the high expression of A1AR in the renal vasculature, our initial studies in A1AR knockout animals were aimed at defining the effect of A1AR deficiency on renal hemodynamics, specifically on TGF regulation of afferent arteriolar tone.
TGF is a mechanism in which an increase in NaCl concentration at the macula densa cells of the distal nephron causes vasoconstriction of the afferent arterioles (13). There is evidence to show that this response is blocked by inhibitors of NaCl transport such as loop diuretics, suggesting that an increase in NaCl transport initiates vasoconstriction (14). In view of the anatomical discontinuity between macula densa and underlying mesangial and smooth muscle cells, it has been assumed that transport-dependent generation and release of a diffusible mediator is responsible for the vascular effect. The identity of the paracrine agent produced by macula densa cells in a flow-dependent fashion has remained unclear, with possible candidate agents including thromboxane, 20-HETE, prostaglandins, nitric oxide, dopamine, and ATP (15). In addition, we noted in previous studies that the luminal administration of methylxanthines such as theophylline or 3-isobutyl-1-methylxanthine completely blocked TGF-induced vasoconstriction (16). Based on this and additional evidence, it has been suggested that methylxanthines, potent inhibitors of P1 purinoceptors, affect the TGF mechanism by preventing a paracrine action of adenosine (9). A role of adenosine in TGF response mediation is consistent with its well established effect to cause vasoconstriction. Adenosine reduces renal blood flow predominantly of the outer cortex and elicits a persistent decrease in glomerular filtration rate (17, 18). Furthermore, adenosine and A1AR agonists elicit persistent vasoconstriction of renal afferent arterioles in the hamster cheek pouch preparation, in the hydronephrotic kidney, in isolated perfused afferent arterioles, and in the juxtamedullary nephron preparation (19–22). Afferent arteriolar vasoconstriction is accompanied by a peak and sustained increase in cytosolic free calcium concentration (23). Specific A1AR blockers have been shown to inhibit TGF responses when delivered to the tubular lumen or the peritubular blood (24). However, in other studies, adenosine receptor blockers had none or only small inhibitory actions when given by the i.v. route, raising doubts about the importance of A1AR in TGF mediation (25, 26).
The current results from A1AR-deficient mice show unequivocally that TGF responses to a saturating increase in macula densa flow are completely abolished. Thus, functional A1AR are a necessary requirement for TGF responsiveness. By implication, adenosine as the natural ligand of A1AR appears to be a required constituent and likely mediator of the TGF response. Mediation of TGF responses by adenosine is also supported by a recent study showing effective attenuation of TGF responses during 5′-nucleotidase inhibition and constant administration of an A1AR agonist (27). Our results do not address the question of the origin of the adenosine involved in the TGF response. It is possible that adenosine is released by macula densa cells into the juxtaglomerular apparatus interstitium and that it reaches A1AR on smooth muscle or possibly mesangial cells by diffusion. Because of the well established relation between TGF response magnitude and NaCl transport, it is reasonable to suggest that adenosine production is determined by metabolic rate-dependent hydrolysis of ATP. The molecular identity of the cytosolic nucleotidases and of the nucleoside transporters in macula densa cells remains to be determined. Alternatively, adenosine may be formed from extracellular ATP by ecto-ATPases, ecto-ATP-diphosphohydrolases, and ecto-5′-nucleotidases. This possibility is supported by the recent studies suggesting that ATP is released across the basolateral membrane of macula densa cells and that this release is increased by increasing luminal NaCl concentration (28). ATP directly constricts afferent arterioles by activating P2 receptors presumably of the P2x subtype, but the current studies show that this pathway cannot substitute for A1AR deficiency in sustaining TGF responsiveness (29). We believe that our results provide strong evidence for the concept that adenosine via A1AR acts in a circumscribed region of the kidney, the juxtaglomerular interstitium, as a paracrine mediator of a kidney-specific and local vascular response.
It is of interest to note that chronic absence of TGF control of afferent arteriolar tone in A1AR−/− mice is not associated with drastic alterations in body salt balance or renal function under resting conditions. Specifically, glomerular filtration rate was not found to be significantly different between wild-type and A1AR knockout mice. Failure to observe an increase of GFR in animals without TGF regulation indicates that TGF does not appear to exert a major tonic GFR-suppressing effect in mice under resting conditions. Earlier observations in which SNGFR was determined by paired collections in distal and proximal segments, i.e., in the presence and acute absence of TGF control, also did not reveal the marked increase in SNGFR on removal of the macula densa signal typically seen in rats (30–32). Thus, it seems that the operating point of the TGF system in the mouse is located near the upper shoulder of the TGF relationship so that its predominant effect is vasoconstriction, not vasodilatation. Further studies are needed to verify this notion. Overall, A1AR knockout mice will be useful to determine the role of the TGF control system during physiological and pathophysiological challenges.
Previous studies from our laboratory have shown that targeted deletions of the angiotensin II type 1A receptor (AT1A) or angiotensin converting enzyme genes are also associated with inhibition of TGF responses (12, 33). Thus, the presence of both AT1A receptors and A1AR seems to be necessary for full TGF responsiveness. The nature of the interaction between angiotensin II and adenosine is still unclear, but considerable experimental evidence supports its existence. The reduction in renal blood flow caused by adenosine has been found to be more pronounced in high renin than in low renin states, and inhibition of converting enzyme attenuates adenosine-induced blood flow reductions (34). Furthermore, vasoconstriction in response to A1AR activation is reduced in AT1A knockout mice, and pharmacological blockade of either AT1A or A1AR reduced the vasoconstrictor response to the other ligand (11, 35). Conversely, fixation of angiotensin II at a higher level augments TGF responses (36, 37). Augmentation of TGF responses during a fixed increase in angiotensin II level indicates that variations in angiotensin II are not responsible for causing the variable vascular TGF reaction. Rather, the ambient level of angiotensin II appears to importantly modify the graded response to the flow-dependent TGF mediator. In light of the present data, it appears that angiotensin II modulates TGF by determining the response magnitude to adenosine. In addition, a reduced blood pressure as found in AT1A receptor or angiotensin converting enzyme knockout mice in itself reduces TGF responses (38). Finally, it is possible that blunting of TGF responses in AT1A knockout mice results from a reduction in angiotensin II-dependent production of superoxide (39). This may be associated with higher levels of nitric oxide, a known inhibitory modulator of TGF responses (40).
In conclusion, targeted deletion of A1AR in mice is not associated with gross developmental or morphological abnormalities. Thus, A1AR knockout mice promise to be a useful model to study the role of A1AR in behavioral, neurological, cardiovascular, and renal phenotypes. Absence of TGF responses in the present study strongly indicates that expression of functional A1AR is necessary for macula densa control of renal vascular tone. Data are consistent with the notion that adenosine is a critical and possibly the sole direct mediator of the TGF response.
Acknowledgments
We gratefully acknowledge the assistance of Jean Lay with Southern analyses and of Elizabeth Hughes and the University of Michigan Transgenic Animal Model Core with ES cell culture and blastocyst microinjections. We thank Jolene Gouin and Gina Bane for mouse genotyping. This work was supported by National Institutes of Health Grant DK 37448 and by intramural funds from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Abbreviations
- GFR
glomerular filtration rate
- SNGFR
single nephron filtration rate
- A1AR
A1 adenosine receptors
- TGF
tubuloglomerular feedback
- PSF
stop flow pressure
- RT
reverse transcription
- ES
embryonic stem
- EPFR
early proximal flow rate
- AT1A
angiotensin II type 1A receptor
- RT-PCR
reverse transcription–PCR
References
- 1.Conant A R, Jarvis S M. Biochem Pharmacol. 1994;48:873–880. doi: 10.1016/0006-2952(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 2.Londos C, Cooper D M, Wolff J. Proc Natl Acad Sci USA. 1980;77:2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Calker D, Muller M, Hamprecht B. J Neurochem. 1979;33:999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 4.Gerwins P, Fredholm B B. Naunyn-Schmiedeberg's Arch Pharmacol. 1995;351:186–193. doi: 10.1007/BF00169332. [DOI] [PubMed] [Google Scholar]
- 5.Olah M E, Stiles G L. Annu Rev Pharmacol Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- 6.Weaver D R, Reppert S M. Am J Physiol. 1992;263:F991–F995. doi: 10.1152/ajprenal.1992.263.6.F991. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi S, Umemura S, Tamura K, Iwamoto T, Nyui N, Ishigami T, Ishii M. Hypertension. 1995;26:1181–1185. doi: 10.1161/01.hyp.26.6.1181. [DOI] [PubMed] [Google Scholar]
- 8.Spielman W S, Thompson C I. Am J Physiol. 1982;242:F423–F435. doi: 10.1152/ajprenal.1982.242.5.F423. [DOI] [PubMed] [Google Scholar]
- 9.Osswald H, Hermes H H, Nabakowski G. Kidney Int Suppl. 1982;12:S136–S142. [PubMed] [Google Scholar]
- 10.Beck E, Ludwig G, Auerswald E A, Reiss B, Schaller H. Gene. 1982;19:327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- 11.Traynor T, Yang T, Huang Y G, Arend L, Oliverio M I, Coffman T, Briggs J P, Schnermann J. Am J Physiol. 1998;275:F922–F927. doi: 10.1152/ajprenal.1998.275.6.F922. [DOI] [PubMed] [Google Scholar]
- 12.Schnermann J B, Traynor T, Yang T, Huang Y G, Oliverio M I, Coffman T, Briggs J P. Am J Physiol. 1997;273:F315–F320. doi: 10.1152/ajprenal.1997.273.2.F315. [DOI] [PubMed] [Google Scholar]
- 13.Schnermann J, Briggs J P. In: The Kidney Physiology and Pathophysiology. Seldin D W, Giebisch G, editors. Vol. 1. Philadelphia: Lippincott; 2000. pp. 945–980. [Google Scholar]
- 14.Wright F S, Schnermann J. J Clin Invest. 1974;53:1695–1708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navar L G, Inscho E W, Majid D S A, Imig J D, Hariison-Bernard L M, Mitchell K D. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 16.Schnermann J, Osswald H, Hermle M. Pflügers Arch. 1977;369:39–48. doi: 10.1007/BF00580808. [DOI] [PubMed] [Google Scholar]
- 17.Tagawa H, Vander A J. Circ Res. 1970;26:327–338. doi: 10.1161/01.res.26.3.327. [DOI] [PubMed] [Google Scholar]
- 18.Osswald H, Spielman W S, Knox F G. Circ Res. 1978;43:465–469. doi: 10.1161/01.res.43.3.465. [DOI] [PubMed] [Google Scholar]
- 19.Joyner W L, Mohama R E, Myers T O, Gilmore J P. Microvasc Res. 1988;35:122–131. doi: 10.1016/0026-2862(88)90055-6. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich M S, Endlich K, Parekh N, Steinhausen M. Microvasc Res. 1991;41:275–288. doi: 10.1016/0026-2862(91)90028-a. [DOI] [PubMed] [Google Scholar]
- 21.Weihprecht H, Lorenz J N, Briggs J P, Schnermann J. Am J Physiol. 1992;263:F1026–F1033. doi: 10.1152/ajprenal.1992.263.6.F1026. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama A, Inscho E W, Navar L G. Am J Physiol. 2001;280:F406–F414. doi: 10.1152/ajprenal.2001.280.3.F406. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez A M, Kornfeld M, Persson A E G. Acta Physiol Scand. 1999;166:175–181. doi: 10.1046/j.1365-201x.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- 24.Schnermann J, Weihprecht H, Briggs J P. Am J Physiol. 1990;258:F553–F561. doi: 10.1152/ajprenal.1990.258.3.F553. [DOI] [PubMed] [Google Scholar]
- 25.Franco M, Bell P D, Navar L G. Am J Physiol. 1989;257:F231–F236. doi: 10.1152/ajprenal.1989.257.2.F231. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox C S, Welch W J, Schreiner G F, Belardinelli L. J Am Soc Nephrol. 1999;10:714–720. doi: 10.1681/ASN.V104714. [DOI] [PubMed] [Google Scholar]
- 27.Thomson S, Bao D, Deng A, Vallon V. J Clin Invest. 2000;106:289–298. doi: 10.1172/JCI8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell P, Lapointe J Y, Sabirov R, Hayashi S, Okada Y. FASEB J. 2000;14:A314. [Google Scholar]
- 29.Inscho E W, Cook A K, Mui V, Miller J. Am J Physiol. 1998;274:F718–F727. doi: 10.1152/ajprenal.1998.274.4.F718. [DOI] [PubMed] [Google Scholar]
- 30.Lorenz J N, Schultheis P J, Traynor T, Shull G E, Schnermann J. Am J Physiol. 1999;277:F447–F453. doi: 10.1152/ajprenal.1999.277.3.F447. [DOI] [PubMed] [Google Scholar]
- 31.Schnermann J, Chou C-L, Ma T, Traynor T, Knepper M A, Verkman A S. Proc Natl Acad Sci USA. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallon V, Traynor T, Barajas L, Huang Y G, Briggs J P, Schnermann J. J Am Soc Nephrol. 2001;12:1599–1606. doi: 10.1681/ASN.V1281599. [DOI] [PubMed] [Google Scholar]
- 33.Traynor T, Yang T, Huang Y G, Krege J H, Briggs J P, Smithies O, Schnermann J. Am J Physiol. 1999;276:F751–F757. doi: 10.1152/ajprenal.1999.276.5.F751. [DOI] [PubMed] [Google Scholar]
- 34.Osswald H, Schmitz H J, Heidenreich O. Pflügers Arch. 1975;357:323–333. doi: 10.1007/BF00585986. [DOI] [PubMed] [Google Scholar]
- 35.Weihprecht H, Lorenz J N, Briggs J P, Schnermann J. Am J Physiol. 1994;266:F227–F239. doi: 10.1152/ajprenal.1994.266.2.F227. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell K D, Navar L G. Am J Physiol. 1988;255:F383–F390. doi: 10.1152/ajprenal.1988.255.3.F383. [DOI] [PubMed] [Google Scholar]
- 37.Schnermann J, Briggs J P. Miner Electrolyte Metab. 1989;15:103–107. [PubMed] [Google Scholar]
- 38.Schnermann J, Briggs J P. Am J Physiol. 1989;256:F421–F429. doi: 10.1152/ajprenal.1989.256.3.F421. [DOI] [PubMed] [Google Scholar]
- 39.Griendling K K, Minieri C A, Ollerenshaw J D, Alexander R W. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox C S, Welch W J, Murad F, Gross S S, Taylor G, Levi R, Schmidt H H. Proc Natl Acad Sci USA. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]